Abstract
Aims
Dry mouth is a common side-effect seen with immediate-release oxybutynin (IR-Oxy). Ditropan XL® [(Oxy-XL), a controlled-release formulation of oxybutynin chloride, is a once-daily oral dosage form that incorporates the OROS® technology. Dry mouth as the pharmacodynamic measure was compared between Oxy-XL and IR-Oxy administration. The steady state stereospecific pharmacokinetics were also established for the two formulations and the kinetic-dynamic relationship of oxybutynin was examined.
Methods
This was a randomized, repeated-dose, double-blind, two-treatment, two-period, crossover study. After a baseline assessment day, volunteers were randomly assigned to one of two treatment sequences and received 4 days of each treatment with a washout period of 7 days between treatments. The treatments were: 1) Oxy-XL 10 mg in the morning and placebo 8 h later, and 2) IR-Oxy 5 mg in the morning and again 8 h later. Volunteers assessed dry mouth severity subjectively using a 100 mm visual analogue scale, VAS (Baseline, treatment days 1 and 4) and objectively by collecting saliva (Baseline and treatment day 4) before dosing and every hour after the morning dose for ∼16 h. Several blood samples were collected during each treatment, with frequent sampling on day 4 to analyse for plasma R- and S-oxybutynin and R- and S-desethyloxybutynin concentrations.
Results
Relatively constant plasma concentrations of oxybutynin and its metabolite were seen over 24 h following Oxy-XL administration with the degree of fluctuation being much lower (P = 0.001; 66% to 81% reduction for the various analytes) than IR-Oxy. Compared with IR-Oxy, Oxy-XL yielded higher (131% and 158% for the R- and S-isomer, respectively) oxybutynin and lower (62% and 78% for the R- and S-isomer, respectively) desethyloxybutynin bioavailability, suggesting reduced first-pass metabolism. Saliva output (area under the effect curve) was significantly higher [P = 0.001; 37% (95% confidence interval: 24, 51%)] with Oxy-XL than with IR-Oxy and, accordingly, dry mouth severity (VAS) integrated over the day was significantly lower with Oxy-XL. The decrease in saliva output and the consequent increase in dry mouth severity correlated with the metabolite R-desethyloxybutynin concentration, and no apparent relationship was observed with the R-oxybutynin concentration. This suggests that the metabolite may contribute to the dry mouth. Therefore, the reduction in metabolite exposure with Oxy-XL may be a possible explanation for the observed decrease in dry mouth severity with OXY-XL compared with IR-Oxy.
Conclusions
Oxy-XL maintains relatively constant plasma drug and metabolite concentrations and minimizes first-pass metabolism of oxybutynin. The metabolite appears to contribute to dry mouth associated with oxybutynin, and following Oxy-XL metabolite exposure is reduced compared with IR-Oxy. Consequently less dry mouth was observed with Oxy-XL as compared with IR-Oxy.
Keywords: anticholinergic, controlled-release, Ditropan XL®, dry mouth, OROS® systems, oxybutynin, urinary incontinence
Introduction
Oxybutynin chloride is commonly prescribed to treat symptoms of urge incontinence, urgency, and frequency arising from overactivity of the detrusor muscle (overactive bladder) [1]. Anticholinergic (antimuscarinic) agents, such as oxybutynin chloride, inhibit the binding of acetylcholine to the cholinergic receptor and suppress involuntary bladder contractions. The primary adverse events associated with immediate-release oxybutynin chloride (IR-Oxy) are anticholinergic in nature and may limit the drug's therapeutic applications [2]. Of the anticholinergic effects, dry mouth is among the most commonly cited reasons patients either discontinue oxybutynin treatment or have to titrate to a lower dose [2, 3].
Oxybutynin is a tertiary amine ester with anticholinergic (antimuscarinic), spasmolytic (muscle relaxant) and local anaesthetic properties. It is a chiral compound with anticholinergic activity that resides predominantly in the R-isomer [2, 4]. Its metabolite, desethyloxybutynin, has demonstrated equipotent anticholinergic activity in vitro [2, 5]. Oxybutynin has a short half-life (2–5 h) and is rapidly cleared (25–35 l h−1) on intravenous administration [6]. When administered orally, oxybutynin is rapidly absorbed from the gastrointestinal tract but undergoes extensive first-pass metabolism. Absolute bioavailability is reported to be only about 6% [6]. Oxybutynin appears to be metabolized by cytochrome P450 3A4 in the liver and the gut wall [11]. IR-Oxy must be given frequently, up to four times a day.
