Abstract
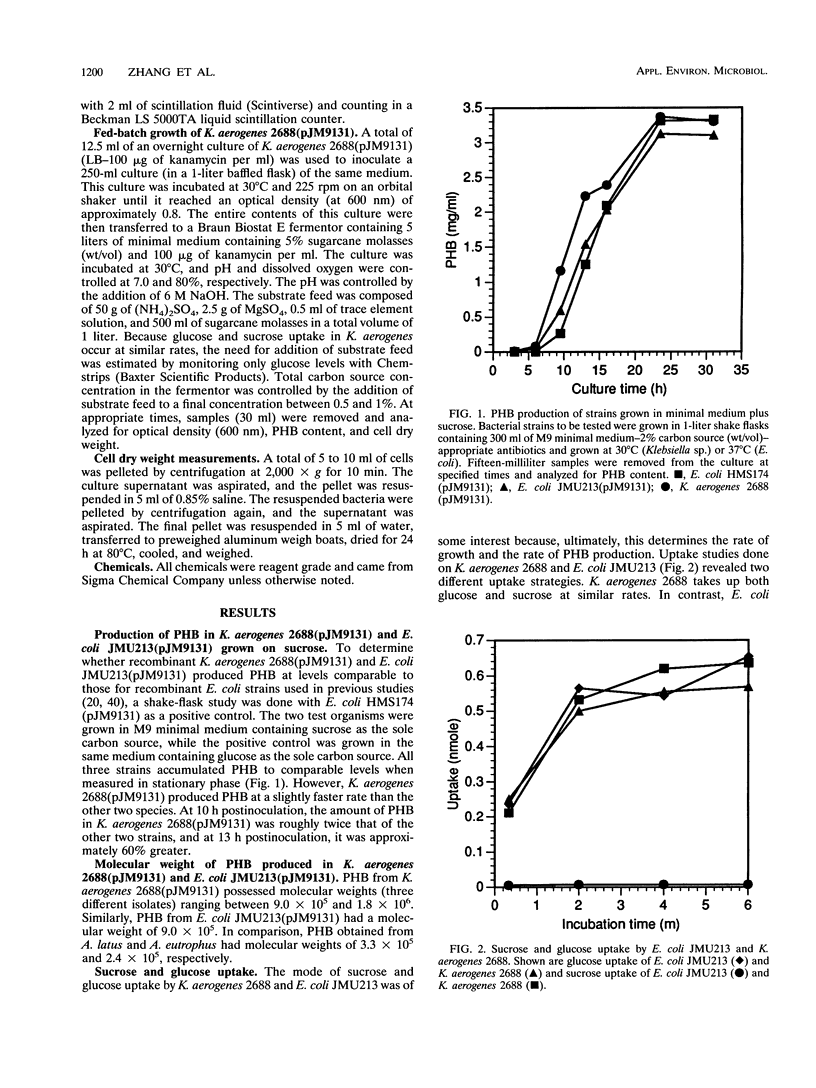
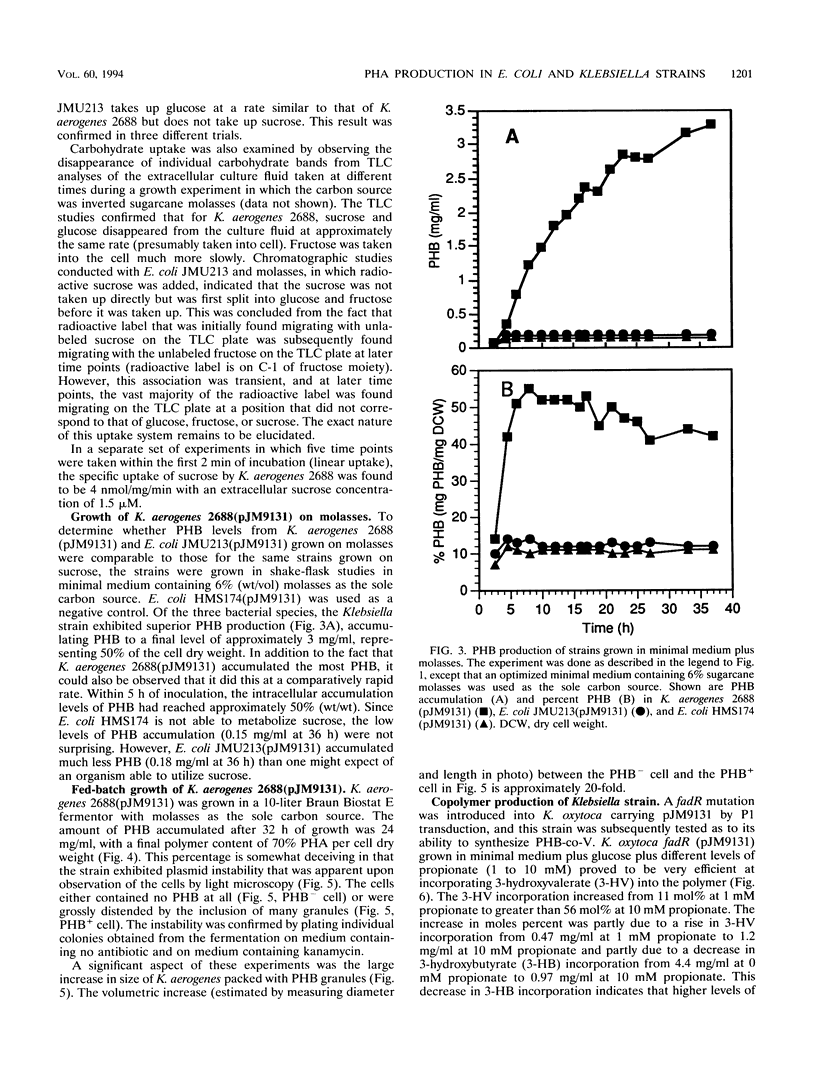
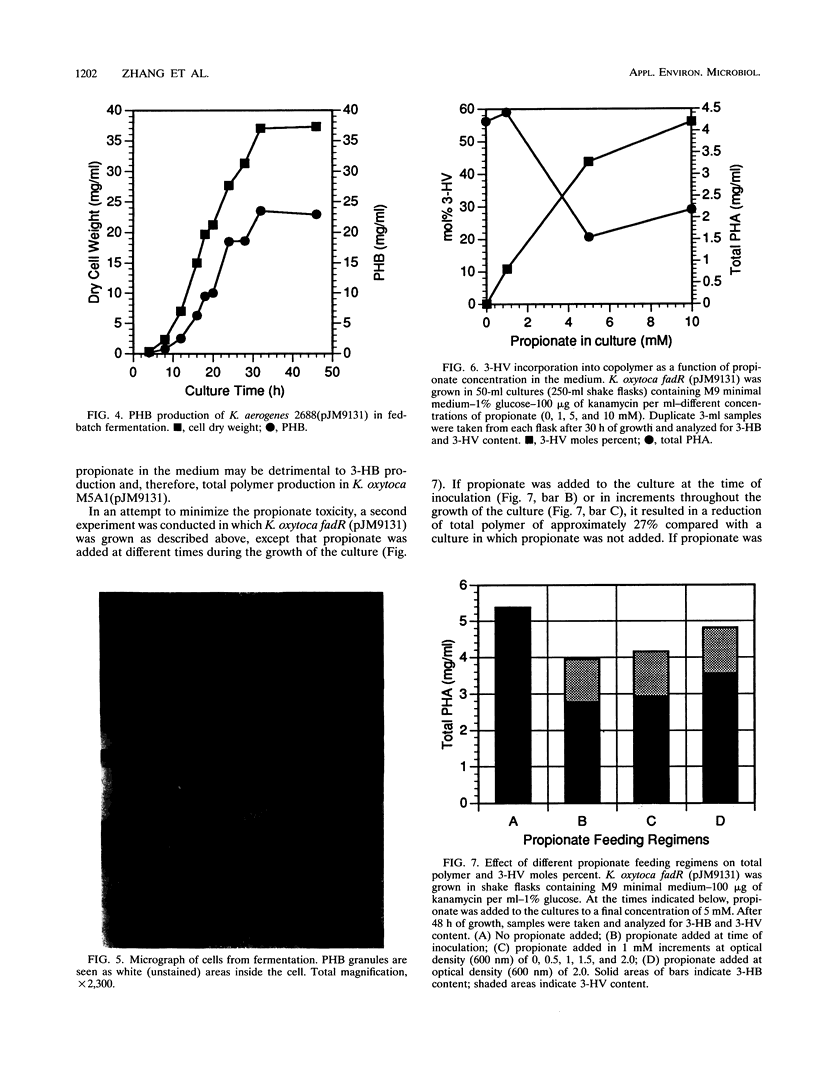
The cloned poly-3-hydroxybutyrate (PHB) synthesis pathway from Alcaligenes eutrophus has been introduced into sucrose-utilizing strains of Escherichia coli, Klebsiella aerogenes, and Klebsiella oxytoca. The plasmid-borne genes were well expressed in these environments and were able to mediate the production of significant amounts of PHB when the bacteria were grown with sucrose as the sole carbon source. The molecular weight of the PHB polymer made in K. aerogenes and E. coli was approximately 1 x 10(6) to 2 x 10(6). Sucrose uptake in K. aerogenes was measured and found to be similar to that found for other Klebsiella strains, but sucrose uptake in the E. coli strain was not detectable. K. aerogenes is able to utilize sugarcane molasses as the sole carbon source to accumulate PHB at the rate of approximately 1 g of PHB per liter of culture fluid per h. A K. oxytoca fadR strain was able to incorporate 3-hydroxyvalerate into a poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB-co-V) polymer to levels as high as 56 mol% when grown in a medium containing propionate. Total PHB-co-V levels could be enhanced by adding propionate at the beginning of stationary phase rather than at the time of inoculation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Dawes E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990 Dec;54(4):450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmann J., Heuel H., Lengeler J. W. Characterization of a chromosomally encoded, non-PTS metabolic pathway for sucrose utilization in Escherichia coli EC3132. Mol Gen Genet. 1992 Oct;235(1):22–32. doi: 10.1007/BF00286177. [DOI] [PubMed] [Google Scholar]
