Abstract
Changes in synaptic efficacy are crucial for the development of appropriate neural circuits and brain information storage. We have investigated mechanisms underlying long-term depression (LTD) at glutamatergic synapses in the striatum, a brain region important in motor performance and cognition, and a target for Huntington and Parkinson diseases. Induction of striatal LTD is dependent on postsynaptic depolarization and calcium influx through L-type channels. Surprisingly, LTD maintenance appears to involve a decrease in the probability of neurotransmitter release from presynaptic terminals as evidenced by increases in paired-pulse facilitation and the coefficient of variation of synaptic responses that are tightly associated with LTD expression. Furthermore, both the apparent probability of neurotransmitter release and the magnitude of LTD decrease concomitantly during postnatal development, consistent with the idea that striatal LTD is involved in a developmental decrease in the probability of neurotransmitter release at corticostriatal synapses. The presynaptic changes that underlie striatal LTD may also be important for motor performance and certain forms of learning and memory.
Activity-dependent changes in synaptic efficacy, such as long-term potentiation (LTP) and long-term depression (LTD), are critical for the development of appropriate neural circuits and for many forms of neural information storage (1, 2). Expression of both LTP and LTD at different synapses has been attributed to alterations in neurotransmitter release or the postsynaptic response to neurotransmitter (2–12). In the CA1 region of the hippocampus, both pre- and postsynaptic mechanisms have been suggested to play a role in LTP and LTD (3–14). Extensive evidence supporting presynaptic mechanisms for LTD, including evidence consistent with decreased probability of neurotransmitter release, has been observed in hippocampus in neonatal and young rats (5, 13). However, changes in paired-pulse facilitation (PPF), a sensitive measure of altered release probability, have not been observed during expression of LTD in the hippocampus (13, 15). At present the role of changes in release probability in LTD expression remains unclear.
The development of appropriate neural circuits involves activity-dependent processes (16). Forms of synaptic plasticity such as LTP and LTD have been suggested as cellular mechanisms for this activity-dependent process. For example, maximal susceptibility to LTP and LTD coincides with critical periods for cortical plasticity (17–19). The induction mechanisms for LTP and LTD in immature cortex are dependent on N-methyl-d-aspartate receptors (20). In the CA1 region of hippocampus, a postsynaptic expression mechanism underlying LTP appears to be involved in the activity-dependent formation of conducting glutamatergic synapses (21).
The striatum is a brain region with important roles in motor sequencing and control (22, 23). In humans, nonhuman primates, and rodents the striatum appears to participate, along with the frontal cortex, in neural circuitry that is important for learning and memory distinct from the types of memory encoded in the hippocampus (24, 25). In humans, the striatum appears to be important for the gradual, incremental learning of associations that is characteristic of habit learning (26).
Corticostriatal glutamatergic fibers represent the major excitatory input to the striatum (27). Repetitive activation of corticostriatal glutamatergic synapses produces LTD of excitatory synaptic transmission in the striatum as demonstrated both in vivo (28) and in vitro (29–31). Interestingly, coactivation of D1 and D2 dopamine receptors is involved both in motor control and generation of striatal LTD (27). This unique form of LTD may represent the cellular substrate for long-term behavioral changes, and it appears to be altered by different psychoactive drugs (27). Although induction of striatal LTD has been shown to be dependent on activation of L-type calcium channels, metabotropic glutamate receptors and dopamine receptors (27), little is known about the mechanisms involved in maintained expression of striatal LTD.
In this study we provide evidence that striatal LTD results, at least in part, from a long-lasting decrease in the probability of transmitter release brought about by prior postsynaptic events. Furthermore, our findings suggest that postnatal development of corticostriatal synapses involves a decrease in the probability of neurotransmitter release induced by striatal LTD or a mechanistically related process.
METHODS
Slice Preparation.
Brain slices were prepared from 10- to 27-day-old Sprague–Dawley rats using previously described techniques (30) with the exception that coronal brain slices (300 or 400 μm thick) were cut in ice-cold modified artificial cerebrospinal fluid (aCSF) containing 194 mM sucrose, 30 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, and 10 mM d-glucose adjusted to pH 7.4 by bubbling with 95% O2/5% CO2. A hemislice containing the cortex and striatum just anterior to the globus pallidus was completely submerged and continuously superfused with aCSF at 32.5 ± 0.5°C. The flow rate was 2–3 ml/min. Brain slices from 10- to 19-day-old rats were used for the results shown in Figs. 1, 2, 3, 4, 5.
