Abstract
Aims
Inherited differences in thiopurine methyltransferase (TPMT) activity are an important factor in the wide interindividual variations observed in the clinical response to thiopurine chemotherapy. The aim of this study was to establish a population range for red blood cell (RBC) TPMT activity in children with acute lymphoblastic leukaemia (ALL) at disease diagnosis. An additional aim was to investigate factors that can influence TPMT activity within the RBC.
Methods
Blood samples were collected from children with ALL at disease diagnosis, prior to any blood transfusions, as part of the nationwide UK MRC ALL97 therapeutic trial. RBC TPMT activity was measured by h.p.l.c. RBCs were age-fractionated on Percoll density gradients.
Results
Pretreatment blood samples were received from 570 children within 3 days of venepuncture. TPMT activities at disease diagnosis ranged from 1.6 to 23.6 units/ml RBCs (median 7.9) compared with 0.654–18.8 units (median 12.9), in 111 healthy control children (median difference 4.5 units, 95% CI 3.9, 5.1 units, P < 0.001). A TPMT quality control sample, aliquots of which were assayed in 60 analytical runs over a 12 month period, contained a median of 11.98 units with a CV of 11.6%. Seven children had their RBCs age-fractionated on density gradients. TPMT activities in the top gradient (young cells) ranged from 4.2 to 14.1 units (median 7.5) and in the bottom gradient (old cells) 1.5–12.6 units (median 4.7 units), median difference 2.3 units, 95% CI 0.7, 4.1, P = 0.035.
Conclusions
Circulating RBCs do not constitute a homogeneous population. They have a life span of around 120 days and during that time undergo a progressive ageing process. The anaemia of ALL is due to deficient RBC production. The results of this study indicate that RBC TPMT activities are significantly lower in children with ALL at disease diagnosis. This may be due, at least in part, to a relative excess of older RBCs.
Keywords: acute lymphoblastic leukaemia, mercaptopurine, red blood cells, thioguanine nucleotides, thioguanine, thiopurine methyltransferase
Introduction
Thiopurine methyltransferase (TPMT) is an important enzyme in the biotransformation of the thiopurine drugs 6-mercaptopurine (6MP) and 6-thioguanine (6TG). Inherited differences in TPMT activity are an important factor in the wide interindividual variations observed in the clinical response to thiopurine chemotherapy. TPMT activity in the red blood cell and other human tissues, including the liver, is under the control of a common genetic polymorphism [1, 2]. The frequency distribution of TPMT activity in Caucasian populations is trimodal: approximately 89% of the population have high enzyme activity and are homozygous for the wild-type allele (TPMTH), 11% inherit intermediate levels of enzyme activity with one wild-type and one variant allele (heterozygous TPMTH/TPMTL) whilst 1 in 300 subjects have no functional activity (two variant alleles, homozygous TPMTL). A number of variant TPMT alleles have now been described [3–5].
Competing with methylation in the biotransformation of thiopurine drugs is oxidation and nucleotide metabolite formation. Oxidation is a catabolic route leading to the formation of hydroxy-thiopurines which include thiouric acid. Nucleotide formation, catalysed via hypoxanthine phosphoribosyltransferase, is a prerequisite for active thiopurine metabolites. Both 6MP and 6TG eventually form the same abnormal nucleotides, the thioguanine nucleotides (TGNs), but the way in which they are metabolized differs. 6TG forms TGNs directly whereas 6MP forms a variety of active nucleotide metabolites. The initial 6MP nucleotide (thioinosine monophosphate) is oxidized to thioxanthine nucleotide (thioxanthosine monophosphate) which is subsequently converted to TGNs. 6MP nucleotide is a good substrate for TPMT, some 18-fold better than the TGNs which function as poor substrates [6].
