Abstract
Aims
To determine the basal pharmacokinetics, lung uptake and plasma cortisol suppression for two commonly prescribed inhaled corticosteroids.
Methods
Twenty-one subjects (13 healthy and 8 mild asthmatic patients) received fluticasone propionate via a chlorofluorocarbon-propelled pressurized metered-dose inhaler (pMDI) (healthy subjects only) and Diskus® and budesonide via Turbuhaler®, 1000 µg twice daily for 7 days. Intravenous doses (200 µg) of both compounds were used as references. Plasma concentrations of fluticasone and budesonide were determined during 48 h by liquid chromatography plus tandem mass spectrometry (LC-MS-MS). Plasma concentrations of cortisol were determined by LC-MS every second hour for 24 h at baseline, and following each treatment.
Results
The volume of distribution was found to be larger and the elimination half-life and mean absorption time longer for fluticasone than for budesonide. The systemic availability of budesonide via Turbuhaler (39%) was significantly higher than that of fluticasone via Diskus (13%) (ratio 3.0 [2.5, 3.6] with 95% confidence interval [CI]), and via pMDI (21%) (ratio 1.8 [1.3, 2.3]). In addition, at steady state the systemic availability of fluticasone via pMDI was significantly higher than via Diskus (ratio 1.6 [1.1, 2.2]). The lung deposition of budesonide via Turbuhaler was 2.2-fold [1.7, 2.9] higher than that of fluticasone pMDI and 3.4-fold [2.8, 4.0] higher than that of fluticasone Diskus. In addition, the lung deposition of fluticasone via pMDI was 1.5-fold [1.1, 2.9] higher than that via the Diskus inhaler. Plasma cortisol (24 h) was significantly reduced vs baseline for all three treatments. The cortisol concentration vs baseline was 12% for fluticasone pMDI, which was significantly lower (ratio 0.32 [0.24, 0.42]) than that for fluticasone Diskus (39%), and for budesonide Turbuhaler (46%) (ratio 0.27 [0.21, 0.37]). The plasma cortisol concentration did not differ significantly between treatments with fluticasone Diskus and budesonide Turbuhaler (ratio 0.87 [0.65; 1.15]).
Conclusions
Budesonide and fluticasone differ in their pharmacokinetic properties in that although clearance is the same, the rate of uptake and elimination is slower for fluticasone. Despite a significantly higher pulmonary availability of budesonide via Turbuhaler, the plasma cortisol suppression is less than that of fluticasone via pMDI and similar to that of fluticasone via Diskus. There is no indication of any difference between healthy subjects and mild asthmatic patients in the pharmacokinetics and plasma cortisol suppression of fluticasone and budesonide.
Keywords: asthma, budesonide, fluticasone, healthy, pharmacokinetics, plasma cortisol
Introduction
Fluticasone propionate (hereafter fluticasone) and budesonide are inhaled corticosteroids used for the local treatment of inflammatory diseases in the airways, e.g. asthma and rhinitis. The desired properties of an inhaled corticosteroid for asthma include a high glucocorticoid receptor binding affinity, a high lung deposition, and a long pulmonary residence time. To minimize the systemic exposure, systemic clearance should be high and volume of distribution low, leading to a rapid systemic elimination. Both drugs are rapidly metabolized, with a total blood clearance approaching the hepatic blood flow [1, 2]. The oral systemic bioavailability is about 1% for fluticasone [3] and about 10% for budesonide [2]. For neither drug is there any evidence of local metabolic inactivation in the lungs. Therefore, for well-performing devices, the fraction of inhaled drug reaching the lungs contributes substantially to the systemic availability, and subsequently to systemic effects such as plasma cortisol suppression.
Fluticasone has been claimed to be slowly absorbed over a long period of time after deposition in the lungs, and thus a slow systemic absorption from the lungs has been suggested to be the rate-limiting step in the elimination of the drug [4]. If so, the terminal half-life would be longer after inhalation than after intravenous administration. There are studies indicating a shorter half-life after intravenous administration (7–8 h) [5] than after inhalation (10–12 h) [6], but determination of the terminal half-life may have been inaccurate in the intravenous studies due to plasma concentrations going below the lower limit of quantification (LOQ). An improved bioassay with a lower LOQ, in combination with high intravenous doses and longer sampling time, would enable a more correct estimation of the terminal phase of the plasma concentration curve.
Inhaled fluticasone has been shown to give a more pronounced plasma cortisol suppression after repeated administration than a single dose, whereas budesonide did not show such a marked difference [7]. This discrepancy between single and repeated dosing for cortisol suppression may be explained by the pharmacokinetic properties of the drugs, since fluticasone has been found to have a slower systemic elimination than budesonide, leading to accumulation and doubling of plasma concentrations after repeated dosing [3]. The elimination half-life of fluticasone is considerably longer than for many other inhaled corticosteroids; elimination half-lives in the range of 1.5–2.5 h have been reported for triamcinolone acetonide [8], flunisolide [9], and budesonide [10]. Thus, an extensive distribution into tissues, as indicated by a high volume of distribution, appears to be the cause of this slow elimination of fluticasone.
Available documentation on pharmacokinetics and systemic effects of fluticasone and budesonide is mainly based on studies in healthy subjects [1, 5, 6, 10, 11]. One reason for this is to minimize the between-subject variability [12]. Recently, a correlation between asthma severity and cortisol suppression was shown for fluticasone [13], although for budesonide, such a relationship is much less clear [14]. As a majority of asthmatic patients suffer from a mild form of the disease and as the variability in pharmacokinetics and dynamics of inhaled corticosteroids probably is lower in these patients than in those with severe asthma, this group of patients is highly relevant to select for comparisons of pharmacokinetics and systemic side-effects between different drug-inhaler combinations.