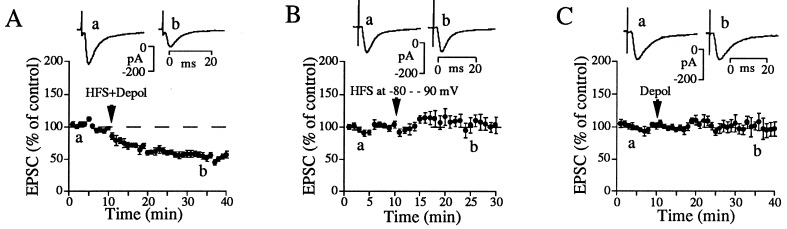
Figure 1.
Dependence of striatal LTD induction on postsynaptic depolarization (Depol). (A) LTD was induced by stimulating corticostriatal afferents at 100 Hz for 1 sec along with simultaneous 1-sec depolarization to −10 or 0 mV, repeated four times at 10-sec intervals (n = 11). The pipette contained a standard intracellular solution. (B) HFS alone in voltage-clamp condition at −80 or −90 mV failed to induce LTD (n = 5). (C) Depolarization to 0 mV (1-sec duration, repeated four times at 10-sec intervals) in the absence of HFS failed to induce LTD (n = 8). Points in each graph are values averaged over 1-min time epochs. EPSCs shown above the graph are recorded at times indicated by letters. Waveforms in A–C are averages of 20 individual EPSCs.
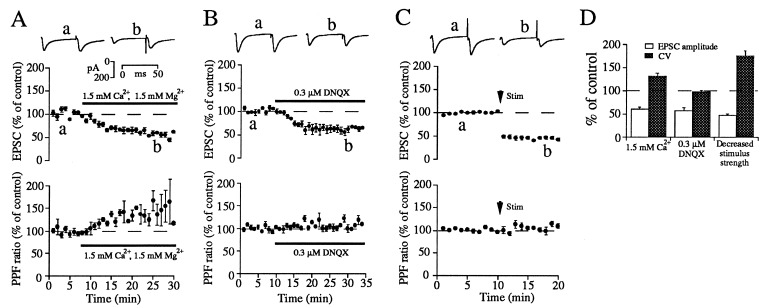
Figure 2.
Changes in PPF ratio and CV values induced by manipulations that alter presynaptic but not postsynaptic function at corticostriatal synapses. (A) Decreasing Ca2+/Mg2+ ratio in the external solution increased both the PPF ratio and CV value (n = 4). PPF ratio after decreasing Ca2+/Mg2+ ratio in the external solution was 141.1 ± 12.7% of control (two-tailed repeated measures t test, P < 0.05). (B) Partial blockade of AMPA/kainate receptors did not alter either the PPF ratio or the CV value (n = 4). PPF ratio after partially blocking AMPA/kainate receptors was 106 ± 2.7% of control (P > 0.1). (C) Decreasing stimulus (Stim) strength increased CV values, but did not change PPF (n = 6). PPF ratio after decreasing stimulus strength was 102.8 ± 4.7% (P > 0.5). (D) Summary of effects of pre- or postsynaptic modulations on average peak EPSC amplitudes and CV values. The CV value after decreasing Ca2+/Mg2+ ratio, partially blocking AMPA/kainate receptors and decreasing stimulus intensities, was 132 ± 6.1, 97.5 ± 3.8, and 175.2 ± 10.9 of control, respectively (P < 0.05, P > 0.5, and P < 0.005, respectively). Points in A–C are values averaged over 1-min time epochs. EPSCs shown above the graph are recorded at times indicated by letters. Waveforms in A–C are averages of 20 individual paired EPSCs. DNQX, 6,7-dinitroquinoxaline-2,3(1H,4H)-dione.
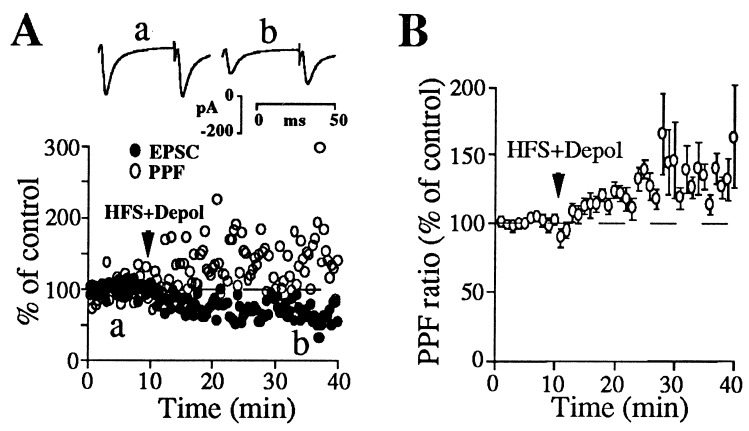
Figure 3.
