Abstract
Background & purpose:
The therapeutically available quinolone antibiotic moxifloxacin has been used as a positive control for prolonging the QT interval in both clinical and non-clinical studies designed to assess the potential of new drugs to delay cardiac repolarization. Despite moxifloxacin prolonging QT, it has not been shown to cause torsades de pointes arrhythmias (TdP). Azithromycin is a macrolide antibiotic that has rarely been associated, clinically, with cases of proarrhythmia. As there is a lack of clinical data available, the cardiac safety of these drugs was assessed in a TdP-susceptible animal model by evaluating their repolarization and proarrhythmia effects.
Experimental approach & Key results:
In transfected HEK cells, the IC50s for I hERG were 45±6 and 856±259 μg ml-1 for moxifloxacin and azithromycin, respectively. Intravenous administration of 2 and 8 mg kg-1 moxifloxacin (total peak-plasma concentrations 4.6±1.5 and 22.9±6.8 μg ml-1) prolonged the QTc in 6 anaesthetized dogs with chronic AV block by 7±3 and 21±19%, respectively. Similar intravenous doses of azithromycin (total peak-plasma concentrations 5.4±1.3 and 20.8±4.9 μg ml-1) had no electrophysiological effects in the same dogs. The reference compound, dofetilide (25 μg kg-1 i.v.) caused QTc prolongation (29±15%) and TdP in all dogs. Beat-to-beat variability of repolarization (BVR), quantified as short-term variability of the left ventricular monophasic action potential duration, was only increased after dofetilide (1.8±0.7 to 3.8±1.5 ms; P<0.05).
Conclusion & implications:
As neither moxifloxacin nor azithromycin caused TdP or an increase in the BVR, we conclude that both drugs can be used safely in clinical situations.
Keywords: electrophysiology, acquired long QT, proarrhythmia, beat-to-beat variability of repolarization, short-term variability, action potential, torsades de pointes
Introduction
Drug-induced prolongation of the QT interval is traditionally regarded as a reliable predictive measure for the risk of torsades de pointes arrhythmias (TdP). The majority of drugs associated clinically with TdP prolong the cardiac QT interval through inhibition of the rapidly activating delayed rectifier potassium current (IhERG (human ether-a-go-go-related gene)). However, the opposite, that most IhERG blockers cause TdP, is incorrect (Hondeghem et al., 2001; van Opstal et al., 2001b; Martin et al., 2004; Thomsen et al., 2004). Proarrhythmic indicators other than IhERG block or QT prolongation have been proposed, but although promising none have been shown to be infallible (Hondeghem et al., 2001; Thomsen et al., 2004; Antzelevitch, 2005). Until such an indicator is found, drugs need to be analysed in models with TdP occurrence as a potential end point when cardiac safety and proarrhythmia properties are being evaluated (Thomsen et al., 2006a).
Moxifloxacin is a quinolone antibiotic, the antimicrobial activity of which depends on inhibition of DNA gyrase, a bacterial enzyme needed for DNA replication, transcription repair and recombination (Kim et al., 2001). Moxifloxacin is available as a 400 mg oral compound that is given for a maximum treatment period of 14 days. The antibiotic is also known to prolong the QT interval in both experimental and clinical situations; this effect is probably mediated through inhibition of IhERG as IC50 values have been found between 30 and 52 mg ml−1 (Bischoff et al., 2000; Kang et al., 2001; Lacroix et al., 2003; Chen et al., 2005). Furthermore, it is the preferred drug for use as a positive control in clinical studies designed to assess the potentials of new drugs to delay cardiac repolarization, as it has a reproducible mean 5–10 ms QT prolongation after 400 mg kg−1 oral ingestion in healthy volunteers (Demolis et al., 2000). Two large clinical trials with more than 30000 patients showed no indication of ventricular arrhythmias (Faich et al., 2004; Andriole et al., 2005). In 2001, a retrospective database analysis of the Adverse Events Reporting System of the American Food and Drug Administration found no reports of moxifloxacin-induced TdP (Frothingham, 2001). Proarrhythmia has only been shown in in vitro proarrhythmia models when plasma concentrations of the drug 10–30 times those encountered clinically were attained (Chen et al., 2005; Fish et al., 2005; Milberg et al., 2005; Wu et al., 2006). Also, in one in vivo model when four times the clinically used plasma concentration was present after oral administration of more than 10 times the therapeutic dose (Chiba et al., 2004).
