Abstract
The human CYP2Cs are an important subfamily of P450 enzymes that metabolize approximately 20% of clinically used drugs. There are four members of the subfamily, CYP2C8, CYP2C9, CYP2C19, and CYP2C18. Of these CYP2C8, CYP2C9, and CYP2C19 are of clinical importance. The CYP2Cs also metabolize some endogenous compounds such as arachidonic acid. Each member of this subfamily has been found to be genetically polymorphic. The most well-known of these polymorphisms is in CYP2C19. Poor metabolizers (PMs) of CYP2C19 represent approximately 3–5% of Caucasians, a similar percentage of African-Americans and 12–100% of Asian groups. The polymorphism affects metabolism of the anticonvulsant agent mephenytoin, proton pump inhibitors such as omeprazole, the anxiolytic agent diazepam, certain antidepressants, and the antimalarial drug proguanil. Toxic effects can occur in PMs exposed to diazepam, and the efficacy of some proton pump inhibitors may be greater in PMs than EMs at low doses of these drugs. A number of mutant alleles exist that can be detected by genetic testing. CYP2C9 metabolizes a wide variety of drugs including the anticoagulant warfarin, antidiabetic agents such as tolbutamide, anticonvulsants such as phenytoin, and nonsteroidal anti-inflammatory drugs. The incidence of functional polymorphisms is much lower, estimated to be 1/250 in Caucasians and lower in Asians. However, the clinical consequences of these rarer polymorphisms can be severe. Severe and life-threatening bleeding episodes have been reported in CYP2C9 PMs exposed to warfarin. Phenytoin has been reported to cause severe toxicity in PMs. New polymorphisms have been discovered in CYP2C8, which metabolizes taxol (paclitaxel). Genetic testing is available for all of the known CYP2C variant alleles.
Keywords: CYP2C, CYP2C9, CYP2C19, mephenytoin, omeprazole, phenytoin, polymorphisms, tolbutamide, warfarin
Introduction
The purpose of this report is to review the clinical impact of polymorphisms of the CYP2C subfamily. The CYP2C subfamily consists of four members in humans (CYP2C8, CYP2C9, CYP2C18, CYP2C19). All members of this subfamily exhibit genetic polymorphisms. Genetic polymorphisms of CYP2C9 and CYP2C19 have been shown to have clinical consequences resulting in toxicity of some drugs in the affected individual, and may alter efficacy of other drugs. Historically, the first polymorphism discovered in the CYP2Cs subfamily, was the well-known polymorphism in the metabolism of the anticonvulsant drug mephenytoin which was discovered in the 1980s [1]. Mephenytoin exists as two optically active enantiomers (the R- and S-enantiomers). The S-enantiomer is much more rapidly 4′-hydroxylated than the R-enantiomer and it is this pathway which is polymorphic. Population studies have shown that individuals can be categorized as extensive (EMs) or poor metabolizers (PMs) of this drug [2]. The PM trait is inherited as an autosomal recessive trait. PMs represent only 3–5% of Caucasians, but from 12–23% of most Asian populations. The PM phenotype is even more common throughout Polynesia and Micronesia [3] with a very high incidence (38–79%) in certain Vanuatu islands in eastern Melanesia. This polymorphism has been shown to be due to genetic polymorphisms in CYP2C19. The most predominant polymorphisms are two null alleles, one exhibiting a splice mutation in exon 5 and the second containing a premature stop codon in exon 4 [4, 5]. At least seven different inactivating mutations in CYP2C19 have been described (Figure 1). These alleles include null mutations which prevent expression of the protein, as well as amino acid changes that affect catalytic activity of the protein [6–9]. Rare defective alleles have also been described for CYP2C9 [10, 11]. Despite their low incidence, these polymorphic alleles are important because CYP2C9 metabolizes many clinically important drugs. Polymorphisms in CYP2C9 have the potential to affect the toxicity of CYP2C9 drugs with somewhat lower therapeutic indices such as warfarin, phenytoin, and certain antidiabetic drugs. Genetic tests have been described for all known CYP2C9 and CYP2C19 alleles. Table 1 shows a list of drugs for which significant interactions have been demonstrated in individuals who have polymorphisms in CYP2C enzymes. Recently two new polymorphisms have been described in CYP2C8 [12] which metabolizes taxol and other drugs [13]. The consequences of these polymorphisms have not been examined clinically. Polymorphisms of CYP2C18 have also been described [14, 15], but CYP2C18 protein has not yet been found in detectable amounts in any tissues. Until this protein is shown to have some function in vivo, the polymorphisms in this enzyme are primarily of intellectual interest.