Ditropan XL® (Oxy-XL), a controlled-release formulation of oxybutynin chloride, is a once-daily dosage form that incorporates the OROS® technology for drug delivery to the gastrointestinal tract. Oxy-XL delivers oxybutynin by an osmotic process at a controlled rate over approximately 24 h. A previous study [7] demonstrated that the system provides relatively constant oxybutynin concentrations throughout the day and night, minimizing the peak/trough fluctuations associated with medications given several times a day. It has also been shown that using Oxy-XL, the mean relative bioavailability was higher (153%) for racemic oxybutynin and lower (69%) for racemic N-desethyloxybutynin compared with IR oxybutynin [7]. This increased parent drug bioavailability may be due to reduced gut-wall first-pass metabolism. Within 3–5 h after dosing, Oxy-XL systems are thought to reach the colon, where cytochrome P450-mediated oxidation, the primary metabolic pathway, appears to be less extensive than in the small intestine [12–14].
The objective of this study was to compare the severity of dry mouth following Oxy-XL and IR-Oxy administration. Steady state stereospecific pharmacokinetics were also studied for the two formulations, as was the relationship between the pharmacokinetics and pharmacodynamics.
Methods
Study design
This was a randomized, double-blind, repeated-dose, two-treatment, two-period, crossover study. After a baseline assessment day, volunteers were randomly assigned to one of two treatment sequences. Volunteers received 4 days of each treatment with a washout period of 7 days between treatments. The treatments were: 1) Oxy-XL 10 mg in the morning and a placebo capsule 8 h later, and 2) IR-Oxy 5 mg in the morning and again 8 h later.
Study participants
Twenty-nine healthy volunteers between 19 and 45 years of age, within 15% of ideal weight for height were enrolled. Prior to study initiation, the study protocol was reviewed and approved by the Institutional Review Board of the study centre. All volunteers were required to provide written consent to study procedures.
Pharmacokinetic measurements
Serial blood samples were drawn before the morning dose on days 1, 2, 3, and 4 and 12 h after the first dose on day 1. On day 4, samples were also drawn at 0.5, 1, 2, 3, 5, 8, 8.5, 9, 10, 11, 13, 16, 18, 21, 24 h after the first dose. Plasma was analysed for R- and S-oxybutynin and R- and S-desethyloxybutynin concentrations. A validated, stereospecific, high-performance liquid chromatography assay was used to simultaneously determine the plasma concentrations of R- and S-oxybutynin and active metabolite R- and S-desethyloxybutynin [18]. The lower limit of quantification was 0.025 ng ml−1 and 0.125 ng ml−1 for the oxybutynin and desethyloxybutynin enantiomers, respectively. Calibration curves were linear in the range of 0.025–5 ng ml−1 for R- and S-oxybutynin and in the range of 0.125–12.5 ng ml−1 for R- and S-desethyloxybutynin. Quality control (QC) samples were included in each analytical run to ensure the accuracy and precision of the analytical method. The interday precision (% coefficient of variation, CV) for the QC samples ranged from 5.6% to 13.9% CV across all analytes. The interday method bias for the QC samples ranged from −3.3% to 5.9% across all analytes. The intraday precision (% CV) for the QC samples ranged from 0% to 44.8% CV across all analytes. The intraday bias for the QC samples ranged from −25.3% to 26.7% across all analytes.
Pharmacodynamic measurements
On days 1 and 4, volunteers provided a subjective assessment of dry mouth severity before dosing and every hour after the morning dose (except following meals) for ∼16 h. Dry mouth severity (no dry mouth to severe dry mouth) was rated by volunteers on a 100 mm visual analogue scale. On day 4, saliva flow, an objective measure of dry mouth, was measured. The subjects were asked to swallow saliva and were then instructed to start chewing on a 1 inch× 1 inch square of Parafilm® for 2 min. During this period minutes, the subjects were asked to provide saliva continuously into a clean, dry, pre-weighed beaker. Saliva output was measured before dosing and every hour after the morning dose (except after meals) for about 16 h. On the baseline assessment day (prior to receiving any treatment), dry mouth severity and saliva output were measured in the same manner as on day 4 of the study.