The objective of the present study in patients with asthma and in healthy subjects was to determine the pharmacokinetics and lung uptake of fluticasone from a pressurized metered-dose inhaler (pMDI) (only healthy subjects) and Diskus and of budesonide from Turbuhaler. The influence of the three different drug-inhaler combinations on plasma cortisol concentrations (area under the curve [AUC]) after multiple-dose inhalations was also determined.
Methods
The study was an open, crossover trial conducted in Lund, Sweden. The trial was randomized between the three inhalation treatments and baseline cortisol. The two intravenous, single dose administrations, were separately randomized at the end of the study. The subjects received fluticasone via Diskus or pMDI (only healthy), or budesonide via Turbuhaler, as a single-dose inhalation on day 1 and as repeated-dose inhalations, twice daily, during day 3–9, with a wash-out of at least 2 weeks between treatments. The trial was approved by the research ethics committee in Lund/Malmö, Sweden.
Subjects
The subjects were either healthy, as determined by medical history, physical examination and clinical laboratory tests, or diagnosed with asthma as defined by the American Thoracic Society [15], and currently not being treated with glucocorticosteroids by any route of administration. Demographics, asthma duration and morning peak expiratory flow during the two treatment periods are given in Table 1. All subjects received full verbal and written information about the study, and provided written informed consent before inclusion.
Table 1.
Demographics, duration of asthma and morning peak expiratory flow during the two treatment periods.
| Subject category | Sex | Age (years) | Weight (kg) | Height (cm) | Atopy | Asthma duration (years) | Morning PEF at baseline T1 and T2* | |
|---|---|---|---|---|---|---|---|---|
| Healthy | Male | 30 | 85 | 180 | No | NA | Not determined | |
| Healthy | Male | 27 | 76 | 191 | No | NA | Not determined | |
| Healthy | Male | 23 | 95 | 186 | No | NA | Not determined | |
| Healthy | Female | 24 | 83 | 173 | No | NA | Not determined | |
| Healthy | Female | 24 | 63 | 159 | No | NA | Not determined | |
| Healthy | Male | 30 | 80 | 176 | No | NA | Not determined | |
| Healthy | Male | 27 | 67 | 178 | No | NA | Not determined | |
| Healthy | Female | 19 | 59 | 166 | No | NA | Not determined | |
| Healthy | Male | 22 | 72 | 178 | No | NA | Not determined | |
| Healthy | Female | 27 | 73 | 170 | No | NA | Not determined | |
| Healthy | Male | 23 | 75 | 184 | No | NA | Not determined | |
| Healthy | Female | 28 | 70 | 164 | No | NA | Not determined | |
| Healthy | Female | 29 | 53 | 170 | No | NA | Not determined | |
| With asthma | Male | 24 | 91 | 186 | Yes | 14 | 84 | 82 |
| With asthma | Male | 28 | 65 | 167 | Yes | 21 | 94 | 98 |
| With asthma | Male | 24 | 75 | 176 | Yes | 14 | 97 | 84 |
| With asthma | Male | 32 | 84 | 185 | Yes | 6 | 93 | 81 |
| With asthma | Male | 20 | 80 | 174 | Yes | 15 | 112 | 101 |
| With asthma | Male | 51 | 95 | 183 | Yes | 44 | 67 | 63 |
| With asthma | Female | 30 | 82 | 166 | Yes | 15 | 108 | 89 |
| With asthma | Female | 43 | 72 | 171 | Yes | 22 | 98 | 105 |
| Mean (s.d.) | M = 13, F = 8 | 28 (7) | 76 (11) | 175 (9) | – | 19 (11) | 94 (14) | 88 (14) |
T1=treatment period 1 and T2=treatment period 2.
NA = Not applicable.
Study procedures
On the study days, the subjects arrived at the clinic in the morning after fasting overnight (no food or beverage after 22.00 h). No extensive physical exercise or alcohol consumption was allowed for 48 h before, and during each treatment period. The subjects were served a regular breakfast at the clinic 30 min before drug administration, and then had to abstain from beverages for 2 h and from food for 4 h. They stayed overnight at the clinic on the first night after the intravenous infusions, after the single-dose inhalations, and between days 9 and 10 during repeated administration.
Fluticasone pMDI was not administered to the asthmatic patients, as it was thought that any possible differences between pMDI and Diskus in the pharmacokinetics and systemic activity of fluticasone would be sufficiently reflected in the healthy subjects.
Intravenous dosing
The intravenous administrations were given at approximately 08.00 h as single doses of 200 µg of fluticasone and budesonide. Plasma (100 ml) was extracted from each individual subject into a sterile plastic bag. A volume of 2.0 ml of fluticasone (250 µg ml−1) or 0.5 ml of budesonide (1000 µg ml−1) in 70% ethanol was infused into the plasma bag during continuous mixing. Thereafter, 40 ml of the resulting solution was infused using an infusion pump (240 ml h−1) over 10 min into an antecubital vein in the arm not used for blood sampling. After infusion, the indwelling catheter was rinsed with saline. The exact dose was determined by weighing the syringe before and after dosing, and analysing the concentration of fluticasone or budesonide in the plasma remaining in the syringe. Blood samples (5 ml) for determination of fluticasone or budesonide in plasma were obtained at predecided timepoints; immediately before and at 5, 10, 15, 20, and 40 min, and 1, 1.5, 2, 4, 6, 8, 12, 16, 24, 36 and 48 h after the start of infusion. To achieve an adequate sensitivity in the bioanalytical assays, a larger sample volume (10 ml) was taken at the sampling points at 16–48 h.
