Abstract
Background and purpose:
Pharmacological resultant analysis is a technique that can detect secondary effects of competitive antagonists in vitro. The utility of pharmacological resultant analysis as a potential tool for the investigation of antagonist interactions in vivo was examined in the present study using two opioid antagonists, naltrexone and CTAP.
Experimental approach:
Using the experimental design of pharmacological resultant analysis, the well-characterized opioid antagonist naltrexone was examined in the presence of multiple doses of CTAP to block the antinociceptive effects of morphine in the rat warm-water (55oC), tail-withdrawal assay.
Key results:
Alone, all doses of naltrexone, CTAP, and CTOP examined blocked the antinociceptive effects of morphine. In the presence of fixed doses of 1 or 10 μg CTAP, increasing doses of naltrexone produced dose-dependent shifts to the right in the morphine dose-response curve. However, a lower dose of naltrexone in combination with 1 or 10 μg CTAP failed to alter the morphine dose-response curve. In the presence of a fixed dose of 0.1 mg kg−1 naltrexone, CTAP doses produced irregular shifts to the right in the morphine dose-response curves.
Conclusions and implications:
Resultant analysis was applied and an apparent pKC value for CTAP was found to be one log unit higher than the apparent pA2 value for CTAP, evidence that CTAP may have secondary actions or that a signal transducer function may be altered by the combinations of these antagonists. Taken together, these data suggest pharmacological resultant analysis can reveal novel interactions between antagonists in vivo.
Keywords: antinociception, CTAP, CTOP, morphine, naltrexone, pharmacologic resultant, rats, Schild regressions
Introduction
Competitive, reversible antagonism studies are the defining pharmacological tool for characterizing opioid ligands and their receptors. When an antagonist produces a competitive reversible antagonism of an agonist, the receptor agonist occupancy-effect curve is shifted to the right in a parallel, dose-dependent manner and dose ratios can be calculated according to null methodology (Tallarida and Murray, 1987; Kenakin, 1997). A specific potency value, called the pA2 value, is defined as the negative logarithm of the molar concentration of the antagonist that produces a two-fold shift to the right in the agonist dose–response curve (Arunlakshana and Schild, 1959). This quantitative model of competitive antagonism has been the cornerstone of early opioid receptor characterization in vitro and in vivo (e.g., Takemori, 1974; Tallarida and Murray, 1987). Indeed, the opioid antagonists naloxone and naltrexone have been extensively characterized by apparent pA2 analysis to define receptor, ligand and pharmacological effects in a variety of animal models (Holtzman, 1982; Young et al., 1992; Picker et al., 1993; Walker et al., 1994).
According to prevailing theory, when an agonist binds to a receptor, the receptor shifts to an activated form that can interact with components of signal transduction pathways to initiate a response. Some G-protein-coupled receptors, such as the δ-opioid receptor, may also exist in a constitutively active form capable of regulating signal transduction systems independently of agonists (Costa and Herz, 1989; Neilan et al., 1999). Although unaffected by agonists, constitutive receptor activity can be reduced by some receptor antagonists. Under these circumstances, such antagonists are said to display negative intrinsic efficacy and are therefore referred to as ‘inverse agonists' because they produce effects opposite to those of agonists. A small minority of other antagonists referred to as ‘neutral antagonists' have no apparent effect on basal activity while occupying the receptor, yet block the effects of both agonists and inverse agonists (Kenakin, 2004). In vitro, traditional antagonists, such as naloxone, naltrexone, β-chloronaltrexamine (β-CNA), 7-benzylidenenaltrexone (BNTX) and diprenorphine were labeled inverse agonists because these antagonists produced effects opposite to those of agonists. Other antagonists such as 6β-naltrexol, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) and D-Phe-Cys-Tyr-D-Tryp-Lys-Thr-Pen-Thr-NH2 (CTAP) were been identified as possible neutral antagonists since these compounds fail to produce effects and CTAP blocked the effect of both naloxone and morphine in cells (Wang et al., 1994, 2001; Liu et al., 2001).
Data distinguishing among antagonists in regards to negative intrinsic efficacy is very limited in functional assays. In vivo, naltrexone, naloxone, CTAP and CTOP appear to be selective μ-opioid receptor antagonists in non-dependent subjects (Gulya et al., 1988; Kramer et al., 1989; Adams et al., 1994; Handler et al., 1994), yet the pharmacological profiles for these antagonists may be differentiated under some circumstances. In an antinociceptive assay, naltrexone was essentially equipotent against both peptide and alkaloid agonists, whereas CTAP was significantly more potent as an antagonist of D-Ala2-NMePhe4-Gly(ol)enkephalin (DAMGO) than any other μ-opioid agonist in non-dependent rats. High concentrations of CTAP appeared to produce a noncompetitive antagonism of some opioid agonists as indicated by increasingly shallow agonist dose–response curves (Sterious and Walker, 2003). CTAP, CTOP and 6β-naltrexol are unique in morphine-dependent mice in that these antagonists failed to produce significant withdrawal jumping, one of the most characteristic signs of opiate withdrawal (Gulya et al., 1988; Wang et al., 1994, 2001, 2004). Interestingly, CTAP as well as nalorphine and naloxonazine blocked withdrawal jumping produced by naloxone (Wang et al., 1994; Bilsky et al., 1996; Walker and Sterious, 2005) further supporting the notion these compounds may be neutral antagonists. Yet, in morphine-treated rats or guinea-pig ileum CTAP produced moderate withdrawal or naloxone-like effects (Maldonado et al., 1992; Mundey et al., 2000; Szucs et al., 2004). The discrepancies between these studies may be due to different dependence states of the preparations or perhaps to secondary actions of these antagonists.
In recent decades, the study of inverse agonism has had an impact on almost every G-protein-coupled receptor family yet the availability of in vivo data on inverse agonism, antagonist classification and antagonist interactions is notably scarce. A further limitation to the study of antagonists and inverse agonists is that quantitative techniques to analyze multiple antagonist combinations have not been tested or applied in vivo. The purpose of the present study was to test the utility of an in vitro quantitative technique called pharmacological resultant analysis as a potential tool for the investigation of antagonist interactions in vivo using two opioid antagonists, naltrexone and CTAP. Pharmacological resultant analysis is a technique developed to detect and eliminate secondary effects of competitive antagonists that may interfere with accurate determinations of affinity estimates and characterization of antagonists (Black et al., 1986). As defined by Black et al. (1986) a pharmacological resultant is ‘the net effect of a single compound resulting from the simultaneous expression of two or more specific actions'. It has been used to detect purely syntopic actions for antagonists with mixed actions (Trist et al., 1987), allosteric interactions (Kenakin and Boselli, 1989; Christopoulos and Mitchelson, 1997), functional antagonism (Goodall et al., 1985) and assay selectivity issues (Kenakin and Beek, 1987; Trist et al., 1987). Pharmacologic resultant analysis allows the examination of one competitive antagonist in the presence of another (Hughes and Mackay, 1985; Black et al., 1986). One antagonist is generally a well-known ‘reference antagonist' and the other the ‘test antagonist'. Several Schild regressions for the reference antagonist are obtained in the presence of different concentrations of the test antagonist and the displacement of the Schild plots along the concentration axis for the reference antagonist are called the resultant plots. Based on dose-ratio analysis (Paton and Rang, 1965), if the two antagonists compete for the same site on the receptor, the changes on the resultant plots are proportional and the antagonists are considered competitive. However, if the slope of the Schild or resultant plots deviate significantly from −1, the test antagonist possesses another property such allosterism, functional antagonism, uptake inhibition, or antagonism through an additional receptor (Kenakin, 1997). Therefore, pharmacological resultant analysis provides a further test of the assumption that antagonists and agonists interact in a simple, competitive manner.
