Abstract
Aims
Since suspicion has been raised that administration of an oral live attenuated rotavirus vaccine may increase the risk of intussusception in young children, there has been concern about the possible effects of oral polio vaccine. The aim of the study was to evaluate the relationship between oral attenuated polio vaccine and intussusception in children below the age of 1 year.
Methods
We conducted a nested case-control analysis based on data from the General Practice Research Database which encompassed 133 children who developed documented intussusception during the first year of life and 515 controls.
Results
The time from oral polio vaccine to the index date was similar in cases and controls. Relative risk estimates for intussusception ranged from 0.7 (95% CI 0.2, 2.1) for babies whose last oral polio vaccine was given 29-35 days before their index date to 1.0 (95% CI 0.4, 2.3) for those whose last oral polio vaccine was given 15-21 days before their index date (compared with babies vaccinated more than 43 days before their index date).
Conclusions
There is no suggestion that oral polio vaccine increases the risk for intussusception.
Keywords: intussusception, live attenuated polio vaccine
Introduction
Sufficient concern has been raised in the United States that administration of an oral live attenuated rotavirus vaccine may increase the risk of intussusception in young children [1–3] and consequently its use has now been suspended in the United States. The cases of intussusception reported regularly occurred within 2 weeks of vaccination [1, 2]. This issue has raised additional concerns as to whether oral attenuated live polio vaccine, another live enteric virus vaccine, which is regularly used in the United Kingdom and other countries, may also increase the risk of intussusception. To provide information on this matter we conducted a study based on the General Practice Research Database (GPRD).
Methods
The GPRD provides computerized information on demographics, drugs and vaccines given to some 3.5 million patients encompassing some 350 general practices in the United Kingdom. The quality and completeness of the information has been confirmed in over 70 published studies in peer review journals [4–7]. Data on all drugs, vaccines, clinical diagnoses, hospitalizations and referrals to specialists are included, together with comments which often provide details of the diagnosis and treatment.
For the current study we identified all babies (n = 207 524) enrolled in the GPRD born between January 1, 1988 and October 31, 1998. We also identified all babies with a diagnosis of intussusception (ICD 560.0) recorded on computer prior to 1 year of age. The computer record of these babies was reviewed by one of the authors to evaluate the reliability of the diagnosis and, if given, the treatment given for intussusception. Where there were any doubts about diagnosis, we sent for hospital discharge letters from the appropriate general practitioner (n = 53). Confirmed cases were those in which the intussusception was treated surgically, reduced by enema, or otherwise listed as a discharge diagnosis. All information received by us was anonymized with regard to the patients and general practices.
We excluded two subjects who had an illness which predisposes to intussusception, namely with malrotation of the bowel or Hirschsprung′s disease. We also excluded, after review of all available information, those subjects where the diagnosis of intussusception was not confirmed. The time (in days) from the last polio vaccination to the date of diagnosis in the remaining subjects was determined for each confirmed case.
We then conducted a nested case-control evaluation to determine whether there was a relationship between time since last polio vaccine and the risk of intussusception. Up to four babies in the base population who did not have the illness were matched at random to each case with respect to month of birth, sex and age of the mother (within 1 year). The date of the diagnosis in the cases was used in their matched controls as the index date from which the time from the last vaccination was computed. Ninety-five percent of babies in the GPRD were recorded as receiving at least one polio vaccine. Each baby received, on average, 2.7 doses of polio vaccine before one year of age. All cases and controls included in this study had received at least one dose of polio vaccine at some time during their first year of birth.
After excluding the two babies with a predisposing illness, we identified 161 babies with a computer-recorded diagnosis of intussusception. After review of all of the available information, including hospital discharge letters, we excluded 19 additional subjects where the diagnosis was not confirmed in the clinical record by operation or diagnostic tests. For the remaining cases, we excluded from analysis nine further cases where the diagnosis was made prior to receiving any polio vaccine.
The study protocol was approved by the Scientific and Ethical Advisory Group of the General Practice Research Database.
Results
We first examined the age distribution among 142 cases of intussusception occurring in babies up to 1 year of age. The greatest number of cases, 30 (21% of total) occurred in babies 6 months of age. A further 96 cases (68%) occurred in the age range 3–7 months, with only 12 cases (8%) occurring in younger babies and 34 cases (24%) occurring in 8–11 month old babies.
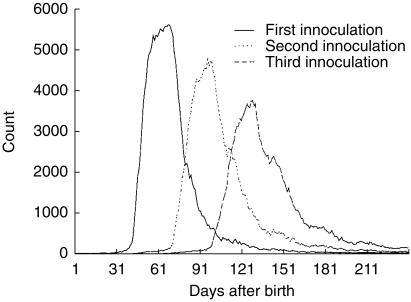
The study population encompassed 133 documented cases of intussusception in babies who had received at least one dose of polio vaccine, yielding an estimated risk of 60 per 100 000 babies less than 1 year of age. Males comprised 62% of the cases. The distribution of polio vaccines in the general population of babies less than 1 year of age according to age in days of receipt of each of the three vaccines is provided in Figure 1.
Figure 1.