Inhalation
The subjects were instructed and trained to use each inhaler according to the instructions supplied by the manufacturer. The subjects had to breathe out and to inhale via Diskus and Turbuhaler at an equivalent inspiratory effort, i.e. at a flow of 70 l min−1 via Diskus and 60 l min−1 via Turbuhaler. The target flow for inhalations via pMDI was 30 l min−1. Peak inspiratory flow and inspiratory volume of each inhalation of fluticasone and budesonide at the clinic were monitored with a pneumotachygraph. The first dose was inhaled at the clinic at 08.00 h on day 1. After inhalation, the subjects were instructed to hold their breath for 5 s, and then to exhale. A total dose of 1 mg was inhaled either as 4 × 250 µg of fluticasone, or as 5 × 200 µg of budesonide. After drug inhalation, the subjects rinsed their mouths with 2 × 10 ml of water, which was collected and analysed for fluticasone or budesonide content. Subsequently, each subject self-administered a dose of 1 mg of fluticasone or budesonide at home, twice daily (08.00 h and 20.00 h), starting with the morning dose of day 3 and continuing during a period of 7 days, using a diary to record the inhalations. The last three doses were also inhaled at the clinic under supervision. Drug inhalations had to be carried out satisfactory before proceeding with blood sampling. Blood samples for determination of inhaled drug in plasma were obtained at predecided timepoints; immediately before and at 10, 20, 40 min, and 1, 2, 4, 6, 8, 12, 16, 24, 36, and 48 h following start of inhalation of the morning dose on days 1 and 9. Samples for the measurement of plasma cortisol concentrations were taken every second hour during the first 24 h after the morning dose on day 9, with an additional sample taken at 36 h, and compared with baseline concentrations obtained on a separate occasion.
Assays
The delivered dose (dose leaving the inhaler) was assessed by sampling the dose onto a filter (Turbuhaler and pMDI) or as the total amount of drug found in a 5-stage Multistage Liquid Impinger (Diskus), and subsequent determination by u.v. spectrophotometry (budesonide) or liquid chromatography (fluticasone). The dose-to-subject was then calculated as the delivered dose subtracted with the amount of drug recovered in the mouth-rinsing water.
Budesonide or fluticasone was isolated from plasma or mouth-rinsing water by solid phase extraction and analysed by liquid chromatography plus tandem mass spectrometry with athmospheric pressure chemical ionization. The LOQ was 25 pmol l−1 for both budesonide and fluticasone, with a coefficient of variation of 11.2% at 36 pmol l−1 for budesonide and 17.3% at 25 pmol l−1 for fluticasone.
Plasma cortisol concentrations were analysed using a radioimmunoassay (Orion Diagnostica). The LOQ was 20 nmol l−1 with a coefficient of variation of 24.3% at 40 nmol l−1.
Data analysis
The AUC of plasma concentration vs time, volume of distribution at steady state, clearance, mean residence time, mean absorption time, and systemic availability were calculated according to routine methods. The pulmonary availability of the dose-to-subject was estimated from the systemic availability with a method described previously [16].
The fraction of the dose deposited in and absorbed from the lung (flung) was calculated from the systemic availability (Fsystemic) on the assumption that oral availability (Foral) was 10% for budesonide and 1% for fluticasone, and that no metabolism occurred locally in the lungs, using the following equation:
where ‘fret’ is the fraction of the nominal dose retained in the device. The pulmonary deposition in percent of the nominal dose (Flung, nominal) was then obtained as flung, nominal × 100.
The plasma variability (ΔC) was calculated as (Cmax−Cmin)/Css. The accumulation index (Racc) was calculated from the single-dose inhalations as AUC(0, 12 h)/∫
The mean plasma cortisol concentrations during the defined sampling period, defined as the AUC (trapezoidal rule) divided by observational time, were compared between inhalation treatments and baseline.
Sample size determination was based on the comparison of the systemic availability of the different drug-inhaler combinations of fluticasone and budesonide. It was estimated that with 12 healthy subjects there was 80% probability to detect a difference in systemic availability between the drug-inhaler combinations, if the true value lies outside 62–160% for one combination of drug-inhaler relative to another. This assumes a within-subject standard deviation of 0.36 for the log of the estimated systemic availability. The corresponding figures with six patients were 50–200%.
Results
Intravenous kinetics
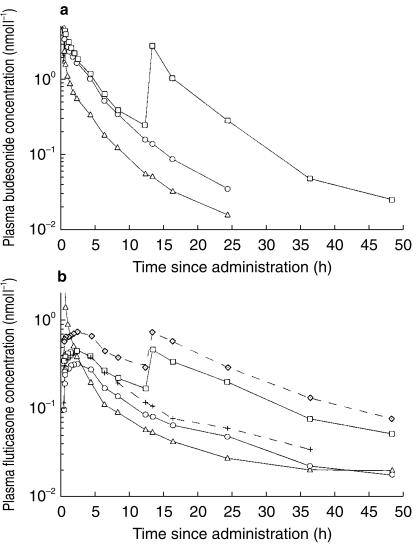
The plasma concentration curves after intravenous administration of budesonide and fluticasone are shown in Figure 1a and b. The plasma concentrations of budesonide could be monitored for at least 16 h in all but one healthy and one asthmatic subject. The plasma concentrations of fluticasone could be monitored for 24 h in all subjects after intravenous administration and for 36 h in about half of the subjects. The pharmacokinetic parameters of distribution and elimination of budesonide and fluticasone, derived from the intravenous administrations in healthy subjects and in asthmatic patients, are given in Table 2. The elimination rate was estimated individually from the terminal phase of the plasma concentration vs time curves using log-linear regression, with a user-independent algorithm for inclusion of timepoints in the analysis. The estimated terminal half-lives of fluticasone did not differ significantly between the intravenous administration and the inhalation treatments (Table 3).
