Abstract
Aims
To evaluate the potency and specificity of valproic acid as an inhibitor of the activity of different human CYP isoforms in liver microsomes.
Methods
Using pooled human liver microsomes, the effects of valproic acid on seven CYP isoform specific marker reactions were measured: phenacetin O-deethylase (CYP1A2), coumarin 7-hydroxylase (CYP2A6), tolbutamide hydroxylase (CYP2C9), S-mephenytoin 4′-hydroxylase (CYP2C19), dextromethorphan O-demethylase (CYP2D6), chlorzoxazone 6-hydroxylase (CYP2E1) and midazolam 1′-hydroxylase (CYP3A4).
Results
Valproic acid competitively inhibited CYP2C9 activity with a Ki value of 600 µm. In addition, valproic acid slightly inhibited CYP2C19 activity (Ki = 8553 µm, mixed inhibition) and CYP3A4 activity (Ki = 7975 µm, competitive inhibition). The inhibition of CYP2A6 activity by valproic acid was time-, concentration- and NADPH-dependent (KI = 9150 µm, Kinact=0.048 min−1), consistent with mechanism-based inhibition of CYP2A6. However, minimal inhibition of CYP1A2, CYP2D6 and CYP2E1 activities was observed.
Conclusions
Valproic acid inhibits the activity of CYP2C9 at clinically relevant concentrations in human liver microsomes. Inhibition of CYP2C9 can explain some of the effects of valproic acid on the pharmacokinetics of other drugs, such as phenytoin. Co-administration of high doses of valproic acid with drugs that are primarily metabolized by CYP2C9 may result in significant drug interactions.
Keywords: CYP2C9, cytochrome P450, inhibition, interaction, valproic acid
Introduction
Valproic acid is a widely used anticonvulsant agent. It can affect the pharmacokinetics of several drugs including phenytoin [1–3], phenobarbitone [4–7], diazepam [8], nimodipine [9], amitriptyline [10] and clomipramine [11], consistent with inhibition of their metabolism. These drugs are mainly metabolized by different cytochrome P450 (CYP) isoforms. However, only limited studies about the inhibitory effects of valproic acid on the activities of CYP isoforms in vitro have been published [12, 13].
Valproic acid is eliminated via extensive hepatic metabolism to several metabolites [14]. Its major metabolic pathways are glucuronidation and mitochondrial β-oxidation, while CYP-dependent oxidation is only a minor pathway [15]. The steady-state plasma concentrations of the metabolites are at least 100-fold lower than those of the parent compound [16]. Thus, it is unlikely that the metabolites would significantly alter CYP-dependent drug metabolism in vivo.
In this study, we investigated the in vitro inhibitory effects of valproic acid on major CYP isoform activities in human liver microsomes using selective marker reactions for CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4.
Methods
Materials
Valproic acid (sodium salt), phenacetin, paracetamol, coumarin, 7-hydroxycoumarin, tolbutamide, chlorzoxazone, quinidine, sulfaphenazole, tranylcypromine, pyridine, troleandomycin (TAO) and NADPH were purchased from Sigma Chemical Co. (St Louis, MO, USA). Hydroxytolbutamide, 6-hydroxychlorzoxazone, S-mephenytoin, 4′-hydroxymephenytoin and furafylline were purchased from Ultrafine Chemicals (Manchester, UK). Dextromethorphan and dextrorphan were obtained from Orion Pharma (Espoo, Finland). Midazolam and 1′-hydroxymidazolam were kindly provided by Hoffmann-La Roche (Basel, Switzerland). Pooled human liver microsomes (prepared from five male and five female human liver microsomal samples) known to contain high levels of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 were obtained from Gentest Corp. (Woburn, MA, USA). The liver samples were obtained from several organ procurement organizations, which collect tissues in accordance with all pertinent regulations and obtain permission from the donors' families prior to organ collection. The procedures of these organizations have all been reviewed and approved by their respective institutional Human Subjects Committee. Other chemicals and reagents were obtained from Merck (Darmstadt, Germany).