Azithromycin is the most commonly prescribed macrolide antibiotic with a yearly prescription rate in the US around 30 million (Shaffer et al., 2002). Macrolide antibiotic activity is through the inhibition of protein synthesis by binding to the 50S subunit of the bacterial ribosome (Retsema and Fu, 2001). From spontaneous reporting, the clinical proarrhythmic propensity of azithromycin has been estimated to be 0.06 TdP per million prescriptions and isolated accounts of proarrhythmia and QT prolongation are available in the scientific literature (Samarendra et al., 2001; Kim et al., 2005). Azithromycin has only been tested in one proarrhythmia model where it did not induce TdP even at very high concentrations (Milberg et al., 2002).
The objective of this study was to test the electrophysiological and proarrhythmic potential of moxifloxacin and azithromycin in a sensitive animal model using plasma concentrations relevant to the clinical situation. For this purpose, we used anaesthetized dogs with chronic atrioventricular block (CAVB), an animal model with a high proarrhythmia-predictive value, where serial testing in the same animal is possible (van Opstal et al., 2001a; Thomsen et al., 2003, 2004; Detre et al., 2005). Furthermore, we evaluated the effects of these drugs on beat-to-beat variability of repolarization duration (BVR), a novel indicator of proarrhythmic effects (Thomsen et al., 2004).
Methods
Animal handling was in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The Committee for Experiments on Animals of Utrecht University approved all experiments. For the pharmacological study, AV block was induced in six dogs (Marshall, NY,USA) by radiofrequency ablation according to methods previously described (Detre et al., 2005). Experiments were performed more than 4 weeks after the ablation; this allowed cardiac remodelling to complete.
Complete anaesthesia was induced by thiopental (15 mg kg−1 intravenous (i.v.)) and maintained by isoflurane (1.5% in O2 and N2O, 1:2). In addition to ECG recordings, monophasic action potentials (MAP) from the endocardium of the free walls of the left (LV) and right ventricle (RV) were recorded. Perioperative care, signal processing and data recording were as described in detail previously (van Opstal et al., 2001a). All drugs were administered i.v. over a period of 5 min. Plasma samples for drug-concentration determination were taken from a contra-lateral vein.
In vitro, human embryonic kidney (HEK) 293 cells were stably transfected with hERG providing a transmembrane current attributed to the human rapidly activating delayed rectifier potassium current. Standard voltage-clamp protocols, patch electrodes and solutions were used at room temperature. Peak tail IhERG were measured at control and after 5-min incubation with moxifloxacin or azithromycin (30–300 μM). IC50 values were obtained by fitting the data to a two-parameter sigmoidal curve (I=cn (cn+IC50n)−1) based on the assumption that 10 M antibiotic would block IhERG completely.
Dose selection
Moxifloxacin (Avelox; MW 438 g mol−1) and azithromycin (Zithromac; MW 785 g mol−1) were obtained from a commercial source and dissolved in 0.5 ml kg−1 0.5% lactic acid. Dofetilide was dissolved in 0.1 ml 0.1 mM HCl and diluted in 0.5 ml kg−1 0.9% saline.