6MP was first used in the treatment of childhood acute lymphoblastic leukaemia (ALL) in the 1950s [7]. Since then the drug has become enshrined in all conventional protocols as part of long-term continuing chemotherapy. The response to 6MP therapy, in terms of marrow suppression, is highly variable and the ability of the child to form TGN metabolites from the parent pro-drug is also important in terms of the risk of disease relapse [8]. Children with very high TPMT activities form low concentrations of TGNs from standard dosages of mercaptopurine and have a higher subsequent relapse rate [9]. Although 6MP is the traditional drug used in continuing chemotherapy protocols there may be some advantages in the use of 6TG, particularly in those children with high TPMT activity. A back-to-back comparison of the two thiopurines is the subject of a number of current clinical trials [10].
Factors that can influence the efficacy of oral 6MP therapy are many and include compliance, drug absorption and transport and variable metabolism. The latter has been shown to influence the antileukaemic effect of 6MP [9]. In a pilot study, red blood cell (RBC) TPMT activity, measured at disease diagnosis in children with ALL, has been shown to reflect thiopurine metabolism and toxicity during continuing chemotherapy [11], and pretreatment RBC TPMT activity has been reported to correlate with leukaemic blast TPMT activity [12]. Therefore a knowledge of enzyme activity before beginning therapy with 6MP may help in clinical management, particularly of those children with very low or very high TPMT activities. Approximately 400 children per year enter UK ALL protocols which contain 2 or 3 years antimetabolite based continuing chemotherapy. Thus, at any one time two or three children taking thiopurines will be TPMT deficient. Such children can be managed by the use of heavily attenuated dosage regimens [13–15].
The primary aim of this study was to establish a population range of TPMT activities in children with ALL at disease diagnosis as part of the UK MRC ALL97 trial. RBCs are used as an easily accessible tissue in which to monitor TPMT activity. An additional aim was to investigate factors which could influence the stability of TPMT activity within the RBC. This study formed part of a larger programme to assess the importance of TPMT activity in the clinical management of ALL.
Methods
Patients and study design
Blood samples were forwarded from children with ALL at disease diagnosis, prior to any blood transfusions, as part of the MRC ALL97 therapeutic trial [10]. The UK MRC ALL trials, and the thiopurine studies within them, were approved by the local ethics committees of all the participating clinical centres and full informed written consent was obtained from the parents. Blood samples (3 ml) were taken into lithium heparin tubes and forwarded by first class post to the Sheffield centre for the preparation of red cell lysates for the assay of TPMT activity. In addition, red cell lysates prepared from 44 children who were long-term survivors of ALL, were assayed. This subgroup who were in full remission and no longer receiving chemotherapy, formed part of a separate study. TPMT activities measured in both these groups of children were compared with those measured in a previously published control group of 111 healthy children [16] and 104 long-term survivors of ALL [9].
TPMT stability studies
To study the stability of RBC TPMT activity in heparinized blood samples, control blood was taken from a healthy adult volunteer. The whole blood sample was kept at room temperature (20 ° C) for 7 days, with gentle mixing for 5 min every 12 h, and aliquots removed daily for the assay of RBC TPMT activity. This study was repeated in patients when excess blood samples were provided. In an attempt to study the effect of delays due to the mailing of samples on TPMT activity 5 ml aliquots of control blood in lithium heparin tubes were forwarded by first-class post to a second centre. On receipt the blood samples were kept on the laboratory bench and mailed back to the Sheffield centre, on a daily basis, over a period of time when the maximum daily ambient temperature during postage was 27 ° C. On receipt of the whole blood sample in Sheffield, RBC lysates were prepared and stored at −80 ° C prior to assay for TPMT activity.
Blood fractionation
To investigate the influence of RBC age on TPMT activity leucocyte depleted blood was age fractionated on percoll gradients. Two groups of subjects were studied. Firstly, a group of healthy adult volunteers and secondly, children with ALL when excess (5 ml) blood samples were provided for the measurement of TPMT at disease diagnosis. This technique has been described in detail elsewhere [17]. Briefly, leucocytes are removed from whole blood (4 ml) by filtering through cellulose and the eluted RBCs were washed and resuspended in saline to a haematocrit of 40%. The leucocyte depleted blood (500 µl per gradient) was then layered onto discontinuous (50, 57%, 60%, 63%, 66% and 69%) Percoll gradients and the 12 ml conical disposable test-tubes centrifuged at 1100 g for 10 min at 20 ° C. After centrifugation the 50% layer, which contained no RBCs, was discarded Each gradient fraction removed contained the cells in that fraction and those lying on the interface layer beneath. All fractions of the same Percoll concentration, from the same individual, were pooled and washed twice in saline prior to resuspension of the packed RBCs in 1 volume of saline. The haematocrit of the resuspended cells was recorded prior to preparation of RBC lysates.
