Abstract
Aims
Because of the widespread use of aspirin for prevention of cardiovascular diseases, side-effects associated with thromboprophylactic doses are of interest. This study summarizes the relative risk (RR) for serious upper gastrointestinal complications (UGIC) associated with aspirin exposure in general and with specific aspirin doses and formulations in particular.
Methods
After a systematic review, 17 original epidemiologic studies published between 1990 and 2001 were selected according to predefined criteria. Heterogeneity of effects was explored. Pooled estimates were calculated according to different study characteristics and patterns of aspirin use.
Results
The overall relative risk of UGIC associated with aspirin use was 2.2 (95% confidence interval (CI): 2.1, 2.4) for cohort studies and nested case-control studies and 3.1 (95% CI: 2.8, 3.3) for non-nested case-control studies. Original studies found a dose–response relationship between UGIC and aspirin, although the risk was still elevated for doses lower or up to 300 mg day−1. The summary RR was 2.6 (95% CI: 2.3, 2.9) for plain, 5.3 (95% CI: 3.0, 9.2) for buffered, and 2.4 (95% CI: 1.9, 2.9) for enteric-coated aspirin formulations.
Conclusions
Aspirin was associated with UGIC even when used at low doses or in buffered or enteric-coated formulations. The latter findings may be partially explained by channeling of susceptible patients to these formulations.
Keywords: aspirin, complications, epidemiology, meta-analysis
Introduction
Safety data from randomized, controlled, trials showed that aspirin use increases about two-fold the risk of severe gastrointestinal events and suggested a lower, but persistent, risk associated with low doses [1–5]. Based on the general population, early observational studies have reported risks of upper gastrointestinal complications (UGIC) from 1 to 10 times higher among aspirin users, with an estimated pooled relative risk between 2 and 3 [6, 8], Nonetheless, the fact that aspirin is widely available over-the-counter without prescription complicates the assessment of its effects in observational studies.
During the last years, aspirin has been increasingly used in a long-term fashion for primary and secondary prevention of cardiovascular diseases. Since the dose required for thromboprophylaxis (≤ 300 mg day−1) is lower than that needed for analgesic or anti-inflammatory indications [2], the assessment of side-effects associated with low doses is particularly important. Moreover, to diminish gastric damage, enteric-coated and buffered aspirin formulations have been suggested as alternatives to plain aspirin. Endoscopic studies showed a reduction in gastric and duodenal injury with the use of enteric-coated aspirin, but not with buffering [9–12]; whether these preparations are associated with lower risks of UGIC than plain aspirin outside an experimental setting is still unclear.
Our objective was to systematically review the literature on serious gastrointestinal complications associated with aspirin use and to evaluate the influence of dose and formulation of aspirin as well as the effect of study design. Since studies published before 1990 were included in previous reviews [6–8], this paper summarizes the main results from observational epidemiologic studies published from 1990 to 2001.
Methods
To be considered, a publication had to meet predefined inclusion criteria: Articles had to be case-control or cohort studies on aspirin use and UGIC (defined as bleeding, perforation, or other serious upper gastrointestinal event resulting in hospitalization or visit to specialist), and the articles had to provide valid relative risk estimates or enough data for us to estimate a relative risk comparing aspirin users with nonusers.
We conducted a MEDLINE search from 1990 to February 2001 searching for the terms: ‘anti-inflammatory nonsteroidal agents’ (both overall and aspirin), ‘adverse effects’, and ‘toxicity’ combined with ‘peptic ulcer’, ‘stomach ulcer’, ‘duodenal ulcer’, or ‘gastrointestinal diseases’ (including haemorrhage and perforation). The search was restricted to human studies on adults.
We identified 2477 entries and examined their abstracts. Studies on any nonsteroidal anti-inflammatory drug were considered in this first screening to avoid missing those in which aspirin was one among other drugs. When the abstract had no clear reason for exclusion, the full article was obtained. We also examined the references of previous reviews. Inclusion criteria were applied independently by two of us and decisions regarding inclusion of studies were reached by consensus. When two articles reported results from the same study population, the most recent version was chosen. However, if the earliest version provided additional subanalyses, they were considered.
A total of 46 original research articles were examined, but 20 of them did not provide specific data on aspirin [13–32]. Among the remaining 26 studies, four were rejected for the following reasons: inappropriate reference group for this particular analysis [33], the outcome was identification of gastrointestinal bleeding with endoscopy rather than the presence of serious gastrointestinal complications [34], the outcome combined upper and lower gastrointestinal bleeding [35], or methodological concerns regarding both the design (i.e. patients with ulcer history excluded only from cases) and the analysis (i.e. unclear interpretation of discordant pairs for McNemar's test) [36]. From the 22 published epidemiologic studies fulfilling all the inclusion criteria, one reported the same results in a different language [37, 38], three reported results from the same study population as more recently published articles [39–41], and one presented additional analyses from a sample that overlapped with a previous article [42]. Hence, the final number of analysed studies was 17 [38, 43–58].