In the present study, we used naltrexone as the reference antagonist and CTAP as the test antagonist. We examined the capacity of multiple doses of naltrexone to block the antinociceptive effects of morphine in the presence of a constant dose of CTAP. To further test the generality of the procedure, we also examined CTAP as the reference antagonist and naltrexone as the test antagonist. Therefore, we examined the capacity of multiple doses of CTAP to block the antinociceptive effects of morphine in the presence of a constant dose of naltrexone. Finally, we tested a combination of naltrexone, CTAP and CTOP for the capacity to block the antinociceptive effects of morphine. In each set of experiments we tested the hypothesis that naltrexone, CTAP and CTOP would maintain a competitive relationship with morphine independent of the presence of additional antagonists and this relationship would be revealed quantitatively through pharmacological resultant analysis.
Methods
Animals
Male, Sprague–Dawley rats (N=56) (Ace Animals Inc. Boyertown, PA, USA) were housed individually in a colony room maintained under a 12-h light–dark cycle. Water was freely available and rats were fed 25 g Purina rat chow daily to maintain body weights averaging approximately 340 g for the course of the experiments (approximately 8 weeks per group). Before testing, rats were habituated to the restraint tubes for 30 min in the experimental room on two separate days. Cumulative dose testing of morphine, s.c., either alone or in combination with antagonists occurred once a week for a given group of rats. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
For central drug administration, a permanent in-dwelling cannula (Plastics One, Roanoke, VA, USA) was placed into the lateral ventricle of each rat using a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). Each rat was anesthetized with 100 mg kg−1 ketamine and 10 mg kg−1 xylazine i.m. Patency of each cannula was tested by injecting 100 ng of angiotensin II and checking for vigorous drinking. These patency tests occurred before, after and periodically throughout the experiments. If the cannula was no longer patent, the rat was removed from the experiment and only those data obtained between positive patency tests were used in the data analysis.
Apparatus
Eight rodent restraint tubes (Harvard Apparatus, Braintree, MA, USA) were used to lightly restrain the rats during tail-withdrawal studies. A model 280 Series water bath (Precision Scientific, Winchester, VA, USA) with two compartments maintained water temperatures of 40 and 55°C. A hand-held thermos was used to contain the water temperature to be tested. An HI 9060 model microcomputer thermometer (Hanna Instruments, Vila do Conde, Portugal) was used to measure the temperature of the water. Tail-withdrawal latencies were measured by visual observation and recorded manually through a hand-operated digital stopwatch with a time resolution of 1 per 100 s.
Procedures
Dose–response curves for morphine were determined using a multiple-trial, cumulative dosing procedure in the warm-water, tail-withdrawal procedure as reported previously (Walker et al., 1994; Sterious and Walker, 2003). Briefly, rats (6–8) were lightly restrained in rodent restraint tubes with their tails hanging freely. The distal 6–12 cm of their tails was immersed into a thermos containing either 40 or 55°C water and the latency for tail-withdrawal was measured. If the rat failed to remove its tail within 15 s, the experimenter removed the thermos to prevent tissue damage. The first stimulus presentations were control water temperature of 40°C. All rats in these studies kept their tails in the 40°C water for 15 s for two out of three stimulus presentations. A 2-min interval occurred between each stimulus presentation. A baseline control latency value for tail-withdrawal from 55°C water was then obtained for each rat.
After baseline tail-withdrawal latencies were determined, each rat was removed from the restraint tube, injected with the first dose of morphine, s.c., and placed back into the restraint tube. After a 15-min pretreatment period, tail-withdrawal latencies for 40 and 55°C were re-determined, once for each rat, with 2 min between the temperature presentations. The order of 40 and 55°C was varied randomly from trial to trial. At the conclusion of the 10-min testing period, the rat was removed from the restraint tube and the next dose of morphine was administered so that the total morphine dose was increased 0.25–0.5 log10 unit. After another 15 min pretreatment period, the tail-withdrawal latencies for 40 and 55°C were determined during the 10 min testing period. The entire test consisted of four to seven trials, each consisting of a 15-min pretreatment period and a 10-min testing period. The test sessions continued until the majority of rats failed to remove their tails from the 55°C water within 15 s.
During the antagonism experiments, the first trial of the multiple-trial test session examined the antinociceptive effects of the antagonists alone or the antagonist combination alone. Doses of CTAP, CTOP or naltrexone i.c.v., were administered into the lateral ventricle. The i.c.v. injections were performed using a hand-held 50 μl Hamilton syringe over a period of 1 min. For the first 30 s, 5 μl of drug was infused into the lateral ventricle. After an additional 30 s, the injector was removed from the guide cannula and the dummy cannula replaced. In the combination experiments, the i.c.v. dose was administered first. Immediately after i.c.v. injection, the dummy cannula was replaced and the rat was injected with naltrexone s.c. All antagonists or antagonist combinations were administered 25 min before the establishment of the morphine dose–response curve. Over the 6–8 week testing period, neither baseline tail-withdrawal latencies nor the sensitivity to morphine changed for an individual group.
Data analysis
The latencies for tail-withdrawal were converted into percentage of maximum effect by the formula: using the control baseline latency for 55°C water measured at the beginning of each experiment for each individual rat. A value of zero was assigned if the rat withdrew its tail faster than the control latency.
Schild regression of a single antagonist
All dose–response curves were analyzed by linear regression and tested for parallelism with the morphine control dose–response curve. Apparent pA2 values for a single antagonist were then calculated (PharmToolsPro, v1.1.27, Philadelphia, PA, USA) using the Schild equation (Arunlakshana and Schild, 1959) with drug doses substituted for drug concentrations (Takemori, 1974). Schild plot slopes were considered to be significantly different from unity if the 95% CL did not include −1. If single antagonist Schild plots slopes were not significantly different from unity, they were constrained to −1. In the event that only a single antagonist dose was administered alone or in combination with another antagonist dose and morphine, an apparent pKB value was determined using the equation pKB=−log(B (DR−1)−1) (Tallarida et al., 1979). In the present study, as described in Negus et al. (1993), the antagonist concentration ‘B' equals the dose of antagonist in moles per kilogram.
Pharmacological resultant analysis
As described by Black et al. (1986), to test whether a second antagonist (C) expresses both a competitive antagonism and an additional action on the transducer function of the concentration of agonist-occupied receptors, the competitive element of C can be estimated by measuring concentration ratios produced by a standard antagonist B, in the presence of C. In this model, concentration ratio data are fitted to the equation: where rcB+C defines the concentration ratio of agonist required to surmount the additional competition of the standard antagonist B in the presence of the test antagonist C; KB and KC are the dissociation constants for B and C, respectively; n and m correspond to the slopes of two plots log(rcB+C−1) vs log([B]) and log(y−1) vs log([C]), respectively; and y is the ratio by which KB′ in the presence of C exceeds KB, that is, (KB′ KB−1). If the antagonist of B and C are simply competitive, n and m will not be significantly different from unity. A plot of log(y−1) vs [C] against the concentrations of C provides an estimate of pKC in a manner similar to the way that a conventional Schild plot provides antagonist affinities to be estimated by rightward displacements of the agonist concentration curves. Therefore, the displacement of these Schild regressions should allow an estimate of the remaining competitive element for the test antagonist, C.