Striatal LTD is closely associated with increases in PPF ratio. (A Upper) Paired EPSCs recorded at the indicated times before and after LTD induction. (Lower) EPSC peak amplitude and PPF ratio from the same neuron plotted against time before and after LTD induction. (B) Summary graph showing increases in PPF ratio after LTD induction (n = 11). PPF ratio after LTD induction was 137.9 ± 7.3% of control. Note that the change in PPF ratio associated with LTD expression is similar to that observed after decreasing Ca2+/Mg2+ ratio in the external solution (see Fig. 2A). Waveforms in A are averages of 20 individual paired EPSCs. Points in B are values averaged over 1-min time epochs.
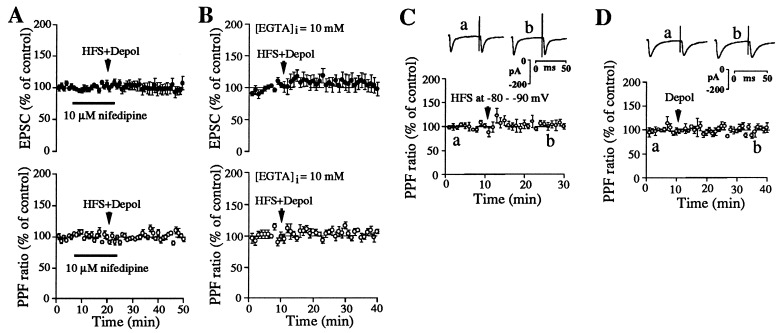
Figure 4.
Increases in PPF ratio are strongly associated with striatal LTD. (A) Effect of 10 μM nifedipine on LTD induction and PPF ratio (n = 6). (B) Effect of 10 mM EGTA applied in the intracellular solution on LTD induction and PPF ratio (n = 5). (C and D, Upper) Paired EPSCs taken at the indicated times before and after either HFS or depolarization (Depol) alone, respectively. (Lower) PPF ratio plotted against time before and after HFS alone (C, n = 5) or depolarization (D, n = 8). Waveforms in C and D are averages of 20 individual paired EPSCs. Points in A–D are values averaged over 1-min time epochs.
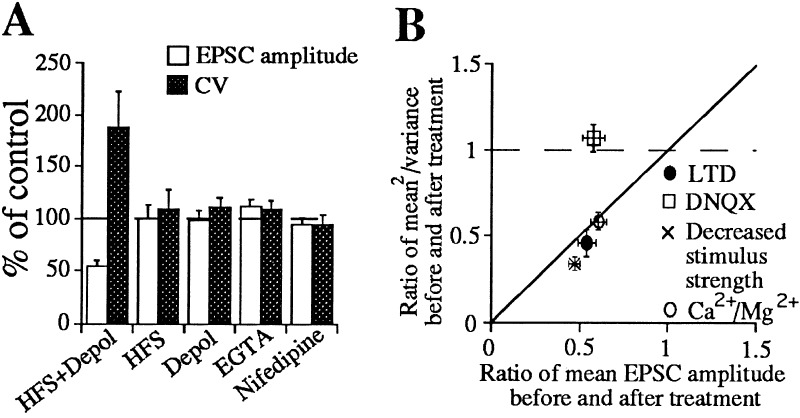
Figure 5.
The increase in CV associated with striatal LTD is sufficient to explain the magnitude of synaptic depression. (A) Summary of results using different protocols comparing changes in average peak EPSC amplitudes and CV values. (B) Graph summarizing the effects on μ2/σ2 (1/CV2) of all of the manipulations that affected synaptic strength. These include DNQX (n = 4), decreased stimulus intensity (n = 6), decreased Ca2+/Mg2+ ratio (n = 4), and LTD (n = 11). If the mechanism of LTD expression is due predominantly to the change in Pr or n then values either on the diagonal line or below would be expected, whereas, if changes in postsynaptic response to transmitter account for LTD expression then no change in μ2/σ2 would be expected. Depol, depolarization.
Whole-Cell Recording.