TPMT assay
RBC lysates, for the assay of TPMT activity, were prepared as previously described [16] using 200 µl of resuspended RBCs, at an haematocrit of approximately 40%, lysed in 800 µl of ice-cold water. RBC lysates were stored at −80 ° C prior to the assay of TPMT activity, which was measured by a modification of the radiochemical assay described by Weinshilboum and coworkers [1, 16]. The assay is based on the TPMT-catalysed conversion of 6MP to 6-methylmercaptopurine using nonradioactive S-adenosyl-l-methionine as the methyl donor. Methylmercaptopurine is extracted from the incubate and the concentration measured by reverse phase h.p.l.c. with u.v. detection. One unit of TPMT activity represents the formation of 1 nmol methylmercaptopurine h−1 ml−1 of packed RBCs at 37 ° C. TPMT activity as assayed in quadruplicate using 100 µl lysate per assay [16].
Statistical analysis
Population differences were assessed by the Kruskal–Wallis one-way analysis of variance by ranks. Statistical comparisons were made by the Mann–Whitney test, and for paired data by the Wilcoxon signed-ranks test.
Results
TPMT assay
Standard curves ranging from 3 ng to 30 ng (18–180 pmol) methylmercaptopurine/100 µl spiked lysate were taken through every analytical run. Evaluated over 10 assays the interassay coefficient of variation (CV) ranged from 9 to 5%. The intra-assay CV for the healthy adult volunteer TPMT quality control sample was 6.3% (activity 11.9 units, n = 6).
TPMT stability studies
Prior to the study of TPMT activity in patient samples the stability of TPMT, at ambient temperature and during shipment, was investigated. Quality control blood left at room temperature, from which aliquots were removed and lysates prepared daily, over 7 days gave 12.0 units TPMT/ml RBCs (CV 7.1%, n = 7). Quality control blood posted back to the Sheffield centre was received on a daily basis from day 2 (48 h post blood sampling) to day 7. On receipt no blood sample had lysed or clotted. TPMT activity was 12.65 units (CV 5.6% n = 6).
The stability of TPMT activity was then studied in 20 patients to confirm that the enzyme stability observed in healthy adults was reflected children with ALL. Blood sample aliquots were processed within 24 h of blood sampling and after 3 days and 6 days at room temperature. There was no statistical difference between TPMT activities measured at 24 h and 3 days but by day 6 TPMT activities had decreased. At 24 h TPMT activities ranged from 5.4 to 18.4 units (median 9.3) compared with 4.6–17.6 units (median 8.2) at 6 days (median difference 1.19 units, 95% CI 0.4, 1.8, P = 0.001). The stability of TPMT was studied in an additional five patients whose whole blood sample was processed in two aliquots, one removed 24 h after sampling and the second after 5 days at room temperature. At 24 h TPMT activities ranged from 7.6 to 14.8 units (median 10.2) compared with 7.3–14.2 units (median 9.0) at 5 days. In this small sample group the difference in TPMT activities approached, but did not reach significance (P = 0.06, median difference 1.0 unit, 95% CI 0.3, 1.8). After 5 days at room temperature all the blood samples showed some red cell lysis.