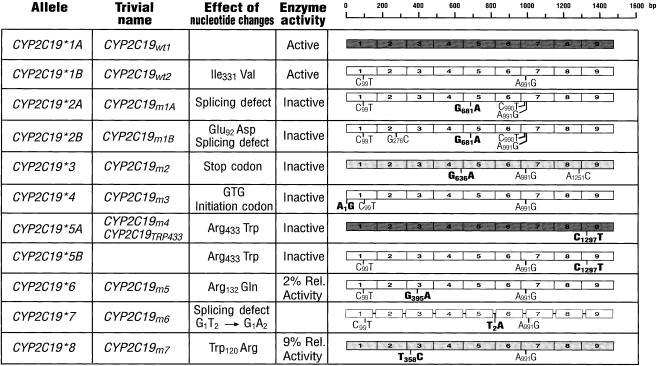
Figure 1.
CYP2C19 alleles [4–9, 63, 64]. The background of the wild-type CYP2C19*1A allele is indicated by dark shading while the clear background represents the wild-type CYP2C19*1B allele which differs by a noncoding change at bp 99 and an Ile331Val substitution. The two intermediate shaded alleles contain the amino acid difference but not the noncoding change at bp 99. The nomenclature is according to the Human Cytochrome P450 (CYP) allele nomenclature committee at http://www.imm.ki.se/CYPalleles/
Table 1.
Effects of CYP2C polymorphisms on clinical consequences of drugs.
| Enzyme | Drug | Interaction |
|---|---|---|
| CYP2C9 | Warfarin | Prolonged bleeding time, increased incidence of severe bleeding episodes in PMs [61] |
| Glipizide, tolbutamide | Possibility of low blood sugar levels in PMs [54] | |
| Phenyoin | Signs of overdose: ataxia, disturbances of consciousness, mental confusion [55] | |
| CYP2C19 | Diazepam | Unacceptable prolonged sedation in PMs, unconsciousness noted more frequently in Asian populations [29] |
| Omeprazole | Reports of decreases in cure rates at low dosage in homozygous or heterozygous EMs [31] |
CYP2C19
CYP2C19 has been shown to metabolize not only S-mephenytoin but also diverse pharmacologically important therapeutic agents including the antiulcer drug omeprazole and other important proton pump inhibitors [16], certain tricyclic antidepressants such as imipramine [17–20] some barbiturates [1, 21]. It is also partially responsible for the metabolism of certain β-adrenoceptor blockers such as propranolol [22]. The biotransformation of the antimalarial drug proguanil to its active metabolite cycloguanil is partially dependent on CYP2C19 [23].
Omeprazole is a proton pump blocker which acts on the H+/K + adenosine triphosphatase in gastric mucosa [24]. This drug was the first proton pump inhibitor to be registered to treat duodenal ulcers, peptic ulcers, reflux oesophagitis and other hyperacidic conditions. However, a number of other proton pump inhibitors have been recently introduced for similar purposes [25–27]. They are often used in combination with antibacterial treatments to eradicate H. pylori infections, which are closely associated with this disease. The 5-hydroxylation of omeprazole is metabolized by CYP2C19 [28], and omeprazole metabolism segregates with the metabolism of S-mephenytoin [16, 29]. After administration of omeprazole, the area under the plasma concentration vs time curves (AUC) is much higher in PMs of mephenytoin than EMs, and there is approximately a 10-fold difference in the oral clearance of omeprazole in PMs of mephenytoin due to impaired 5-hydroxylation. Individuals who are PMs of mephenytoin also have higher serum omeprazole/5-hydroxyomeprazole ratios than EMs [30]. Most PMs of omeprazole have been found to be homozygous for known PM alleles of CYP2C19. Recently, studies suggested that the CYP2C19 genotype may affect the cure rates for H. pylori infection in peptic ulcer patients. In one study, Japanese patients with peptic or duodenal ulcers received dual therapy with a low dose of omeprazole (20 mg day−1 for 6–8 weeks) and amoxicillin (2000 mg day−1 for 2 weeks) [31]. The cure rate for gastric ulcers and duodenal ulcers was 100% in CYP2C19 PMs, 60% for heterozygotes containing one mutant allele and only 29% in individuals who were homozygous for the wild-type CYP2C19*1 allele. The high cure rate in CYP2C19 PMs was suggested to be the consequence of markedly higher plasma concentration time curves of omeprazole in the PM phenotype. After omeprazole administration, gastric pH was highest in CYP2C19 PMs, lowest in homozygous EMs, and intermediate in heterozygous EMs [32]. Amoxicillin is unstable and its antibacterial activity is higher at high pH. Gastrin levels were also higher in CYP2C19 PMs than EMs. Omeprazole has also been reported to have anti-helicobacter activity [33]. However, the authors used a low dose of omeprazole in these studies, and the difference in cure rate would probably not have been observed at a higher dose.