The peak-plasma concentrations of moxifloxacin after a single oral dose of 400 mg to healthy volunteers have been shown to range from 2.5±1.3 to 4.3±1.6 μg ml−1. Previous studies in anaesthetized dogs showed a peak-plasma concentration after 1 mg kg−1 moxifloxacin i.v. of 2.3±0.4 μg ml−1 (Chiba et al., 2004). To cover the concentration range likely to be encountered in clinical situations, 2 mg kg−1 moxifloxacin was administered. To challenge repolarization further, 8 mg kg−1 was administered in a serial, cumulative way separated by 30 min. The clinically used doses of azithromycin are comparable to those of moxifloxacin, despite the resulting clinical plasma concentration only reaches 0.4 μg ml−1 azithromycin (Rapp, 1998). Thus, azithromycin was also administered in cumulative, increasing doses of 2 and 8 mg kg−1 separated by 30 min. Dofetilide (25 μg kg−1) was administered as a positive control for proarrhythmic ventricular remodelling after AVB, similar to earlier experiments (van Opstal et al., 2001a; Thomsen et al., 2003). Experiments with moxifloxacin (n=6 dogs), azithromycin (n=5 dogs) and dofetilide (n=5 dogs) were conducted in a random crossover manner separated by 2 weeks.
In some experiments RV programmed electrical stimulation (twice diastolic threshold, 2 ms pulse width) was performed using an external stimulator. Pacing trains mimicking short–long–short RR intervals were used to probe TdP susceptibility at control and after moxifloxacin (n=3). In other CAVB dogs, abrupt prolongation of the steady-state pacing cycle length from 500 to 1000 ms was used to test the influence of dynamic heart-rate on BVR under drug-free circumstances (n=8).
Analysis
Mean RR and QT intervals from lead LL, a left lateral precordial lead placed in the 6th intercostal space near the sternum, were measured manually (ECGview, Maastricht University, The Netherlands). Durations of the MAP to 90% repolarization (MAPD) were determined semiautomatically (ECG-Auto, EMKA Technologies, France). Interventricular dispersion of repolarization duration (ΔMAPD) was defined as LV minus RV MAPD. QT intervals were corrected for heart rate (QTc) according to Van de Water's formula. Measurements were performed during periods without extrasystolic activity as previously described (Thomsen et al., 2004). BVR was assessed from the duration of 30 consecutive LV MAPs and quantified as short-term variability (STVLV) describing the mean orthogonal distance to the line-of-identity on a Poincaré plot, as described earlier (Thomsen et al., 2004). Extrasystolic activity, defined as beats initiating before the end of the preceding T wave, was quantified. Discrimination was made between couplets and multiples as in earlier publications (Thomsen et al., 2004).
Heparin-treated blood samples were centrifuged at 1000 g for 15 min at 4°C and plasma samples were stored at −18°C. Concentrations of moxifloxacin or azithromycin were determined using high-performance liquid chromatography. Total (protein bound and freely dissolved) plasma concentrations are presented.
Data are means±s.d., unless otherwise noted. Statistical significance was assumed if P<0.05, from one or two-way repeated measures analysis of variance (ANOVA) followed by a Bonferroni t-test, when appropriate. Correlations were evaluated using Pearson product moment correlation.
Results
Electrophysiological effects of moxifloxacin
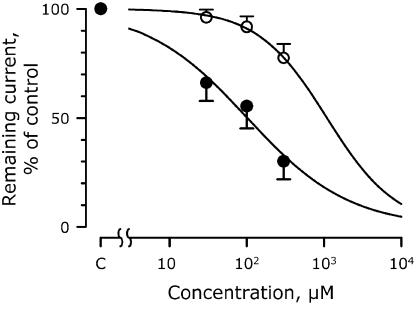
Figure 1 shows the concentration–response curves for IhERG when treated with 30–300 μM moxifloxacin and azithromycin. Moxifloxacin inhibited IhERG in a concentration-dependent manner, with 50% block at 102±14 μM (45±6 μg ml−1).
Figure 1.
The blocking effects of moxifloxacin and azithromycin on IhERG expressed in HEK 293 cells. Data points represent the means of four cells. Concentration–response curves are shown, where the remaining current is plotted relative to its control level. Closed circles, moxifloxacin with an IC20 and IC50 of 12 and 102 μM, respectively, and a Hill coefficient of 0.66. Open circles, azithromycin with corresponding values of 265, 1091 and 0.97 μM.