- Boe L., Gerdes K., Molin S. Effects of genes exerting growth inhibition and plasmid stability on plasmid maintenance. J Bacteriol. 1987 Oct;169(10):4646–4650. doi: 10.1128/jb.169.10.4646-4650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Q., König K. H., Lafferty R. M. Production of poly-D(-)-3-hydroxybutyrate and poly-D(-)-3-hydroxyvalerate by strains of Alcaligenes latus. Antonie Van Leeuwenhoek. 1991 Jul;60(1):61–66. doi: 10.1007/BF00580443. [DOI] [PubMed] [Google Scholar]
- Cooper N. S., Brown M. E., Caulcott C. A. A mathematical method for analysing plasmid stability in micro-organisms. J Gen Microbiol. 1987 Jul;133(7):1871–1880. doi: 10.1099/00221287-133-7-1871. [DOI] [PubMed] [Google Scholar]
- Feldmann S. D., Sahm H., Sprenger G. A. Cloning and expression of the genes for xylose isomerase and xylulokinase from Klebsiella pneumoniae 1033 in Escherichia coli K12. Mol Gen Genet. 1992 Aug;234(2):201–210. doi: 10.1007/BF00283840. [DOI] [PubMed] [Google Scholar]
- Fidler S., Dennis D. Polyhydroxyalkanoate production in recombinant Escherichia coli. FEMS Microbiol Rev. 1992 Dec;9(2-4):231–235. doi: 10.1016/0378-1097(92)90314-e. [DOI] [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto F., Gomi H., Kanaoka M., Nakatani T., Ito A., Katoh T., Agui H., Sumida S., Ogino S. Direct expression of urogastrone gene in Escherichia coli. Gene. 1986;45(3):311–316. doi: 10.1016/0378-1119(86)90029-6. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorrow I., Chin D. T., Fiebig K., Pierce J. L., Wilson D. M., Reeve E. C., Wilson T. H. The lactose carrier of Klebsiella pneumoniae M5a1; the physiology of transport and the nucleotide sequence of the lacY gene. Biochim Biophys Acta. 1988 Nov 22;945(2):315–323. doi: 10.1016/0005-2736(88)90494-4. [DOI] [PubMed] [Google Scholar]