There are several other proton pump inhibitors including pantoprazole, rabeprazole (E3810) [34] and lansoprazole whose metabolism has also been shown to be dependent on CYP2C19. For example, pantoprazole has a 6-fold increase in its plasma AUC, and a 5-fold shorter half-life in EMs of mephenytoin than in PMs [26]. Pantoprazole lacks the 5-methyl group on the pyridine ring of omeprazole which is hydroxylated by CYP2C19. However, the demethylation of the 4-position of the pyridine ring is affected in CYP2C19PMs. Lansoprazole is structurally related to omeprazole and its 5-hydroxylation is mediated by CYP2C19. The oral clearance of lansoprazole was about 6.5 times lower in PMs of mephenytoin than EMs, and the AUC was also greater [25]. These data indicate that metabolism of lansoprazole, like that of omeprazole, is highly dependent on the CYP2C19 genotype.
A recent review [27] cited studies showing that individual variability in plasma concentrations of various proton pump inhibitors can be accounted for by genetically determined expression of CYP2C19 as determined by S-mephenytoin 4′-hydroxylation status. However, the magnitude of the contribution of CYP2C19 to metabolism of these proton pump inhibitors differed. The ratios of the AUC values in PMs vs EMs for omeprazole, pantoprazole, lansoprazole and rabeprazole were 6.3, 6.0, 4.7, and 1.8, respectively [35]. The metabolism of rabeprazole, which reacts nonenzymatically to produce a major metabolite, is less dependent on CYP2C19 status than other proton pump inhibitors. All these data suggest CYP2C19 genetic status could contribute to some interindividual variability in responsiveness to proton pump inhibitors particularly at low doses.
Proguanil is an antimalarial drug which is used for prophylaxis [36]. CYP2C19 has been proposed to be the principal enzyme involved in cycloguanil formation using inhibitor studies in vivo as well as in vitro [35]. However, CYP3A4, CYP1A2, and CYP2C19 have all been proposed to activate proguanil to its active metabolite cycloguanil [37, 38]. CYP2C19 and CYP3A4 accounted for ∼73% and 16% of the conversion of proguanil to cycloguanil in human liver microsomes. Several studies have shown that the formation of cycloguanil cosegregates with PMs of mephenytoin, and the proguanil/cycloguanil ratio tends to be higher but the demarcation of the antimode is not clear [36, 38–41]. Therefore, the proguanil/cycloguanil ratio is not a good probe for the CYP2C19 polymorphism. An early study showed a significant correlation of mephenytoin S/R ratios and chloroguanide/cycloguanil ratios and suggested a correlation between breakthrough parasitaemia episodes and the chloroguanide/cycloguanil ratio [42]. In a later study, the antimalarial efficacy in 62 individuals genotyped as CYP2C19 PMs was reported to similar to that of 33 patients with an EM genotype [3]. These authors suggested that the parent compound has intrinsic efficacy against the parasite and that metabolism is not necessary for activation. Since cycloguanil was seen in all subjects in the earlier study, another plausable explanation of the results of the later study might be the fact that other P450s such as CYP3A4 and CYP1A2 contribute to formation of the metabolite. Therefore, the clinical implications of CYP2C19 genotype on clinical efficacy of proguanil are uncertain.