Inhibition studies
The effects of valproic acid on seven different CYP isoform-specific marker reactions were studied. Phenacetin O-deethylation was used to probe for CYP1A2, coumarin 7-hydroxylation for CYP2A6, tolbutamide hydroxylation for CYP2C9, S-mephenytoin 4′-hydroxylation for CYP2C19, dextromethorphan O-deethylation for CYP2D6, chlorzoxazone 6-hydroxylation for CYP2E1 and midazolam 1′-hydroxylation for CYP3A4. The incubation conditions used to study the metabolism of the various substrates were adapted from reported procedures [17–20] and are specified in Table 1. The time of incubation (see Table 1) and concentration of microsomal protein (100 µg ml−1) were determined to be in the linear range for the rate of metabolite formation for each substrate.
Table 1.
Summary of the reaction conditions and estimated Michaelis-Menten parameters of the CYP isoform-selective marker reactions
| CYP | Substrate/solvent Inhibitor/solvent | Substrate concentration in assay (range) (µm)a | Incubation time(min) | Quenching method | Km1 (Km2)(µm)b | Vmax1 (Vmax2)(pmol mg−1 min−1)b |
|---|---|---|---|---|---|---|
| CYP1A2 | Phenacetin/methanol Furafylline/methanol | 10 (5–250) | 30 | 100 µl acetonitrile | 19 (556) | 836 (824) |
| CYP2A6 | Coumarin/methanol NAc | 0.5, 1, 5 (0.1–50) | 10 | 20 µl 70% perchloric acid | 1.1 | 1303 |
| CYP2C9 | Tolbutamide/ethanol Sulfaphenazole/methanol | 25, 50, 100, 250 (10–250) | 60 | 20 µl 85% phosphoric acid | 65 | 194 |
| CYP2C19 | S-mephenytoin/methanol Tranylcypromine/water | 25, 50, 75, 100 (10–250) | 60 | 20 µl 85% phosphoric acid | 39 | 102 |
| CYP2D6 | Dextromethorphan/water Quinidine/methanol | 1.5 (0.5–50) | 20 | 20 µl 70% perchloric acid | 1.5 | 227 |
| CYP2E1 | Chlorzoxazone/methanol Pyridine/water | 30 (5–250) | 30 | 20 µl 85% phosphoric acid | 27 | 807 |
| CYP3A4 | Midazolam/acetonitrile Troleandomycin/methanol | 2, 5, 10, 15 (0.5–50) | 6 | 200 µl methanol | 1.4 | 1858 |
Concentrations used in the inhibition study were chosen around appropriate apparent Km values. Values in parenthesis are the substrate concentration ranges used for characterization of Km and Vmax.
A two-enzyme Michaelis-Menten equation was best fitted to data for phenacetin O-deethylation (the apparent kinetic data for CYP1A2 are shown without parentheses), in agreement with previous studies. A single Michaelis-Menten equation was best fitted to data for the other marker reactions.
Chemical (8-methoxypsoralen) not available.
Valproic acid (sodium salt) (final concentrations ranged from 0 to 25 mm) was dissolved in 0.1 m sodium phosphate buffer (pH 7.4). Addition of valproic acid (sodium salt) had no effect on the pH of the incubation matrix, which was between 7.4 and 7.5 in all incubations. The unbound concentrations of valproic acid in the incubation medium were determined using an ultrafiltration method [21, 22]. The unbound concentrations were essentially unaffected by the addition of human liver microsomes (protein concentration 0.1 mg ml−1) when studied at the valproic acid concentrations used (50 µm–25 mm), suggesting that nonspecific microsomal binding did not affect our results.
The same pooled batch of human liver microsomes was used in all experiments. All our results represent the average of duplicate determinations, and data showing more than a 10% difference between the duplicate assays were excluded, to assure reproducibility of the experiments. Briefly, each incubation consisted of 20 µg pooled human microsomal protein in an incubation medium containing 0.1 m phosphate buffer (pH 7.4), 5 mm MgCl2 and 1.0 mm NADPH. The final incubation volume was 0.2 ml. Valproic acid was added to the incubation medium and the reaction was started by the addition of the substrate either without or after a 15 min preincubation at 37 ° C, except in studies with coumarin 7-hydroxylase, when valproic acid was preincubated for 0, 2, 5, 10 or 15 min. The substrate stock solutions were prepared in different solvents as specified in Table 1 and the final organic solvent content in each sample did not exceed 1% (v/v). An equal volume of solvent was added to control incubations not containing inhibitor. Reactions were terminated as indicated in Table 1. After centrifugation at 10 000 g for 5 min, an aliquot of the supernatant was subjected to analysis by high-performance liquid chromatography (h.p.l.c.).