Subjects were monitored frequently throughout the study to assess safety. Heart rate and blood pressure were measured and adverse events were recorded by routinely questioning subjects.
Data analysis
The steady state pharmacokinetic parameters [maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), and minimum plasma concentration (Cmin) were determined from the observed day 4 plasma concentration-time data. The steady state AUC(0, 24 h) was calculated by the linear trapezoidal method. The degree of fluctuation (DF) was estimated for both formulations from the following equation:
where Cavg = AUC(0, 24 h)÷24 h.
The relative bioavailability (BA) of oxybutynin and N-desethyloxybutynin enantiomers following Oxy-XL administration as compared with IR oxybutynin was estimated from the following equation:
The AUEC (area under the effect curve) over a 24 h period was calculated using the linear trapezoidal method for both severity of dry mouth and salivary output.
Statistical procedures to compare the parameters (log transformed AUC, untransformed DF, dry mouth severity and saliva output AUEC) between treatments included analysis of variance and the Student–Newman–Keuls multiple comparison procedure. The 90% confidence interval (CI) for the log transformed AUC was computed using the standard two one-sided test procedure [19]. For the untransformed parameters the confidence interval was computed around the difference (percent of reference) between treatments. To evaluate the concentration-effect relation, the mean observed effect following treatment at any time was corrected for the mean effect observed on baseline day at the corresponding time. The concentration-effect relation was then examined by fitting various functions described below to the data.
Linear:
Log-linear:
Sigmoid Emax:
Simple Emax:
where, C = concentration; SL=slope; INT=intercept; Emax = maximum effect; EC50 = concentration at which 50% of the maximum effect is observed; n = Hill coefficient.
Results
A total of 28 volunteers (20 men, 8 women) aged 20–43 years completed the study. One volunteer discontinued after the baseline day assessment and did not receive any treatment. Therefore data from that volunteer were not included in any analysis.
Pharmacokinetics
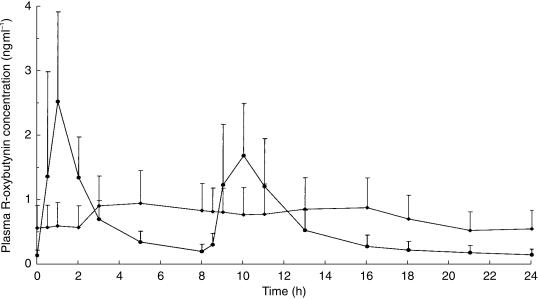
Rapid drug absorption was seen with IR-Oxy, resulting in marked peaks and troughs in plasma oxybutynin (Figure 1) and desethyloxybutynin (Figure 2) concentrations. Only the R-isomer is shown, because that of the S-isomer profile is similar. In contrast, relatively constant plasma concentrations of oxybutynin and its metabolite were seen over 24 h with Oxy-XL (Figures 1 and 2). The mean (s.d.) oxybutynin and desethyloxybutynin pharmacokinetic parameters are presented in Table 1. The degree of fluctuation for Oxy-XL was observed to be significantly lower (P = 0.001; 66% to 81% reduction for the various analytes) compared to IR-Oxy.
Figure 1.
Mean (s.d.) steady state plasma R-oxybutynin concentration-time profile after oral administration of the Oxy-XL (✦) and IR-Oxy (•) formulations of racemic oxybutynin.
Figure 2.
Mean (s.d.) steady state plasma R-desethyloxybutynin concentration-time profile after oral administration of the Oxy-XL (✦) and IR-Oxy (•) formulations of racemic oxybutynin.
Table 1.
Mean (s.d.) oxybutynin and desethyloxybutynin pharmacokinetic parameters (n = 28) after the administration of the Oxy-XL and IR-Oxy formulations.
