Abstract
Aims
To implement and monitor the effectiveness of a strategy to curb unnecessary use of vancomycin and teicoplanin for inpatients in a teaching hospital/tertiary referral centre where 33% of S. aureus isolates (72% from ICU patients) were methicillin resistant.
Methods
A sample of 182 vancomycin/teicoplanin inpatient prescriptions surveyed, revealed that only 31 (17%) conformed with Centre for Disease Control (CDC) guidelines. Following education (ward-rounds, bulletins) on appropriate CDC based guidelines for prescribing glycopeptides directed at relevant clinicians, ‘Immediate Concurrent Feedback’ (ICF) was gradually deployed throughout the hospital. This entailed review of respective inpatient records on the next working day. If the indication was deemed not to conform with our guidelines, the prescriber was issued a memo (copied to the supervising doctor). Each memo detailed the ‘errant’ incident, listed appropriate indications and explicitly advised desisting from such prescribing and suggested alternative therapy if necessary. Corresponding glycopeptide usage data for our hospital and others in Hong Kong were retrieved and analysed as were samples of records of our inpatients with staphylococcal septicaemia (pre and during ICF).
Results
Compared with baseline values, during 2 years of ICF, inpatient prescribing of vancomycin and teicoplanin deemed to conform increased to 71% (773/1086); difference 54% (P < 0.0001, 95% CIs 47–62%). Corresponding average monthly usage (DDDs/1000 admissions) decreased from 76 (pre-ICF) to 45; mean difference 31 (P < 0.0001, 95% CIs 24, 38). Mortality from staphylococcal bacteraemia remained unchanged. No comparable changes in glycopeptide usage ensued in comparator hospitals.
Conclusions
ICF can be used safely to curb irrational overuse of vancomycin and teicoplanin in a hospital with high methicillin resistant S. aureus infection rates.
Keywords: immediate concurrent feedback, implementing guidelines, methicillin resistant Staphylococcus aureus (MRSA), vancomycin
Introduction
Recognition that the widespread prescribing of glycopeptides in hospitals is related to a corresponding increase in the incidence of vancomycin resistant enterococcus (VRE) infections [1] has prompted the CDC and other authorities to issue explicit guidelines to control the use of these drugs [2, 3]. Variably effective strategies to curtail their overuse have been published [4–7], but do not describe the impact of such policies on the outcome of methicillin resistant S. aureus (MRSA) infections. Understandably, experts in the field remain cautious about introducing such controls, lest over restriction (particularly of empirical therapy) could result in inadvertent mortality or morbidity, especially where MRSA infection rates are high. Whereas such caution is not disputed, even in institutions with high MRSA infection rates, it is critical to limit the unnecessary overuse of glycopeptides. Ironically, though vancomycin continues to be the final bastion of sepsis treatment under such circumstances, conserving its efficacy depends on reducing usage.
In anticipation of such problems in our teaching hospital, a tertiary referral centre where 33% of S. aureus isolates (72% from ICU patients) were already methicillin resistant, we set out to study the possible indiscriminate use of vancomycin and teicoplanin. Over a 9 week period from July to September 1996, vancomycin prescribing for hospital inpatients was audited by our Infection Control Team (microbiologist and specially trained research nurses and clinical pharmacists). The audit's primary purpose was to determine how much corresponding prescribing in different clinical specialties conformed to the above mentioned guidelines [2, 3]. The extent to which such prescriptions did not conform (151/182 or 83%) was extremely alarming; the Department of Medicine being the main offender. With the support and endorsement of our hospital's Quality Improvement and Management Committees a strategy to curb unwarranted inpatient prescribing of vancomycin and teicoplanin was therefore developed. Our strategy was based on immediate concurrent feedback (ICF) and after implementation, we set out to determine whether it could achieve a clinically and statistically significant reduction in corresponding inappropriate prescribing and overall usage, without jeopardy to patients.
Methods
Based on CDC guidelines for the rational use of vancomycin and teicoplanin, a list of seven indications and seven situations for withholding such therapy were drawn up (Table 1) in consultation with relevant clinical departments. After publicising these recommendations at departmental rounds/meetings, a strategy based on ICF was gradually introduced throughout the hospital, starting in August 1997. The programme was first introduced in the wards of the Departments of Medicine (including Medical ICU), Orthopaedics & Trauma, Obstetrics and Gynaecology and other smaller specialties. In January 1998 it was extended to inpatients of the Departments of Surgery (including ICU) and to Paediatric ward patients in June 1998. Thus, only patients in the Neonatal and Paediatric ICUs, and Bone Marrow Transplant centre (BMTC) remained exempted. The entire strategy was endorsed by the Hospital Management Committee and the Infection Control Team.