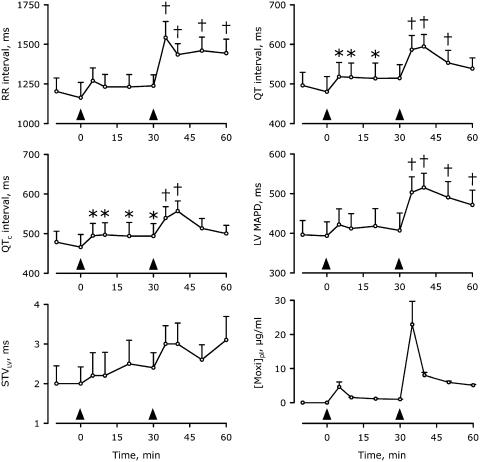
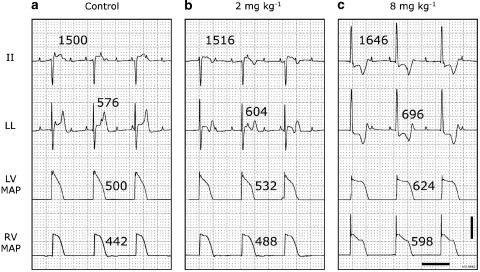
In the anaesthetized CAVB dogs, cumulative doses of moxifloxacin produced total peak-plasma concentrations of 4.6±1.5 and 22.9±6.8 μg ml−1 at the end of infusion of 2 and 8 mg kg−1 moxifloxacin, respectively, whereas maximally prolonged QTc intervals were observed 10 min after the start of moxifloxacin administration (Figure 2, Table 1). A representative, individual example of the electrophysiological effects is shown in Figure 3. After 2 mg kg−1 moxifloxacin QTc was prolonged from 466±78 to 497±75 ms (7%; P<0.05). At this dose, the RR interval was not significantly changed, whereas the QT interval was prolonged from 480±95 to 517±88 ms (8%; P<0.05). The LV and RV MAPD changed from 394±86 and 350±61 ms to 412±92 (5%) and 381±72 ms (9%), respectively (both P=NS) and ΔMAPD was not altered. The high dose delayed repolarization further, and both atrial and ventricular cycle lengths were prolonged (Table 1). Furthermore, the origin of ventricular activation changed in the majority of dogs following administration of the high dose (Figure 3). The prolongation of RR, QT and QTc intervals alongside LV MAPD showed clear dose-dependent properties (Figure 2). A general tendency towards an increase in STVLV was seen, but this was neither statistically significant nor did it seem to develop parallel to the dose-dependent prolongation of repolarization duration. Extrasystolic activity was confined to couplets and neither dose of moxifloxacin was associated with induction of TdP (Table 1).
Figure 2.
Time-dependent electrophysiological effects of cumulative moxifloxacin administration in anaesthetized CAVB dogs. Arrowheads indicate the start of the 5-min infusion of 2 and 8 mg kg−1 moxifloxacin. [Moxi]pl, plasma concentration of moxifloxacin. *P<0.05 versus control (−10 or 0 min); †P<0.05 versus control and 30 min. Mean±s.e. are shown. All plasma concentrations were significantly different from control (not shown). n=6 anaesthetized CAVB dogs.
Table 1.