| Parameters (units) | Oxy-XL | IR-Oxy | Oxy-XL | IR-Oxy |
|---|---|---|---|---|
| R-Oxybutynin | S-Oxybutynin | |||
| Cmax (ng ml−1) | 1.14 (0.52) | 2.85 (1.52) | 1.96 (0.86) | 5.19 (2.97) |
| tmax (h) | 4.29 (2.33) | 1.20 (0.57) | 4.21 (3.19) | 0.93 (0.44) |
| Cmin (ng ml−1) | 0.32 (0.21) | 0.09 (0.06) | 0.59 (0.40) | 0.13 (0.10) |
| AUC (ng ml−1 h) | 18 (9) | 14 (6) | 31 (16) | 20 (9) |
| Ratio (90% CI)a | 126.9 (116.5, 138.2) | Reference | 153.0 (139.8, 167.3) | Reference |
| Degree of fluctuation (%) | 1.1 (0.3) | 4.5 (1.0) | 1.1 (0.3) | 6.0 (1.5) |
| Difference (90% CI)b | −75.2 (−82.0, −68.5) | Reference | −81.2 (−89.6, −72.8) | Reference |
| Bioavailability (%) | 131 (32) | Reference | 158 (39) | Reference |
| R-Desethyloxybutynin | S-Desethyloxybutynin | |||
| Cmax (ng ml−1) | 8.3 (2.97) | 30.29 (7.28) | 4.78 (2.37) | 15.24 (4.55) |
| tmax (h) | 5.78 (2.71) | 1.11 (0.39) | 5.71 (2.71) | 1.04 (0.30) |
| Cmin (ng ml−1) | 2.28 (1.38) | 0.92 (1.24) | 1.42 (1.06) | 0.40 (0.57) |
| AUC (ng ml−1 h) | 132 (56) | 217 (82) | 79 (43) | 100 (43) |
| Ratio (90% CI)a | 60.3 (55.6, 65.4) | Reference | 75.9 (70.6, 81.5) | Reference |
| Degree of fluctuation (%) | 1.2 (0.46) | 3.5 (0.8) | 1.1 (0.4) | 3.8 (1.0) |
| Difference (90% CI)b | −66.1 (−73.1, −59.0) | Reference | −71.3 (−79.8, −62.8) | Reference |
| Bioavailability (%) | 62 (16) | Reference | 78 (16) | Reference |
Confidence interval (CI) estimated with log transformed data.
Confidence interval (CI) estimated around difference which is calculated as [(Test −Reference)/Reference].
For both formulations, metabolite concentrations were much higher than parent drug concentration. This suggests that oxybutynin undergoes significant first-pass metabolism. Compared with IR-Oxy, administration of Oxy-XL treatment resulted in higher oxybutynin relative bioavailability (AUC) and lower desethyloxybutynin relative bioavailability (P = 0.001 for all comparisons between the two formulations; Table 1) suggesting less first-pass metabolism using Oxy-XL than IR-Oxy. With both formulations, S-oxybutynin concentrations and the resultant AUCs were higher than those of R-oxybutynin and the reverse was true for the metabolite enantiomers.
Pharmacodynamics
On day 1 of treatment, the mean AUEC for dry mouth severity was significantly higher for IR-Oxy than during baseline day (Figure 3). In contrast the day 1 mean AUEC for dry mouth severity for Oxy-XL was not significantly different from the baseline day. On day 4, when drug concentrations were at steady state, the mean AUEC for dry mouth severity with Oxy-XL was significantly lower (i.e. less dry mouth) than that with IR-Oxy (Figure 3).
Figure 3.
Dry mouth severity and saliva output following administration of Oxy-XL and IR-Oxy. a) Mean (s.e. mean) for dry mouth severity AUEC on day 1 and at steady state; b) Mean (s.e. mean) for saliva output AUEC at steady state. (aSignificantly different from baseline day; bSignificantly different from Oxy-XL; P < 0.05).
The mean AUEC for salivary output on day 4 (Figure 3, Table 2) was significantly lower than that on the baseline day for both treatments. Treatment with Oxy-XL resulted in the production of more saliva output than did IR-Oxy.
Table 2.
Mean (s.d.) salivary output AUEC (n = 28) after oral administration of the Oxy-XL and IR-oxy formulations.
| Contrast Test treatment | Reference treatment | Difference (% of reference)a | 95% Confidence Intervala | Power (%) | P value |
|---|---|---|---|---|---|
| Oxy-XL | Baseline | −15.5 | −23.8, −7.1 | > 99 | 0.001 |
| 41.9 (23.8) | 49.5 (25.2) | ||||
| IR-Oxy | Baseline | −38.4 | −46.8, −30.0 | > 99 | 0.001 |
| 30.6 (15.7) | 49.5 (25.2) | ||||
| Oxy-XL | IR-Oxy | −37.2 | 23.6, 50.8 | 75 | 0.001 |
| 41.9 (23.8) | 30.6 (15.7) |
Calculated as [(Test − Reference)/Reference].