TPMT activity in ALL at disease diagnosis
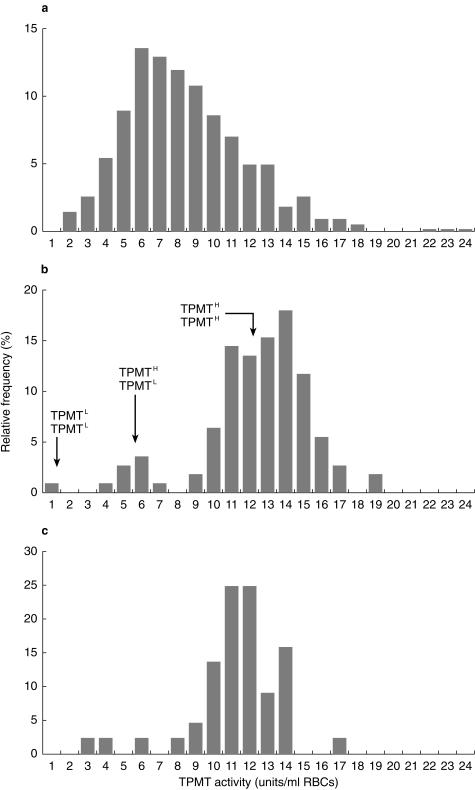
TPMT activity was measured at disease diagnosis in pretransfusion blood samples taken from 570 children entered onto the MRC ALL97 trial whose red cell lysates were prepared within 3 days of blood sampling. At diagnosis TPMT activities ranged from 1.6 to 23.6 units, median 7.9, compared with 0.65–18.8 units, median 12.9, in 111 healthy control children (median difference 4.5 units, 95% CI 3.9, 5.1 units, P < 0.001). The TPMT activities measured in 44 long-term survivors of ALL ranged from 3.1 to 16.8 units (median 11.5) (Figure 1). TPMT activities were lower in the long term survivors when compared with the healthy control children (median difference 1.1 units, 95% CI 0.3–1.9 units, P < 0.005). The TPMT activities in these 44 long-term survivors was no different to the range of 5.7–16.4 recorded in a previously published group of 104 long-term survivors of ALL [9]. A TPMT quality control sample, aliquots of which were assayed in 60 analytical runs over a 12 month period, gave a value of 11.98 units, CV 11.6%.
Figure 1.
TPMT frequency distribution histograms for 570 children with ALL at disease diagnosis (a), 111 healthy children ( b) and 44 long-term survivors of ALL (c). Presumed genotypes at the locus TPMT are indicated on the middle histogram.
TPMT activity in age-fractionated RBCs
Adult controls
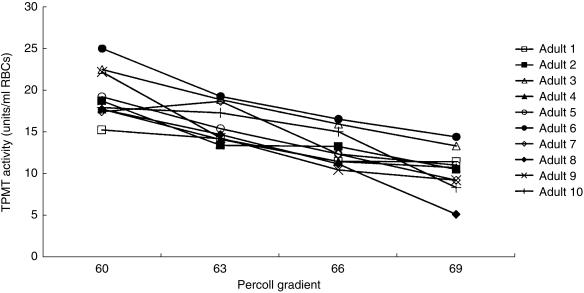
RBC TPMT activities were studied in 10 healthy adults (aged 22–46 years, median 23). TPMT activities in RBC lysates prepared from whole blood ranged from 13.3 to 18.3 units/ml RBCs (median 15.9) for the 10 adults. The 57% gradient contained insufficient RBCs for assay. TPMT activities in the 60%, 63%, 66% and 69% gradients for each of the 10 adults were analysed by the Kruskal–Wallis test. The RBC TPMT activities in each of the four gradients differed (P < 0.001). When age fractionated, TPMT activities in the top gradient (60% Percoll, younger cells) ranged from 15.2 to 25.1 units/ml RBCs (median 18.3) compared with 5.2–14.5 units (median 10.7) in the bottom gradient (69% Percoll, oldest cells). In the 10 adults young RBCs had higher TPMT activities than old cells, Wilcoxon median difference 8.8 units (95% CI 7.2–10.8, P = 0.006) (Figure 2).
Figure 2.
TPMT activities in age fractionated RBCs for the 10 healthy adults. The whole blood TPMT activities ranged from 13.3 to 18.3 units/ml RBCs (median 15.9).