Six isoform-selective CYP inhibitors were used as positive controls at appropriate concentrations (Table 1). The final concentrations of the inhibitors used in the incubations were chosen according to previous publications [23–26]. Furafylline and troleandomycin were preincubated with the incubation medium for 15 min. The apparent kinetic parameters (Km, Vmax) of the pooled human liver microsomes used in these experiments (Table 1) and the effects of isoform-selective inhibitors on CYP activities were similar to those reported previously [17–20, 23–26].
H.p.l.c. analysis
Metabolites were assayed by h.p.l.c., as described previously [17–19, 27, 28]. The intraday and interday coefficients of variation (CV) were < 7% at relevant concentrations (n = 6).
Analysis of data
The apparent kinetic parameters, i.e. Vmax and Km for each specific CYP isoform-catalysed marker reaction and the inhibitory constant (Ki) values were calculated using the nonlinear regression analysis program Enzfitter (Biosoft, Cambridge, UK). An assessment of goodness of fit of the models was made using the size of the residual sum of squares and the random distribution of the residuals, the standard error and the 95% confidence interval of the parameter estimates. When necessary, an F-test was performed to determine whether there was a significant difference in the size of the residual sum of squares between models [29]. In the case of time-dependent CYP inactivation, the apparent half-life for inactivation (t½) was estimated from linear regression analysis of the natural logarithm of residual enzyme activity against preincubation time. The concentration required for half-maximal inactivation (KI) and the maximal rate of inactivation at saturation (Kinact) were calculated from the double-reciprocal plot of the rate of inactivation of metabolite formation as a function of inhibitor concentration.
Results
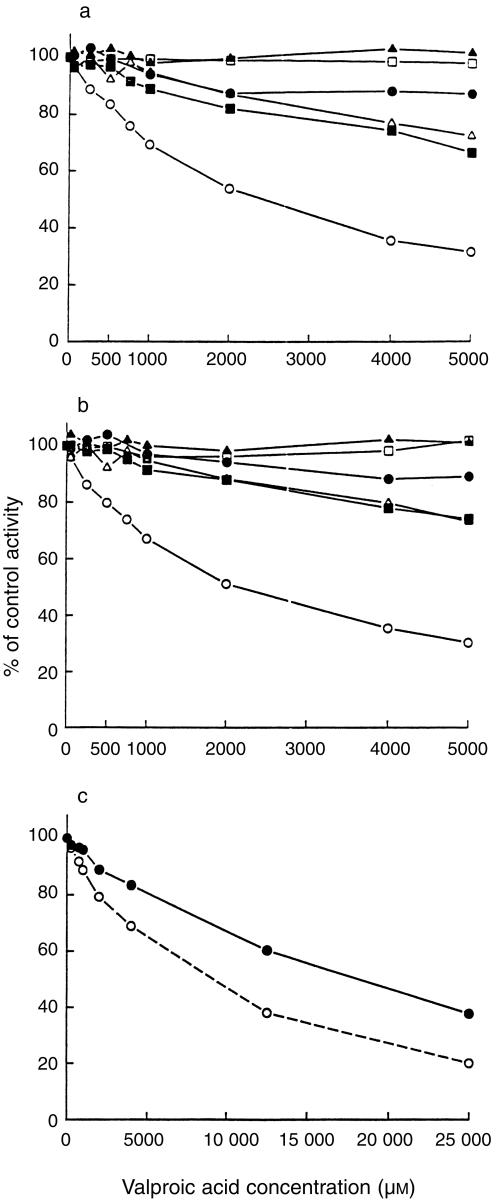
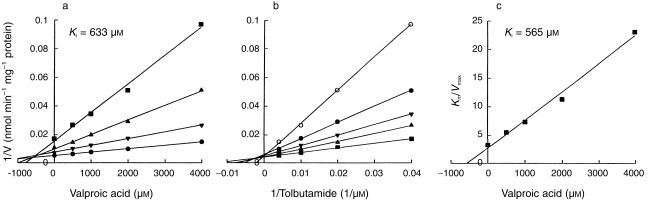
Valproic acid preferentially inhibited CYP2C9-catalysed tolbutamide hydroxylation (Figure 1). The double reciprocal plots, Dixon plots and the secondary plot of the slopes of double reciprocal plots vs valproic acid concentration indicated that valproic acid competitively inhibited CYP2C9 activity, with an apparent Ki value of 600 µm (Figure 2, Table 2).