For these in vivo experiments, concentration ratios were replaced with molar dose ratios. Increasing doses of naltrexone [B] (0.0032, 0.01, 0.1, 0.18 or 0.32 mg kg−1, s.c.) were studied in the presence of a fixed dose of CTAP [C] (1.0 μg, i.c.v.). These experiments were repeated with a second fixed dose of CTAP [C] (10 μg, i.c.v.) and a series of in vivo Schild regressions were constructed as a function of naltrexone [B] in the presence of different doses of CTAP. As the slopes of the naltrexone Schild regressions in the presence of CTAP did not differ from the slopes of the Schild regression with naltrexone alone, a common slope was calculated for all Schild regressions so that resultant plots could be estimated. The distance between each displaced naltrexone Schild regression in the presence of 1.0 or 10 μg CTAP and the control naltrexone Schild regression was estimated at the level log (rcB+C−1)=1. From these values, molar dose ratios (y) were determined and plotted as a function of log CTAP for the in vivo resultant plots. In additional experiments, CTAP served as the reference antagonist [B] and naltrexone was the test antagonist [C]. Therefore, increasing doses of CTAP (1.0, 3.2 and 10 μg, i.c.v.) were evaluated in the presence of a fixed dose of naltrexone (0.1 mg kg−1, s.c.). To control for route of administration and because 0.0032 mg kg−1 naltrexone appeared less potent in combination with CTAP, 1.0 μg naltrexone i.c.v. was tested with 0.0032 mg kg−1 naltrexone s.c. as a combined pretreatment to a morphine dose–response curve. In the last experiment, a dose of 1 μg CTOP i.c.v. was examined alone in the presence of combined doses of 0.18 mg kg−1 naltrexone s.c., and 1.0 μg CTAP i.c.v. to antagonize the effects of morphine. The potency of CTOP in the presence of two antagonists (naltrexone and CTAP) was estimated by apparent pKB analysis as described above. In addition, the slopes of the morphine dose–response curve alone and in the presence of 1 μg CTOP alone, 1 μg CTAP alone, 0.18 mg kg−1 naltrexone alone and CTOP, CTAP and naltrexone combined were analyzed by one-way analysis of variance with post hoc Dunnett multiple comparisons test. Significance was set at P<0.05.
Drugs
The following compounds were used: morphine sulfate, naltrexone hydrochloride, CTAP and CTOP (supplied by National Institute on Drug Abuse, Rockville, MD, USA). For systemic injection, morphine and naltrexone were dissolved in physiological saline and injected s.c. into the dorsal flank. For central administration, naltrexone, CTAP, CTOP or a combination of CTAP and CTOP were dissolved in filtered, sterile water in a concentration allowing a 5 μl volume.
Results
Antagonists alone
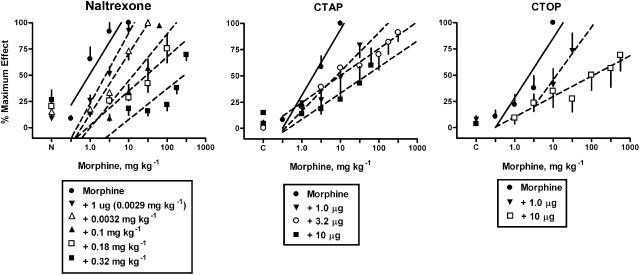
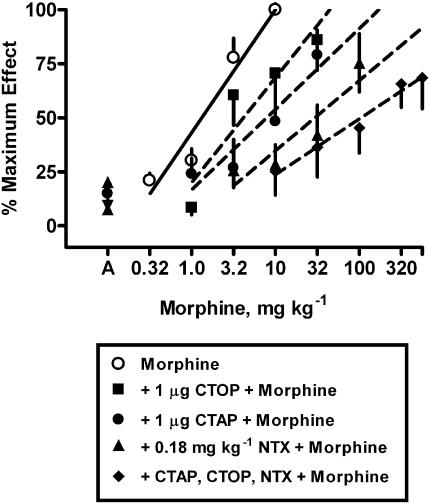
Morphine produced dose-dependent increases in tail-withdrawal latencies until near maximal effects were obtained (Figures 1, 2, 3, 4 and 5). The average ED50 value for morphine across the eight experiments was 1.73±0.31 mg kg−1 (mean±s.e.m.). Naltrexone pretreatments (0.0032–0.32 mg kg−1, s.c.) produced dose-dependent, parallel shifts in the morphine dose–response curve (Figure 1, left panel). Additionally, an injection of 1 μg naltrexone i.c.v., equivalent to a 0.0029 mg kg−1 dose based on the average rat weight of 340 g, produced slightly less antagonism (2.5-fold) than a systemic injection of 0.0032 mg kg−1 naltrexone (4.4-fold). CTAP (1–10 μg, i.c.v.) pretreatments produced dose-dependent shifts in the morphine dose–response curves. CTOP (1 and 10 μg, i.c.v.) pretreatments produced dose-dependent shifts in the morphine dose–response curve.
Figure 1.
Naltrexone s.c. or i.c.v., CTAP i.c.v. and CTOP i.c.v. antagonism of the antinociceptive effects of morphine-induced antinociception in the warm-water (55°C) tail-withdrawal assay. Ordinate: latency measures converted to % maximum effect. Abscissa: cumulative dose of morphine in mg kg−1. Each point represents the mean of one observation in 6–8 rats. All antagonist doses were administered 25 min before the determination of the morphine-dose–response curve. Points above N or C are the effects of naltrexone, CTAP or CTOP alone.
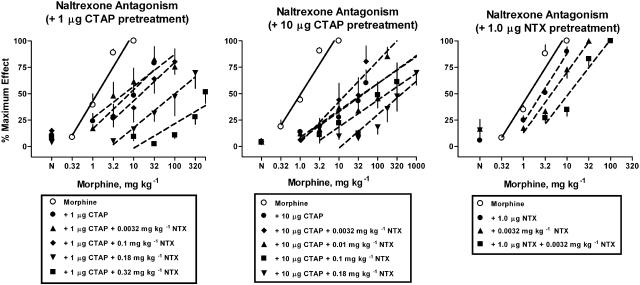
Figure 2.
Effects of naltrexone s.c. in the presence of a fixed dose of CTAP i.c.v. (left and middle panels) or naltrexone i.c.v. (right panel), respectively. Naltrexone s.c., CTAP i.c.v. or naltrexone i.c.v. were coadministered 25 min before determination of the morphine dose–response curve. Ordinate: latency measures converted to % maximum effect. Abscissa: cumulative dose of morphine in mg kg−1. Each point represents the mean of one observation in 6–8 rats. Points above N are the effects of naltrexone alone or the effects of naltrexone in combination with CTAP or naltrexone alone.
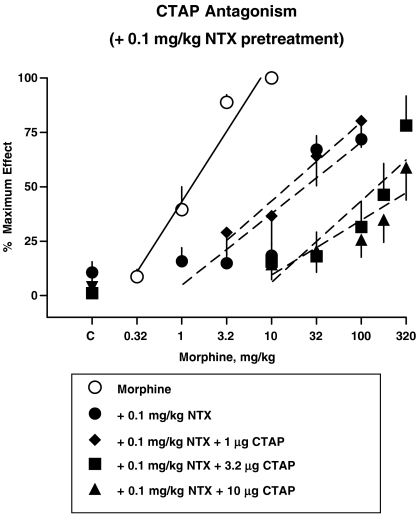
Figure 3.