Table 1.
CDC based agreed guidelines for prescribing or withholding.
| Intervention with vancomycin (or teicoplanin) considered appropriate |
|---|
| 1 Treatment of infections (not colonization) attributed to β-lactam resistant Gram +ve organisms (mainly MRSA & MRSE, viz: (a) confirmed by culture (b) suspected prosthetic valve IE (awaiting microbiology) |
| 2 Empiric treatment of fever in (a) neutropenic patients and (b) ICU patients: whenever they have (i) central line inflammation, or (ii) gram +ve organism revealed by blood culture or appropriate smear* (until confirmed to be b-lactam sensitive); otherwise consider appropriate alternative therapy such as cloxacillin** |
| 3 Treatment of ‘Antibiotic Colitis’ not responding to metronidazole or if it is life-threatening |
| 4 Treatment of serious gram +ve infections if there is β-lactam ‘hypersensitivity’ |
| 5 As an additional antibiotic in the empirical treatment of presumed pneumococcal meningitis |
| 6 Prophylaxis against IE during potentially bacteraemic procedures/episodes in certain high risk patients (with prosthetic heart valves/devices, vascular shunts or prior IE) and hypersensitivity or recent exposure to penicillin |
| 7 Prophylaxis against wound infection during major surgery for insertion of foreign body (prosthetic valve, shunt, joint) |
| Intervention with vancomycin (or teicoplanin) considered inappropriate |
| 1 If only one blood culture is +ve for S. epidermidis; two cultures should normally be taken from different sites at around the same time |
| 2 Empiric intervention in febrile neutropenic patient, unless there is ‘central’ i.v line inflammation |
| 3 Continued empirical treatment of ‘sepsis’/fever (> 48 h), if cultures yield no β-lactam resistant gram +ve microbe |
| 4 Against β-lactam-sensitive microbial infections in renal failure patients |
| 5 Primary treatment (orally) of ‘antibiotic’ colitis; metronidazole preferred, unless life-threatening |
| 6 Eradication of MRSA from colonized surfaces and/or any other form of topical application/irrigation |
| 7 Routine prophylaxis against infection/colonization: (i) systemically with central or peripheral lines (ii) locally (antibiotic lock) (iii) during surgery (iv) for gut decontamination (v) for CAPD/–haemodialysis, or Tenckhoff insertion (vi) for low birth weight infant |
If from pus, deep wound swab or tissues
Vancomycin may be considered for patient who is seriously ill or deteriorating. (Adapted from CDC guidelines (2), which did not refer to telcoplanin).
On every working day (week-days other than bank-holidays) the pharmacy computer generated a list of all inpatients newly prescribed vancomycin or teicoplanin (i.e since the last list was generated, usually within the previous 24 h). Every name appeared with the patient's ward and bed number. That morning each patient was seen by a specially trained research nurse or clinical pharmacist, who examined the respective medical record to ascertain the possible reason for prescribing either drug. Later that day, findings pertinent to each patient were reviewed by a panel consisting of clinicians with a special interest, microbiologists, infection control nurses and clinical pharmacists. For patients whose prescribing was deemed not to conform with the agreed guidelines, the prescribing doctor and supervising medical officer were given ICF on the very same day. This entailed issue of a memo signed by a consultant physician or microbiologist, detailing the suspected ‘errant’ prescribing for the specific patient, together with an explanation. Depending on what was appropriate, the memo also included explicit advice to (i) discontinue such treatment entirely, (ii) prescribe an alternative medication/intervention (pending further information if necessary), or (iii) avoid such prescribing in the future under similar circumstances. The memo also provided contact telephone/fax numbers to enable the relevant doctors to seek clarification, justify or challenge the advice given. Patients for whom prescribing prompted memos with a suggestion to discontinue treatment with either drug were also followed up (repeatedly if necessary) to document whether the advice had been complied with. The ICF program was introduced gradually throughout the hospital. Monthly statistics for the usage of vancomycin and teicoplanin were collected from the Hospital's Pharmacy. Corresponding usage figures in all major public hospitals in Hong Kong were also retrieved, courtesy of the Hong Kong Hospital Authority (HKHA) Computerized Pharmaceutical Supplies System for financial years ending in 1997, 1998 and 1999. All usage was expressed as defined daily doses (DDDs)/month, DDDs/1000 admissions or DDDs/bed/year. Findings pertaining to glycopeptide prescribing and usage pre vs during ICF were subjected to appropriate statistical analysis.