Figure 1.
Effects of valproic acid on CYP-catalysed reactions in pooled human liver microsomes incubated with phenacetin (10 µm), coumarin (1 µm), tolbutamide (50 µm), S-mephenytoin (50 µm), dextromethorphan (1.5 µm), chlorzoxazone (30 µm) or midazolam (2 µm) with valproic acid (0–5000 µm) either with (b) or without (a) a 15 min preincubation as described in the Methods. The enzyme reactions evaluated were CYP1A2-catalysed phenacetin O-deethylation (•), CYP2C9-catalysed tolbutamide hydroxylation (○), CYP2C19-catalysed S-mephenytoin 4′-hydroxylation (▪), CYP2D6-catalysed dextromethorphan O-demethylation (□), CYP2E1-catalysed chlorzoxazone 6-hydroxylation (▴) and CYP3A4-catalysed midazolam 1′-hydroxylation (▵). (c) Inhibition of CYP2A6-catalysed coumarin 7-hydroxylation by valproic acid (0 – 25 000 µm) with (○) or without (•) a 15 min preincubation in the presence of NADPH. Each data point represents the mean of duplicate determinations.
Figure 2.
Inhibitory effect of valproic acid on CYP2C9-catalysed tolbutamide hydroxylation in pooled human liver microsomes. (a) A representative Dixon plot obtained from a 60 min incubation with 25 µm (▪), 50 µm (▴), 100 µm (▾) and 250 µm (•) of tolbutamide in the absence or presence of valproic acid (500–4000 µm). (b) A double-reciprocal plot obtained from a 60 min incubation of human liver microsomes with NADPH and tolbutamide (25–250 µm) in the absence (▪) or presence of 500 µm (▴), 1000 µm (▾), 2000 µm (•) or 4000 µm (○) valproic acid. (c) A secondary plot of slopes taken from double-reciprocal plots vs valproic acid concentration. Each data point represents the mean of duplicate determinations.
Table 2.
Inhibitory types and kinetic constants of valproic acid for human CYP activities
| CYP | Kia (µm) [KI,Kinact]b | Type of inhibition |
|---|---|---|
| CYP2A6 | 12372 (αc = 3.2) | mixed |
| [9150 µm, 0.048 min−1]b | [mechanism-based] | |
| CYP2C9 | 600 | competitive |
| CYP2C19 | 8553 (αc = 2.5) | mixed |
| CYP3A4 | 7975 | competitive |
Values are derived from nonlinear regression analysis based on coincubation of the respective CYP specific substrates with various concentrations of valproic acid without preincubation at 37 ° C (see the Methods and Table 1 for details).
Inhibition of CYP2A6 was preincubation-time dependent. The parameters for mechanism-based inhibition are given in parenthesis (see Methods for details).
The factor by which Km changes when inhibitor occupies the enzyme site.
With concentrations ranging from 50 to 1000 µm, valproic acid showed minimal inhibitory effects on CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 activities (Figure 1). However, with concentrations higher than 1000 µm, valproic acid exhibited weak reversible inhibitory effects on CYP2C19 and CYP3A4 activities. The apparent Ki values of valproic acid for these CYP activities and the types of inhibition are listed in Table 2.