A number of drugs are partially metabolized by CYP2C19. Diazepam, an anxiolytic drug is demethylated by CYP2C19. Plasma half-lives of diazepam were dramatically longer in individuals genotyped as homozygous for the defective CYP2C19*2 allele (84 h) than in individuals who were homozygous for the wild-type CYP2C19*1 allele (20 h), or individuals heterozygous for one defective CYP2C19 allele (64 h) [43, 44]. The half-life of the metabolite desmethyldiazepam was also longer in homozygous CYP2C19PMs. Asian populations in general have been reported to have slower metabolism of diazepam than Caucasians, which is attributed to the high frequency of the mutant CYP2C19*2 and CYP2C19*3 alleles in Asians [29]. Toxic doses of diazepam may occur as the result of this slower metabolism in PMs. As a consequence, more care must be taken with the dosage of diazepam in Asian individuals. The side-chain oxidation of propranolol is also metabolized partly by CYP2C19 [22], but only a small correlation between mephenytoin metabolism and side chain oxidation was observed in vivo [45]. This presumably reflects the fact that side chain cleavage is also carried out by CYP1A2, and ring hydroxylation by CYP2D6.
CYP2C19 also metabolizes the HIV protease inhibitor nelfinavir to its major circulating metabolite. However, the metabolite has an antiviral activity similar to that of nelfinavir itself [46, 47]. As a consequence, the polymorphism does not appear to have a clinical effect on drug toleration or antiviral response to nelfinavir.
CYP2C9
CYP2C9 is the principal CYP2C in human liver [28]. It metabolizes many clinically important drugs including the diabetic agent tolbutamide, the anticonvulsant phenytoin, the S-enantiomer of the anticoagulant warfarin, Δ1-tetrahydrocannabinol and numerous anti-inflammatory drugs such as ibuprofen, diclofenac, piroxicam, tenoxicam, mefenamic acid [28], the antihypertensive losartan [48], and several new drugs including the antidiabetic drug glipizide and the diuretic torasemide [49, 50]. A rare polymorphism was reported in the metabolism of tolbutamide and phenytoin as early as the 1970s [51, 52]. Subsequently, the tolbutamide polymorphism was shown to be due a rare allele, CYP2C9*3, which carries an Ile359Leu mutation [10]. The same defect was identified in two individuals who were homozygous PMs of losartan. One of these was phenotyped for tolbutamide and was shown to be a PM for this drug. Another individual who was homozygous for the CYP2C9*3 allele was found to have diminished clearance of S-warfarin and an exacerbated response to warfarin [53]. In addition, a PM metabolizer of phenytoin, glipizide and tolbutamide was found to be homozygous for the CYP2C9*3 allele [54]. Clinical problems with toxicity and dosage adjustment of both warfarin and phenytoin have been found in CYP2C9 PMs [53, 55]. This allele has a frequency of approximately 6% in Caucasians (the frequency of homozygous CYP2C9*3 PMs is ∼0.3%) [10]. The frequency in Asians is probably fairly similar. CYP2C19 also metabolizes phenytoin to a lesser extent and metabolism of phenytoin in CYP2C19 PMs has been reported to be slightly lower than that of EMs [56]. However, CYP2C9 probably accounts for 80–90% of the metabolism of phenytoin and therefore polymorphisms in CYP2C9 have a larger affect on clinical toxicity of the drug (Figure 2).
Figure 2.
Recombinant CYP2C9*3 has a higher Km for tolbutamide and S-warfarin 7-hydroxylation, torasemide metabolism, diclofenac 4′-hydroxylation, and a lower Vmax for piroxicam 5′-hydroxylation, tenoxicam 5′-hydroxylation, mefenamic acid 3′-hydroxylation [10, 50, 57]. These changes resulted in lower intrinsic clearance values for all of the CYP2C9 substrates studied to date. In some cases, the effect appears to be the result of altered affinity of the enzyme for the substrate and in other cases, the mutation affects the Vmax. Although the effects of the CYP2C9 polymorphisms on metabolism of these anti-inflammatory drugs have not been studied in vivo, the in vitro results suggest the possibility that individuals who are homozygous for CYP2C9*3 could have slower metabolism of these drugs. Since therapeutic indices of these drugs are relatively high, these polymorphisms would be less likely to have clinical consequences.