Effects of CTAP i.c.v. in the presence of a fixed dose of 0.1 mg kg−1 naltrexone s.c. Naltrexone s.c. and CTAP i.c.v. were coadministered 25 min before determination of the morphine dose–response curve. Points above C are the effects of CTAP alone or the effects of CTAP in combination with naltrexone alone. Each point represents the mean of one observation in 6–8 rats. Other details as in Figure 1.
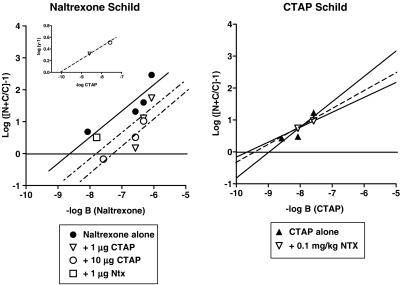
Figure 4.
Secondary Schild plots for naltrexone (left panel) or CTAP (right panel) as antagonists of the antinociceptive effects of morphine. Left panel: Schild plots for naltrexone alone and in the presence of 1 or 10 μg CTAP i.c.v. Individual potency estimate for naltrexone in the presence of a single dose of 1 μg naltrexone (open squares). Slopes of the regression lines were not significantly different so regressions lines were drawn with a common slope calculated to be −0.87 (for details see ‘Data Analysis'). Right panel: Schild plots of CTAP alone and in the presence of 0.1 mg kg−1 naltrexone. Ordinate: logarithm of the quantity [(N+C (A50 of the agonist in the presence of a constant dose of CTAP and increasing dose of naltrexone) divided by C (A50 of the agonist plus a constant dose of CTAP)]-1). A50 values are determined from Figures 2, 3. Abscissa: negative logarithm of molar doses of naltrexone (left panel) or CTAP (right panel). Inset: resultant plot for CTAP. Ordinate: logarithm of the quantity (y−1), where y is the equal dose ratio for naltrexone in the presence of 1.0 or 10 μg CTAP. Abscissa: negative logarithm of molar doses of CTAP.
Figure 5.
Effects of CTOP i.c.v. in the presence of a fixed dose of 0.18 mg kg−1 naltrexone s.c. and 1 μg CTAP i.c.v. CTOP i.c.v., naltrexone s.c. or CTAP i.c.v. were administered alone or in combination 25 min before determination of the morphine dose–response curve. Points above A are the effects of the antagonist or antagonist combination alone. Each point represents the mean of one observation in 6–8 rats. Other details as in Figure 1.
Schild regressions for antagonists alone
Schild regression analysis of naltrexone (Figure 4, left panel) revealed an apparent pA2 value of 8.9 and a slope of −0.72 (Table 1). As the variance on the slope of the naltrexone Schild regression included −1, a constrained pA2 value of 8.3 was calculated. For the single dose of naltrexone i.c.v. (1 μg), a comparable value for apparent pA2 was obtained (Table 1). Schild regression analysis of CTAP (Figure 4, right panel) revealed an apparent pA2 value of 9.0 and a slope whose variance included −1, allowing a constrained pA2 value to be calculated (Table 1). Higher doses of CTAP were not tested because these doses produce a noncompetitive antagonism of morphine and etorphine (Sterious and Walker, 2003). Although only two pretreatment doses of CTOP were available for analysis, it was possible to estimate an apparent pA2 value with a corresponding slope, as shown in Table 1.
Table 1.
Apparent pA2 values for naltrexone, CTAP and CTOP as antagonists of morphine antinociception
| Antagonist | pA2 (+95% CL) | Slope (+95% CL) | Slope constrained pA2 (+95% CL) |
|---|---|---|---|
| Naltrexone s.c. | 8.9 (5.1–13) | −0.72 (−1.9 to 0.48) | 8.3 (7.6–9.0) |
| Naltrexone i.c.v. | 8.5a | —c | —c |
| CTAP i.c.v. | 9.0 (2.6–15) | −0.80 (−6.0 to 4.4) | 8.8 (8.2–9.4) |
| CTOP i.c.v. | 8.9b | −1.54 | —c |
Apparent pKB calculation.
Values determined using only two pretreatment doses of antagonist.
Value cannot be determined.
Naltrexone s.c. (reference antagonist) in the presence of fixed doses of CTAP i.c.v. (test antagonist) or naltrexone i.c.v. (control)
Cumulative doses of morphine produced dose-dependent increases in antinociceptive effect (open circle, dotted lines) that were blocked by a single dose of 1 μg CTAP i.c.v., 10 μg CTAP i.c.v. or 1 μg naltrexone i.c.v. (Figure 2). In the presence of a fixed dose of 1 μg CTAP i.c.v., increasing doses of naltrexone (0.1–0.32 mg kg−1, s.c.) produced dose-dependent, parallel shifts to the right in the morphine dose–response curve (Figure 2, left panel). However, a low dose of 0.0032 mg kg−1 naltrexone s.c. in the presence of 1.0 μg CTAP i.c.v. produced a 2.6-fold shift to the left of the morphine dose–response curve. Therefore, this dose–response curve could not be used for further Schild analysis. In the presence of a fixed dose of 10 μg CTAP i.c.v. doses of naltrexone (0.01–0.1 mg kg−1, s.c.) produced dose-dependent, parallel shifts to the right in the morphine dose–response curve (Figure 2, middle panel). However, a low dose of 0.0032 mg kg−1 naltrexone s.c. in the presence of 10 μg CTAP i.c.v. produced a 1.8-fold shift to the left of the morphine dose–response curve. Therefore, this dose–response curve could not be used for further Schild analysis. As a control experiment, a dose of 0.0032 mg kg−1 naltrexone s.c., was examined in combination with 1 μg naltrexone i.c.v. to determine if 0.0032 mg kg−1 naltrexone s.c. will produce a leftward shift in the morphine dose–response curve when combined with any low dose of i.c.v. antagonist. In contrast to the results obtained with CTAP i.c.v. pretreatment of 0.0032 mg kg−1 naltrexone s.c. produced a 4.2-fold further shift to the right in the morphine dose–response curve (Figure 2, right panel).
CTAP i.c.v. (reference antagonist) in the presence of a fixed dose of naltrexone s.c. (test antagonist)
Cumulative doses of morphine produced dose-dependent increases in antinociceptive effect that were blocked by a single dose of 0.1 mg kg−1 naltrexone s.c. (Figure 3). In the presence of a fixed dose of 0.1 mg kg−1 naltrexone s.c., CTAP doses (3.2 and 10 μg i.c.v.) produced parallel shifts to the right in the morphine dose–response curve. A lower dose of 1.0 μg CTAP i.c.v., in the presence of 0.1 mg kg−1 naltrexone s.c. produced a 1.5-fold shift to the left of the morphine dose–response curve. Therefore, this dose–response curve could not be used for further Schild analysis. Higher doses of CTAP were not tested because these doses produce a noncompetitive antagonism of morphine and etorphine (Sterious and Walker, 2003).