Figure 1.
a) Mean plasma concentrations of budesonide after intravenous administration of 200 µg (n = 21), and after a single dose inhalation of 1000 µg and repeated dose inhalations of 1000 µg twice daily for 7 days via Turbuhaler (n = 21). ▵=Intravenous administration, ○=Budesonide Turbuhaler, single dose inhalation, (□=Budesonide Turbuhaler, repeated dose inhalation.b) Mean plasma concentrations of fluticasone after intravenous administration of 200 µg (n = 21) and after a single dose inhalation of 1000 µg after inhalation and repeated dose inhalations of 1000 µg twice daily for 7 days via pMDI (n = 13) and Diskus (n = 21). ▵=Intravenous administration, ○=Fluticasone Diskus, single dose inhalation, □=Fluticasone Diskus, repeated dose inhalation, +=Fluticasone pMDI, single dose inhalation, ◊=Fluticasone pMDI, repeated dose inhalation.
Table 2.
Pharmacokinetic parameters of distribution and elimination of budesonide and fluticasone after intravenous administration of 200 µg, respectively.
| Substance | Subjects | n | Dose (nmol)* | t½ (h) | AUC (nmol l−1 h) | MRT (h) | CL (ml min−1) | Vss (l) |
|---|---|---|---|---|---|---|---|---|
| Budesonide | Healthy | 13 | 406 (49) | 4.4 (3.7; 5.4) | 5.0 (4.5; 5.6) | 3.5 (3.0; 4.0) | 1334 (1213; 1466) | 280 (245; 321) |
| With asthma | 8 | 397 (24) | 4.6 (3.5; 6.1) | 4.9 (4.2; 5.8) | 3.8 (3.1; 4.7) | 1344 (1167; 1548) | 310 (269; 357) | |
| Total | 21 | 403 (39) | 4.5 (3.9; 5.2) | 5.0 (4.6; 5.4) | 3.6 (3.3; 4.0) | 1338 (1245; 1437) | 291 (265; 320) | |
| Fluticasone | Healthy | 13 | 377 (19) | 12.7 (9.4; 17.2) | 5.0 (4.6; 5.5) | 8.0 (5.8; 11.0) | 1245 (1140; 1359) | 599 (448; 800) |
| With asthma | 8 | 386 (26) | 12.0 (9.0; 16.1) | 4.5 (3.6; 5.7) | 7.1 (5.3; 9.5) | 1418 (1134; 1775) | 607 (475; 777) | |
| Total | 21 | 380 (22) | 12.5 (10.2; 15.2) | 4.8 (4.4; 5.3) | 7.7 (6.2; 9.5) | 1308 (1192; 1436) | 602 (500; 726) |
Values given as geometric means (95% CI).
Arithmetic mean (s.d.).
t½=half-life; AUC=area under the curve of plasma concentration vs time; MRT=mean residence time; CL=clearance; Vss=volume of distribution at steady state.
Table 3.
Terminal half-lives of fluticasone after intravenous administration and after inhalation via Diskus and pMDI (only healthy subjects).
| Dosing regimen | Subjects | Diskus (h) | pMDI (h) | Intravenous (h) |
|---|---|---|---|---|
| Single dose | Healthy | 11.4 (8.4–17.5) | 12.8 (7.1–41.4) | 12.7 (6.8–29.0) |
| With asthma | 9.4 (3.4–17.7) | – | 12.0 (7.1–19.5) | |
| Total | 10.7 (3.4–17.7) | – | 12.5 (6.8–29.0) | |
| Repeated dosing | Healthy | 11.1 (8.3–15.4) | 12.4 (8.1–18.9) | – |
| With asthma | 11.2 (9.9–14.6) | – | – | |
| Total | 11.1 (8.3–15.4) | – | – |
Values are given as geometric means (range).
Inhalation kinetics
The total amount of drug in the mouth washings was 173 µg of budesonide, 221 µg of fluticasone after inhalation via Diskus, and 196 µg of fluticasone after inhalation via pMDI. The mean dose-to-subject was comparable in molar amounts for the three formulations with 1327 nmol (572 µg) of budesonide via Turbuhaler, 1510 nmol (755 µg) of fluticasone via Diskus, and 1336 nmol (668 µg) of fluticasone via pMDI.
The plasma concentration curves after inhalation of budesonide and fluticasone are shown in Figure 1a and b. It was possible to monitor the plasma concentrations of budesonide for 24 h after the single dose in all but two healthy subjects and in all asthmatic patients, and for 36 h in all subjects but one after repeated doses. The plasma concentrations of fluticasone were possible to monitor for 36 h after the single dose via Diskus in all but two healthy and four asthmatic subjects, and in all subjects for 48 h after repeated doses. After inhalation via the pMDI in the healthy subjects, the plasma concentrations of fluticasone could be monitored for 36 h in all but three subjects after the single dose and for 48 h in all subjects after repeated doses. The pharmacokinetic parameters of fluticasone and budesonide, derived from the inhalations, are given in Tables 4a and 4b.
Tables 4a and 4b.
Pharmacokinetic parameters derived from repeated dose inhalations (1000 µg twice daily) of budesonide via Turbuhaler and of fluticasone via pMDI and Diskus.