Whole-cell voltage clamp recordings were obtained using pipettes made from borosilicate glass capillaries pulled on a Flaming–Brown micropipette puller. Pipette resistances ranged from 2.5–5 MΩ, when filled with internal solution containing 120 mM CsMeSO3, 5 mM NaCl, 10 mM tetraethylammonium chloride, 10 mM Hepes, 3–5 mM QX-314 (Br salt), 1.1 mM EGTA, 4 mM ATP (Mg2+ salt), and 0.3 mM GTP (Na+ salt), pH adjusted to 7.2 with CsOH, and osmolarity adjusted to 297–300 mmol/kg with sucrose. Recordings were made primarily using the blind whole-cell patch-clamp recording technique in 400-μm-thick slices (32). However, 20% of the recordings in Fig. 6 were also made under differential interference contrast-enhanced visual guidance from neurons three to four layers below the surface using 300- or 400-μm-thick slices. There were no differences in the results obtained using the two techniques in terms of the magnitude of LTD and basal PPF ratio across age. It is highly probable that most or all of the cells recorded cells were medium spiny neurons because these cells make up >95% of the striatal neuronal population (33). In past studies, we have observed that the large majority of cells examined with whole-cell recording exhibited firing properties consistent with those of medium spiny neurons (30). In studies from other laboratories, the majority of intracellularly stained cells in the striatum were shown to be of this type (34). Medium-sized neurons that were visually identified in striatal slices also appeared to be of this type (35). Cells were voltage clamped at −60 or −70 mV during test periods before and after LTD induction. To evoke synaptic currents, stimuli were delivered through bipolar tungsten electrodes placed in corpus callosum. Paired stimuli (interstimulus interval of 50 msec) were delivered at a frequency of 0.05–0.033 Hz. Whole-cell currents recorded with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) were filtered at 2 KHz, digitized at up to 50 KHz, and stored on a microcomputer using pclamp software. The series resistance, which was not compensated and was typically between 5 and 30 MΩ (35% of cells below 10 MΩ), was monitored continuously. Normal aCSF and drugs were delivered to slices via superfusion by gravity flow.
Figure 6.
A developmental increase in basal PPF ratio is associated with a decrease in the susceptibility to striatal LTD. (A) Basal PPF ratio plotted as a function of age (n = 171 cells). PPF ratio measured in P10–P15 group (n = 68) and P23–P27 group (n = 42) averaged 0.97 ± 0.02 and 1.29 ± 0.03, respectively (unpaired t test, P < 0.0001). There was no significant difference between the two age groups in average peak EPSC amplitude of the first pulse of the paired responses. The range of stimulus intensity used to evoke the response and series resistance of whole-cell patch-clamp recordings did not differ across age. (B) Summary of bar graph comparing changes induced by LTD induction in average peak EPSC amplitudes, PPF ratio, and CV values in P10–P19 group (n = 11) and P23–P27 group (n = 13). The difference in the magnitude of LTD and the increase in PPF after LTD induction between the two age groups was statistically significant (two-tailed, unpaired t test; ∗, P < 0.005; ∗∗, P < 0.05). There was no significant difference in the increase in CV after LTD induction between the two groups. PPF ratio and CV value after LTD induction in the P23–P27 group were 119.3 ± 3.8% and 139.9 ± 15.9% of control, respectively (P < 0.0005 and P < 0.05, respectively). Note that we failed to induce LTD in 4 out of 17 slices in the P23–P27 group and only the values from the 13 slices showing LTD are included in the graph. Basal PPF ratio in the neurons from the P10–P19 and P23–P27 groups in which LTD was examined averaged 1.017 ± 0.054 and 1.255 ± 0.056, respectively (two-tailed, unpaired test, P < 0.01).
LTD Induction.
LTD was induced by pairing high frequency stimulation (four 1-sec duration, 100-Hz trains delivered at a frequency of one train every 10 sec) with 1-sec depolarization of the postsynaptic neuron to −10 or 0 mV. LTD could be elicited using this pairing protocol as long as 30 min after initiation of whole-cell recording even when access resistance was low (<10 MΩ). LTD in slices from young rat brains appears to be similar to LTD previously described in adult striatum. In preliminary studies, striatal LTD recording in slices from young rats using field potential recording was blocked by 1 μM sulpiride, a D2 dopamine receptor antagonist (data not shown).
Data Analysis.
EPSC amplitudes were measured using peak detection software provided by pclamp6. Synaptic responses recorded during a 10-min period 20–30 min after LTD induction were used to calculate PPF ratio and coefficient of variation (CV). In the case of other treatments, synaptic responses that had stabilized after particular treatments were used to calculate PPF ratio and CV. PPF was expressed as the ratio of the amplitude of the second excitatory postsynaptic current (EPSC) to that of the first EPSC. CV was calculated as σ/μ in which σ and μ are the standard deviation and mean of 30 successive EPSCs, respectively. PPF ratios were calculated from the same cells used to calculate CV values in Figs. 2, 3, 4, 5. Standard deviation was calculated from the variance taken as the total amplitude variance minus the variance due to background noise. Background noise variance was calculated using the same method used to measure the EPSC amplitude, but during a portion of each sweep free of synaptic current. All averaged values are given as mean ± SEM. The statistical criterion for significance was P < 0.05.