A second mutant allele of CYP2C9 (CYP2C9*2 containing an Arg144Cys substitution) has been reported to have decreased catalytic activity in vitro toward some substrates including warfarin but may have less effect on catalytic activity toward other substrates such as tolbutamide [10, 11]. The effect on catalytic activity in vitro appears to be dependent on the recombinant system used and has been attributed to an impaired ability of CYP2C9*2 to associate with reductase [58]. CYP2C9*2 has a frequency of approximately 8% in Caucasians but has a lower frequency in African-Americans and appears to be virtually absent in Asians [10]. A recent preliminary study indicated that phenytoin metabolism was affected in a compound heterozygote (CYP2C9*2/CYP2C9*3) and a CYP2C9*2 homozygote although the details of this study are not yet available [59]. An earlier study [60] reported a slightly higher incidence of the CYP2C*2 allele in individuals who required a lower dose of warfarin for maintenance for anticoagulative therapy. A later study [61] reported a higher incidence of variant CYP2C9 alleles (CYP2C9*2 and CYP2C9*3) in individuals requiring a low dose of warfarin to maintain optimum anticoagulation than in those who required a high dose. The incidence of serious and life-threatening bleeding episodes was four times higher in the group of patients requiring a low dose of warfarin. There was also increased difficulty in establishing optimum coagulation in this group with delayed discharges from an anticoagulant clinic, multiple visits to the clinic, and additional studies to explain unusual warfarin sensitivity. Unfortunately, the PCR primers used in the genetic tests in the latter two studies were not specific for CYP2C9, but have been shown to amplify CYP2C19 [62]. Since CYP2C19, like wild-type CYP2C9, contains an Arg144 and Ile359, results of these studies may in fact under represent the incidence of the PM allele. Despite these problems, the latter study is consistent with serious effects of the polymorphic CYP2C9 alleles on coagulation times after administration of warfarin.
CYP2C8
CYP2C8 metabolizes the anticancer drug paclitaxel [13]. A recent preliminary report from our laboratory has shown that CYP2C8 is also polymorphic [12]. One of the polymorphic alleles is defective in metabolizing paclitaxel in vitro. Future studies will address whether this polymorphism has clinical consequences on the CYP2C8 substrates such as paclitaxel in vivo.
Conclusions
Drugs for which CYP2C polymorphisms have been reported to impact clinical effects are documented in Table 1. CYP2C9 has been shown to metabolize many clinically used drugs. Polymorphisms in this enzyme seriously affect the toxicity of drugs such as the anticonvulsant phenytoin, and the common anticoagulant warfarin. The polymorphism in CYP2C19 has been shown to markedly affect the half-life of the common anxiolytic drug diazepam, with a the result that a much lower dose is required in many Asians. Individuals homozygous for the CYP2C19 polymorphism have a markedly longer half-life of the antiulcer drug omeprazole with increased efficacy at low doses of the drug. These polymorphisms continue to be studied with respect to their effects on toxicity and efficacy of clinically used drugs. The varied incidence of these alleles results in variations in risk and efficacy in different racial populations. New polymorphisms in CYP2C8 are of potential interest in future clinical studies.
References
- 1.Kupfer A, Branch RA. Stereoselective mephobarbital hydroxylation cosegregates with mephenytoin hydroxylation. Clin Pharmacol Ther. 1985;38:414–418. doi: 10.1038/clpt.1985.196. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson GR, Guengerich FP, Branch RA. Genetic polymorphism of S-mephenytoin hydroxylation. Pharmacol Ther. 1989;43:53–76. doi: 10.1016/0163-7258(89)90047-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko A, Lum JK, Yaviong L, et al. High and variable frequencies of CYP2C19 mutations: medical consequences of poor drug metabolism in Vanuatu and other Pacific islands. Pharmacogenetics. 1999;9:581–590. [PubMed] [Google Scholar]
- 4.de Morais SM, Wilkinson GR, Blaisdell J, et al. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 5.de Morais SM, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 6.Ferguson RJ, de Morais SM, Benhamou S, et al. A new genetic defect in human CYP2C19: mutation of the initiation codon is responsible for poor metabolism of S-mephenytoin. J Pharmacol Exp Ther. 1998;284:356–361. [PubMed] [Google Scholar]