| a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regimen | Subjects | n | AUC (nmol l−1 h) | MAT (h) | Cmax (nmol) | ΔC(%) | tmax (min) | Racc [ratio] |
| Budesonide | Healthy | 13 | 10.5 (8.5; 13.2) | 0.6 (0.3; 0.9) | 3.8 (3.1; 4.6) | 414 (342; 500) | 17 (10; 24) | 1.09 (1.07; 1.10) |
| Turbuhaler | With asthma | 8 | 12.9 (11.4; 14.5) | 1.0 (0.7; 1.3) | 4.3 (3.1; 5.9) | 379 (288; 499) | 15 (6; 24) | 1.14 (1.09; 1.20) |
| Total | 21 | 11.4 (9.9; 13.1) | 0.8 (0.6; 1.0) | 4.0 (3.4; 4.6) | 400 (346; 462) | 16 (11; 21) | 1.11 (1.09; 1.13) | |
| Fluticasone pMDI | Healthy | 13 | 5.5 (4.5; 6.9) | 7.1 (5.6; 8.5) | 0.7 (0.6; 0.9) | 104 (94; 14) | 113 (84; 143) | 1.63 (1.55; 1.70) |
| Fluticasone Diskus | Healthy | 13 | 3.5 (2.9; 4.3) | 5.3 (4.0; 6.6) | 0.5 (0.4; 0.6) | 112 (102; 124) | 100 (66; 135) | 1.66 (1.54; 1.78) |
| With asthma | 8 | 3.0 (2.2; 4.1) | 6.9 (4.5; 9.3) | 0.4 (0.3; 0.5) | 108 (86; 136) | 79 (14; 144) | 1.63 (1.48; 1.80) | |
| Total | 21 | 3.3 (2.9; 3.9) | 5.9 (4.8; 7.0) | 0.4 (0.4; 0.5) | 111 (101; 122) | 92 (63; 122) | 1.65 (1.56; 1.74) | |
| b) | ||||||||
| Regimen | Subjects | n | Dose-to-subject (%) (µg)* | Fnominal (%) | Flung, nominal (%) | Flung, DTS (%) | ||
| Budesonide | Healthy | 13 | 572 (77) | 36 (29; 46) | 32 (24; 43) | 59 (41; 82) | ||
| Turbuhaler | With asthma | 8 | 587 (64) | 45 (36; 55) | 42 (32; 53) | 82 (66; 102) | ||
| Total | 21 | 578 (72) | 39 (34; 46) | 36 (29; 43) | 68 (55; 83) | |||
| Fluticasone pMDI | Healthy | 13 | 668 (95) | 21 (17; 25) | 20 (16; 25) | 31 (25; 37) | ||
| Fluticasone Diskus | Healthy | 13 | 755 (102) | 13 (11; 16) | 12 (10; 15) | 17 (14; 21) | ||
| With asthma | 8 | 768 (109) | 13 (10; 17) | 12 (9; 16) | 19 (14; 26) | |||
| Total | 21 | 760 (105) | 13 (11; 15) | 12 (10; 14) | 18 (15; 21) | |||
Values are given as geometric means (95% CI).
Arithmetic mean (s.d.).
AUC=area under the curve of plasma concentration vs time; MAT=mean absorption time; Cmax=maximum concentration
C=plasma variability; tmax=time for Cmax; Racc=accumulation index; F=systemic availability; DTS=dose-to-subject.
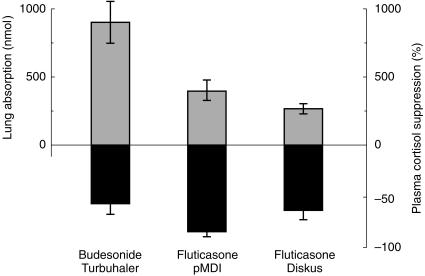
The systemic availability of budesonide via Turbuhaler was significantly higher than that of fluticasone via Diskus (ratio 3.0 [2.3, 5.6] with 95% confidence interval [CI]), and via pMDI (ratio 1.8 [1.3, 2.3]). In addition, at steady state the systemic availability of fluticasone via pMDI was significantly higher than via Diskus (ratio 1.6 [1.1, 2.2]). With the assumption of an oral availability of 1% for fluticasone [3] and 10% for budesonide [2], the pulmonary absorption at steady state was 134 [115, 156] µg of fluticasone via Diskus, 202 [164, 249] µg of fluticasone via pMDI, and 388 [314, 440] µg of budesonide via Turbuhaler. Expressed in molar dose, which normalizes for the differences in molecular weight, the lung deposition of budesonide via Turbuhaler was 2.2-fold [1.2, 7.9] higher than that of fluticasone pMDI and 3.4-fold [2.8; 4.0] higher than that of fluticasone Diskus. The lung deposition of fluticasone via pMDI was 1.5-fold [1.1, 2.9] that via the Diskus inhaler (Figure 2). The pulmonary absorption of budesonide via Turbuhaler accounted for about 2/3 of the dose-to-subject, whereas only about 1/3 of the dose-to-subject of fluticasone via pMDI and 1/5 via Diskus was absorbed via the lungs (Table 4b). It is evident that Turbuhaler directs the major fraction of budesonide retained by the subject to the lungs, whereas only a minor fraction of fluticasone that is retained by the subject is directed to the lungs via pMDI and Diskus.
Figure 2.
Lung dose and plasma cortisol suppression after repeated dose inhalations (1000 µg twice daily for 7 days) of budesonide via Turbuhaler (n = 21) and fluticasone via pMDI (n = 13) and Diskus (n = 21).
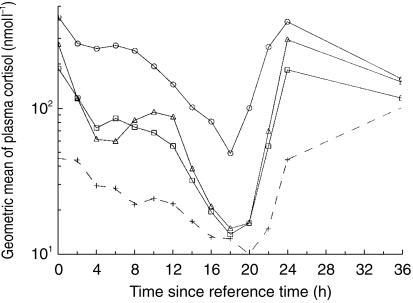
Plasma cortisol
The concentrations of cortisol in plasma over time are shown in Figure 3. Mean plasma cortisol suppression is illustrated in Figure 2 and tabulated in Table 5. Plasma cortisol (24 h) was significantly reduced vs baseline for all three treatments. The cortisol concentration vs baseline was 12% for fluticasone pMDI, which was significantly lower (ratio 0.32 [0.24, 0.42, 95% CI]) than that for fluticasone Diskus (39%), and for budesonide Turbuhaler (46%) (ratio 0.27 [0.21, 0.37]). The plasma cortisol concentration did not differ significantly between treatments with fluticasone Diskus and budesonide Turbuhaler (ratio 0.87 [0.65, 1.15]).