RESULTS
Induction of Striatal LTD.
Cortical afferents make direct monosynaptic connections with neurons in the dorsal striatum and elicit an excitatory postsynaptic potential mediated mainly by α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/kainate glutamate receptors (27, 36). To selectively stimulate cortical afferents projecting to striatal neurons in coronal brain slices, a bipolar tungsten electrode was placed in the corpus callosum dorsal to the striatum (37). EPSCs were recorded in striatal cells in slices from young rats with whole-cell voltage-clamp methods. At holding potentials of −60 to −80 mV, synaptic transmission was mediated mainly through AMPA/kainate receptors with very little contribution of N-methyl-d-aspartate receptors, and was stable during 60- to 90-min recordings.
To induce LTD reliably at modest stimulation intensities, repetitive high frequency stimulation (HFS) of cortical afferents was paired with postsynaptic depolarization (Fig. 1A). Synaptic depression persisted throughout recording periods lasting at least 30 min posttetanus with an average depression of the EPSC amplitude to 54.3 ± 5.3% of its control value 20–30 min after pairing HFS and depolarization. HFS itself failed to produce LTD when striatal neurons were voltage-clamped at −80 to −90 mV, supporting the idea that the induction mechanism of striatal LTD is dependent on postsynaptic depolarization (Fig. 1B). However, postsynaptic depolarization itself without HFS was not sufficient to generate LTD, suggesting that both depolarization and HFS are necessary for LTD induction (Fig. 1C).
Mechanism of LTD Expression.
To determine the site of LTD expression, we used two alternative approaches. One approach was to examine the association of LTD with changes in PPF ratio, a phenomenon in which activation of a synapse twice within a short time period results in a presynaptic facilitation of transmitter release in response to the second stimulus (38). At most synapses, PPF is thought to reflect an increase in the probability of neurotransmitter release (38). This idea is supported by the observation that manipulations which alter probability of transmitter release induce predictable changes in PPF ratio (38). However, postsynaptic manipulations can alter PPF ratio at some synapses (39), and thus it may not be the case that only genuinely presynaptic changes alter PPF ratio. A second approach was to measure changes in the CV of EPSC amplitudes in relation to expression of LTD. Changes in the CV value (σ/μ, where σ is the standard deviation of EPSC amplitude and μ is the mean EPSC amplitude), provide an independent measure of changes in presynaptic function (6, 7, 13), although alternative interpretations of this measurement have been suggested (40, 41). The parameters that determine CV are Pr, the average probability of release, and n, a parameter estimating the number of presynaptic release sites.
To test the utility of these measures in detecting presynaptic changes in synaptic efficacy, we first examined the effects of manipulations known to result in presynaptic changes in transmitter release to determine if PPF ratio and CV would be altered in the expected direction as a result of these manipulations. When we decreased Pr by lowering extracellular calcium, PPF ratio and CV values were considerably increased (Fig. 2 A and D). Furthermore, the time course of the increase in PPF ratio corresponded closely to the decrease in EPSC amplitude. In contrast, decreasing postsynaptic responsiveness had no effect on PPF ratio and CV values as predicted. Antagonizing postsynaptic AMPA/kainate receptors with a submaximal concentration of DNQX, reduced the amplitude of the evoked EPSC to 57.6 ± 6.4% of control without any change in PPF ratio or CV (Fig. 2 B and D). Reducing the intensity of afferent stimulation, a manipulation that alters the number of activated afferent fibers and hence reduces n, altered only CV values, with no change in PPF ratio (Fig. 2 C and D). Taken together, our results support previous observations that increases in both CV and PPF ratio are expected only when Pr is decreased.
To evaluate whether striatal LTD is associated with a decrease in Pr, the PPF ratio and CV were measured in the same population of synapses before and after LTD induction. As shown in Fig. 3, induction of LTD increased the PPF ratio in parallel with the depression of EPSC amplitude. Similar increases in PPF ratio were observed when synaptic efficacy was reduced to a similar extent either after LTD induction or in the presence of lowered extracellular calcium (compare Figs. 2A and 3B). This observation suggests that the change in Pr associated with LTD is sufficient to account for the depression of synaptic transmission. The CV was also increased after LTD induction [186.9 ± 35.2% of control (n = 11)].