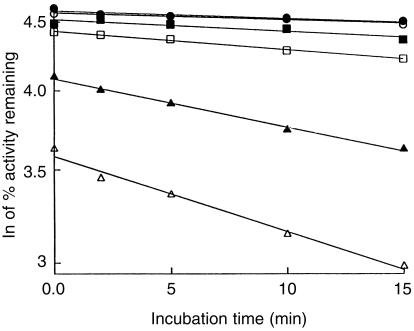
Inhibition of coumarin 7-hydroxylation by valproic acid was found to be time-and concentration-dependent (Figure 3), indicating a probable mechanism-based inhibition of CYP2A6 activity. In addition, the presence of NADPH was found to be a necessary prerequisite for CYP2A6 inactivation by valproic acid (data not shown). The Kinact and KI were estimated to be 0.048 min−1 and 9150 µm, respectively. The time required for half of the enzyme to become inactivated (t½) was 14 min. The inhibitory effects of valproic acid on the other CYP activities were not significantly altered by preincubation of microsomes with valproic acid and NADPH for 15 min (Figure 1).
Figure 3.
Time- and concentration-dependent inactivation of the CYP2A6-mediated 7-hydroxylation of coumarin by valproic acid in pooled human liver microsomes. The incubation medium was preincubated with 0 mm (•), 1 mm (○), 2 mm (▪), 4 mm (□), 12.5 mm (▴) and 25 mm (▵) valproic acid for 0, 2, 5, 10 and 15 min at 37 ° C in the presence of NADPH before adding 1 µm coumarin. Each data point represents the mean of duplicate determinations.
Discussion
The results of the present study showed that valproic acid competitively inhibited CYP2C9 with a Ki of 600 µm, which is in the range of plasma concentrations of valproic acid observed in clinical practice (< 1000 µm). In addition, valproic acid exhibited weak reversible inhibitory effects on CYP2C19 and CYP3A4 activities and probable mechanism-based inhibition of CYP2A6, but the Ki values were at least an order of magnitude higher than the Ki for CYP2C9. Valproic acid had no appreciable effect on CYP1A2, CYP2D6 and CYP2E1 activities even at 5000 µm. The results are in agreement with previous in vitro studies showing that 100 µm and 250 µm valproic acid had no effect on the CYP3A4-mediated cyclosporin oxidation [13] and on the CYP2D6-mediated hydroxylation of mexiletine [12], respectively. It should be noted that because we used pooled microsomes, any conclusions about interindividual variability in the inhibitory potency of valproic acid cannot be drawn from this study.
In in vivo studies, valproic acid has been found to decrease the metabolic clearance of the CYP2C9 substrate phenytoin [1–3]. In one study, valproic acid at a serum concentration of about 400 µm reduced the systemic clearance of unbound phenytoin by 23% [2]. This decrease is at least three times larger than what would be expected on the basis of unbound valproic acid concentrations (assuming a 10% unbound fraction [14]) and a Ki of 600 µm for CYP2C9 [30]. Thus, the concentrations of valproic acid to which the enzyme is exposed in the liver may be even higher than its unbound plasma concentrations. Thus, predictions based on unbound plasma concentrations of valproic acid may underestimate its inhibitory potency.
Some patients may require valproic acid concentrations even higher than 700 µm, the upper limit of the therapeutic range, to achieve seizure control [14]. Because the free fraction of valproic acid increases nonlinearly when the total plasma concentrations exceed 550 µm [14], it is likely that the concentrations of valproic acid at the enzyme in the liver could be disproportionately higher with large doses of valproic acid, leading to even greater reduction in hepatic CYP2C9 activity. Furthermore, as CYP2C9 is expressed in human small intestine [31], valproic acid may also cause interactions by inhibiting intestinal CYP2C9.
The observation that inactivation of CYP2A6 activity by valproic acid is time-, concentration- and NADPH-dependent suggests that it is a mechanism-based inactivator of this CYP isoform, a conclusion supported further by the finding that the formation of 4-ene-valproic acid, a minor metabolite of valproic acid, was mediated by CYP2A6 and CYP2C9 in vitro [32]. However, as the inhibitory effect was weak (KI 9150 µm and Kinact 0.048 min−1), it is unlikely that valproic acid would significantly inactivate CYP2A6 at clinically relevant concentrations.