A standardized data extraction form was designed to collect information on study methodology and objective quality-related characteristics. The list of characteristics was based on literature about the methods of epidemiologic studies in general and on previous meta-analyses on anti-inflammatory drugs and UGIC [6, 7, 59]. Data from articles was abstracted in duplicate and entered into a database.
To determine whether it was appropriate to pool the individual results into one common summary measure, the heterogeneity in effects between studies was analysed using the DerSimonian & Laird's test statistic for heterogeneity (Q) [60]. We calculated a summary relative risk (RR) and 95% confidence interval (CI), weighting study estimates by the inverse of the variance and estimating linear predictors for the log effect measure [61, 62]. In addition to these fixed effects estimates, we also calculated the corresponding random effects models. The odds ratio from case-control studies was assumed to provide a valid estimate of the relative risk [63]. We explored potential publication bias qualitatively using a ‘funnel plot’ [64].
Results
The relative risks of UGIC associated with aspirin use reported in the original studies are shown in Table 1 and Figure 1. The pooled RR was 2.6 (95 CI: 2.4, 2.7). However, the individual RR estimates were heterogeneous (P < 0.01) and varied from 1.4 to 11.2. We explored sources of variability among results and estimated specific RRs.
Table 1.
Pooled and individual relative risk (RR) and 95% confidence intervals (CIs) of UGIC associated with aspirin use. Studies published from 1990 to 2001.
| Study | Cases (n) | Controls (n) | RR* | 95% CI |
|---|---|---|---|---|
| Laporte et al. [43] | 875 | 2682 | 7.2 | 5.4, 9.6 |
| Holvoet et al. [44] | 161 | 161 | 2.2 | 1.3, 4.0 |
| Nobili et al. [38] | 441 | 1323 | 11.2 | 7.8, 16.9 |
| Keating J, [45]† | 77 | 77 | 2.6 | 1.0, 7.3 |
| Henry et al. [46] | 644 | 1268 | 2.4 | 1.9, 3.0 |
| Savage et al. [47]† | 494 | 972 | 2.1 | 1.5, 3.0 |
| Weil et al. [48] | 1121 | 2115 | 3.0 | 2.5, 3.7 |
| Hallas et al. [49] | 183 | NA | 1.9 | 1.2, 2.9 |
| Kelly et al. [51]† | 550 | 1202 | 2.4 | 2.0, 3.0 |
| Matikainen et al. [50]† | 48 | 156 | 1.5 | 0.6, 3.4 |
| Pérez Gutthann et al. [54] | 1377 | 10 000 | 1.4 | 1.0, 1.8 |
| McMahon et al. [52] | 172 | NA | 2.3 | 1.4, 3.8 |
| Wilcox et al. [53] | 461 | 1895 | 3.0 | 2.4, 3.7 |
| García Rodríguez et al. [55] | 1505 | 20 000 | 2.3 | 1.7, 3.2 |
| Lanas et al. [56] | 1122 | 2231 | 2.4 | 1.8, 3.3 |
| Sorensen et al. [57] | 804 | NA | 2.6 | 2.2, 2.9 |
| De Abajo et al. [58] | 2105 | 11 500 | 2.0 | 1.7, 2.3 |
| Pooled RR: Fixed effects | 2.6 | 2.4, 2.7 | ||
| Random effects | 2.7 | 2.2, 3.2 | ||
| P value test for heterogeneity: < 0.001 | ||||
Relative risk estimate and 95% CIs provided in the publication.n: number of cases or controls. NA: not applicable, cohort study.
Estimated from raw data provided in the publication.
Figure 1.
Relative risks and 95% confidence interval reported in original publications on aspirin use and UGIC during 1990–2001, stratified by study design.
Methodological factors
The main study characteristics are summarized in Table 2. Among the 16 studies considered, three were cohorts and 14 were case-control studies. Nonetheless, three case-control studies were nested in a well-defined cohort [54, 55, 58]. Ten case-control studies used matched designs. The nested case-control studies obtained their control subjects from registries; the other case-control studies ascertained controls from hospitals (n = 7), communities (n = 1), or both (n = 3). Study years ranged from 1982 to 1998. Three studies restricted their sample to elderly populations. Seven studies used computerized records as the source of exposure and outcome information (all cohort and nested case-control studies and one hospital-based case-control study); the rest were based on interviews. Nine studies specifically excluded oesophageal lesions and only considered lesions located in the stomach or duodenum. Studies often had the following exclusion criteria: cancer (n = 10), oesophageal varices (n = 10), Mallory-Weiss disease (n = 10), alcoholism (n = 7), chronic liver disease (n = 7) or/and coagulopathies (n = 6). Aspirin exposure was defined as use during the last week in nine studies, use in the last month in three studies, and use reaching the index date or prescriptions that would cover the index date in the other five studies. Aspirin use was the main exposure of interest in four studies, was one among other anti-inflammatory drugs in 10, and was only considered as a potential confounder for other main associations in three studies.
Table 2.