Induction of LTD can be prevented by a variety of treatments based on the fact that LTD initiation requires postsynaptic depolarization, activation of L-type calcium channels and an increase in postsynaptic calcium concentration (27). To determine if manipulations that block LTD induction also prevent the increase in PPF ratio, we examined the effect on LTD and PPF ratio of blocking L-type calcium channels with nifedipine and preventing postsynaptic calcium increases by inclusion of 10 mM EGTA in the patch pipette solution (Fig. 4 A and B). These manipulations blocked LTD induction and prevented the associated increase in PPF ratio. In addition, changes in PPF ratio were not observed after manipulations that were insufficient to induce LTD (e.g., HFS with postsynaptic hyperpolarization or depolarization in the absence of HFS, Fig. 4 C and D). These findings indicate that the observed increases in PPF were specific to LTD expression.
Increases in CV also appeared to be specific to LTD expression. Manipulations that blocked LTD induction prevented increases in CV, and manipulations that were insufficient to induce LTD did not produce increases in CV (Fig. 5A). Moreover, increases in CV were sufficient to explain the decrease in synaptic efficacy by a presynaptic mechanism (e.g., decreases in Pr or n). If the mechanism of LTD expression is due predominantly to a change in Pr or n then the ratio of 1/CV2 values before and after LTD induction should be similar to or greater than the change in ratio of EPSC amplitudes before and after LTD as shown in Fig. 5B. If changes in postsynaptic response to transmitter account for LTD expression then the 1/CV2 value would not change after LTD induction. Fig. 5B illustrates that presynaptic manipulations alter 1/CV2 in proportion to the change in EPSC amplitude while postsynaptic receptor blockade alters EPSC amplitude with no change in 1/CV2 as predicted. The asynchrony of AMPA receptor-mediated EPSCs in our experimental conditions could not account for the increase in CV after LTD induction, since alternative measures of CV, based on charge transfer during the EPSC (data not shown), gave similar values for CV increases during LTD expression. The combined increases in CV and PPF after LTD induction suggest that LTD involves a decrease in Pr rather than a decrease in n since PPF should not change when n changes.
Postnatal Developmental Changes in PPF and LTD at Corticostriatal Synapses.
One potential physiological role for striatal LTD might be to alter the efficacy of corticostriatal synaptic transmission during postnatal development. To assess this possibility we examined the magnitude of LTD as well as the PPF ratio prior to LTD induction in striatal slices prepared from animals at different postnatal ages. As shown in Fig. 6A, basal PPF ratio was close to 1 at postnatal days 10–19 (P10–P19) and began to increase after P20, suggesting that the development of corticostriatal synapses is associated with a decrease in Pr.
To determine if the magnitude of LTD induced by pairing HFS and depolarization changed over this same developmental time course, we examined LTD at two different ages (P10–P19 and P23–P27) at which a significant difference in basal PPF ratio was observed. Unlike P10–P19 slices, in which we induced LTD in 11 of 11 slices, we failed to induce LTD in 4 of 17 slices in the P23–P27 group. The increase in PPF ratio after LTD induction as well as the magnitude of LTD in the 13 slices in the P23–P27 group that exhibited LTD were significantly smaller than in P10–P19 slices (Fig. 6B). Thus, both the apparent probability of neurotransmitter release and susceptibility to LTD decrease concomitantly with age. It should be noted that both PPF and CV were increased after LTD induction in the 13 slices in the P23–P27 group that exhibited LTD, suggesting that the mechanism underlying the LTD expression is similar at both developmental stages. These findings provide evidence that a decrease in Pr, perhaps brought about by LTD, is important for proper development of corticostriatal synapses.
DISCUSSION
The present findings suggest that a decrease in the probability of glutamate release from presynaptic terminals contributes to expression of LTD at corticostriatal synapses in young animals. Other forms of LTD described to date have not been shown to involve changes in PPF ratio, a reliable indicator of changes in Pr. For example, LTD at the Schaffer collateral–CA1 synapses in hippocampus of neonatal rats appears to involve postsynaptic induction and presynaptic expression (13); however, it has not been established whether this form of LTD involves a change in PPF ratio. Both pre- and postsynaptic expression mechanisms have been suggested to be involved in hippocampal LTD at CA3–CA1 synapses in young rats (5, 14). However, this form of LTD is not accompanied by any changes in PPF ratio, despite the fact that changes in CV have been detected during LTD. These observations suggest that the expression mechanism for this form of LTD is different from that of striatal LTD (15, 42). Cerebellar LTD results from a decrease in the postsynaptic response of Purkinje neurons to glutamate, although induction of this form of LTD involves mechanisms similar to those implicated in striatal LTD (2). To date, the only forms of central nervous system long-term synaptic plasticity shown to be accompanied by changes in PPF are LTP phenomena at the mossy fiber synapses and cerebellar parallel fiber synapses (3, 43). However, mechanisms underlying induction of these forms of LTP appear to take place at presynaptic sites in contrast to the postsynaptic site of striatal LTD induction. Striatal LTD thus appears to be a unique form of synaptic plasticity that is induced postsynaptically and is maintained by a decrease in Pr.