- Osuna R., Boylan S. A., Bender R. A. In vitro transcription of the histidine utilization (hutUH) operon from Klebsiella aerogenes. J Bacteriol. 1991 Jan;173(1):116–123. doi: 10.1128/jb.173.1.116-123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Rahn S., Santos D. S., Maas W. K. Characterization of plasmids in a sucrose-fermenting strain of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1402–1403. doi: 10.1128/jb.130.3.1402-1403.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L., Fu T. F., Liu S. F., Chang H. Y. Cloning and expression of the Klebsiella pneumoniae galactose operon. J Biochem. 1992 Nov;112(5):604–608. doi: 10.1093/oxfordjournals.jbchem.a123947. [DOI] [PubMed] [Google Scholar]
- Ramsay B. A., Lomaliza K., Chavarie C., Dubé B., Bataille P., Ramsay J. A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl Environ Microbiol. 1990 Jul;56(7):2093–2098. doi: 10.1128/aem.56.7.2093-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Altenbuchner J., Schmitt R., Lengeler J. W. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol. 1988 Jan;2(1):1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle R. R., Coyne V. E., Maharaj R., Robb F. T., Woods D. R. Expression and regulation of a Vibrio alginolyticus sucrose utilization system cloned in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2685–2690. doi: 10.1128/jb.169.6.2685-2690.