In this study, the estimated apparent Ki values of valproic acid for CYP3A4 (7975 µm) and CYP2C19 (8553 µm) were about 10 times as high as the Ki for CYP2C9. Furthermore, valproic acid had virtually no effect on CYP1A2, CYP2D6 and CYP2E1 activities. Therefore, valproic acid is not expected to inhibit hepatic drug metabolism mediated by these CYP isoforms. This is also in agreement with studies showing that valproic acid had no effect on the plasma concentrations of the CYP1A2 substrates caffeine [33] and clozapine [34] or the CYP2D6 and CYP3A4 substrate haloperidol [35]. However, the concentration of valproic acid may be very high in the enterocytes during absorption and consequently inhibition of intestinal CYP3A4 activity cannot be totally excluded. In one study in epileptic patients and control subjects, treatment with valproic acid was associated with an increase in the AUC (about 50%), but not in the half-life, of nimodipine [9], suggesting inhibition of CYP3A4-mediated presystemic metabolism of nimodipine.
Valproic acid decreases the metabolic clearances of diazepam, phenobarbitone and amitriptyline by 25–40% [4–8, 10]. The metabolism of diazepam is mediated mainly by CYP2C19 and CYP3A4, but CYP2C9 may also be involved [36]. Therefore, inhibition of CYP2C9 by valproic acid may explain the reported modest reduction in the clearance of unbound diazepam [8]. The metabolism of phenobarbitone involves glucosidation, glucuronidation [37] and CYP2C19-mediated oxidation [38], but the contributions of other CYP isoforms are not known. CYP2C19, CYP2D6 and CYP3A4 seem to be the major enzymes mediating the metabolism of amitriptyline, but CYP2C9 is also involved [39] and a small fraction of the dose is eliminated via direct glucuronidation [40]. Consequently, the inhibitory effects of valproic acid on CYP2C9 and UDP-glucuronyltransferases [37, 41] probably explain the valproic acid-phenobarbitone [4–7] and valproic acid–amitriptyline interactions [10].
In conclusion, valproic acid inhibits CYP2C9 activity in vitro in human liver microsomes, with an apparent Ki value of 600 µm. It may also inhibit CYP2C9 activity in vivo especially in patients requiring high doses of valproic acid. Although valproic acid is a weak inhibitor of CYP3A4, the possibility of inhibition of the intestinal enzyme can not be completely excluded. With the absence of an inhibitory effect on CYP1A2, CYP2D6 and CYP2E1 activities, and weak inhibition of CYP2A6 and CYP2C19 activities, valproic acid is unlikely to produce clinically relevant interactions by inhibiting these CYP isoforms.
Acknowledgments
We would like to thank Mr Jouko Laitila and Mrs Kerttu Mårtensson for skilful technical assistance. This study was supported by grants from the Helsinki University Central Hospital Research Fund and the National Technology Agency of Finland (Tekes).
References
- 1.Bruni J, Gallo JM, Lee CS, Perchalski RJ, Wilder BJ. Interactions of valproic acid with phenytoin. Neurology. 1980;30:1233–1236. doi: 10.1212/wnl.30.11.1233. [DOI] [PubMed] [Google Scholar]
- 2.Perucca E, Hebdige S, Frigo GM, Gatti G, Lecchini S, Crema A. Interaction between phenytoin and valproic acid: plasma protein binding and metabolic effects. Clin Pharmacol Ther. 1980;28:779–789. doi: 10.1038/clpt.1980.235. [DOI] [PubMed] [Google Scholar]
- 3.Lai ML, Huang JD. Dual effect of valproic acid on the pharmacokinetics of phenytoin. Biopharm Drug Dispos. 1993;14:365–370. doi: 10.1002/bdd.2510140409. [DOI] [PubMed] [Google Scholar]
- 4.Levy RH, Koch KM. Drug interactions with valproic acid. Drugs. 1982;24:543–556. doi: 10.2165/00003495-198224060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Suganuma T, Ishizaki T, Chiba K, Hori M. The effect of concurrent administration of valproate sodium on phenobarbital plasma concentration/dosage ratio in pediatric patients. J Pediatr. 1981;99:314–317. doi: 10.1016/s0022-3476(81)80488-x. [DOI] [PubMed] [Google Scholar]