- 7.Xiao ZS, Goldstein JA, Xie HG, et al. Differences in the incidence of the CYP2C19 polymorphism affecting the S-mephenytoin phenotype in Chinese Han and Bai populations and identification of a new rare CYP2C19 mutant allele. J Pharmacol Exp Ther. 1997;281:604–609. [PubMed] [Google Scholar]
- 8.Ibeanu GC, Goldstein JA, Meyer U, et al. Identification of new human CYP2C19 alleles (CYP2C19*6 and CYP2C19*2B) in a Caucasian poor metabolizer of mephenytoin. J Pharmacol Exp Ther. 1998;286:1490–1495. [PubMed] [Google Scholar]
- 9.Ibeanu GC, Blaisdell J, Ferguson RJ, et al. A novel transversion in the intron 5 donor splice junction of CYP2C19 and a sequence polymorphism in exon 3 contribute to the poor metabolizer phenotype for the anticonvulsant drug S-mephenytoin. J Pharmacol Exp Ther. 1999;290:635–640. [PubMed] [Google Scholar]
- 10.Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–349. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rettie AE, Wienkers LC, Gonzalez FJ, et al. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics. 1994;4:39–42. doi: 10.1097/00008571-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Dai D, Blaisdell JA, Chanas B, Ghanayem BI, Goldstein JA. Genetic polymorphisms of human CYP2C8 and their effects on metabolism of anticancer drug: paclitaxel. FASEB J. 2000;14:A1338. doi: 10.1097/00008571-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A, Korzekwa KR, Grogan J, et al. Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res. 1994;54:5543–5546. [PubMed] [Google Scholar]
- 14.Tsuneoka Y, Matsuo Y, Okuyama E, et al. Genetic analysis of the cytochrome P-45OIIC18 (CYP2C18) gene and a novel member of the CYP2C subfamily. FEBS Lett. 1996;384:281–284. doi: 10.1016/0014-5793(96)00329-8. [DOI] [PubMed] [Google Scholar]
- 15.Komai K, Sumida K, Kaneko H, et al. Identification of a new non-functional CYP2C18 allele in Japanese: substitution of T204 to A in exon2 generates a premature stop codon. Pharmacogenetics. 1996;6:117–119. doi: 10.1097/00008571-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Andersson T, Regardh CG, Lou YC, et al. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992;2:25–31. doi: 10.1097/00008571-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Baumann P, Jonzier-Perey M, Koeb L, et al. Amitriptyline pharmacokinetics and clinical response: II. Metabolic polymorphism assessed by hydroxylation of debrisoquine and mephenytoin. Int Clin Psychopharmacol. 1986;1:102–112. doi: 10.1097/00004850-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Skjelbo E, Brosen K, Hallas J, et al. The mephenytoin oxidation polymorphism is partially responsible for the N-demethylation of imipramine. Clin Pharmacol Ther. 1991;49:18–23. doi: 10.1038/clpt.1991.4. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen K, Brosen K, Hansen J, Gram LF. Single-dose kinetics of clomipramine: relationship to the sparteine/debrisoquine and S-mephenytoin oxidation polymorphisms. Clin Pharmacol Ther. 1994;55:518–527. doi: 10.1038/clpt.1994.65. [DOI] [PubMed] [Google Scholar]
- 20.Sindrup SH, Brosen K, Hansen MGJ, et al. Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit. 1993;15:11–17. doi: 10.1097/00007691-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Adedoyin A, Prakash C, O'Shea D, et al. Stereoselective disposition of hexobarbital and its metabolites: relationship to the S-mephenytoin polymorphism in Caucasian and Chinese subjects. Pharmacogenetics. 1994;4:27–38. [PubMed] [Google Scholar]
- 22.Ward SA, Walle T, Walle UK, et al. Propranolol metabolism is determined by both mephenytoin and debrisoquin hydroxylase-activities. Clin Pharmacol Ther. 1989;45:72–79. doi: 10.1038/clpt.1989.11. [DOI] [PubMed] [Google Scholar]
- 23.Ward SA, Helsby NA, Skjelbo E, et al. The activation of the biguanide antimalarial proguanil co-segregates with the mephenytoin oxidation polymorphism – a panel study. Br J Clin Pharmacol. 1991;31:689–692. doi: 10.1111/j.1365-2125.1991.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs G, Wallmark B. The gastric H+,K+-ATPase: the site of action of omeprazole. Scand J Gastroenterol Suppl. 1989;166:3–11. [PubMed] [Google Scholar]