An alternative mechanism for this novel form of LTD expression would be a preferential loss of responsiveness at synapses with high Pr, leading to a bias toward activation of low Pr synapses. Such a change could occur by postsynaptic “silencing” of high Pr synapses. However, changes in the number of apparently silent synapses associated with Schaffer collateral–CA1 LTP (3, 12) are accompanied by changes in CV, but not changes in PPF (41). This finding suggests that changes in the number of silent synapses do not differentially affect synapses exhibiting different baseline Pr values, at least in the CA1 region of hippocampus. At present it is not clear to what extent changes in the number of silent synapses account for LTP expression. Postsynaptic silencing of high Pr synapses during striatal LTD is still a viable alternative to a decrease in Pr. Further studies will be needed to determine whether any postsynaptic changes contribute to striatal LTD.
The findings reported at present suggest a scenario for LTD induction and maintained expression that involves (i) repetitive transmission leading to depolarization of the postsynaptic striatal neuron and to the activation of dopamine and metabotropic glutamate receptors, (ii) activation of voltage-gated L-type calcium channels and increased intracellular calcium in the postsynaptic neuron, and (iii) coordination of these initiation events with presynaptic mechanisms leading to decreased glutamate release at some or all of the synapses experiencing high frequency activation. The molecular basis for this suppression of release probability has not been established, but could involve a retrograde messenger, as has been suggested to be the case for hippocampal LTP and LTD as well as LTD at the neuromuscular junction (9–11, 13, 44, 45).
Striatal LTD can be induced by high frequency afferent activation in vivo (28), and patterns of neuronal activity needed to induce striatal LTD occur in both cortical and striatal neurons in vivo. For example, Cowan and Wilson (46) have recently shown that identified corticostriatal neurons can fire action potentials at frequencies that sometimes reach values near 100 Hz for seconds. Also, a “depolarized state” in striatal neurons has been observed in vivo and is thought to be determined primarily by the pattern of coordinated excitatory inputs impinging on the striatal neuron (47). During this depolarized state, striatal neurons show a tonic firing pattern that could provide sufficient depolarization for the activation of L-type calcium channels. In fact, activation of L-type channels by moderate postsynaptic depolarization has been observed in striatal neurons (48). Thus, high frequency activation of certain populations of cortical afferents along with postsynaptic depolarization driven by coordinated inputs is likely to happen in vivo, leading to a long-term decrease in transmitter release. Interestingly, previous studies suggest that the induction of striatal LTD involves dopamine release and activation of D1 and D2 dopamine receptors (29). Activation of D1 dopamine receptors potentiates L-type channel currents, suggesting a possible role for D1 receptors in LTD induction (49). The combination of the dopaminergic activation and an up-state transition in response to cortical input may set the stage for striatal LTD in vivo.
Little is known about the physiological significance of LTD in vivo. Here we provide evidence that a decrease in Pr is associated with postnatal development of corticostriatal synapses and striatal LTD. Furthermore, susceptibility to LTD decreases concomitantly with the developmental decrease in Pr, consistent with the involvement of striatal LTD in postnatal development of corticostriatal synapses. Although developmental down-regulation of the components that are necessary for LTD induction and/or expression may be responsible for the decrease in the susceptibility to LTD, it is more likely that striatal LTD is occluded by the mechanism underlying postnatal development of corticostriatal synapses because PPF increases are a common feature of both development and LTD.
It is also important to note that the developmental increase in PPF occurs over a time course during which the depolarized state in striatal neurons first appears (50). Furthermore, bursting activity of cortical neurons also begins to appear during this developmental stage (51). Thus, the appearance of both pre- and postsynaptic activities that can trigger striatal LTD in vivo coincides closely with the developmental increase in PPF. PPF at corticostriatal synapses appears to be important for striatal physiology in adult rats. Corticostriatal synapses, but not thalamostriatal synapses, are responsible for PPF at striatal synapses in adult rats (52). Decreased PPF at corticostriatal synapses is associated with the aging process, which may contribute to motor deficits (53). Our findings are most consistent with the idea that a decrease in Pr induced by striatal LTD or a mechanistically similar process is involved in postnatal development of corticostriatal synapses. However, striatal LTD can occur in adult animals (27). Thus, LTD may also play a role in integrative functions such as learning, memory, and alterations of motor function in adult animals.
Acknowledgments
We thank Drs. Nevin A. Lambert and Lee Limbird for comments on the manuscript. This research was supported by National Institutes of Health Grant NS30470.