Figure 3.
Mean plasma cortisol concentrations after repeated dose inhalations (1000 µg twice daily for 7 days) of budesonide via Turbuhaler (n = 21) and fluticasone via pMDI (n = 13) and Diskus (n = 21). ○ = No treatment, ▵=Budesonide Turbuhaler, □=Fluticasone Diskus, +=Fluticasone pMDI.
Table 5.
Plasma cortisol (AUC(0,24 h)) suppression vs baseline after repeated dose inhalations (1000 µg twice daily) of budesonide via Turbuhaler and of fluticasone via pMDI and Diskus.
| Regimen | Subjects | n | Plasma cortisol suppression (%) |
|---|---|---|---|
| Budesonide | Healthy | 13 | 54 (39, 66) |
| Turbuhaler | With asthma | 8 | 59 (34, 74) |
| Total | 21 | 56 (46, 65) | |
| Fluticasone pMDI | Healthy | 13 | 88 (84, 91) |
| Fluticasone Diskus | Healthy | 13 | 61 (48, 70) |
| With asthma | 8 | 68 (50, 80) | |
| Total | 21 | 64 (55,71) |
Values are given as geometric means (95% CI).
Discussion
Although fluticasone and budesonide were shown to have similar systemic clearances, which predicts a similar plasma exposure at steady state, the present study shows that the pharmacokinetics of the two inhaled corticosteroids differ in many respects; budesonide via Turbuhaler resulted in a high lung deposition, a rapid systemic absorption and moderate systemic activity, whereas fluticasone via Diskus and pMDI gave a low to moderate lung deposition, a slow systemic absorption, and an equal (Diskus) or higher (pMDI) systemic activity.
An extensive distribution into tissues, as indicated by a high volume of distribution, appears to be the cause of the slow elimination of fluticasone, and the elimination half-life of 12 h in the present study is in agreement with the 10–14 h found in earlier studies [3, 5, 17]. The slow elimination results in a significant (1.7-fold) accumulation of fluticasone following repeated dosing. As the terminal elimination phase is entered at a late stage, around 8–16 h after administration (Figure 1), it is difficult to make proper estimates of the terminal elimination half-life if plasma is sampled during a time period that is too short. This is a likely explanation as to why shorter half-lives, ranging from 3.1 to 8.3 h for intravenous administrations, were found in previous studies [1, 6, 18, 19]. In those studies, the estimates were made from plasma concentrations obtained only up to 24 h, at most, after administration. In the present study, where the plasma concentrations could be monitored for a longer time, there was no difference in the elimination half-lives of fluticasone after intravenous administration and inhalation. Hence, the slow absorption of fluticasone from the lungs did not determine the terminal elimination rate of fluticasone. Therefore, systemic absorption of fluticasone from the lungs is not the rate-limiting step in the elimination of the drug, as has been claimed previously [4].
The rate of systemic absorption of fluticasone, with a mean absorption time (MAT) of 5–7 h was, however, considerably slower than for budesonide (∼1 h), thereby indicating a longer pulmonary residence time. This is consistent with the relatively low aqueous solubility of fluticasone and may suggest a more extended local availability of undissolved fluticasone in the lungs. Intracellular fatty acid esterification of budesonide may also provide a prolonged pulmonary residence time [20]. Since the amount of glucocorticoid receptors in normal cells are limited, only a small fraction of the inhaled, dissolved and unbound fraction of the steroid dose is likely to bind to the receptor. Hence, the fate within the lung of this fraction, which eventually determines the extent and duration of pharmacological activity, cannot be deduced from plasma determinations of MAT, as has been implied by some investigators [19].
The elimination half-life of budesonide was close to 5 h in the present study, which is longer than the 2–3 h found in earlier studies [2, 10]. This discrepancy is likely due to shorter sampling times and a lower sensitivity in the bioassays used in earlier studies. In any case, the slower elimination of budesonide found in the present study did not cause any significant accumulation.
A long elimination half-life will also reduce the peak vs trough plasma concentration ratio. The amplitude of the plasma concentrations was lower for fluticasone than for budesonide in the present study, with a plasma peak/trough variability (ΔC) of 111% for fluticasone via Diskus and 400% for budesonide. Little is known of the clinical consequences of this. It is, however, evident that the different plasma concentration profiles of fluticasone and budesonide in the present study are reflected in the dynamics of the plasma cortisol suppression. It is apparent that the recovery from cortisol suppression after the last dose differs between the three formulations, with budesonide Turbuhaler having the fastest recovery and fluticasone pMDI the slowest (Figure 3). This is consistent with a previous study in which plasma cortisol was statistically significantly suppressed compared with baseline up to 24 h following the last dose of fluticasone via Diskhaler (1000 µg twice daily) but not for budesonide via Turbuhaler (800 µg twice daily) [11].
Although it is difficult to show a dose-response in clinical efficacy for inhaled corticosteroids, due to the shallow dose–response curve [21], the effect elicited in the target organ is probably related to the amount of drug reaching that site [22]. Thus, due to a significant difference in lung deposition, it is recommended that a lower dose of budesonide be tried when changing from pMDI to Turbuhaler [23]. A comparison of the lung deposition with different formulations of fluticasone has not been published. About a twofold difference in systemic availability between the Diskus and pMDI formulations does, however, indicate a substantial difference in lung deposition [24]. The marked difference in systemic availability between different formulations of fluticasone and budesonide should also be kept in mind when comparing efficacy and safety in clinical trials.