- 25.Sohn DR, Kwon JT, Kim HK, et al. Metabolic disposition of lansoprazole in relation to the S-mephenytoin 4′-hydroxylation phenotype status. Clin Pharmacol Ther. 1997;61:574–582. doi: 10.1016/S0009-9236(97)90137-5. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka M, Ohkubo T, Otani K, et al. Metabolic disposition of pantoprazole, a proton pump inhibitor, in relation to S-mephenytoin 4′-hydroxylation phenotype and genotype. Clin Pharmacol Ther. 1997;62:619–628. doi: 10.1016/S0009-9236(97)90081-3. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors-emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13(Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein JA, de Morais SM. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics. 1994;4:285–299. doi: 10.1097/00008571-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Bertilsson L. Geographical interracial differences in polymorphic drug oxidation-current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokin. 1995;29:192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- 30.Balian JD, Sukhova N, Harris JW, et al. The Hydroxylation of Omeprazole Correlates With S-Mephenytoin Metabolism – a Population Study. Clin Pharmacol Ther. 1995;57:662–669. doi: 10.1016/0009-9236(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 31.Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027–1030. doi: 10.7326/0003-4819-129-12-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–561. doi: 10.1016/S0009-9236(99)70075-5. [DOI] [PubMed] [Google Scholar]
- 33.Midolo PD, Turnidge JD, Lambert JR, et al. Oxygen concentration influences proton pump inhibitor activity against Helicobacter pylori in vitro. Antimicrob Agents Chemother. 1996;40:1531–1533. doi: 10.1128/aac.40.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuda S, Horai Y, Tomono Y, et al. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin Pharmacol Ther. 1995;58:143–154. doi: 10.1016/0009-9236(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 35.Funck-Brentano C, Becquemont L, Lenevu A, et al. Inhibition by omeprazole of proguanil metabolism: mechanism of the interaction in vitro and prediction of in vivo results from the in vitro experiments. J Pharmacol Exp Ther. 1997;280:730–738. [PubMed] [Google Scholar]
- 36.Brosen K, Skjelbo E, Flachs H. Proguanil metabolism is determined by the mephenytoin oxidation polymorphism in Vietnamese living in Denmark. Br J Clin Pharmacol. 1993;36:105–108. doi: 10.1111/j.1365-2125.1993.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkett DJ, Rees D, Andersson T, et al. In vitro proguanil activation to cycloguanil by human liver microsomes is mediated by CYP3A isoforms as well as by S-mephenytoin hydroxylase. Br J Clin Pharmacol. 1994;37:413–420. doi: 10.1111/j.1365-2125.1994.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coller JK, Somogyi AA, Bochner F. Comparison of (S)-mephenytoin and proguanil oxidation in vitro: contribution of several CYP isoforms. Br J Clin Pharmacol. 1999;48:158–167. doi: 10.1046/j.1365-2125.1999.00005.x. 10.1046/j.1365-2125.1999.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kortunay S, Basci NE, Bozkurt A, et al. The hydroxylation of omeprazole correlates with S-mephenytoin and proguanil metabolism. Eur J Clin Pharmacol. 1997;53:261–264. doi: 10.1007/s002280050373. [DOI] [PubMed] [Google Scholar]
- 40.Basci NE, Bozkurt A, Kortunay S, et al. Proguanil metabolism in relation to S-mephenytoin oxidation in a Turkish population. Br J Clin Pharmacol. 1996;42:771–773. doi: 10.1046/j.1365-2125.1996.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funck-Brentano C, Bosco O, Jacqz-Aigrain E, et al. Relation between chloroguanide bioactivation to cycloguanil and the genetically determined metabolism of mephenytoin in humans. Clin Pharmacol Ther. 1992;51:507–512. doi: 10.1038/clpt.1992.55. [DOI] [PubMed] [Google Scholar]
- 42.Skjelbo E, Mutabingwa TK, Bygbjerg I, et al. Chloroguanide metabolism in relation to the efficacy in malaria prophylaxis and the S-mephenytoin oxidation in Tanzanians. Clin Pharmacol Ther. 1996;59:304–311. doi: 10.1016/S0009-9236(96)80008-7. [DOI] [PubMed] [Google Scholar]
- 43.Wan J, Xia H, He N, et al. The elimination of diazepam in Chinese subjects is dependent on the mephenytoin oxidation phenotype. Br J Clin Pharmacol. 1996;42:471–474. doi: 10.1046/j.1365-2125.1996.42712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin XP, Xie HG, Wang W, et al. Effect of the gene dosage of CgammaP2C19 on diazepam metabolism in Chinese subjects. Clin Pharmacol Ther. 1999;66:642–646. doi: 10.1016/S0009-9236(99)90075-9. [DOI] [PubMed] [Google Scholar]
- 45.Xie HG, Xu ZH, Huang SL, et al. No correlation between side-chain of propranolol oxidation and S-mephenytoin 4′-hydroxylase activity. Acta Pharmacologica Sinica. 1997;18:216–218. [PubMed] [Google Scholar]