- 6.Bruni J, Wilder BJ, Perchalski RJ, Hammond EJ, Villarreal HJ. Valproic acid and plasma levels of phenobarbital. Neurology. 1980;30:94–97. doi: 10.1212/wnl.30.1.94. [DOI] [PubMed] [Google Scholar]
- 7.Patel IH, Levy RH, Cutler RE. Phenobarbital–valproic acid interaction. Clin Pharmacol Ther. 1980;27:515–521. doi: 10.1038/clpt.1980.72. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon S, Richens A. Valproic acid and diazepam interaction in vivo. Br J Clin Pharmacol. 1982;13:553–560. doi: 10.1111/j.1365-2125.1982.tb01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartara A, Galimberti CA, Manni R, et al. Differential effects of valproic acid and enzyme-inducing anticonvulsants on nimodipine pharmacokinetics in epileptic patients. Br J Clin Pharmacol. 1991;32:335–340. doi: 10.1111/j.1365-2125.1991.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SL, Cavanaugh J, Shi H, Awni WM, Granneman GR. Effects of divalproex sodium on amitriptyline and nortriptyline pharmacokinetics. Clin Pharmacol Ther. 1996;60:48–53. doi: 10.1016/S0009-9236(96)90166-6. [DOI] [PubMed] [Google Scholar]
- 11.Fehr C, Grunder G, Hiemke C, Dahmen N. Increase in serum clomipramine concentrations caused by valproate. J Clin Psychopharmacol. 2000;20:493–494. doi: 10.1097/00004714-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Broly F, Libersa C, Lhermitte M, Dupuis B. Inhibitory studies of mexiletine and dextromethorphan oxidation in human liver microsomes. Biochem Pharmacol. 1990;39:1045–1053. doi: 10.1016/0006-2952(90)90283-q. [DOI] [PubMed] [Google Scholar]
- 13.Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 14.Davis R, Peters DH, McTavish D. Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;47:332–372. [PubMed] [Google Scholar]
- 15.Rettie AE, Sheffels PR, Korzekwa KR, Gonzalez FJ, Philpot RM, Baillie TA. CYP4 isozyme specificity and the relationship between omega-hydroxylation and terminal desaturation of valproic acid. Biochemistry. 1995;34:7889–7895. doi: 10.1021/bi00024a013. [DOI] [PubMed] [Google Scholar]
- 16.Levy RH, Rettenmeier AW, Anderson GD, et al. Effects of polytheraphy with phenytoin, carbamazepine, and stiripentol on formation of 4-ene-valproate, a hepatotoxic metabolite of valproic acid. Clin Pharmacol Ther. 1990;48:225–235. doi: 10.1038/clpt.1990.144. [DOI] [PubMed] [Google Scholar]
- 17.Chauret N, Gauthier A, Martin J, Nicoll-Griffith DA. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab Dispos. 1997;25:1130–1136. [PubMed] [Google Scholar]
- 18.Ko JW, Sukhova N, Thacker D, Chen P, Flockhart DA. Evaluation of omeprazole and lansoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab Dispos. 1997;25:853–862. [PubMed] [Google Scholar]
- 19.Wester MR, Lasker JM, Johnson EF, Raucy JL. CYP2C19 participates in tolbutamide hydroxylation by human liver microsomes. Drug Metab Dispos. 2000;28:354–359. [PubMed] [Google Scholar]
- 20.Koenigs LL, Peter RM, Thompson SJ, Rettie AE, Trager WF. Mechanism-based inactivation of human liver cytochrome P450 2A6 by 8-methoxypsoralen. Drug Metab Dispos. 1997;25:1407–1415. [PubMed] [Google Scholar]
- 21.Ward ES, Pollack GM, Brouwer KL. Probenecid-associated alterations in valproic acid pharmacokinetics in rats: can in vivo disposition of valproate glucuronide be predicted from in vitro formation data? Drug Metab Dispos. 2000;28:1433–1439. [PubMed] [Google Scholar]
- 22.Liu MJ, Brouwer KL, Pollack GM. Pharmacokinetics and pharmacodynamics of valproate analogs in rats. III. Pharmacokinetics of valproic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexanecarboxylic acid in the bile-exteriorized rat. Drug Metab Dispos. 1992;20:810–815. [PubMed] [Google Scholar]
- 23.Hargreaves MB, Jones BC, Smith DA, Gescher A. Inhibition of p-nitrophenol hydroxylase in rat liver microsomes by small aromatic and heterocyclic molecules. Drug Metab Dispos. 1994;22:806–810. [PubMed] [Google Scholar]