We were intent on ensuring that our restrictive policy on the use of glycopeptides should not have a detrimental effect on patient outcome. We therefore remained open to feedback from front line doctors and whenever necessary organized meetings with relevant medical specialists to allow contentious issues and available evidence to be discussed. We also showed willingness to heed possible complaints and fine tune or modify our agreed criteria for prescribing vancomycin under special circumstances. Furthermore, to allay concerns that our programme could be having an adverse impact in patients with MRSA infections, a number of retrospective audits were carried out. These entailed: (i) retrieval of the names of all inpatients with confirmed S. aureus bacteremia (MRSA or MSSA positive blood cultures) in 1996 (pre-ICF) and in 1998 (during ICF) from the Microbiology Laboratory computer, (ii) using the centralized Hospital Admissions computer determining which of the corresponding patients died, and (iii) manually checking individual medical records of patients who had died, to log their demographic data, underlying disease(s), and time elapsing from documentation of bacteremia till death.
Results
Pre-ICF, 31/182 (17%) of prescriptions conformed to our guidelines compared with 773/1086 (71%) during the first 24 months of our ICF programme (Table 2). This 54% difference was both clinically and statistically significant (P < 0.0001, 95% CIs 47, 62). Among ICF patients audited, 57 instances of empirical therapy were sanctioned as conforming because patients were seriously ill or deteriorating. Both before and in the course of ICF, prescribing that did not conform was mainly due to inappropriate empirical treatment. ICF to withhold vancomycin or teicoplanin was complied with in 83% (259/313) of instances and in 7%, the response was ‘unevaluable’ (once only prescribing had ensued, patients had died or records were unavailable).
Table 2.
Vancomycin/teicoplanin prescribing for inpatients not conforming to agreed guidelines.
| Number of prescriptions not conforming (Number of prescriptions not conforming/1000 prescriptions audited) | |||
|---|---|---|---|
| Category of prescribing confirmed as not conforming | Pre-ICF Audit-9 weeks | During ICF-104 weeks | P value |
| Empirical treatment | 94 (516) | 157 (145) | < 0.0001 |
| Prophylaxis | 25 (137) | 26 (24) | < 0.0001 |
| β-lactam sensitive isolate* | 8 (44) | 8 (7) | < 0.0005 |
| Coagulase-negative staphylococcus in only one blood culture | 10 (55) | 40 (37) | NS |
| Primary treatment of antibiotic colitis | 11 (60) | 17 (16) | < 0.0005 |
| Treatment of MRSA colonization | 3 (16) | 65 (60) | < 0.05 |
| All the above† | 151 (830) | 313 (288) | < 0.0001 |
N.B. Patients in the Bone Marrow Transplant Centre and Paediatric and Neonatal ICU were not audited. During ICF intervention for MRSA, colonization became most common presumably due to indiscriminate dependence on laboratory reports by clinicians.
From patients without ‘penicillin allergy’.
Among the 182 patients in the pre ICF audit and the 1086 audited during the conduct ofICF, the remainder were deemed to have conformed; 31 and 773, respectively. However, 3and 51, respectively, of the latter were actually ‘unevaluable’.
P values refer to two tailed χ sqauare tests; NS = not significant.
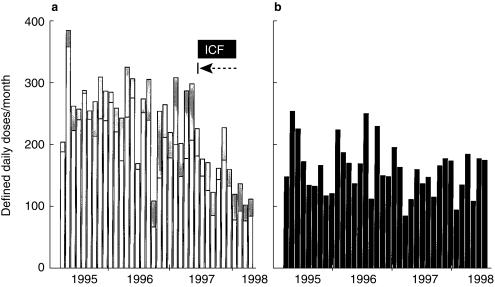
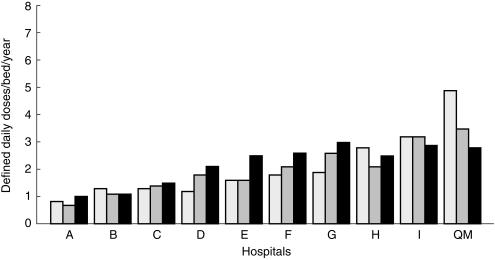
Figure 1 shows that coinciding with the institution of education followed by ongoing ICF, monthly usage of the two glycopeptides declined dramatically and consistently in the corresponding wards but not elsewhere in the hospital. Regarding total hospital usage of vancomycin and teicoplanin, average monthly DDDs/1000 admissions decreased from 76 (in 1996/97, pre-ICF) to 45 during 2 years of ICF (53 and 41 in successive years); mean difference 31 (P < 0.0001; 95% CI 24, 38). Figure 2 shows that over 1997–99, usage decreased consistently only in Queen Mary Hospital.