Electrophysiological properties and proarrhythmic potential of 8 mg kg−1 moxifloxacin (n=6), 8 mg kg−1 azithromycin (n=5) and 25 μg kg−1 dofetilide (n=5) in isoflurane-anaesthetized dogs with CAVB (mean±s.d.)
| Control 1 | Moxifloxacin | Control 2 | Azithromycin | Control 3 | Dofetilide | |
|---|---|---|---|---|---|---|
| RR (ms) | 1162±238 | 1436±167*† | 1021±182 | 1044±149 | 1159±198 | 1251±252 |
| QT (ms) | 480±95 | 594±75* | 452±49 | 420±52† | 478±84 | 622±143* |
| QTc (ms) | 466±78 | 556±63* | 450±42 | 416±48† | 464±73 | 600±126* |
| LV MAPD (ms) | 394±86 | 515±89* | 369±36 | 345±57† | 418±75 | 607±139* |
| RV MAPD (ms) | 350±61 | 451±99* | 323±37 | 315±37† | 352±82 | 479±156* |
| ΔMAPD (ms) | 43±40 | 64±63 | 46±13 | 30±37 | 66±42 | 128±43* |
| STVLV (ms) | 2.0±0.9 | 3.0±1.3 | 2.2±0.6 | 2.3±0.5 | 1.8±0.7 | 3.8±1.5* |
| PP (ms) | 597±137 | 681±112* | 531±53 | 521±38† | 573±46 | 666±82* |
| Couplets | 0.3±0.5 | 9.5±14 | 0.0±0.0 | 1.2±1.6 | 2.4±3.6 | 77±75 |
| Multiples | 0.3±0.8 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 2.5±3.5 |
| TdP incidence | 0 | 0 | 0 | 0 | 0 | 100% |
| [Drug]pl (μg ml−1)a | 0±0 | 8.0±0.8 | 0±0 | 4.7±0.8 | 0±0 | 0.035±0.005b |
Abbreviations: CAVB, chronic atrioventricular block; MAP, monophasic action potentials; MAPD, MAP to 90% repolarization; STV, short-term variability; TdP, torsades de pointes.
Analyses of experiments with moxifloxacin or azithromycin were performed before and 10 min after start of infusion of 8 mg kg−1, whereas dofetilide experiments were analysed before administration and before the first TdP (3±1 min). No statistically significant differences were identified within the control data. Singles and multiples, mean frequency of single and coupled (>2) extrasystoles, respectively, determined over 20 min. TdP incidence is relative to group size.
*P<0.05 versus control; †P<0.05 versus dofetilide-induced change.
Concentrations at 10 min are not peak-plasma concentrations (see text for details);
Plasma concentrations of dofetilide were acquired from another study performed under comparable conditions Thomsen et al. (2003).
Figure 3.
Electrophysiological effects of cumulative doses of moxifloxacin in a representative anaesthetized dog with CAVB. Two ECG leads, LV and RV MAP recordings are shown in each panel. RR intervals are above lead II and QT times above lead LL. MAPDs are next to the action potentials. ECG calibrated to 1 mV cm−1. Scale bar, 20 mV on the MAP signals. Horizontal scale bar, 1 s. (a) Control situation before moxifloxacin administration. (b) 10 min after start of 2 mg kg−1 moxifloxacin infusion. (c) 10 min after start of 8 mg kg−1 moxifloxacin infusion. The low dose causes a change in the T-wave morphology, whereas the high dose alters the ventricular origin of activation. No TdP was observed with any dose of moxifloxacin. In this example, the plasma concentrations measured at the time of these tracings were 1.6 and 8.6 μg ml−1 after 2 and 8 mg kg−1, respectively.
Electrophysiological effects of azithromycin and dofetilide
IhERG was inhibited by 22.5±6.5%, by 300 μM azithromycin, the highest concentration tested. If it is assumed 100% block occurs at 10 M, IC50 was 1091±330 μM (856±259 μg ml−1; Figure 1). In vivo, cumulative doses of 2 and 8 mg kg−1 azithromycin created total peak-plasma concentrations of 5.4±1.3 and 20.8±4.9 μg ml−1, respectively, but did not produce any significant electrophysiological effects. Furthermore, the drug induced no TdP (Table 1).