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle R. R., Steffen H. E., Goodman H. J., Woods D. R. Expression and regulation of a Bacteroides fragilis sucrose utilization system cloned in Escherichia coli. Appl Environ Microbiol. 1990 Jun;56(6):1944–1948. doi: 10.1128/aem.56.6.1944-1948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S., Gallaher T., Dennis D. Production of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) in a recombinant Escherichia coli strain. Appl Environ Microbiol. 1992 Apr;58(4):1089–1094. doi: 10.1128/aem.58.4.1089-1094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger G. A., Lengeler J. W. Analysis of sucrose catabolism in Klebsiella pneumoniae and in Scr+ derivatives of Escherichia coli K12. J Gen Microbiol. 1988 Jun;134(6):1635–1644. doi: 10.1099/00221287-134-6-1635. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Schlegel H. G. Physiology and molecular genetics of poly(beta-hydroxy-alkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991 Mar;5(3):535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tsunekawa H., Azuma S., Okabe M., Okamoto R., Aiba S. Acquisition of a sucrose utilization system in Escherichia coli K-12 derivatives and its application to industry. Appl Environ Microbiol. 1992 Jun;58(6):2081–2088. doi: 10.1128/aem.58.6.2081-2088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. E., Ingram L. O. Ethanol production from cellobiose, amorphous cellulose, and crystalline cellulose by recombinant Klebsiella oxytoca containing chromosomally integrated Zymomonas mobilis genes for ethanol production and plasmids expressing thermostable cellulase genes from Clostridium thermocellum. Appl Environ Microbiol. 1992 Jul;58(7):2103–2110. doi: 10.1128/aem.58.7.2103-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]