Description of studies on UGIC and aspirin use published from 1990 to 2001.
| Study | Design | Period | Location | Exposure assessment | Exposure window | Outcome |
|---|---|---|---|---|---|---|
| Laporte et al. [43] | Case-control | 87–88 | Spain | Interview | Last week | Hospitalization for gastric or duodenal bleeding |
| Holvoet et al. [44] | Case-control | 87–89 | Belgium | Interview | Last week | Hospitalization for upper GI tract bleeding |
| Nobili et al. [38] | Case-control | 87–88 | Italy | Interview | Last week | Hospitalization for upper GI tract bleeding+ |
| Keating [45] | Case-control | 87–91 | New Zealand | Records | Index day | Hospitalization for upper GI tract bleeding or perforation |
| Henry et al. [46] | Case-control | 85–89 | Australia | Interview | Last week | Hospitalization for upper GI tract bleeding or perforation |
| Savage et al. [47] | Case-control | 86–90 | New Zealand | Interview | Last week | Hospitalization for gastric or duodenal bleeding or perforation |
| Weil et al. [48] | Case-control | 87–91 | UK | Interview | Last month | Hospitalization for gastric or duodenal bleeding |
| Hallas et al. [49] | Cohort | 91–92 | Denmark | Records | Prescription coverage | Hospitalization for gastric or duodenal bleeding |
| Kelly et al. [51] | Case-control | 87–94 | US | Interview | Last week | Hospitalization for gastric or duodenal bleeding |
| Matikainen et al. [52] | Case-control | 92–93 | Finland | Interview | Last week | Hospitalization for upper GI tract bleeding |
| Pérez Gutthann et al. [54] | Nested Case-control | 82–86 | Canada | Records | Prescription last month | Hospitalization for gastric or duodenal bleeding or perforation |
| McMahon et al. [52] | Cohort | 89–92 | UK | Records | Prescription coverage | Hospitalization for upper GI tract bleeding or perforation |
| Wilcox et al. [53] | Case-control | 91–93 | US | Interview | Last week | Hospitalization for upper GI tract bleeding |
| García Rodríguez et al. [55] | Nested Case-control | 91–95 | Italy | Records | Prescription coverage | Hospitalization for gastric or duodenal bleeding or perforation |
| Lanas et al. [56] | Case-control | 95–98 | Spain | Interview | Last week | Hospitalization for upper GI tract bleeding |
| Sorensen et al. [57] | Cohort | 91–95 | Denmark | Records | Prescription coverage | Hospitalization for upper GI tract bleeding |
| De Abajo et al. [58] | Nested Case-control | 93–98 | UK | Records | Last month | Hospitalization for gastric or duodenal bleeding or perforation |
Study design was associated with differences in RRs. Cohort studies and nested case-control studies (n = 6) had a significantly lower summary estimate (RR = 2.2, 95% CI: 2.1, 2.4) than non-nested case-control studies (RR = 3.1, 95% CI: 2.8, 3.3). All nested case-control and cohort studies used computerized records as the source of exposure and outcome information, vs only one non-nested case-control study [45]. Exposure was defined as prescriptions that would cover the month before the index date or the index date itself in the six cohort studies or nested case-control studies. Once design was accounted, the other methodological characteristics mentioned in the paragraph above did not significantly affect the summary estimate of aspirin.
Heterogeneity of results within study design was mainly due to two non-nested case-control studies with high RR estimates (Figure 1) [38, 43]. Yet, even excluding these ‘outliers’, non-nested case-control studies had still a significantly higher average RR (RR = 2.6, 95% CI: 2.4, 2.9).
In addition, since aspirin has been widely used for cardioprotection (i.e. at lower doses) only in recent years, we estimated summary RRs for studies conducted only before and studies conducted at least in part after 1991. The pooled RR was 2.9 (95% CI: 2.6, 3.3) for earlier studies and 2.4 (95% CI: 2.2, 2.6) for later ones.
Regarding quality-related characteristics, all the studies had adequate definitions of exposure and outcome, five had slightly different inclusion criteria for cases and controls, and one had dissimilar ascertainment of compared groups. Thirteen studies verified the outcome with endoscopies, and the 6 studies using computerized records verified the information by chart review. All but two studies attempted to control for potential confounders. The most frequent confounders considered were age (n = 15), sex (n = 15), prior ulcer history (n = 9), and concomitant medication (n = 9). Among the 10 matched case-control studies, five utilized statistical analysis for matched data, three considered the matching factors in the multivariate model and two did not consider the matching factors during the analysis. Restricting the analysis to those publications with best quality did not substantially change the results.
Aspirin use factors
Five studies addressed the effect of different daily doses of aspirin in their analyses [46–48, 51, 58]; all of them found greater risks of UGIC for aspirin doses above 300 mg day−1 than for lower doses. However, the risk was still elevated for doses up to 300 mg day−1. Studies reported a significantly increased risk of UGIC with daily doses below 300 mg, [47, 56] 150 mg [46, 57], and even as low as 75 mg [48, 58] (Table 3).
Table 3.