Figure 1.
Vancomycin and teicoplanin usage per month. (a) Department of Medicine (excepting ICU and BMTC) (□) and Orthopedics and Trauma ( ), where the ICF programme was first implemented from August 1997. (b) Other departments in the hospital (▪), where the ICF programme was only introduced gradually over several months starting in January 1998. |→–– represents initiation of immediate concurrent feedback (ICF).
), where the ICF programme was first implemented from August 1997. (b) Other departments in the hospital (▪), where the ICF programme was only introduced gradually over several months starting in January 1998. |→–– represents initiation of immediate concurrent feedback (ICF).
Figure 2.
Vancomycin and teicoplanin usage in major Hong Kong hospitals, 1997–1999. Consistent with the highest level of tertiary activity in HK (management of the vast majority of patients with complex haematological disorders and renal, bone marrow and liver transplants), in 1997, Queen Mary (QM) hospital exhibited the highest usage of glycopeptides; about 30% more than Hospital I which ranked second highest. Despite continuing the same order of tertiary activity, by 1999 corresponding QM usage had decreased to the same level as in Hospital I, but usage elsewhere increased or remained unchanged.
Audit of inpatient bacteraemias logged by the Microbiology laboratory revealed that there were 106 and 43 due to methicillin sensitive S.aureus (MSSA) and MRSA, respectively, in 1996 and the corresponding numbers for 1998 were 91 and 60. Among these, corresponding numbers of patients who subsequently died were 25 (24%) and 21 (49%) in 1996, and 16 (19%) and 30 (50%) in 1998. Regarding the patients who died with MRSA bacteremia (21 pre-ICF in 1996) and (30 during ICF in 1998), their demographic features were very similar and all had serious underlying diseases. Thus, in the former group the mean age was 56 years and the male/female ratio was 15/6, 7 had terminal malignancies, 7 died within 3 days and 2 more than 100 days after their bacteraemia. For those whose prescribing was subjected to ICF, corresponding values were 60, 23/7, 15, 7 and 10, respectively. Moreover, since the institution of our programme, not a single death was attributed to ICF, nor was there any complaint that action taken in response to an ICF memo had had a detrimental influence on the course of a patient's illness.
Discussion
Administrative restrictions achieving a 50% reduction in vancomycin use in one US hospital were associated with a corresponding reduction in VRE isolation rates from the stools of high risk patients [4]. Elsewhere, minimal reductions in vancomycin usage had no such effect [5]. Thus, there is a general consensus that only substantial reductions in the indiscriminate overuse of antibiotics can curb the selective pressure favouring the development of bacterial resistance. Based on this premise, we set out to achieve a corresponding reduction in unnecessary glycopeptide use in our hospital, with the ultimate aim of curtailing the emergence of vancomycin resistant infections but without jeopardizing patients. That this objective was achieved in successive years is evident from Figure 2, which also shows that, if anything, there was a converse trend in other major HA hospitals. The consistent decrease in glycopeptide usage which coincided with implementation of ICF in our institution is all the more remarkable, as it ensued against a background of increasing tertiary activity as well as increasing usage in most of the other hospitals. These observations are all consistent with our ICF program being effective.
Most variably successful programmes to influence the prescribing of vancomycin reported by others, almost always entailed education coupled with administrative restrictions applied to all prescribers [4–9]. These included: automatic stop orders, revising routine perioperative orders, alerts attached to patient medical records and computer alerts to prescribers, computerized prescribing according to limited options. However, success has not been demonstrated in the longer term (> 6 months) [9].