ABBREVIATIONS
- LTD
long-term depression
- LTP
long-term potentiation
- aCSF
artificial cerebrospinal fluid
- EPSC
excitatory postsynaptic current
- HFS
high frequency stimulation
- Pr
probability of transmitter release
- n
number of presynaptic release sites
- PPF
paired-pulse facilitation
- CV
coefficient of variation
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- DNQX
6,7-dinitroquinoxaline-2,3(1H,4H)-dione
- P
postnatal day
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 3.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 4.Kullmann D M, Siegelbaum S A. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 5.Stevens C F, Wang Y. Nature (London) 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- 6.Malinow R, Tsien R W. Nature (London) 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 7.Bekkers J M, Stevens C F. Nature (London) 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 8.Larkman A, Hannay T, Stratford K, Jack J. Nature (London) 1992;360:70–73. doi: 10.1038/360070a0. [DOI] [PubMed] [Google Scholar]
- 9.Stevens C F, Wang Y. Nature (London) 1993;364:147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo M, Small S A, Kandel E R, Hawkins R D. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 11.Schuman E M, Madison D V. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 12.Liao D, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 13.Bolshakov V Y, Siegelbaum S A. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 14.Oliet S H R, Malenka R C, Nicoll R A. Science. 1996;271:1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- 15.Mulkey R M, Malenka R C. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 16.Goodman C S, Shatz C J. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 17.Crair M C, Malenka R C. Nature (London) 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood A, Rioult M G, Bear M F. Nature (London) 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 19.Dudek S M, Friedlander M J. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 20.Fox K. Neuron. 1995;15:485–488. doi: 10.1016/0896-6273(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 21.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 22.Delong M R. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 23.Graybiel A M. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 24.Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta J C, Fazio F. Nature (London) 1994;371:600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- 25.Graybiel A M, Aosaki T, Flaherty A W, Kimura M. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 26.Knowlton B J, Mangels J A, Squire L R. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 27.Calabresi P, Pisani A, Mercuri N B, Bernardi G. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Munoz M, Young S J, Groves P M. NeuroReport. 1992;3:357–360. doi: 10.1097/00001756-199204000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Calabresi P, Maj R, Pisani A, Mercuri N B, Bernardi G. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovinger D M, Tyler E C, Meritt A. J Neurophysiol. 1993;70:1937. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- 31.Walsh J P. Brain Res. 1993;608:123–128. doi: 10.1016/0006-8993(93)90782-i. [DOI] [PubMed] [Google Scholar]
- 32.Blanton M G, Lo Turco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 33.Kemp J M, Powell T P S. Philos Trans R Soc London B. 1971;262:383–457. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguch Y, Wilson C J, Emson P C. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- 35.Cepeda C, Chandler S H, Shumate L W, Levine M S. J Neurophysiol. 1995;74:1343–1348. doi: 10.1152/jn.1995.74.3.1343. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Z G, North R A. J Physiol (London) 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malenka R C, Kocsis J D. J Neurosci. 1988;8:3750–3756. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 39.Clark K A, Randall A D, Collingridge G L. Exp Brain Res. 1994;101:272–278. doi: 10.1007/BF00228747. [DOI] [PubMed] [Google Scholar]
- 40.Korn H, Faber D. Trends Neurosci. 1991;14:439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- 41.Manabe T, Willie D J A, Perkel D J, Nicoll R A. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 42.Selig D K, Hjelmstad G O, Herron C, Nicoll R A, Malenka R C. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 43.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 44.Malenka R C. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 45.Cash S, Zucker R S, Poo M. Science. 1996;272:998–1001. doi: 10.1126/science.272.5264.998. [DOI] [PubMed] [Google Scholar]
- 46.Cowan R H, Wilson C J. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- 47.Wilson C J. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- 48.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier D J. J Neurosci. 1991;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Surmeier D J, Bargas J, Hemmings H C, Jr, Nairn A C, Greengard P. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 50.Tepper J M, Trent F. Prog Brain Res. 1993;99:35–50. doi: 10.1016/s0079-6123(08)61337-0. [DOI] [PubMed] [Google Scholar]
- 51.Kasper E M, Larkman A U, Lubke J, Blakemore C. J Comp Neurol. 1994;339:475–494. doi: 10.1002/cne.903390403. [DOI] [PubMed] [Google Scholar]
- 52.Cromwell H C, Buchwald N A, Levine M S. Neurosci Lett. 1995;192:213–217. doi: 10.1016/0304-3940(95)11633-8. [DOI] [PubMed] [Google Scholar]
- 53.Walsh J P, Qu X. Synapse. 1994;17:36–42. doi: 10.1002/syn.890170105. [DOI] [PubMed] [Google Scholar]