Original relative risks (RR) and 95% confidence interval (CI) of UGIC comparing aspirin users with nonusers according to aspirin dose, 1990–2001 studies.
| Articles | Cutoff points | RR | 95% CI |
|---|---|---|---|
| Henry et al. [46] | |||
| ≤ 150 mg day−1 | 1.4 | 1.0, 2.1 | |
| > 150 mg day−1 | 2.7 | 2.0, 3.5 | |
| Savage et al. [47] | |||
| ≤ 300 mg day−1 | 1.3 | 0.8, 1.9 | |
| > 300 mg day−1 | 3.1 | 3.1, 5.1 | |
| Weil et al. [48] | |||
| 75 mg day−1 | 2.3 | 1.2, 4.4 | |
| 150 mg day−1 | 3.2 | 1.7, 6.5 | |
| 300 mg day−1 | 3.9 | 2.5, 6.3 | |
| Kelly et al. [51] | |||
| ≤ 325 mg day−1 | 2.1 | 1.5, 2.9 | |
| > 325 mg day−1 | 4.3 | 3.1, 6.0 | |
| Lanas et al. [56] | ≤ 300 mg day−1 | 2.4 | 1.8, 3.3 |
| Sorensen et al. [57] | |||
| 100 mg day−1 | 2.6 | 1.8, 3.5 | |
| 150 mg day−1 | 2.6 | 2.2, 3.0 | |
| De Abajo et al. [58] | |||
| 75 mg day−1 | 1.9 | 1.6, 2.4 | |
| 150 mg day−1 | 2.1 | 1.6, 2.7 | |
| 300 mg day−1 | 1.9 | 1.3, 2.7 | |
| > 600 mg day−1 | 4.0 | 1.4, 11.5 | |
Only four studies reported data on aspirin formulation [48, 51, 57, 58]. The pooled RRs were 2.4 (95% CI: 1.9, 2.9) for coated and 2.6 (95% CI: 2.3, 2.9) for plain preparations. Two studies found buffered aspirin not to be associated with a lower UGIC risk than regular aspirin; the pooled RRs were 4.1 (95% CI: 3.2, 5.1) for plain and 5.3 (95% CI: 3.0, 9.2) for buffered aspirin in those two studies (Table 4).
Table 4.
Specific pooled relative risks (RR) and 95% confidence interval (CI) of UGIC comparing aspirin users with nonusers according to patterns of use and other factors, 1990–2001 studies.
| Factors | Number of studies | P value* | RR | 95% CI |
|---|---|---|---|---|
| Formulation | ||||
| Plain | 4 | < 0.001 | 2.6 | 2.3, 2.9 |
| Coated | 4 | 0.515 | 2.4 | 1.9, 2.9 |
| Buffered | 2 | 0.572 | 5.3 | 3.0, 9.2 |
| Frequency | ||||
| Occasional use | 2 | 0.047 | 2.1 | 1.7, 2.6 |
| Regular use | 2 | 0.389 | 3.2 | 2.6, 3.9 |
| Duration of use | ||||
| < 1 month | 3 | 0.859 | 4.4 | 3.2, 6.1 |
| > 1 month | 3 | 0.152 | 2.6 | 2.1, 3.1 |
| Site of the lesion | ||||
| Gastric | 8 | < 0.001 | 2.9 | 2.5, 3.2 |
| Duodenal | 8 | < 0.001 | 2.6 | 2.2, 2.9 |
| Type of lesion | ||||
| Bleeding | 2 | 0.256 | 2.1 | 1.8, 2.5 |
| Perforation | 2 | 0.737 | 1.7 | 1.1, 2.5 |
| Gender | ||||
| Women | 4 | < 0.001 | 3.0 | 2.6, 3.6 |
| Men | 4 | < 0.001 | 3.0 | 2.7, 3.4 |
| Age | ||||
| < 60 years | 4 | < 0.001 | 5.0 | 4.1, 6.1 |
| > 60 years | 4 | < 0.001 | 4.0 | 3.3, 4.8 |
P value test for heterogeneity.
When frequency of exposure was investigated, the RR was higher for patients using aspirin regularly (RR = 3.2; 95% CI: 2.6, 3.9) than for patients using aspirin occasionally (RR = 2.1; 95% CI: 1.7, 2.6) [48, 51]. The risk of UGIC associated with aspirin was higher during the first month of use (RR = 4.4; 95% CI: 3.2, 6.1) than in the subsequent months of treatment (RR = 2.6; 95% CI: 2.1, 3.1) [46, 48, 58].
Other factors
The relative risk associated with aspirin use was not significantly different in women than in men [43, 44, 46, 57]; nor for patients below or above 60 years of age [38, 43, 44, 46, 57].
Studies that looked at different sites of bleeding found similar relative risks for gastric (RR = 2.9; 95% CI: 2.5, 3.2) and duodenal lesions (RR = 2.6; 95% CI: 2.2, 2.9) [43, 44, 46, 48, 50, 51, 53, 58]. Estimates of RR were not much different between bleeding (RR = 2.1; 95% CI: 1.8, 2.5) and perforation (RR = 1.7; 95% CI: 1.1, 2.5) [46, 58].