- 46.Sogawa K, Gotoh O, Kawajiri K, et al. Complete nucleotide sequence of a methylcholanthrene-inducible cytochrome P-450 (P-450d) gene in the rat. J Biol Chem. 1985;260:5026–2031. [PubMed] [Google Scholar]
- 47.Lillibridge JH, Liang BH, Kerr BM, et al. Characterization of the selectivity and mechanism of human cytochrome P450 inhibition by the human immunodeficiency virus-protease inhibitor nelfinavir mesylate. Drug Metab Dispos. 1998;26:609–616. [PubMed] [Google Scholar]
- 48.McCrea JB, Cribb A, Rushmore T, et al. Phenotypic and genotypic investigations of a healthy volunteer deficient in the conversion of losartan to its active metabolite E-3174 [clinical conference] Clin Pharmacol Ther. 1999;65:348–352. doi: 10.1016/S0009-9236(99)70114-1. [DOI] [PubMed] [Google Scholar]
- 49.Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 1999;9:71–80. doi: 10.1097/00008571-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Miners JO, Coulter S, Birkett DJ, et al. Torsemide metabolism by CYP2C9 variants and other human CYP2C subfamily enzymes [In Process Citation] Pharmacogenetics. 2000;10:267–270. doi: 10.1097/00008571-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Scott J, Poffenbarger PL. Pharmacogenetics of tolbutamide metabolism in humans. Diabetes. 1979;28:41–51. [PubMed] [Google Scholar]
- 52.Page MABJS, Shenfield GM. A screening test for slow metabolizers of tolbutamide. Br J Clin Pharmacol. 1991;46:341–347. doi: 10.1111/j.1365-2125.1991.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steward DJ, Haining RL, Henne KR, et al. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics. 1997;7:361–367. doi: 10.1097/00008571-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kidd RS, Straughn AB, Meyer MC, Goldstein JA, Dalton JT. Pharmacokinetics of clorpheniramine, phenytoin, glipizide, and nefedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 1999;9:71–80. doi: 10.1097/00008571-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Ninomiya H, Mamiya K, Matsuo S, et al. Genetic polymorphism of the CYP2C subfamily and excessive serum phenytoin concentration with central nervous system intoxication. Ther Drug Monit. 2000;22:230–232. doi: 10.1097/00007691-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Odani A, Hashimoto Y, Otsuki Y, et al. Genetic polymorphism of the CYP2C sub family and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther. 1997;62:287–292. doi: 10.1016/S0009-9236(97)90031-X. [DOI] [PubMed] [Google Scholar]
- 57.Takanashi K, Tainaka H, Kobayashi K, et al. CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with seven substrates. Pharmacogenetics. 2000;10:95–104. doi: 10.1097/00008571-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Crespi CL, Miller VP. The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH. cytochrome P450 oxidoreductase. Pharmacogenetics. 1997;7:203–210. doi: 10.1097/00008571-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Caraco Y, Muszkat M, Wood AJJ. Phenytoin metabolic ratio: a putative probe of CYP2C9 activity. Clin Pharmacol Ther. 2000;67:98. [Google Scholar]
- 60.Furuya H, Fernandez-Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics. 1995;5:389–392. doi: 10.1097/00008571-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Aithal GP, Day CP, Kesteven PJ, et al. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications [see comments] Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 62.Yasar U, Eliasson E, Dahl ML, et al. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun. 1999;254:628–631. doi: 10.1006/bbrc.1998.9992. 10.1006/bbrc.1998.9992. [DOI] [PubMed] [Google Scholar]
- 63.Ibeanu GC, Blaisdell J, Ghanayem BI, et al. An additional defective allele, CYP2C19*5, contributes to the S-mephenytoin poor metabolizer phenotype in Caucasians. Pharmacogenetics. 1998;8:129–135. doi: 10.1097/00008571-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Ibeanu GC, Goldstein JA, Meyer U, et al. Identification of new human CYP2C19 alleles (CYP2C19*6 and CYP2C19*2B) in a Caucasian poor metabolizer of mephenytoin. J Pharmacol Exp Ther. 1998;286:1490–1495. [PubMed] [Google Scholar]
- 65.Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin–drug interactions. Chem Res Toxicol. 1992;5:54–59. doi: 10.1021/tx00025a009. [DOI] [PubMed] [Google Scholar]
- 66.Kimura S, Pastewka J, Gelboin HV, et al. cDNA and amino acid sequences of two members of the human P450IIC gene subfamily. Nucl Acids Res. 1987;15:10053–10054. doi: 10.1093/nar/15.23.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]