In vivo pharmacological resultant analysis
Competition experiments for naltrexone s.c. and CTAP i.c.v. combinations were analyzed as described by Black et al. (1986) with drug doses substituted for drug concentrations. Dose ratios were calculated between the morphine dose–response curve obtained in the presence of 1.0 μg CTAP i.c.v. alone and the morphine dose–response curves obtained in the presence of both 1.0 μg CTAP i.c.v. and increasing doses of naltrexone (Figure 2, left panel). The dose ratios were plotted as a function of log dose of naltrexone (Figure 4, left panel) yielding an apparent pA2 value for naltrexone in the presence of 1.0 μg CTAP i.c.v. of 6.6 and a significantly steeper slope of −3.7 (Table 2). Constraining the slope of this regression line yielded an apparent pA2 value of 7.4 for naltrexone s.c. in the presence of 1.0 μg CTAP i.c.v. Similarly, dose ratios were calculated between the morphine dose–response curve obtained in the presence of 10 μg CTAP i.c.v. alone and the morphine dose–response curves obtained in the presence of both 10 μg CTAP i.c.v. and increasing doses of naltrexone s.c. (Figure 2, middle panel). The dose ratios were plotted as a function of log dose of naltrexone (Figure 4, left panel; Table 2) yielding an apparent pA2 value for naltrexone in the presence of 10 μg CTAP i.c.v. and a slope close to unity. Constraining the slope of this regression line yielded an apparent pA2 value of 7.3 (Table 2).
Table 2.
Apparent pA2 or pKB values for naltrexone in the presence of CTAP, CTAP in the presence of naltrexone, and CTOP in the presence of naltrexone and CTAP
| Antagonist | pA2 (+95% CL) | Slope (+95% CL) | Slope constrained pA2 (+95% CL) |
|---|---|---|---|
| Naltrexone s.c. | 8.9 (5.1–13) | −0.72 (−1.9 to 0.48) | 8.3 (7.6–9.0) |
| +1.0 μg CTAP | 6.6 (6.4–6.8) | −3.7c (−6.0 to −1.4) | —d |
| +10 μg CTAP | 7.3 (5.0–9.7) | −0.88 (−3.6 to 1.9) | 7.3 (6.9–7.7) |
| +1 μg Naltrexone | 8.6a | —d | —d |
| CTAP i.c.v. | 9.0 (2.6–15) | −0.80 (−6.0 to 4.4) | 8.8 (8.2–9.4) |
| +0.1 mg kg−1 naltrexone | 9.7b | −0.46 | —d |
| CTOP i.c.v. | 8.9 | −1.54 | —d |
| +Naltrexone and CTAP | 9.5a | —d | —d |
Apparent pKB calculation using a dose of antagonist or antagonist combination.
Apparent pA2 and slope values determined using only two dose–response curves.
Slope significantly different than naltrexone s.c. in the presence of 10 μg CTAP i.c.v.
Value cannot be determined.
Schild regressions of naltrexone s.c. alone and in the presence of two doses of CTAP i.c.v. were analyzed for parallelism (Figure 4, left panel). Neither the slope of the naltrexone Schild regression in the presence of 1.0 μg CTAP i.c.v. nor the slope of the naltrexone Schild regression in the presence of 10 μg CTAP i.c.v. were significantly different from the slope of the naltrexone Schild regression alone. Therefore, a common slope was calculated and equal dose ratios (y) for naltrexone in the presence of 1.0 μg CTAP i.c.v. and in the presence of 10 μg CTAP were determined and plotted on a resultant plot (Figure 4, left panel inset). From the resultant plot, an apparent pKC value for CTAP i.c.v. was estimated as −10 with a slope value of −0.19. As only two CTAP i.c.v. values were available for analysis, a variance estimate was not obtained. Although the slopes of the naltrexone Schild regressions in the presence of CTAP were not different from those for the Schild regression on naltrexone alone, the slopes of naltrexone Schild regressions in the presence of 1.0 and 10 μg CTAP i.c.v. were different from each other.
Competition experiments for CTAP i.c.v. and naltrexone s.c. combinations were also analyzed as described by Black et al. (1986) with drug doses substituted for drug concentrations. Dose ratios were calculated between the dose–response curve obtained in the presence of 0.1 mg kg−1 naltrexone s.c. alone and the dose–response curves obtained in the presence of 0.1 mg kg−1 naltrexone s.c. alone and doses of 3.2 or 10 μg CTAP i.c.v. (Figure 3). The dose ratios were plotted as a function of log dose of CTAP i.c.v. (Figure 4, right panel) yielding an apparent pA2 value for CTAP in the presence of 0.1 mg kg−1 naltrexone of 9.7 and a slope of −0.46. As only two morphine dose–response curves in the presence of 0.1 mg kg−1 naltrexone s.c. and CTAP i.c.v. were available for analysis in this experiment, a variance estimate was not obtained. As the elevations of the CTAP Schild regression in the presence of 0.1 mg kg−1 naltrexone s.c. were not significantly different from that of the Schild regression with CTAP alone, resultant plots could not be determined.
Combinations of CTAP, CTOP and naltrexone
Alone, 1 μg CTOP i.c.v. produced a 3.5-fold shift in the morphine dose–response curve. In the presence of combined doses of 0.18 mg kg−1 naltrexone s.c. and 1.0 μg CTAP i.c.v., a dose of 1 μg CTOP i.c.v., produced a 31-fold further shift in the morphine dose–response curve (Figure 5). Using this single dose of CTOP in the presence of naltrexone and CTAP, an apparent pKB value for CTOP was calculated as 9.5. However, the slope of the morphine dose–response curve in the presence of the three antagonists was significantly shallower (P<0.01) than the morphine control dose–response curve (F(4,16)=7.14; P<0.002). Although the addition of each dose of antagonist produced further shifts to the right in the morphine dose–response curves, the slope of each morphine dose–response curve became progressively more shallow (morphine alone=1.4±0.42; +1.0 μg CTOP i.c.v.=1.1±0.42; +1 μg CTAP i.c.v.=0.77±0.19; +0.18 mg kg−1 naltrexone s.c.=0.66±0.20; +CTAP, CTOP, naltrexone=0.48±0.042).
Discussion
In the present study, the antagonist potencies of three opioid antagonists, naltrexone, CTAP and CTOP, were examined alone and in combination, to further elucidate the competitive nature of the peptide antagonists CTAP and CTOP. Given alone, naltrexone, CTAP and CTOP were potent antagonists of the antinociceptive effects of morphine with apparent pA2 or pKB values of 8.5–8.9, 9.0 and 8.9, respectively. These values for naltrexone, CTAP and CTOP are similar to potency estimates obtained in previous antinociception assays in rats and mice (Gulya et al., 1988; Adams et al., 1994; Garner et al., 1997; Sterious and Walker, 2003). However, higher apparent pA2 values (11) were obtained for CTAP and CTOP as antagonists of PL017 in the mouse hot-plate assay (Kramer et al., 1989) and for CTAP as an antagonist of DAMGO (13) in the rat warm-water, tail-withdrawal assay (Sterious and Walker, 2003). High doses of CTAP produced noncompetitive antagonism of morphine and etorphine in rats (Sterious and Walker, 2003) and lower doses of CTAP produced a noncompetitive antagonism of DPDPE (D-Pen2-D-Pen5)enkephalin) in mice (Kramer et al., 1989). Although CTAP and CTOP appear to produce a competitive antagonism in most assays (Kramer et al., 1989) others are equivocal and suggest interactions with other receptors (He and Lee, 1998; Sterious and Walker, 2003). In the present study, although the slopes of the CTAP and CTOP pretreatment to morphine dose–response curves were not significantly different than the control morphine dose–response curve, clearly a trend toward shallower dose–response curves was observed. These data suggest CTAP may possess actions that interfere with the competitive antagonism of morphine. Therefore, we used pharmacological resultant analysis to quantitatively assess the interaction of CTAP with the μ-opioid receptor in presence of the well-characterized antagonist naltrexone.