By contrast, our long-term strategy to curb inappropriate glycopeptide utilization (based on education followed by ICF) was unique in several respects. First, prescribers were not subjected to any form of direct or indirect administrative restrictions or sanctions. Second, the ICF programme was introduced into our hospital gradually in order to (i) test its acceptability (ii) cause minimal acute disruption throughout the hospital whilst giving our research nurses/clinical pharmacists sufficient time to familiarize themselves with their tasks. Third, we remained open to communication and special requests from our front-line doctors and showed willingness to fine tune or modify our agreed criteria for prescribing these drugs, if that became necessary. This also entailed informal meetings with various specialists to discuss available evidence pertinent to contentious issues. Fourth, a multidisciplinary team (Clinical Pharmacologist, Microbiologists, Paediatrician, Infection Control Nurses, Pharmacists) met almost daily to review every instance of vancomycin or teicoplanin prescribing and decide whether a memo should be issued. Whilst this strategy might be perceived as labour intensive and expensive, most of the time expended was by appropriately trained, junior team members (Infection Control Nurse, Clinical Pharmacist) collecting relevant data at the bedside. No extra staff were deployed by the hospital and the full team needed to meet only briefly (30 min day−1 on average).
The special challenge we faced was to curb glycopeptide usage safely in a hospital with high MRSA infection rates. For the management of suspected or confirmed nosocomial infections with staphylococci, it is generally recommended that the prescribing of vancomycin in the first instance should only be restricted if the risk of methicillin resistance is low [2, 10–13]. If not (as in our institution), such restrictions are generally not applied. For several reasons, we nevertheless opted for a policy of vancomycin (and teicoplanin) restraint. Firstly, unlike sepsis due to gram-negative bacilli which is regarded as liable to rapidly progressive endotoxic shock, multiorgan failure and death [14, 15], the progression of staphylococcal infections is usually more gradual even in neutropaenic patients [10]. Secondly, some benefit may accrue even when cloxacillin is used to treat infections which eventually turn out to be associated with MRSA, as some of the relevant bacterial population could be moderately susceptible [16, 17]. We therefore inferred that in patients who were otherwise stable, delaying intervention with vancomycin would not jeopardise lives. Accordingly, vancomycin (or teicoplanin) was only advocated in line with the criteria listed in Table 1. We were also able to reinforce some of this advice, as it turned out to be consistent with various new sets of guidelines published during the implementation of our ICF program [13, 18, 19]. Moreover, in anticipation of special situations such as infections due to rare strains of MRSA causing toxic shock syndrome [20], there was a proviso for sanctioning vancomycin for any seriously ill or deteriorating patient. That there was no change in the case fatality rate associated with staphylococcal bacteremias in 1998 compared with 1996, seems to have vindicated our confidence in the safety of this approach. Thus, the latter observation taken together with our MRSA mortality data, appears to confirm that even among corresponding patients with MRSA bacteremia, outcomes were no less favourable since institution of ICF.
Whether this strategy actually prevented VRE infections in our hospital remains a matter of speculation. However, substantial reduction in inpatient vancomycin usage by others has been associated with declining VRE isolation rates [9]. During the first 2 years of ICF, VRE infections were detected in two of our inpatients and a third had VRE isolated from rectal swabs; two of these patients had acquired the organism elsewhere. Spread to other patients in our hospital was not encountered. Be that as it may, despite a high MRSA infection rate, implementation of our ICF based strategy achieved a substantial reduction in glycopeptide usage in our hospital (whilst usage in comparitor hospitals increased) and there were no apparent adverse sequelae.
Acknowledgments
We wish to thank Dr Vivian Wong, Hospital Chief Executive, Mr W.M. Chui, Mr R. Mak and Mr W. Chan of the Department of Pharmacy, Queen Mary Hospital for facilitating our ICF program and retrieving relevant computerized data. We also thank Mr P.W. Lee, the HKHA Chief Pharmacist and staff, Ms Anna Lee and Ms Eileen Ma for retrieving relevant information for all major HA hospitals. We are indebted to Dr W.K. Luk (now microbiologist at the Alice Ho Miu Ling Nethersole Hospital, Tai Po) for helping to develop and initiate our ICF program and Ms A. Chow of the Department of Microbiology for customizing our software.
There was no specific sponsorship earmarked to support this project.