Children with ALL
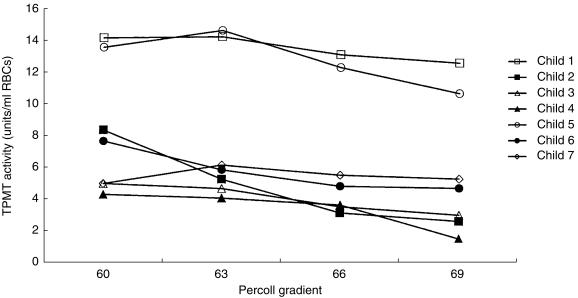
RBC TPMT activities were studied in seven children with ALL (aged 2–11 years, median 5). Whole blood TPMT activity measured ranged from 4.2 to 13.5 units/ml RBCs (median 6.5). Four children had TPMT activities less than 9.0 units, activities which would be classified as a heterozygous TPMT phenotype in healthy children. The 57% gradient contained insufficient RBCs for assay. Analysis of the TPMT activities in the 60%, 63%, 66% and 69% gradients for each of the seven children by the Kruskal–Wallis test did not show that the activities in each of the four gradients differed. Comparison of the enzyme activities in the top (60%) and bottom (69%) gradients indicated that TPMT activities were higher in the young cells than the old cells. TPMT activities in the 60% gradient ranged from 4.2 to 14.1 units (median 7.5) and in the 69% gradient 1.5–12.6 units (median 4.7 units), median difference 2.3 units, 95% CI 0.7, 4.1, P = 0.035 (Figure 3).
Figure 3.
TPMT activities in age fractionated RBCs for seven children with ALL. The whole blood TPMT activities ranged from 4.2 to 13.5 units/ml RBCs (median 6.5).
Discussion
The range of TPMT activities measured in children with ALL at disease diagnosis were significantly lower than those measured in healthy children. Stability studies on human RBC TPMT activity in healthy adults showed that the enzyme was stable within the RBC for several days. TPMT activity within the patient samples was not as robust as the adult controls and activities started to decrease as red cell lysis occurred. Nonetheless, patient TPMT activity was stable for at least 3 days after blood sampling. The enzyme activities recorded in children at disease diagnosis were measured in blood samples processed within 3 days of sampling and therefore, the lower range of TPMT activities recorded in these children was not an artefact produced by a deterioration of enzyme activity post sampling. TPMT activities measured in the long term survivors of lymphoblastic leukaemia, assayed alongside the disease diagnosis samples, showed a similar range of enzyme activities to those previously recorded for this subgroup [9]. Therefore the decrease in measured activities at diagnosis was not due to a systematic decline in the TPMT phenotype assay. Age-fractionation of adult RBCs indicated that TPMT activities were significantly higher in young RBCs when compared to older RBCs. This pattern was also observed in the small group of children studied. The downward shift of the TPMT frequency distribution histogram observed at disease diagnosis may be due in part to an excess of older RBCs.
Circulating RBCs do not constitute an homogeneous population. They have a life span of around 120 days and during that time undergo a progressive ageing process. Glutathione S-transferase shows an age-dependent decay in human RBCs and this reduction in detoxifying ability is thought to play a part in the RBC ageing process [18]. TPMT at disease diagnosis could be present in an abnormally old population of RBCs, because the anaemia of ALL is due to deficient RBC production. At the end of its life-span a RBC is smaller, has a higher concentration of solutes and is heavier than the younger RBC. Density gradient separation of RBCs is a well validated technique which yields cell fractions with a significant progressive shift in the average cell age with increasing gradient density. The fractions isolated are heterogeneous but those fractions at the top are enriched with younger cells whilst the older cells graduate to the bottom of the gradient [19].
The TPMT frequency distribution and range of enzyme activities are similar in adults and children [9, 16], and long-term and circadian variability in healthy human adult TPMT activities are low [20]. In addition to the influence of RBC age, a number of factors have been reported to influence TPMT phenotypic activity. The range of enzyme activities measured in some ethnic groups is higher than others [21–23]. Uraemia is associated with an increased range of RBC TPMT activities [24] and 6MP chemotherapy significantly increases the measured range of TPMT activities when compared to healthy control children. In the latter example, TPMT activities decrease to the healthy control range at the end of 6MP therapy [9]. The shifts of TPMT activities observed in leukaemic children may, in part, be due to the RBC age distribution of the study population.