Pharmacological resultant analysis is a technique developed to detect and eliminate secondary effects of antagonists that may interfere with the characterization of the competitive effects of antagonists (Black et al., 1986). The pharmacological resultant analysis presented here is the first attempt to apply this modeling technique to a pair of antagonists in vivo. In these studies, control naltrexone Schild regressions were displaced by fixed doses of 1.0 and 10 μg CTAP. The apparent pA2 values for naltrexone in the presence of CTAP were approximately one log unit lower (7.4) to those obtained for naltrexone as an antagonist of morphine alone (8.3–8.4) (Sterious and Walker, 2003, present study). Although these intermediate pA2 values are generally not reported in studies using pharmacological resultant analysis (Trist et al., 1987; Kenakin and Boselli, 1989; but see Black et al., 1986), the observation that naltrexone is less potent in the presence of CTAP indicates that these two antagonists are competing for a common receptor as part of their interactions. Additional analysis of the competitive component of CTAP's resultant effect or the apparent pKC was determined by estimating a common slope for all three regressions and plotting the distance of the displaced naltrexone Schild regressions from the control naltrexone Schild regression on a resultant plot. The apparent pKC value for CTAP determined from the resultant plot was 10, a value one log unit higher than the apparent pA2 value obtained for CTAP alone as an antagonist of morphine in this assay (Sterious and Walker, 2003, present study). An initial estimate of the slope of the resultant plot from the two displaced Schild regressions was shallow (−0.19). Taken together, these two observations from the resultant plot indicate that CTAP may not interact with morphine and naltrexone in a purely competitive manner and that CTAP may possess secondary actions. Therefore, the in vivo application of the pharmacological resultant analysis supports previous in vitro and in vivo studies with CTAP (Wang et al., 1994, 2001; Sterious and Walker, 2003).
Pharmacological resultant analysis is derived under the assumptions of occupancy theory and essentially relies on null methodology. However, there are many assumptions and limitations to applying pharmacological resultant analysis or even Schild regression analysis in vivo. To make accurate estimates of pKC and apply pharmacological resultant analysis, parallel rightward displacements of the reference Schild regressions by the test antagonist and a slope of unity on the resultant plot are required (Black et al., 1986; Kenakin and Beek, 1987). In vivo, the variance estimates on Schild plots (Tallarida et al., 1979; Dykstra, 1990; Walker et al., 1994; Negus and Mello, 2002) or resultant plots (present study) slope values are often quite large due to the limited number of antagonist doses that are available for analysis. This variance is certainly the greatest limitation to application of pharmacological resultant analysis in vivo. Even in vitro, resultant analysis requires a sufficient number of displaced Schild regressions which can be difficult to obtain. For example, despite determining 12 additional dose–response curves to construct a resultant plot for a single test antagonist in vitro, too few data points on the resultant plot were available to estimate the error on the slope value (Kenakin and Boselli, 1989). In the present study, more naltrexone Schild regressions in the presence of additional doses of CTAP are required to obtain the required statistical power for precise slope and pKC deviations. However, this is often not be practical because high concentrations of the agonists may produce toxic or competing actions that preclude testing higher combination doses of antagonists even in vitro (Kenakin and Beek, 1987).
A more specific limitation related to the lack of statistical power for in vivo pharmacological resultant analysis for naltrexone and CTAP combinations in the present study was the required exclusion of some dose–response curves because CTAP and naltrexone were not additive at low doses. In single antagonist experiments, doses of 0.0032 mg kg−1 naltrexone, 1 and 10 μg CTAP alone shifted the morphine dose–response curve to the right. Although parallel to the morphine control dose–response curve, 0.0032 mg kg−1 naltrexone in the presence of either 1.0 or 10 μg CTAP shifted the morphine curve 2.6- and 1.8-fold to the left of the morphine dose–response curve, respectively. Similarly, when a lower dose of 1 μg CTAP i.c.v. was examined in the presence of a fixed dose of 0.1 mg kg−1 naltrexone, there was a small, nonsignificant shift to the left. These small, leftward shifts cannot be used in the Schild plots for naltrexone in the presence of CTAP as they violate some of the basic assumptions of Schild analysis (Arunlakshana and Schild, 1959). A priori, one would assume that low doses of competitive antagonists would combine to produce either no change in the potency of an agonist or perhaps to produce an additive antagonism of an agonist greater than either low dose of antagonist alone (Tallarida, 2001; Braverman et al., 2002). For example, in the present study, a combination of 0.0032 mg kg−1 naltrexone s.c. and 1 μg naltrexone i.c.v. shifted the morphine dose–response in the presence of 1 μg naltrexone i.c.v. alone to the right as would be expected by essentially increasing doses of a competitive antagonist (Figure 2, right panel). Apparent pKB estimates of this combination of systemic and central doses of naltrexone fell directly on the Schild regression for naltrexone alone (Figure 4, Table 1). Yet, a low dose of naltrexone administered in the presence of a second, putative competitive antagonist CTAP produces shifts to the left of the morphine dose–response curves. These small shifts to the left were observed in three separate experiments and were only revealed when low doses of naltrexone were combined with CTAP. A limitation to pharmacological resultant analysis is that leftward shifts for combinations of antagonists cannot be evaluated and yet these leftward shifts further support the notion that combinations of CTAP and naltrexone with morphine are not purely competitive.
Pharmacological resultant analysis indicates that CTAP and naltrexone may not interact in a purely competitive manner with the μ-opioid receptor in vivo. There are three possible reasons why combinations of CTAP, CTOP and naltrexone may combine in an irregular manner: (1) multiple opioid receptor interactions; (2) route of administration or time course differences or (3) negative intrinsic efficacy. The antagonist naltrexone does interact with other opioid receptors such that high doses of naltrexone block κ (Brandt and France, 1996; Ko et al., 1998) and δ agonists (Comer et al., 1993) and naltrexone binds to δ and κ receptors albeit with lower affinity than μ receptors (Goldstein and Naidu, 1989; Emmerson et al., 1994). The selectivities of CTAP and CTOP for μ-opioid receptors are high relative to those for traditional opioid antagonists. Radioligand binding data in rat brain membranes indicate a 1300 to 4800 and 1300 to 2500-fold greater μ vs δ selectivity and 8700–11 000 and 4000 fold μ vs somatostatin selectivity for CTOP and CTAP, respectively (Pelton et al., 1986; Kazmierski et al., 1988; Kramer et al., 1989). CTAP and CTOP lack μ-agonist activity, potently antagonize μ opioids (Gulya et al., 1988; Kramer et al., 1989; Adams et al., 1994; Takasuna et al., 1994; Sterious and Walker, 2003), and fail to antagonize κ agonists (Mulder et al., 1991). High doses of CTAP block DPDPE (D-Pen2, D-Pen5)enkephalin and endogenous δ ligands, however (Kramer et al., 1989; Hurley and Hammond, 2001). Thus, the peculiar interaction between low doses of naltrexone and CTAP is unlikely to be due to actions at other opioid receptors.