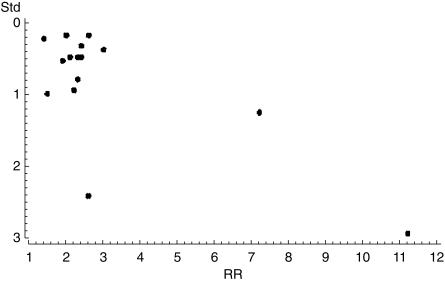
Results were practically unchanged when we used random effect models. Publication bias is unlikely in this meta-analysis, the plot of standard error vs effect size does not quite look as a pyramid but does not suggest a lack of publication of small studies with results closer to the null (Figure 2). Finally, notice that pooled RRs were often different in subanalyses than the overall pooled RR because the former were based on restricted small samples of articles that reported the required data.
Figure 2.
Funnel plot. The RR from each study is plotted on the horizontal axis and an estimate of its precision (in this case the standard error) on the vertical axis.
Discussion
This systematic review confirms that aspirin, as used in the general population, increases the risk of upper gastrointestinal complications. A greater risk is suggested for analgesic/anti-inflammatory (greater than 300 mg daily) doses than for cardioprotective (up to 300 mg) doses. Still, users of low dose of aspirin present a twofold increased risk with no clear dose–response observed under 300 mg daily. Formulation of aspirin has only a minor impact, if any, on serious UGIC. These findings are consistent with a recent meta-analysis of randomized clinical trials that shows an increased incidence of gastrointestinal haemorrhage associated with long-term aspirin, even at low doses or with modified release formulations [5].
Aspirin might induce gastrointestinal damage through several proposed mechanisms: local topical irritation, complete and irreversible impairment of platelet aggregation trough inactivation of the enzyme cyclo-oxygenase (COX-1), and inhibition of COX-1 in the gastroduodenal mucosa [65, 66]. Endoscopic studies found that enteric-coated aspirin, which is designed to reduce local damage, produces fewer gastroduodenal erosions than regular aspirin despite similar serum levels, similar inhibition of gastric mucosal prostaglandin synthesis, and similar suppression of serum thromboxane A2. These findings initially suggested that topical effects of aspirin could be of greater importance than systemic effects [9–12]. A local action would also explain the lesser degree of endoscopic mucosal erosion in the duodenum, which has a more alkaline environment [9, 10, 12]. However, in epidemiologic studies, the similar UGIC risk associated with plain, coated tablets and buffered agents is more supportive of a systemic effect [51, 57, 58]. Another line of evidence supporting a systemic rather than a topical action for serious upper gastrointestinal complications is the similar relative risk showed for duodenal and gastric lesions. Moreover, the elevated risk found with low doses would make biologic sense, since daily doses of aspirin as low as 30/50 mg are sufficient to inactivate platelet thromboxane A2 synthesis, one of the mechanisms implied in the causation of UGIC [66, 67]. Perhaps, coated aspirin is able to reduce the incidence of minor lesions in the upper GI tract, but may not be able to prevent the more serious gastrointestinal events resulting to a large extent from a systemic effect. Channeling of susceptible patients to enteric-coated aspirin may also explain the results, although original studies controlled for prior gastrointestinal history [51, 57, 58]. Regarding buffered preparations, the data from epidemiologic studies suggest that they do not only reduce the risk of upper gastrointestinal complications but appear to be associated with a more elevated risk than plain aspirin. The fact that a number of buffered formulations of aspirin (i.e. Alka-Seltzer®) have ‘heartburn’, ‘acid indigestion’ or ‘upset stomach’ as accepted indications in most countries may help to explain such results [68].
Residual confounding might also bias the overall association. Recent studies have suggested that patients with heart failure or other cardiovascular diseases, the most common indication for low dose aspirin, might be at higher independent risk for gastrointestinal bleeding [32, 56]. Although individual studies did not specifically report the effect of controlling for cardiovascular diseases, we have calculated the RR with and without adjustment for cardiovascular diseases using our data and found no major difference: RRs were 1.8 and 2.0, respectively.
The data from observational studies are rather supportive of a duration response with the highest risk concentrated during the first weeks of treatment. Clinical trials had also suggested a greater aspirin-related UGIC risk early in treatment [4, 46, 48, 58]. Such pattern may be explained by a gastric adaptation phenomena [69]. However, the self-exclusion of patients developing minor gastrointestinal symptoms associated with aspirin throughout their treatment periods could be an alternative explanation.
Summary estimates computed from observational studies with heterogeneous results have been criticized [61, 70]. Heterogeneity among publications may arise from differences in the study design, disease definition, variation in aspirin dose used by the population, occasional vs regular use, etc. In the present analysis, studies with automated databases as the source of information on exposure and outcome provided lower RR estimates than those based on personal interviews. Underestimation of aspirin use, particularly if obtained over-the-counter (OTC), and misclassification of exposed days due to noncompliance is probably greater when computerized prescriptions are used. We did a sensitivity analysis to quantify the impact of nonrecorded aspirin use [71]. With false negative probabilities beyond 50%, the net impact of nondifferential under-recorded use of OTC aspirin with respect to case status would have been a small underestimation of the RR. Moreover, although misclassification of exposures collected prospectively is usually close to nondifferential between cases and controls, we also examined the effects of differential misclassification. Only extreme (50% or over) and unrealistically differential under-recording were able to cancel the elevated risk of UGIC found for aspirin. The limited impact of missing OTC anti-inflammatory drugs use has been previously reported [72, 73]. Conversely, the assessment of exposure in non-nested case-control studies was collected retrospectively through interviews not always blinded to the case status; this may have introduced a differential misclassification of exposure resulting in an overestimation of the RR.