Methylmercaptopurine nucleotide concentrations (products of the TPMT reaction) do not differ between young and old RBCs and this observation has been taken to suggest that TPMT activities do not vary with RBC age [17]. This is in direct contrast with the studies reported in this paper and indicates the complexity of the biochemical background behind the formation of RBC thiopurine metabolites. RBC and liver TPMT activities are correlated [2] and thus RBC TPMT and drug metabolite concentrations may reflect hepatic thiopurine metabolism. Thiopurines are highly metabolized drugs and the liver plays a major role in the ultimate appearance of thiopurine metabolites within the RBC [13, 15, 25]. This could explain why the accumulation profile of 6MP metabolites is the same in all ages of RBCs [17], the function of the RBC may be to form nucleotides from thiopurine metabolites originating elsewhere. In vivo a major function of the RBC is the formation of nucleotides from endogenous purines [17, 25].
In this study a population range for TPMT activity was established in children with ALL at disease diagnosis. A downward shift in TPMT activities was documented which could, in part, be due to an excess of older erythrocytes in the blood samples obtained from the leukaemic child. In childhood ALL thiopurines constitute a major part of long-term continuing chemotherapy and it is important to identify treatment variables that could influence the systemic exposure to active drug metabolites. TPMT activity has been related to the outcome and/or toxicity of therapy retrospectively [9, 11, 26]. For the TPMT deficient child a knowledge of complete absence of TPMT activity can predict dose limiting severe myelotoxicity [13–15]. However, the results of this study indicate that not all children who develop high TPMT activity during chemotherapy, and consequent ‘resistance’ to standard thiopurine dosages, will be predicted at disease diagnosis. With the exception of the identification of children with homozygous low TPMT activity prior to thiopurine therapy, the optimum point in treatment for the measurement of RBC TPMT activity in a prospective study remains to be determined.
Acknowledgments
This work was supported by the Leukaemia Research Fund and a British Pharmacological Society intercalated BSc award (TSC). Blood samples from children on the UK MRC leukaemia trial ALL97 were taken as part of the trial protocol and forwarded by collaborating paediatric oncologists to whom we are grateful.
References
- 1.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 2.Szumlanski CL, Honchel R, Scott MC, Weinshilboum RM. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992;2:148–159. [PubMed] [Google Scholar]
- 3.Szumlanski C, Otterness D, Her C, et al. Thiopurine methyltransferase pharmacogenetics: human gene cloning and characterization of a common genetic polymorphism. DNA Cell Biology. 1996;15:17–30. doi: 10.1089/dna.1996.15.17. [DOI] [PubMed] [Google Scholar]
- 4.Otterness D, Szumlanski C, Lennard L, et al. Human thiopurine methyltransferase pharmacogenetics: Gene sequence polymorphisms. Clin Pharmacol Ther. 1997;62:60–73. doi: 10.1016/S0009-9236(97)90152-1. [DOI] [PubMed] [Google Scholar]
- 5.Otterness DM, Szumlanski CL, Wood TC, Weinshilboum RM. Human thiopurine methyltransferase pharmacogenetics: Kindred with a terminal exon splice junction mutation that results in loss of activity. J Clin Invest. 1998;101:1036–1044. doi: 10.1172/JCI1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deininger M, Szumlanski CL, Otterness DM, van Loon J, Ferber W, Weinshilboum RM. Purine substrates for human thiopurine methyltransferase. Biochem Pharmacol. 1994;11:2135–2138. doi: 10.1016/0006-2952(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 7.Burchenal JH, Murphy ML, Ellison RR, et al. Clinical evaluation of a new anti metabolite, 6-mercaptopurine, in the treatment of leukaemia and allied diseases. Blood. 1953;8:965–999. [PubMed] [Google Scholar]
- 8.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukaemia. J Clin Oncol. 1989;7:1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [published erratum appears in J Clin Oncol 1990; 8: 567] [DOI] [PubMed] [Google Scholar]
- 9.Lennard L, Lilleyman JS, Van Loon JA, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 10. http://www.icnet.uk/trials/children/mrcall97.html.