Atypical interactions between naltrexone and the peptide antagonists CTAP and CTOP may be related to such practical in vivo challenges as route of administration or the time courses of the various antagonists. In the present study, naltrexone was injected s.c. and CTAP and CTOP were administered i.c.v. Morphine is analgesic at multiple points along the pain pathway and the different routes of administration for the antagonists could account for the observed irregular interactions between CTAP and naltrexone. In previous studies using the rat tail-withdrawal assay, the route of administration of naltrexone was varied and the apparent pA2 values for naltrexone i.c.v. and naltrexone s.c. as antagonists of morphine were not different (8.2±0.1; 8.4±0.3, respectively). Although morphine was 32-fold more potent administered i.c.v. vs s.c., naltrexone i.c.v. and s.c. were equipotent (Sterious and Walker, 2003). In the present study, the agonist morphine was used in all experiments and proportionally the same interval of morphine dosing was used throughout the study. In previous experiments using the rat tail-withdrawal assay, a dose of 10 mg kg−1 morphine was fully effective for 160 min suggesting that each acute dose of morphine in the cumulative dose–response curve should be fully effective during each 25 min interval (Walker et al., 1994). In the cumulative dosing procedure, the actual cumulative dose of morphine may be slightly less than the total calculated dose due to dissipation of previously administered doses of morphine. For example, if a previously administered dose of 0.1 mg kg−1 morphine dissipates within 2 h, the final measured antinociceptive effects for a later cumulative dose of 10 mg kg−1 (0.9 mg kg−1 acute dose) morphine is probably not influenced to a great extent. In regards to the time course for naltrexone and CTAP, previous studies in the rat tail-withdrawal assay indicated that doses of 0.032 and 0.1 mg kg−1 naltrexone blocked 10 mg kg−1 morphine for 225 min or 3.75 h (Walker et al., 1994). The cyclic peptide CTAP is stable in the blood and serum of rats with a half-life of 500 min (Abbruscato et al., 1997; Egleton et al., 1998) also supporting the notion that the antagonists CTAP and naltrexone should be effective antagonists for approximately eight cumulative dose trials. Taken together, these data suggest that route of administration and time course are not the decisive variables contributing to the atypical interactions between CTAP and naltrexone in the rat tail-withdrawal procedure.
A final explanation for the noncompetitive interactions between CTAP and naltrexone may be related to differences in negative intrinsic efficacy between the two antagonists. Studies in dependent cells and mice suggest that naltrexone is an inverse agonist whereas CTAP is a neutral antagonist (Wang et al., 1994, 2004; Bilsky et al., 1996). CTAP produces minor withdrawal jumping in morphine-dependent mice, one of the most characteristic signs of opioid-withdrawal, whereas naloxone and naltrexone produce striking withdrawal jumping (Wang et al., 1994, 2001, 2004; Raehal et al., 2005). Interestingly, CTAP blocks the withdrawal jumping produced by naloxone (Wang et al., 1994; Bilsky et al., 1996), however, in morphine-treated rats or guinea-pig ileum CTAP produces moderate withdrawal or naloxone-like effects (Maldonado et al., 1992; Mundey et al., 2000; Szucs et al., 2004). Although the results from these in vitro and in vivo studies appear highly contingent on the different dependence states of the preparations, taken together with the results from the present study, these findings support the notion that CTAP and naltrexone may possess different levels of negative intrinsic efficacy. The μ-opioid receptor is postulated to exhibit low but detectable basal activity in vitro and in vivo under normal conditions (Burford et al., 2000; Wang et al., 2001, 2004). If CTAP is indeed a neutral antagonist and naltrexone is indeed an inverse agonist, one might predict unusual interactions between these two compounds even in non-dependent cells and rats under certain experimental circumstances. Other investigators have demonstrated in vitro that cells may recognize differences between antagonists and inverse agonists even when there is no change in basal responding. For example, chronic treatment with 5-HT2C inverse agonist SB206553 produced sensitization but failed to alter basal tone whereas the neutral antagonist 5-methoxygramine blocked the ability of SB 206553 to produce sensitization (Berg et al., 1999). Although these compounds did not alter basal tone, the combination of the two antagonists revealed differences that were not revealed by studying either antagonist alone. Similarly, in the present study, the combination of CTAP and naltrexone revealed differences that were not revealed by studying either antagonist alone. The resultant plot determined in the present study suggests that CTAP may impede the ability of naltrexone to block the antinociceptive effects of morphine, even under the basal states of μ-opioid receptors. Additionally, combinations of CTAP i.c.v., CTOP i.c.v. and naltrexone s.c. significantly reduced the slope of the morphine dose–response curve indicating that together these antagonists alter some post-receptor, signal transducer function as opposed to competing simply at a common receptor.
In summary, despite the inherent limitations of in vivo data, the results of the present study demonstrate that (1) the opioid antagonists CTAP, CTOP and naltrexone may not interact in a purely competitive manner with morphine even under normal receptor conditions; and (2) the effects of combinations of CTAP and naltrexone may be dose-dependent. This novel approach of studying antagonist combinations appears to identify antagonist interactions that go undetected in single antagonist studies. Although pharmacological resultant analysis is limited in its capacity to identify the particular mechanisms of the observed irregular interactions for antagonists, similar to Schild regression analysis, the results obtained in the present study provide further support for the existence of a continuum of negative intrinsic efficacy for opioid antagonists. The findings presented here suggest that pharmacological resultant analysis, similar to Schild regression analysis, can be applied to in vivo datasets further characterizing and quantifying drug–receptor interactions. Future applications of pharmacological resultant analysis to experiments from other in vivo assays will further support the generality and usefulness of this quantitative tool for pharmacologists.
Acknowledgments
I acknowledge Charlene M Ross, Toniann Razzi, Elizabeth Salmon, Christopher Plachta and Steven N Sterious for their technical assistance. I also thank Peter H Doukas, PhD and Ronald J Tallarida, PhD for their discussion of these data and Robert B Raffa, PhD for review of the manuscript. This research was supported by National Institute on Drug Abuse Grant DA10776.
Abbreviations
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- CTOP
D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2
- DAMGO
D-Ala2-NMePhe4-Gly(ol)enkephalin
Conflict of interest
The authors state no conflict of interest.