Studies that collected data in the eighties reported a greater risk than studies with data collected in the nineties. This could be an indirect reflection of the higher doses of aspirin used in those days for indications other than cardioprotection. This is especially true in the two studies performed in Spain and Italy at a time when prophylactic use with low dose aspirin was materially nonexistent.
In conclusion, epidemiologic studies suggest that aspirin even at daily doses lower or up to 300 mg is still associated with a twofold increased risk of upper gastrointestinal complications and that neither buffered nor enteric-coated formulations appear to materially reduce such a risk.
Acknowledgments
The study was supported in part by a research grant from Pharmacia.
References
- 1.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–226. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson JP, Prentice CRM. Aspirin: benefit and risk in thromboprophylaxis. Quart J Med. 1998;91:523–538. doi: 10.1093/qjmed/91.8.523. [DOI] [PubMed] [Google Scholar]
- 3.Stalnikowicz-Darvasi R. Gastrointestinal bleeding during low-dose aspirin administration for prevention of arterial occlusive events. J Clin Gastroenterol. 1995;21:13–16. [PubMed] [Google Scholar]
- 4.Slattery J, Warlow CP, Shorrock CJ, Langman MJS. Risks of gastrointestinal bleeding during secondary prevention of vascular events with aspirin – analysis of gastrointestinal bleeding during the UK-TIA trial. Gut. 1995;37:509–511. doi: 10.1136/gut.37.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Br Med J. 2000;321:1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115:787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 7.Bollini P, García Rodríguez LA, Pérez Gutthann S, Walker AM. The impact of research quality and study design on epidemiologic estimates of the effect of nonsteroidal anti-inflammatory drugs on upper gastrointestinal tract disease. Arch Intern Med. 1992;152:1289–1295. [PubMed] [Google Scholar]
- 8.Henry D, Lim LL, García Rodríguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. Br Med J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotiezer JW, Silvoso GR, Burks M, Ivey KJ. Comparison of the effects of regular and enteric-coated aspirin on gastroduodenal mucosa of man. Lancet. 1980;ii:609–612. doi: 10.1016/s0140-6736(80)90282-2. [DOI] [PubMed] [Google Scholar]
- 10.Lanza FL, Royer GL, Nelson RS. Endoscopic evaluation of the effects of aspirin, buffered aspirin, and enteric-coated aspirin on gastric and duodenal mucosa. N Engl J Med. 1980;304:136–137. doi: 10.1056/NEJM198007173030305. [DOI] [PubMed] [Google Scholar]
- 11.Hawthorne AB, Mahida YR, Cole AT, Hawkey CJ. Aspirin-induced gastric mucosal damage. prevention by enteric coating and relation to prostaglandin synthesis. Br J Clin Pharmacol. 1991;32:77–83. doi: 10.1111/j.1365-2125.1991.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petroski D. Endoscopic comparison of three aspirin preparations and placebo. Clin Ther. 1993;15:1993. [PubMed] [Google Scholar]
- 13.Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med. 1991;114:257–263. doi: 10.7326/0003-4819-114-4-257. [DOI] [PubMed] [Google Scholar]
- 14.Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: Incidence and risk factor models. Am J Med. 1991;91:213–222. doi: 10.1016/0002-9343(91)90118-h. [DOI] [PubMed] [Google Scholar]
- 15.García Rodríguez LA, Walker AM, Perez Gutthann S. Nonsteroidal antiinflammatory drugs and gastrointestinal hospitalizations in Saskatchewan: a Cohort Study. Epidemiology. 1992;3:337–342. doi: 10.1097/00001648-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Voskuyl AE, Van de Laar MAFJ, Moens HJB, Van der Korst JK. Extra-articular manifestations of rheumatoid arthritis: risk factors for serious gastrointestinal events. Ann Rheum Dis. 1993;53:771–775. doi: 10.1136/ard.52.11.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanas A. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology. 1992;103:862–869. doi: 10.1016/0016-5085(92)90018-t. [DOI] [PubMed] [Google Scholar]
- 18.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med. 1993;153:1665–1670. [PubMed] [Google Scholar]
- 19.Marriott JF, Asquith PA, Shorrock CJ. The use of proprietary medicines by patients presenting with peptic ulcer haemorrhage. Br J Clin Pharmacol. 1993;35:451–455. doi: 10.1111/j.1365-2125.1993.tb04168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- 21.Lanza LL, Walker AM, Bortnichack EA, Dreyer NA. Peptic ulcer and gastrointestinal hemorrhage associated with nonsteroidal anti-inflammatory drug use in patients younger than 65 years. A large health maintenance organization cohort study. Arch Intern Med. 1995;155:1371–1377. [PubMed] [Google Scholar]
- 22.Traversa G, Walker AM, Ippolito FM, et al. Gastroduodenal toxicity of different nonsteroidal antiinflammatory drugs. Epidemiology. 1995;6:49–54. doi: 10.1097/00001648-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol. 1995;141:539–545. doi: 10.1093/oxfordjournals.aje.a117469. [DOI] [PubMed] [Google Scholar]