References
- 1.Murray EB. The life and times of the Enterococcus. Clinical Microbiol Rev. :46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recommendations for preventing the spread of vancomycin resistance. Suppl to MMWR. 1995. pp. 1–13. [PubMed]
- 3.Keane WF, Alexander SR, Bailie GR, et al. Peritonial dialysis – related peritonitis treatment recommendations: 1996 Update. Perit Dial Int. 1996;16:557–573. [PubMed] [Google Scholar]
- 4.Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med. 1997;157:1132–1136. [PubMed] [Google Scholar]
- 5.Lipsky BA, Baker CA, McDonald LL, et al. Improving the appropriateness of vancomycin use by sequential intervention. Am J Infect Control. 1999;27:84–90. doi: 10.1016/s0196-6553(99)70086-6. [DOI] [PubMed] [Google Scholar]
- 6.Singer MV, Haft R, Barlam T, et al. Vancomycin control measures at a tertiary care hospital: impact of interventions on Volume and patterns of use. Infect Control Hosp Epidemiol. 1998;19:248–253. doi: 10.1086/647803. [DOI] [PubMed] [Google Scholar]
- 7.Shojania KG, Yokoe D, Platt R, et al. Reducing vancomycin use utilizing a computer guideline. J Am Med Informatics Assoc. 1998;5:554–562. doi: 10.1136/jamia.1998.0050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belongia EA, Schwartz B. Strategies for promoting judicious use of antibiotics by doctors and patients. Br Med J. 1998;317:668–671. doi: 10.1136/bmj.317.7159.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton CD, Drew R, Janning SW, et al. Excessive use of vancomycin: a successful intervention strategy at an academic medical center. Infect Control Hosp Epidemiol. 2000;21:42–45. doi: 10.1086/501703. [DOI] [PubMed] [Google Scholar]
- 10.Rubin M, Hathorn JW, Marshall D, et al. Gram-positive infections and the use of vancomycin in 550 episodes of fever and neutropenia. Ann Intern Med. 1988;108:30–35. doi: 10.7326/0003-4819-108-1-30. [DOI] [PubMed] [Google Scholar]
- 11.Hughes WT, Armstrong D, Bodey GP, et al. Guidelines for the use of anti-microbial agents in neutropenic patients with unexplained fever. JID. 1990;161:381–396. doi: 10.1093/infdis/161.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Maki DG, Bohn MJ, Stolz SM, et al. Comparative study of cefazolin, cefamandol, and vancomycin for surgical prophylaxis in cardiac and vascular operations; J Thorac Cardiovasc Surg. 1992;104:1423–1434. [PubMed] [Google Scholar]
- 13.Hughes WT, Armstrong D, Bodey GP, et al. 1997 Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. CID. 1997;25:551–573. doi: 10.1086/513764. [DOI] [PubMed] [Google Scholar]
- 14.Mason AD, McManus AT, Pruitt BA. Association of burn mortality and bacteremia. A 25-Year Review. Arch Surg. 1986;121:1027–1031. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 15.Hunt JL, Purdue GF, Tuggle DW. Morbidity and mortality of an endemic pathogen: methicillin-resistant Staphylococcus aureus. Am J Surg. 1988;157:524–527. doi: 10.1016/s0002-9610(88)80545-2. [DOI] [PubMed] [Google Scholar]
- 16.De-Gorgolas M, Aviles P, Verdego C, et al. Treatment of experimental endocarditis due to methicillin-suscetible and methicillin-resistant S.aureus with trimethoprim-sulfamethoxazole and antibiotics that inhibit cell wall synthisis. Antimicrobial Agents Chemother. 1995;39:953–957. doi: 10.1128/aac.39.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Silva N, Mendis LN. Comparison of minnimum inhibitory concentrations to methicillin in heterogenous and homogenous methicillin resistant S. Aureus. Ceylon Med J. 1996;41:144–147. [PubMed] [Google Scholar]
- 18.Alef K. Clostridium difficile-associated disease; implications for midwifery practice. J Nurse-Midwifery. 1999;44:19–29. doi: 10.1016/s0091-2182(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 19.Elting LS, Rubenstein EB, Rolston KVI, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: Observations from two decades of epidemiological and clinical trials. CID. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 20.Cui L, Kasegawa H, Murakami Y, et al. Postoperative toxic shock syndrome caused by a highly virulent methicillin-resistant Staphylococcus aureus strain. Scand J Infect Dis. 1999;31:208–209. doi: 10.1080/003655499750006326. [DOI] [PubMed] [Google Scholar]