- 24.Newton DJ, Wang RW, Lu AY. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1995;23:154–158. [PubMed] [Google Scholar]
- 25.Eagling VA, Tjia JF, Back DJ. Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol. 1998;45:107–114. doi: 10.1046/j.1365-2125.1998.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman D, Wang JP, Wang Y, Unadkat JD. Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos. 1998;26:207–215. [PubMed] [Google Scholar]
- 27.Raucy JL, Schultz ED, Wester MR, et al. Human lymphocyte cytochrome P450 2E1, a putative marker for alcohol-mediated changes in hepatic chlorzoxazone activity. Drug Metab Dispos. 1997;25:1429–1435. [PubMed] [Google Scholar]
- 28.Ha HR, Rentsch KM, Kneer J, Vonderschmitt DJ. Determination of midazolam and its alpha-hydroxy metabolite in human plasma and urine by high-performance liquid chromatography. Ther Drug Monit. 1993;15:338–343. doi: 10.1097/00007691-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- 30.von Moltke LL, Greenblatt DJ, Schmider J, Wright CE, Harmatz JS, Shader RI. In vitro approaches to predicting drug interactions in vivo. Biochem Pharmacol. 1998;55:113–122. doi: 10.1016/s0006-2952(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang QY, Dunbar D, Ostrowska A, Zeisloft S, Yang J, Kaminsky LS. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–809. [PubMed] [Google Scholar]
- 32.Sadeque AJM, Fisher MB, Korzekwa KR, Gonzalez FJ, Rettie AE. Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther. 1997;283:698–703. [PubMed] [Google Scholar]
- 33.Wietholtz H, Zysset T, Kreiten K, et al. Effect of phenytoin, carbamazepine, and valproic acid on caffeine metabolism. Eur J Clin Pharmacol. 1989;36:401–406. doi: 10.1007/BF00558303. [DOI] [PubMed] [Google Scholar]
- 34.Facciola G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its metabolites in patients with schizophrenic or affective disorder. Ther Drug Monit. 1999;21:341–345. doi: 10.1097/00007691-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Hesslinger B, Normann C, Langosch JM, Klose P, Berger M, Walden J. Effects of carbamazepine and valproic acid on haloperidol plasma levels and on psychopathologic outcome in schizophrenic patients. J Clin Psychopharmacol. 1999;19:310–315. doi: 10.1097/00004714-199908000-00005. 10.1097/00004714-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Jung F, Richardson TH, Raucy JL, Johnson EF. Diazepam metabolism by cDNA-expressed human 2C P450s. Identification of P4502C18 and P4502C19 as low KM diazepam N-demethylases. Drug Metab Dispos. 1997;25:133–139. [PubMed] [Google Scholar]
- 37.Bernus I, Dickinson RG, Hooper WD, Eadie MJ. Inhibition of phenobarbitone N-glucosidation by valproate. Br J Clin Pharmacol. 1994;38:411–416. doi: 10.1111/j.1365-2125.1994.tb04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamiya K, Hadama A, Yukawa E, et al. CYP2C19 polymorphism effect on phenobarbitone. Pharmacokinetics in Japanese patients with epilepsy: analysis by population pharmacokinetics. Eur J Clin Pharmacol. 2000;55:821–825. doi: 10.1007/s002280050703. [DOI] [PubMed] [Google Scholar]
- 39.Venkatakrishnan K, Greenblatt DJ, von Moltke LL, Schmider J, Harmatz JS, Shader RI. Five distinct human cytochromes mediate amitriptyline N-demethylation in vitro: dominance of CYP2C19 and CYP3A4. J Clin Pharmacol. 1998;38:112–121. doi: 10.1002/j.1552-4604.1998.tb04399.x. [DOI] [PubMed] [Google Scholar]
- 40.Dahl-Puustinen ML, Aberg-Wistedt A, Bertilsson L. Glucuronidation of amitriptyline in man in vivo. Pharmacol Toxicol. 1989;65:37–39. doi: 10.1111/j.1600-0773.1989.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 41.Yuen AW, Land G, Weatherley BC, Peck AW. Sodium valproate acutely inhibits lamotrigine metabolism. Br J Clin Pharmacol. 1992;33:511–513. doi: 10.1111/j.1365-2125.1992.tb04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]