- 24.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206–210. [PubMed] [Google Scholar]
- 25.Cullen D, Hawkey G, Greenwood D, et al. Peptic ulcer bleeding in the elderly. relative roles of Helicobacter pylori and non-steroidal anti-inflammatory drugs. Gut. 1997;41:459–462. doi: 10.1136/gut.41.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald T, Morant S, Robinson G, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. Br Med J. 1997;315:1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurata J, Nogawa A, Noritake D. NSAIDs increase risk of gastrointestinal bleeding in primary care patients with dyspepsia. J Family Prac. 1997;45:227–235. [PubMed] [Google Scholar]
- 28.Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sainz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683–689. doi: 10.1053/gast.1997.v112.pm9041228. [DOI] [PubMed] [Google Scholar]
- 29.Menniti-Ippolito F, Maggini M, Raschetti R, Da Cas R, Traversa G, Walker AM. Ketorolac use in outpatients and gastrointestinal hospitalization: a comparison with other non-steroidal anti-inflammatory drugs in Italy. Eur J Clin Pharmacol. 1998;54:393–397. doi: 10.1007/s002280050481. [DOI] [PubMed] [Google Scholar]
- 30.Singh G, Ramey DR. NSAID induced gastrointestinal complications. The ARAMIS Perspective −1997. J Rheumatol. 1998;25(Suppl. 51):8–16. [PubMed] [Google Scholar]
- 31.Singh G, Triadafilopoulos G. Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol. 1999;26(Suppl. 26):18–24. [PubMed] [Google Scholar]
- 32.Weil J, Langman M, Wainwright P, et al. Peptic ulcer bleeding, accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46:27–31. doi: 10.1136/gut.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen JM, Hallas J, Lauritsen JM, Bytzer P. Non-steroidal anti-inflammatory drugs and ulcer complications: a risk factor analysis for clinical decision-making. Scand J Gastroenterol. 1996;31:126–130. doi: 10.3109/00365529609031975. [DOI] [PubMed] [Google Scholar]
- 34.Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: Preliminary findings. Am J Gastroenterol. 1997;92:924–928. [PubMed] [Google Scholar]
- 35.Blot WJ, McLaughlin JK. Over the counter non-steroidal anti-inflammatory drugs and risk of gastrointestinal bleeding. J Epidemiol Biostatistics. 2000;5:137–142. [PubMed] [Google Scholar]
- 36.Chan T, Critchley J, Lau J, Sung J, Chung S, Anderson D. The relationship between upper gastrointestinal hemorrhage and drug use: a case control study. Int J Clin Pharmacol Ther. 1996;34:304–308. [PubMed] [Google Scholar]
- 37.Nobili A, Mosconi P. Emorragie del tratto gastroenterico superiore e farmaci anti-infiammatori non steroidei: risultati de una sorvegglianza caso-controllo. Recenti Progressi Med. 1993;84:1–26. [PubMed] [Google Scholar]
- 38.Nobili A, Mosconi P, Franzosi MG, Tognoni G. Non-steroidal anti-inflammatory drugs and upper gastrointestinal bleeding, a post-marketing surveillance case-control study. Pharmacoepidemiology Drug Safety. 1992;1:65–72. [Google Scholar]
- 39.Langman M, Weil J, Wainwright P, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;342:1075–1078. doi: 10.1016/s0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman DW, Kelly JP, Sheehan JE, et al. Nonsteroidal anti-inflammatory drug use in relation to major upper gastrointestinal bleeding. Clin Pharmacol Ther. 1993;53:485–494. doi: 10.1038/clpt.1993.55. [DOI] [PubMed] [Google Scholar]
- 41.Lanas A, Bajador E, Serrano P, Arroyo M, Fuentes J, Santolaria S. Effects of nitrate and prophylactic aspirin on upper gastrointestinal bleeding: a retrospective case-control study. J Int Med Res. 1998;26:120–128. doi: 10.1177/030006059802600302. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman DW, Kelly JP, Wiholm B-E, et al. The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol. 1999;94:3189–3196. doi: 10.1111/j.1572-0241.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 43.Laporte J-R, Carné X, Vidal X, Moreno V, Juan J. Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal antiinflammatory drugs. Lancet. 1991;337:85–89. doi: 10.1016/0140-6736(91)90744-a. [DOI] [PubMed] [Google Scholar]
- 44.Holvoet J, Terriere L, Van Hee W, Verbist L, Fierens E, Hautekeete M. Relation of upper gastrointestinal bleeding to non-steroidal anti-inflammatory drugs and aspirin: a case-control study. Gut. 1991;32:730–734. doi: 10.1136/gut.32.7.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keating J. Antiinflammatory drugs and emergency surgery for peptic ulcers in the Waikato. N Z Med J. 1992;105:127–129. [PubMed] [Google Scholar]
- 46.Henry D, Dobson A, Truner C. Variability in the risk of major gastrointestinal complications from nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology. 1993;105:1078–1088. doi: 10.1016/0016-5085(93)90952-9. [DOI] [PubMed] [Google Scholar]