The effect of Oxy-XL and IR-Oxy on salivary output for each individual is displayed in Figure 4. If the active treatment has no effect on the pharmacodynamic measure, the AUEC following the treatment should be no different than the baseline AUEC, and the data points should be close to or on the unity line. Data points falling below the unity line are indicative of significant treatment effect. A distinct drug effect was observed for most of the subjects using the IR-Oxy formulation, whereas the data are close to the unity line for most subjects who took the Oxy-XL formulation.
Figure 4.
Relationship between area under the effect curve (AUEC) for saliva output at steady state following IR and Oxy-XL treatment compared with that on the baseline day. If the active treatment has no effect on the pharmacodynamic measure, the data should be close to or on the unity line. Data points falling below the unity line are indicative of significant treatment effects.
The pharmacodynamic measures of dry mouth severity (VAS) and salivary output were significantly correlated (Figure 5). The correlation was also significant using individual data at each time point (r = 0.33; P = 0.0001).
Figure 5.
Correlation between severity of dry mouth and salivary output. Data collected at each time point on day 4.
Concentration-effect relationship
These were examined for both saliva output and dry mouth severity as a function of both R-oxybutynin (the isomer possessing most of the anticholinergic effect) and R-desethyloxybutynin concentration. Both salivary output and dry mouth severity seem to relate to plasma R-desethyloxybutynin concentration but not to plasma R-oxybutynin concentration (Figure 6). Linear, log-linear and Emax models were used to correlate R-desethyloxybutynin concentration with the pharmacodynamic effects. In the Emax model, the inclusion of the Hill coefficient (n) did not improve fit and therefore the simple Emax model was used. The Emax (Figure 6) and log–linear model appeared to provide similar fit and performed better than the simple linear model.
Figure 6.
Relationship between mean plasma R-oxybutynin or R-desethyloxybutynin concentration and mean changes from the baseline day in the severity of dry mouth and salivary output.
Tolerability
Twenty-eight subjects completed the two treatment phases. No serious adverse events were reported during the study. The most commonly reported ones that were judged possibly or probably related to study drug were headache (five subjects during Oxy-XL and seven subjects during IR treatment) and dry mouth (three subjects during Oxy-XL and six subjects during IR treatment). Other possibly or probably related events included urinary frequency (three subjects during IR treatment) and dry eyes (two subjects during IR treatment and 1 during Oxy-XL treatment).
Discussion
As expected for a controlled-release product, once-daily treatment with Oxy-XL yielded a slower rise to peak concentration than IR-Oxy and maintained relatively constant plasma drug and metabolite concentrations (Figures 1 and 2). The higher relative bioavailability of the drug isomers and the lower AUC of the metabolite isomers, have also been observed in an earlier study in which concentrations were measured as the racemate [7]. Oxy-XL combines oxybutynin with the OROS® osmotic delivery technology, a nondisintegrating system, which generally reaches the colon within 3–5 h of dosing and where the majority of oxybutynin is likely to be absorbed. In contrast, using IR-Oxy, like most other orally delivered medications, drug is absorbed primarily in the small intestine. Pre-systemic gut wall cytochrome P450-mediated oxidation is more prominent in the small intestine than in the colon [12–14]. Thus with Oxy-XL, oxybutynin delivered to the colon encounters less gut-wall first-pass metabolism, resulting in increased drug bioavailability and decreased total body exposure to the metabolite, desethyloxybutynin.
Oxybutynin enantiomers exhibited stereoselective pharmacokinetics. The total oral clearance of S-oxybutynin was less than that of the R-isomer, regardless of the formulation. However clearance by the gastro-intestine appears to be higher for S-oxybutynin, since its relative bioavailability following Oxy-XL compared with IR oxybutynin was much greater than that of R-oxybutynin (158% vs 131%, respectively). Thus, it can be inferred that the hepatic clearance of R-oxybutynin is much higher than that of the S-isomer. Consistent with the lower oral clearance of S-oxybutynin compared with that of R-oxybutynin, the metabolite AUC was less for the S-isomer compared with the R-isomer with both formulations. The relative availability of the metabolite following Oxy-XL compared with IR-oxybutynin also exhibited stereospecific differences, that of the R-isomer being less than that of the S-isomer.