- 11.Lennard L, Welch J, Lilleyman JS. Thiopurine drugs in the treatment of childhood leukaemia: the influence of inherited thiopurine methyltransferase activity on drug metabolism and cytotoxicity. Br J Clin Pharmacol. 1997;44:455–461. doi: 10.1046/j.1365-2125.1997.t01-1-00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod HL, Relling MV, Liu Q, Pui CH, Evans WE. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995;7:1897–1902. [PubMed] [Google Scholar]
- 13.Lennard L, Gibson BES, Nicole T, Lilleyman JS. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Childh. 1993;69:577–579. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLeod HL, Miller DR, Evans WE. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993;341:1151. doi: 10.1016/0140-6736(93)93168-z. [DOI] [PubMed] [Google Scholar]
- 15.Lennard L, Lewis IJ, Michelangnoli M, Lilleyman JS. Thiopurine methyltransferase deficiency in childhood lymphoblastic leukaemia: 6-mercaptopurine dosage strategies. Med Ped Oncol. 1997;29:252–255. doi: 10.1002/(sici)1096-911x(199710)29:4<252::aid-mpo3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Lennard L, Singleton HJ. High performance liquid chromatographic assay of human red blood cell thiopurine methyltransferase activity. J Chromatog B. 1994;661:25–33. doi: 10.1016/0378-4347(94)00327-0. [DOI] [PubMed] [Google Scholar]
- 17.Rostrami-Hodjegan A, Lennard L, Lilleyman JS. The accumulation of mercaptopurine metabolites in age fractionated red blood cells. Br J Clin Pharmacol. 1995;40:217–222. doi: 10.1111/j.1365-2125.1995.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazi A, Accorsi A, Piatti E, Magnani M. Cell age dependent decay of human erythrocyte glutathione S-transferase. Mech Ageing Dev. 1991;58:255–266. doi: 10.1016/0047-6374(91)90097-j. [DOI] [PubMed] [Google Scholar]
- 19.Piomelli S, Seaman C. Mechanism of red blood cell ageing: relationship of cell density and cell age. Am J Hematol. 1993;42:46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]
- 20.Giverhaug T, Klemetsdal B, Lysaa Aarbakke J. Intraindividual variability in red blood cell thiopurine methyltransferase activity. Eur J Clin Pharmacol. 1996;50:217–220. doi: 10.1007/s002280050095. 10.1007/s002280050095. [DOI] [PubMed] [Google Scholar]
- 21.McLeod HL, Lin J-S, Scott EP, Pui C-H, Evans WE. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther. 1994;55:15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- 22.Park-Hah JO, Klementsdal B, Lyssa R, Choi KH, Aarbakke J. Thiopurine methyltransferase activity in a Korean population sample of children. Clin Pharmacol Ther. 1996;60:68–74. doi: 10.1016/S0009-9236(96)90169-1. [DOI] [PubMed] [Google Scholar]
- 23.Klemetsdal B, Tollefsen E, Loennechen T, et al. Interethnic differences in thiopurine methyltransferase activity. Clin Pharmacol Ther. 1992;51:24–31. doi: 10.1038/clpt.1992.4. [DOI] [PubMed] [Google Scholar]
- 24.Pazmino PA, Sladek SL, Weinshilboum RM. Thiol S-methylation in ureamia: erythrocyte enzyme activities and plasma inhibitors. Clin Pharmacol Ther. 1980;28:356–367. doi: 10.1038/clpt.1980.174. [DOI] [PubMed] [Google Scholar]
- 25.Rowland K, Lennard L, Lilleyman JS. In vitro metabolism of 6-mercaptopurine by human liver cytosol. Xenobiotica. 1999;29:615–628. doi: 10.1080/004982599238434. 10.1080/004982599238434. [DOI] [PubMed] [Google Scholar]
- 26.Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]