- 47.Savage R, Moller P, Ballantyne C, Wells J. Variation in the risk of peptic ulcer complications with nonsteroidal antiinflammatory drug therapy. Arthritis Rheumatism. 1993;36:84–90. doi: 10.1002/art.1780360114. [DOI] [PubMed] [Google Scholar]
- 48.Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. Br Med J. 1995;310:827–830. doi: 10.1136/bmj.310.6983.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallas J, Lauritsen J, Dalsgard Villadsen H, Freng Gram L. Nonsteroidal anti-inflammatory drugs and upper gastrointestinal bleeding, identifying high-risk groups by excess risk estimates. Scand J Gastroenterol. 1995;30:438–444. doi: 10.3109/00365529509093304. [DOI] [PubMed] [Google Scholar]
- 50.Matikaienen M, Kangas E. Is there a relationship between the use of analgesics and non-steroidal anti-inflammatory drugs and acute upper gastrointestinal bleeding? A Finnish case-control prospective study. Scand J Gastroenterol. 1996;31:912–916. doi: 10.3109/00365529609052001. [DOI] [PubMed] [Google Scholar]
- 51.Kelly JP, Kaufman DW, Jugelon JM, Sheehan JE, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996;348:1414–1416. doi: 10.1016/S0140-6736(96)01254-8. [DOI] [PubMed] [Google Scholar]
- 52.McMahon A, Evans J, White G, et al. A cohort study (with re-sampled comparator groups) to measure the association between new NSAID prescribing and upper gastrointestinal hemorrhage and perforation. J Clin Epidemiol. 1997;50:351–356. doi: 10.1016/s0895-4356(96)00361-7. 10.1016/s0895-4356(96)00361-7. [DOI] [PubMed] [Google Scholar]
- 53.Wilcox CM, Alexander LN, Cotsonis GA, Clark WS. Nonsteroidal antiinflammatory drugs are associated with both upper and lower gastrointestinal bleeding. Dig Dis Sci. 1997;42:990–997. doi: 10.1023/a:1018832902287. [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Gutthann S, Garcia Rodriguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997;8:18–24. doi: 10.1097/00001648-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 55.García Rodríguez LA, Cattaruzzi C, Troncon MG, Agostinis L. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33–39. doi: 10.1001/archinte.158.1.33. [DOI] [PubMed] [Google Scholar]
- 56.Lanas A, Bajador E, Serrano P, et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834–839. doi: 10.1056/NEJM200009213431202. [DOI] [PubMed] [Google Scholar]
- 57.Sorensen HT, Mellemkjaer L, Blot WJ, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95:2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 58.De Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol. 2001;1:1. doi: 10.1186/1472-6904-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jick H, Garcia Rodriguez LA, Perez-Guthann S. Principles of epidemiological research on adverse and beneficial drug effects. Lancet. 1998;352:1767–1770. doi: 10.1016/s0140-6736(98)04350-5. 10.1016/s0140-6736(98)04350-5. [DOI] [PubMed] [Google Scholar]
- 60.Takkouche B, Cadarso-Suarez C, Spiegelman D. An evaluation of old and new tests of heterogeneity in meta-analysis in epidemiologic research. Am J Epidemiol. 1999;150:206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 61.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150:469–475. doi: 10.1093/oxfordjournals.aje.a010035. [DOI] [PubMed] [Google Scholar]
- 62.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:77–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 63.Walker AM. Observation and inference. An introduction to the methods of epidemiology. Newton Lower Falls: Epidemiology Resources Inc.; 1991. [Google Scholar]
- 64.Cooper H, Hedges LV. The handbook of research synthesis. New York: Russel Sage Foundation; 1994. [Google Scholar]
- 65.Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 66.Hawkey C. Review article: aspirin and gastrointestinal bleeding. Alimentary Pharmacol Ther. 1994;8:141–146. doi: 10.1111/j.1365-2036.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 67.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 68.Physicians' Desk Reference. 53. Montrale, NJ: Medical Economics Company; 1999. [Google Scholar]
- 69.Olivero J, Graham D. Gastric adaptation to nonsteroidal anti-inflammatory drugs in man. Scand J Gastroenterol. 1992;27(Suppl. 193):53–58. doi: 10.3109/00365529209096006. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro S. Point/Counterpoint: Meta-analysis of observational studies. Am J Epidemiol. 1994;140:771–778. doi: 10.1093/oxfordjournals.aje.a117324. [DOI] [PubMed] [Google Scholar]
- 71.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25:1107–1116. [PubMed] [Google Scholar]
- 72.Drews CD, Greenland S. The impact of differential recall on the results of case-control studies. Int J Epidemiol. 1990;19:1107–1112. doi: 10.1093/ije/19.4.1107. [DOI] [PubMed] [Google Scholar]
- 73.Ulcickas YM, Rothman K, Johnson C, et al. Using prescription claims for drugs available over-the-counter (OTC) Pharmacoepidemiol Drug Safety. 2000;9:S37. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]