This study demonstrated the potential for a subject experiencing dry mouth was much lower with Oxy-XL compared with IR-oxy using two different measures. This supports the outcome of a previous safety and efficacy study in which the patients taking a single daily dose of Oxy-XL reported a lower incidence of dry mouth compared with those taking IR-Oxy one to four times daily. The two groups of patients had similar efficacy in terms of reduction in urge incontinence and return to continence. The percentage of patients reporting continence was also similar when the daily dose of IR-oxy and Oxy-XL administered was the same [9]. A reduction in dry mouth with Oxy-XL may be the result of reduced first-pass metabolism and/or a slower rate of increase in plasma concentration and a lower peak value for the drug and metabolite. Compared with oral administration, anticholinergic side-effects are also reported to be lower with intravesical oxybutynin [10], which also limits first-pass metabolism.
The effect of oxybutynin on the bladder and its main side-effect, dry mouth, are both elicited mainly through its antimuscarinic activity. Literature reports have suggested that both oxybutynin and desethyloxybutynin have similar potency as antimuscarinic agents [5]. However, it appears that oxybutynin has a higher affinity for M2 receptors in Chinese hamster ovary cells than desethyloxybutynin [16]. On the other hand, desethyloxybutynin has a higher affinity than oxybutynin for M3 receptors. It has been suggested that the latter present in the parotid glands may be more sensitive to blockade than those in the bladder, and that M2 receptors mainly contribute to bladder contraction [17]. Therefore the higher affinity of desethyloxybutynin for M3 receptors suggests that it may have a greater role than oxybutynin in causing dry mouth. In another radioligand binding study, both compounds showed similar affinity for the detrusor tissue, but desethyloxybutynin showed a trend towards higher affinity than oxybutynin for the parotid gland [5].
Studies to determine the effect of these compounds separately on human salivary glands have yet to be performed. Ignoring the potentially confounding effects of oxybutynin and desethyloxybutynin on salivary gland function in our subjects, the concentration-effect relationship was examined for both compounds separately. The decrease in salivary output and the consequent increase in dry mouth severity were correlated with plasma R-desethyloxybutynin concentration, but no apparent relationship was observed with plasma R-oxybutynin concentration. Combining the R-oxybutynin and R-desethyloxybutynin data did not improve the correlation observed with the metabolite data alone. This supports the notion that R-desethyloxybutynin may have a larger role in causing dry mouth than the parent drug.
Further evidence of the role of this metabolite comes from the results of another study [15], in which ketoconazole, a selective inhibitor of CYP3A4, was coadministered with Oxy-XL or IR-Oxy, and in which the effect on dry mouth was evaluated. Ketoconazole increased the peak plasma concentration of R-oxybutynin and its AUC following IR-Oxy administration by about 200% but the change in concentration of R-desethyloxybutynin was negligible (about 10%). Interestingly, dry mouth severity was similar when the drugs were administered with and without ketoconazole. The 200% increase in peak concentration of the parent compound (R-oxybutynin) when administered with ketoconazole and the finding that there was no change in dry mouth suggests that the peak and rate of increase in concentration of the parent drug are apparently not directly associated with the occurrence of dry mouth. A smaller effect on salivation by Oxy-XL compared with IR-Oxy was also observed in this drug interaction study. In summary the above in vitro and in vivo findings suggest that the N-desethyl metabolite of R-oxybutynin may be responsible for causing dry mouth. While a slower rate of increase in and lower peak concentration may be contribute to the small effect on salivation observed with Oxy-XL compared with IR-Oxy, the decrease in exposure to metabolite appears to play the major role.
Only one dosage strength of each formulation was evaluated in this study. As a result data are sparse at the higher plasma concentrations (Figure 6) and the Emax model and the log–linear model behaved similarly in describing the concentration–effect data. However, single doses greater than 5 mg IR-Oxy are typically not prescribed. There was a significant correlation between the measures that were used to assess dry mouth (VAS vs saliva output; Figure 5). This suggests that assessment with a VAS is an appropriate means of quantifying the changes in salivary output. The overall outcome and the added information gained from measuring multiple pharmacodynamic parameters should therefore be weighed against the cost and bother of obtaining each measure.