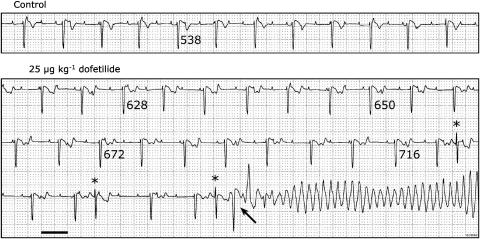
The positive reference compound dofetilide showed significant prolongation of all repolarization parameters including ΔMAPD and STVLV, consistent with reproducible induction of TdP in all dogs (Table 1 and Figure 4). Dofetilide induced significantly larger prolongation of the QT, QTc, LV and RV MAPD than azithromycin (Table 1). Moxifloxacin prolonged the ventricular cycle length more than dofetilide, whereas prolongation of repolarization developed to comparable levels. Dofetilide was the only drug tested to induce multiple extrasystoles.
Figure 4.
Representative examples of the electrophysiological and proarrhythmic effects of dofetilide administration in an anaesthetized CAVB dog. Same dog as in Figure 3. Lead II is shown at control and during dofetilide administration (25 μg kg−1). TdP occurred 2.5 min after the start of administration. QT intervals are indicated below the respective T-wave. Three morphologically comparable single spontaneous extrasystoles (*) occur, the last one triggering a TdP (arrow). ECG calibrated to 1 mV cm−1. Horizontal scale bar, 1 s.
Temporal stability of BVR duration
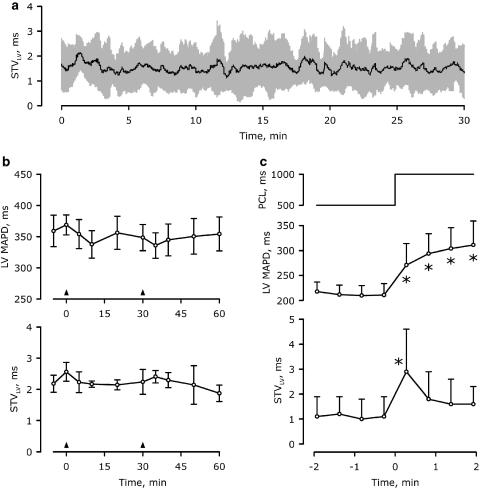
No statistically significant increase in STVLV was seen after moxifloxacin, in concordance with the absence of proarrhythmia (Table 1, Figure 2). Nevertheless, there seem to be a gradual increase in STVLV over the course of the experiment (Figure 2). Representative examples of Poincaré plots before and after moxifloxacin, azithromycin and dofetilide are shown in Figure 5. To investigate the stability of STVLV over prolonged periods, we measured STVLV for 30 min in drug-free conditions (Figure 6a). These results showed that no protracted elevation of STVLV was occurring in control conditions. Furthermore, azithromycin did not induce any time-dependent elevation of STVLV (Figure 6b). To eliminate the possibility that moxifloxacin-induced RR prolongation was indirectly affecting the dynamics of STVLV, we analysed the time-dependent behaviour of STVLV during an abrupt prolongation of the paced cycle length under control conditions (Figure 6c). Upon changing the paced cycle length from 500 to 1000 ms, the LV MAPD prolonged by 102±32 ms, reaching a plateau after about 2 min, whereas the STVLV momentarily increased but reached a new steady-state within 1 min.
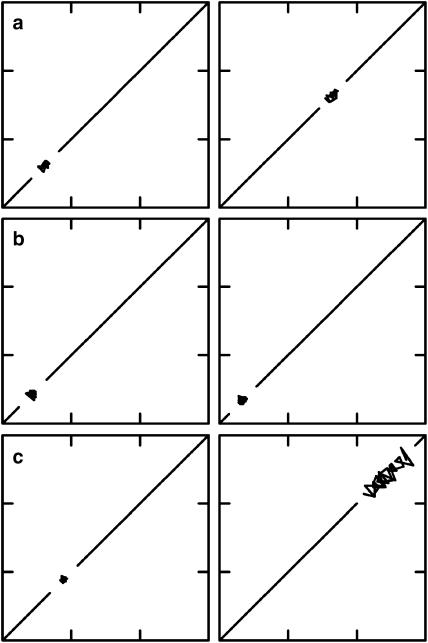
Figure 5.