The fluticasone pMDI and Diskus formulations are claimed to be clinically substitutable, despite this significant difference in systemic availability [25]. Again, the inability to differentiate between the two formulations is probably partly due to the shallow dose–response curve of inhaled corticosteroids in terms of clinical efficacy, which is consistent with the well-known difficulty to discriminate between two adjacent doses of an identical formulation [21], and partly to the use of insensitive techniques to estimate the hypothalamic-pituitary-adrenal HPA-axis effects. The absence of a difference in the outcome may then incorrectly be interpreted as the presence of similarity, or even equivalence. The proper way to compare clinical potency is with dose–response studies, including at least three doses of both formulations, or to use a dose down-titration technique, to find the lowest effective dose of either formulation [21].
It is evident from Figure 2 that the amount of budesonide reaching the lungs via Turbuhaler is more than threefold higher than that of fluticasone via Diskus, and about twofold that of fluticasone via pMDI. Despite this difference in lung delivery, fluticasone via Diskus produces a systemic effect comparable to that of budesonide via Turbuhaler. This similarity in systemic effect is most likely due to a higher glucocorticoid receptor affinity for fluticasone, and to the slow systemic elimination. The in vitro glucocorticoid receptor affinity for fluticasone has been determined to be about twofold that of budesonide [26]. This difference has then erroneously been translated into a twofold difference in clinical efficacy in vivo, as it does not take into account the possible differences in the amount of drug delivered to the lungs. The present pharmacokinetic study better explains the results from a recent effect-controlled study in which there was no difference between fluticasone Diskus and budesonide Turbuhaler used at the same dose in adult asthmatics [27]. In addition, given the ubiquitous distribution of the glucocorticoid receptor, a higher receptor affinity will also increase systemic activity.
In the present study, as shown in Tables 3 and 4, there is no indication of any difference between healthy subjects and patients with mild asthma in the systemic availability, lung deposition, or plasma cortisol suppression of fluticasone and budesonide, which justifies the pooling of all subjects in the analysis. In earlier studies, the total lung deposition of budesonide via Turbuhaler was found to be comparable in healthy subjects (28%) [28] and patients (26%) with mild to moderate asthma (forced expiratory volume in 1 second, 50–92% of predicted) [29], and appears to be more or less unaffected by the disease. A more central deposition was, however, found in the asthmatic patients, with a peripheral vs central ratio of 0.64, compared with 1.72 in healthy subjects, which suggests that peripheral aerosol penetration of budesonide via Turbuhaler is lower in asthmatic patients. In contrast, in a recent study, a more than two-fold difference in systemic availability of fluticasone was found between healthy subjects and patients with severe asthma [30]. Despite this marked difference in systemic availability, the two groups did not differ in plasma cortisol suppression during a 12 h dosing interval. A confounding factor in asthmatic patients is that the HPA-axis function may be affected by previous treatment with oral or inhaled corticosteroids, which makes it difficult to compare the cortisol suppression relative to baseline values between healthy and asthmatic subjects [12]. The present study indicates that the majority of asthmatic patients, i.e. those with milder forms of the disease, do not differ from healthy subjects in terms of total lung deposition and systemic activity of budesonide and fluticasone.
Conclusions
Budesonide and fluticasone differ in their pharmacokinetic properties in that although clearance is the same, the rate of uptake and elimination is slower for fluticasone. Despite a significantly higher pulmonary availability of budesonide via Turbuhaler, the plasma cortisol suppression is less than that of fluticasone via pMDI and similar to that of fluticasone via Diskus. There is no indication of any difference between healthy subjects and patients with mild asthma in the pharmacokinetics and plasma cortisol suppression of fluticasone and budesonide.
Conflicts of interest
The Department of Respiratory Medicine at the University Hospital in Lund, Sweden, has received funding support from AstraZeneca, Glaxo-Wellcome, MSD, and SMKF. Professor Claes-Göran Löfdahl owns no shares in AstraZeneca PLC, and has received lecture honorariums from AstraZeneca, Boehringer Ingelheim, Genentec, Glaxo-Wellcome, Novartis, Orion, and Schering-Plough.
References
- 1.Harding S. The human pharmacology of fluticasone propionate. Respir Med. 1990;84(Suppl A):25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- 2.Ryrfeldt Å, Andersson P, Edsbäcker S, Tönnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;63:86–95. [PubMed] [Google Scholar]
- 3.Thorsson L, Dahlström K, Edsbäcker S, Källén A, Paulson J, Wirén JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esmailpour N, Högger P, Rabe KF, Heitmann U, Nakashima M, Rohdewald P. Distribution of inhaled fluticasone propionate between human lung tissue and serum in vivo. Eur Respir J. 1997;10:1496–1499. doi: 10.1183/09031936.97.10071496. [DOI] [PubMed] [Google Scholar]
- 5.Mackie AE, Ventresca GP, Fuller RW, Bye A. Pharmacokinetics of intravenous fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:539–542. doi: 10.1046/j.1365-2125.1996.36110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie AE, Falcoz C, McDowall JE, Moss J, Ventresca GP, Bye A. Pharmacokinetics of fluticasone propionate inhaled from Diskhaler and Diskus powder devices in healthy subjects. Br J Clin Pharmacol. 1996;43:540P–541P. [Google Scholar]