In conclusion, treatment with Oxy-XL maintains relatively constant plasma oxybutynin and desethyloxybutynin concentrations and minimizes the first-pass metabolism of the drug. The metabolite appears to be responsible for dry mouth associated with oxybutynin administration and plasma metabolite concentrations are lower using the Oxy-XL compared with the IR-Oxy formulation. Consequently less dry mouth was observed with Oxy-XL than with IR-Oxy.
References
- 1.IMS America. 1999. National Prescription Audit Plus Database, Plymouth Meeting, PA.
- 2.Yarker YE, Goa KL, Fitton A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–262. doi: 10.2165/00002512-199506030-00007. [DOI] [PubMed] [Google Scholar]
- 3.Jacquetin B. Review of pharmacological therapy in the treatment of the unstable bladder. Acta Obstet Gynecol Scand. 1997;76:47. (Abstract) [Google Scholar]
- 4.Kachur JF, Peterson JS, Carter JP, et al. R and S enantiomers of oxybutynin: pharmacological effects in guinea pig bladder and intestine. J Pharmacol Exp Ther. 1988;247:867–872. [PubMed] [Google Scholar]
- 5.Waldeck K, Larsson B, Andersson KE. Comparison of oxybutynin and its active metabolite, N-desethyl-oxybutynin, in the human detrusor and parotid gland. J Urol. 1997;157:1093–1097. [PubMed] [Google Scholar]
- 6.Douchamps J, Derenne F, Stockis A, Gangji D, Juvent M, Herchuelz A. The pharmacokinetics of oxybutynin in man. Eur J Clin Pharmacol. 1988;35:515–520. doi: 10.1007/BF00558247. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharm. 1999;39:22–29. [PubMed] [Google Scholar]
- 8.Lukkari E, Aranko K, Juhakoski A, Hakonen T, Neuvonen P. Effect of time interval between food and drug ingestion on the absorption of oxybutynin from a controlled-release tablet. Pharmacol Toxicol. 1997;81:31–34. doi: 10.1111/j.1600-0773.1997.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson R, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once-a-day controlled release versus immediate release oxybutynin chloride in the treatment of urinary urge incontinence. J Urol. 1999;161:1809–1812. [PubMed] [Google Scholar]
- 10.Buyse G, Waldeck K, Verpoorten C, Bjork H, Casaer P, Andersson KE. Intravesical oxybutynin for neurogenic bladder dysfunction: less systemic side effects due to reduced first pass metabolism. J Urol. 1998;160:892–896. doi: 10.1016/S0022-5347(01)62828-3. [DOI] [PubMed] [Google Scholar]
- 11.Yaich M, Popon M, Jacqz AE. The metabolism of oxybutynin is dependent on CYP3A and not on CYP2D6. Therapie. 1995;50(Suppl):67. [Google Scholar]
- 12.Caldwell J, Marsh MV. Metabolism of drugs by the gastrointestinal tracts. In: George CF, Shand DG, editors. Clinical Pharmacology and Therapeutics 1: Presystemic Drug Elimination. London: Butterworth Scientific; 1982. pp. 29–42. [Google Scholar]
- 13.Illett KF, Tee LBG, Reeves PT, Minchin RF. Metabolism of drugs and other xenobiotics in the gut lumen and wall. Pharmacol Ther. 1990;46:67–93. doi: 10.1016/0163-7258(90)90036-2. [DOI] [PubMed] [Google Scholar]
- 14.Paine MF, Khaighi M, Fisher JM, et al. Characterization of interintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–1562. [PubMed] [Google Scholar]
- 15.Sathyan G, Hu W, Chancellor MB, Gupta SK. Comparison of CYP3A4 inhibitor effects on the stereoselective pharmacokinetics of extended release oxybutynin and conventional oxybutynin. Pharm Sci. 1999;1:2056. [Google Scholar]
- 16.Gillberg P, Staffan S. Pharmacological profile of DD01 and desethyloxybutynin (DEOB) – the major metabolite of tolterodine and oxybutynin (OB), respectively. J Urol. 1997;157(4):81. [Google Scholar]
- 17.Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B. Tolterodine – a new bladder selective antimuscarinic agent. Eur J Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- 18.Minser D, Tondeur Y, Davis IM, Warren H, Jersey J. Quantification of oxybutynin enantiomers in human plasma at low pg/ml concentration using solid phase extraction and isotope dilution LC/MS/MS. Pharm Res. 1996;13:S–48. [Google Scholar]
- 19.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]