The distribution of age at polio vaccination in the general population of babies less than 1 year old in the GPRD between 1 January 1988 and 31 October 1998.
All babies received oral polio vaccine before their index date and so we could not use unvaccinated babies as the reference group for a case-control analysis. Instead, we used babies with intussusception who had remote exposure to oral polio vaccine (43 days or more) as the reference group, since if oral polio vaccine were to increase the risk of intussusception, the distribution of time intervals from oral polio vaccine administration to the development of intussusception should be clustered around the induction time for this effect.
The distribution of cases and controls according to time since last vaccine by 7 day periods is given in Table 1, together with relative risk estimates (odds ratios and 95% confidence intervals) using subjects whose illness occurred more than 42 days after receiving a vaccine as the reference group. All of the relative risk estimates were close to 1.0 for each time period. The distribution of the number of vaccines received prior to the index date was similar in cases and controls. Thus 11% of cases and 12% of controls were diagnosed after the first vaccine, 19% of cases and 20% of controls after the second vaccine, and 63% of cases and 59% of controls after the third vaccine. We estimated risks for intussusception separately for cases and their controls who developed intussusception after the first, second or third vaccines. All relative risk estimates were close to 1.0 comparing recently exposed cases with cases occurring more than 28 days after a vaccine.
Table 1.
Time from last polio vaccine to date of diagnosis of intussusception.
| Days from last polio vaccine | Cases(%) | Controls(%) | RR estimatea(Odds ratio) | 95% CI |
|---|---|---|---|---|
| 1–7 days | 12 (9) | 47 (9) | 0.9 | 0.4, 2.0 |
| 8–14 days | 7 (5) | 34 (7) | 0.8 | 0.3, 2.1 |
| 15–21 days | 12 (9) | 45 (9) | 1.0 | 0.4, 2.3 |
| 22–28 days | 12 (9) | 46 (9) | 0.9 | 0.4, 2.0 |
| 29–35 days | 5 (4) | 26 (5) | 0.7 | 0.2, 2.1 |
| 36–42 days | 8 (6) | 32 (6) | 0.9 | 0.2, 2.1 |
| 43–365 daysb | 77 (58) | 285 (55) | 1.0 |
Based on conditional logistic regression.
Reference group.
One baby died as a result of complications from the intussusception. An estimated 71% were treated surgically, 25% were treated by some form of enema and 4% resolved spontaneously.
Discussion
The current study in a large population of babies with well documented intussusception found little, if any, positive association between the time from prior polio vaccination and the risk for this illness.
Study babies with intussusception were carefully matched to babies who did not have this diagnosis (controls) by month of birth of the case baby, sex, and age of the mother. In addition, the date of diagnosis of intussusception in the case baby was used as the date from which exposure to polio vaccine was determined in the controls. Therefore, there was virtually complete control for age, sex and calendar time. Close control of these variables is essential since the recommended time for receipt of the polio vaccine and the observed age of incidence of intussusception are coincidental.
The calculated incidence rate of intussusception in the current population is similar to that reported for England (66/100 000) [8]. We also found that males were at somewhat higher risk than females as was present in another case series [1].
In summary, this carefully controlled study provides persuasive evidence that there is little, if any, positive association between oral attenuated live polio vaccine and intussusception.
Acknowledgments
This study was funded in part by a grant from the UK Medicines Control Agency.
The Boston Collaborative Drug Surveillance Program is supported in part by grants from AstraZeneca, Berlex Laboratories, Boehringer Ingelheim Pharmaceuticals, Boots Healthcare International, Bristol-Myers Squibb Pharmaceutical Research Institute, Glaxo Wellcome Inc., Hoffmann-La Roche Ltd, Janssen Pharmaceutica Products, L.P., RW Johnson Pharmaceutical Research Institute, McNeil Consumer Products Company, and Novartis Farmacéutica S.A.
References
- 1.McCarthy M. Rotavirus vaccine put on hold in USA after interssusception reports. Lancet. 1999;354:309. [Google Scholar]
- 2.Interssusception among recipients of rotavirus vaccine-United States, 1998–1999. Morbidity and Mortality Weekly Report from Centre for disease Control and Prevention. JAMA. 1999;282:521–522. [Google Scholar]
- 3.Centers for Disease. Control. Interssusception among recipients of rotavirus vaccine-United States 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:577–581. [PubMed] [Google Scholar]
- 4.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerised data resource in the United Kingdom. Pharmacoepidemiology and Drug Safety. 1992;1:347–349. [Google Scholar]
- 6.Jick H. A major resource for drug safety studies. The General Practice Research Database (formerly VAMP Research) Carshalton: Centre for Medicines Research; 1995. [Google Scholar]
- 7.Jick H. A database worth saving (commentary) Lancet. 1997;350:1045–1046. doi: 10.1016/S0140-6736(05)70451-7. [DOI] [PubMed] [Google Scholar]
- 8.Gay N, Ramsay M, Waight P. Rotavirus vaccination and interssusception (letter) Lancet. 1999;354:956. doi: 10.1016/S0140-6736(05)75710-X. [DOI] [PubMed] [Google Scholar]