- 7.Lönnebo A, Grahnén A, Jansson B, Brundin RM, Ling-Andersson A, Eckernäs S-Å. An assessment of the systemic activity of single and repeated doses of inhaled fluticasone propionate and inhaled budesonide in healthy volunteers. Eur J Clin Pharmacol. 1996;49:459–463. doi: 10.1007/BF00195931. 10.1007/s002280050050. [DOI] [PubMed] [Google Scholar]
- 8.Möllman H, Rohdewald P, Schmidt EW, Salomon V, Derendorf H. Pharmacokinetics of triamcinolone acetonide and its phosphate ester. Eur J Clin Pharmacol. 1985;29:85–89. doi: 10.1007/BF00547374. [DOI] [PubMed] [Google Scholar]
- 9.Chaplin MD, Rooks WII, Swenson EW, Cooper WC, Nerenberg C, Chu NI. Flunisolide metabolism and dynamics of a metabolite. Clin Pharmacol Ther. 1980;27:402–413. doi: 10.1038/clpt.1980.54. [DOI] [PubMed] [Google Scholar]
- 10.Thorsson L, Edsbäcker S, Conradson T-B. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 11.Grahnén A, Jansson B, Brundin RM, et al. A dose–response study comparing suppression of plasma cortisol induced by fluticasone propionate from Diskhaler and budesonide from Turbuhaler. Eur J Clin Pharmacol. 1997;52:261–267. doi: 10.1007/s002280050287. 10.1007/s002280050287. [DOI] [PubMed] [Google Scholar]
- 12.Boulet LP, Cockcroft DW, Toogood J, Lacasse Y, Baskerville J, Hargreave FE. Comparative assessment of safety and efficacy of inhaled corticosteroids: report of a committee of the Canadian Thoracic Society. Eur Respir J. 1998;11:1194–1210. doi: 10.1183/09031936.98.11051194. [DOI] [PubMed] [Google Scholar]
- 13.Weiner P, Berar-Yanay N, Davidovich A, Magadle R. Nocturnal cortisol secretion in asthmatic patients after inhalation of fluticasone propionate. Chest. 1999;116:931–934. doi: 10.1378/chest.116.4.931. [DOI] [PubMed] [Google Scholar]
- 14.Harrison TW, Wisniewski A, Honour JW, Tattersfield AE. Systemic activity of inhaled fluticasone propionate and budesonide in subjects with and without asthma. Eur Respir J. 1999;14(Suppl 30):466. s, and accepted for publication in Thorax. [Google Scholar]
- 15.American Thoracic Society. Standards for the diagnosis and care of patients with chronic pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 16.Thorsson Edsbäcker S. Lung deposition of budesonide from a pressurized metered-dose inhaler attached to a spacer. Eur Respir J. 1998;12:1340–1345. doi: 10.1183/09031936.98.12061340. [DOI] [PubMed] [Google Scholar]
- 17.Fuller R, Johnson M, Bye A. Fluticasone propionate – an update on preclinical and clinical experience. Respir Med. 1995;89(Suppl A):3–18. doi: 10.1016/0954-6111(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 18.Rohatagi S, Bye A, Falcoz C, et al. Dynamic modelling of cortisol reduction after inhaled administration of fluticasone propionate. J Clin Pharmacol. 1996;36:938–941. doi: 10.1002/j.1552-4604.1996.tb04761.x. [DOI] [PubMed] [Google Scholar]
- 19.Möllman H, Wagner M, Meibohm B, et al. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53:459–467. doi: 10.1007/s002280050407. 10.1007/s002280050407. [DOI] [PubMed] [Google Scholar]
- 20.Edsbäcker S. Pharmacological factors that influence the choice of inhaled corticosteroids. Drugs. 1999;58(Suppl 4):7–16. doi: 10.2165/00003495-199958004-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen S, O'Byrne P. A comparison of the efficacy and safety of inhaled corticosteroids in asthma. Allergy. 1997;52(39 Suppl):1–34. doi: 10.1111/j.1398-9995.1997.tb05047.x. [DOI] [PubMed] [Google Scholar]
- 22.Pauwels R, Newman SP, Borgström L. Airway deposition and airway effects of antiasthma drugs from metered-dose inhalers. Eur Respir J. 1997;10:2127–2138. doi: 10.1183/09031936.97.10092127. [DOI] [PubMed] [Google Scholar]
- 23.Toogood JH, White FA, Baskerville JC, Fraher LJ, Jennings B. Comparison of the antiasthmatic, oropharyngeal, and systemic glucocorticoid effects of budesonide administered through a pressurized aerosol plus spacer or the Turbuhaler dry powder inhaler. J Allergy Clin Immunol. 1997;99:186–193. doi: 10.1016/s0091-6749(97)70094-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson M. Fluticasone propionate: pharmacokinetic and pharmacodynamic implications of different aerosol delivery systems. In: Dalby RN, Byron PR, Farr SJ, editors. Proceedings of Respiratory Drug Delivery VI. South Carolina: Hilton Head; 1998. [Google Scholar]
- 25.Bousquet J, Tisserand B, Medley HV. Eur Respir J. Suppl 19. Vol. 8. 1995. Double-blind parallel group study to compare the long term clinical efficacy and safety of two different methods of administering inhaled fluticasone propionate in chronic severe asthmatic patients; p. 427s. [Google Scholar]
- 26.Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol. 1998;101:S434–S439. doi: 10.1016/s0091-6749(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 27.Kuna P, Magnussen H, Joubert J, Greefhorst APM. Same minimal effective dose of budesonide Turbuhaler and fluticasone Diskus/Accuhaler in adult asthmatics. Am J Respir Crit Care Med. 2001. (Abstract accepted for publication at ATS 2001).
- 28.Borgström L, Bondesson E, Morén F, Trofast E, Newman S. Lung deposition of budesonide inhaled via Turbuhaler: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994;7:69–73. doi: 10.1183/09031936.94.07010069. [DOI] [PubMed] [Google Scholar]
- 29.Thorsson L, Kenyon C, Newman SP, Borgström L. Lung deposition of budesonide in asthmatics: a comparison of different formulations. Int J Pharmaceut. 1998;168:119–127. [Google Scholar]
- 30.Brutsche MH, Brutsche IC, Munavvar M, et al. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356:556–561. doi: 10.1016/S0140-6736(00)02581-2. 10.1016/s0140-6736(00)02581-2. [DOI] [PubMed] [Google Scholar]