References
- Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Blood-brain barrier permeability and bioavailability of a highly potent and mu–selective opioid receptor antagonist, CTAP: comparison with morphine. J Pharmacol Exp Ther. 1997;280:402–409. [PubMed] [Google Scholar]
- Adams JU, Geller EB, Adler MW. Receptor selectivity of icv morphine in the rat cold water tail-flick test. Drug Alcohol Depend. 1994;35:197–202. doi: 10.1016/0376-8716(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol. 1999;55:863–872. [PubMed] [Google Scholar]
- Bilsky EJ, Bernstein RN, Wang Z, Sadee W, Porreca F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J Pharmacol Exp Ther. 1996;277:484–490. [PubMed] [Google Scholar]
- Black JW, Gerskowitch VP, Leff P, Shankley NP. Analysis of competitive antagonism when this property occurs as part of a pharmacological resultant. Br J Pharmacol. 1986;89:547–555. doi: 10.1111/j.1476-5381.1986.tb11155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MR, France CP. Discriminative stimulus effects on enadoline in pigeons. J Pharmacol Exp Ther. 1996;277:960–967. [PubMed] [Google Scholar]
- Braverman AS, Tallarida RJ, Ruggieri MR., Sr Interaction between muscarinic receptor subtype signal transduction pathways mediating bladder contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R663–R668. doi: 10.1152/ajpregu.00116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford NT, Wang D, Sadee W. G-protein coupling of mu-opioid receptors (OP3): elevated basal signalling activity. Biochem J. 2000;348 Pt 3:531–537. [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Mitchelson F. Application of an allosteric ternary complex model to the technique of pharmacological resultant analysis. J Pharm Pharmacol. 1997;49:781–786. doi: 10.1111/j.2042-7158.1997.tb06112.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. Discriminative stimulus effects of BW373U86: a nonpeptide ligand with selectivity for delta opioid receptors. J Pharmacol Exp Ther. 1993;267:866–874. [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA. Butorphanol, levallorphan, nalbuphine and nalorphine as antagonists in the squirrel monkey. J Pharmacol Exp Therapeut. 1990;254:245–252. [PubMed] [Google Scholar]
- Egleton RD, Abbruscato TJ, Thomas SA, Davis TP. Transport of opioid peptides into the central nervous system. J Pharm Sci. 1998;87:1433–1439. doi: 10.1021/js980062b. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Garner HR, Burke TF, Lawhorn CD, Stoner JM, Wessinger WD. Butorphanol-mediated antinociception in mice: partial agonist effects and mu receptor involvement. J Pharmacol Exp Ther. 1997;282:1253–1261. [PubMed] [Google Scholar]
- Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- Goodall J, Hughes IE, Mackay D. Similarity between mu opioid receptors in mouse vas deferens and guinea-pig ileum. Br J Pharmacol. 1985;180:277–283. doi: 10.1111/j.1476-5381.1985.tb08857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K, Krivan M, Nyolczas N, Sarnyai Z, Kovacs GL. Central effects of the potent and highly selective mu opioid antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) in mice. Eur J Pharmacol. 1988;150:355–360. doi: 10.1016/0014-2999(88)90018-0. [DOI] [PubMed] [Google Scholar]
- Handler CM, Piliero TC, Geller EB, Adler MW. Effect of ambient temperature on the ability of mu-, kappa- and delta-selective opioid agonists to modulate thermoregulatory mechanisms in the rat. J Pharmacol Exp Ther. 1994;268:847–855. [PubMed] [Google Scholar]
- He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–1186. [PubMed] [Google Scholar]
- Holtzman SG. Stimulus properties of opioids with mixed agonist and antagonist activity. Fed Proc. 1982;41:2328–2332. [PubMed] [Google Scholar]
- Hughes IE, Mackay D. Quantification of the characteristics of antagonists exhibiting both competitive antagonism and functional interaction. Br J Pharmacol. 1985;85:271–275. doi: 10.1111/j.1476-5381.1985.tb08856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierski W, Wire WS, Lui G. Design and synthesis of somatostatin analogues with topographical properties that lead to highly potent and specific mu opioid receptor antagonists with greatly reduced binding at somatostatin receptors. J Med Chem. 1988;31:2170–2177. doi: 10.1021/jm00119a019. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interactions. Lippincott-Raven: Philadelphia; 1997. [Google Scholar]
- Kenakin T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Boselli C. Pharmacologic discrimination between receptor heterogeneity and allosteric interaction: resultant analysis of gallamine and pirenzepine antagonism of muscarinic responses in rat trachea. J Pharmacol Exp Ther. 1989;250:944–952. [PubMed] [Google Scholar]
- Kenakin TP, Beek D. Measurement of antagonist affinity for purine receptors of drugs producing concomitant phosphodiesterase blockade: the use of pharmacologic resultant analysis. J Pharmacol Exp Ther. 1987;243:482–486. [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Kramer TH, Shook JE, Kazmierski W, Ayres EA, Wire WS, Hruby VJ, et al. Novel peptidic mu opioid antagonists: pharmacologic characterization in vitro and in vivo. J Pharmacol Exp Ther. 1989;249:544–551. [PubMed] [Google Scholar]
- Liu JG, Ruckle MB, Prather PL. Constitutively active mu-opioid receptors inhibit adenylyl cyclase activity in intact cells and activate G-proteins differently than the agonist [D-Ala2, N-MePhe4, Gly-ol5]enkephalin. J Biol Chem. 2001;276:37779–37786. doi: 10.1074/jbc.M106104200. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- Mulder AH, Wardeh G, Hogenboom F, Kazmierski W, Hruby VJ, Schoffelmeer AN. Cyclic somatostatin analogues as potent antagonists at mu-, but not delta- and kappa-opioid receptors mediating presynaptic inhibition of neurotransmitter release in the brain. Eur J Pharmacol. 1991;205:1–6. doi: 10.1016/0014-2999(91)90761-e. [DOI] [PubMed] [Google Scholar]
- Mundey MK, Ali A, Mason R, Wilson VG. Pharmacological examination of contractile responses of the guinea-pig isolated ileum produced by mu-opioid receptor antagonists in the presence of, and following exposure to, morphine. Br J Pharmacol. 2000;131:893–902. doi: 10.1038/sj.bjp.0703659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of mu-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Neilan CL, Akil H, Woods JH, Traynor JR. Constitutive activity of the delta-opioid receptor expressed in C6 glioma cells: identification of non-peptide delta-inverse agonists. Br J Pharmacol. 1999;128:556–562. doi: 10.1038/sj.bjp.0702816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WD, Rang HP. The Uptake of Atropine and Related Drugs by Intestinal Smooth Muscle of the Guinea-Pig in Relation to Acetylcholine Receptors. Proc R Soc Lond B Biol Sci. 1965;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Yarbrough J, Hughes CE, Smith MA, Morgan D, Dykstra LA. Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther. 1993;266:756–767. [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, et al. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Sterious SN, Walker EA. Potency differences for D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 as an antagonist of peptide and alkaloid mu-agonists in an antinociception assay. J Pharmacol Exp Ther. 2003;304:301–309. doi: 10.1124/jpet.102.042093. [DOI] [PubMed] [Google Scholar]
- Szucs M, Boda K, Gintzler AR. Dual effects of DAMGO [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin and CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) on adenylyl cyclase activity: implications for mu-opioid receptor Gs coupling. J Pharmacol Exp Ther. 2004;310:256–262. doi: 10.1124/jpet.104.066837. [DOI] [PubMed] [Google Scholar]
- Takasuna M, Negus SS, DeCosta BR, Woods JH. Opioid pharmacology of the antinociceptive effects of loperamide in mice. Behav Pharmacol. 1994;5:189–195. doi: 10.1097/00008877-199404000-00010. [DOI] [PubMed] [Google Scholar]
- Takemori AE.Determination of pharmacological constants: use of narcotic antagonists to characterize analgesic receptors Narcotic Antagonists 1974Raven Press: New York; 335–344.In: Braude M, Harris LS, May E, Smith JP and Villarreal J (eds) [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tallarida RJ, Cowan A, Adler MW. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979;25:637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. Springer-Verlag: New York; 1987. [Google Scholar]
- Trist DG, Leff P, Black J, Gerskowitch VP, Shankley NP. Resultant action of cimetidine in a cardiac adenylate cyclase assay: its elucidation by concentration-ratios analysis. J Pharmacol Exp Ther. 1987;243:1043–1047. [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Walker EA, Sterious SN. Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol. 2005;145:975–983. doi: 10.1038/sj.bjp.0706247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadee W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, et al. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther. 2004;308:512–520. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:PL339–PL350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Young AM, Masaki MA, Geula C. Discriminative stimulus effects of morphine: effects of training dose on agonist and antagonist effects of mu opioids. J Pharmacol Exp Ther. 1992;261:246–257. [PubMed] [Google Scholar]