Representative examples of Poincaré plots obtained from the same dog used in the other experiments. Left panels are controls, whereas right panels are under the influence of various drugs. Thirty consecutive LV MAPDs are plotted against the value of the previous beat. Each plot is 200–500 by 200–500 ms. All control plots cluster close to the diagonal line. (a) 8 mg kg−1 moxifloxacin provokes prolongation of the LV MAPD but does not elevate BVR as the dimensions of the plot remain unchanged. (b) 8 mg kg−1 azithromycin has no effect on either LV MAPD or BVR. (c) 25 μg kg−1 dofetilide prolongs LV MAPD as well as increasing BVR as is clearly seen by the increased area of the Poincaré plot. Only dofetilide was associated with proarrhythmia.
Figure 6.
Time-dependent behaviour of BVR. (a) Short-term variability calculation continuously for 30 min under control conditions (n=3). Black line, mean data; grey area, mean±s.d. (b) Combined data of the time-dependent electrophysiological effects of cumulative azithromycin administration in anaesthetized CAVB dogs (n=5). Arrowheads indicate the start of the 5-min infusion of 2 and 8 mg kg−1 azithromycin. No statistically significant changes were observed in either LV MAPD (upper panel) or STVLV (lower panel). Mean±s.e. are shown. (c) Dynamicity of LV MAPD and STVLV upon sudden changes in heart rate. Continuous pacing from the right ventricle at 500 ms cycle length for 2 min was followed by an instantaneous change in pacing cycle length (PCL) to 1000 ms (upper panel) for 2 min. LV MAPD and STVLV of the paced periods are depicted in the lower panels. *P<0.05 versus 500 ms PCL.
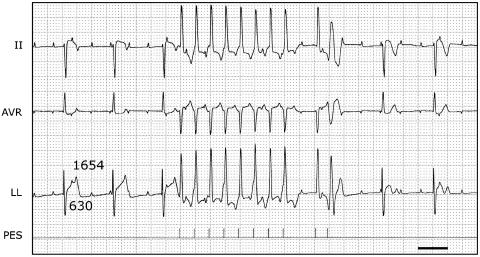
Proarrhythmic programmed electrical stimulation did not induce TdP in either the control or after moxifloxacin. Figure 7 shows a representative example of such a stimulation protocol and the electrophysiological consequences thereof in the presence of moxifloxacin.
Figure 7.
Pacing to induce proarrhythmia after administration of moxifloxacin. Three ECG leads (II, AVR and LL) are shown. After administration of 2 followed by 8 mg kg−1 moxifloxacin, the QT interval is 630 ms at a CL of the idioventricular rhythm of 1654 ms. A programmed electrical stimulation (PES) was performed, resulting in 8 beats with a CL of 500 ms, followed by a beat after 1200 ms and an extra beat after 400 ms. The extra beat was paced close to the refractory period as evidenced by the broad R wave. Neither this nor any other stimulation protocol lead to arrhythmias. Note, however, the different T-wave morphologies of the nonpaced beats before and after the stimulation protocol. Furthermore, a tendency towards T-wave alternans can be observed during pacing with a CL of 500 ms. Horizontal scale bar, 1 s.
Discussion
The present study demonstrates that both moxifloxacin and azithromycin block IhERG at high concentrations. However, from the serial comparison in the anaesthetized CAVB dogs, it was evident that dofetilide and moxifloxacin but not azithromycin prolong the duration of ventricular repolarization. Only dofetilide caused an increase in BVR, which was associated with the occurrence of TdP in all dogs. The two antibiotics showed no increase in BVR and caused no proarrhythmia.
Electrophysiological and proarrhythmic properties of moxifloxacin
Dose-dependent moxifloxacin-induced prolongation of the action potential in vitro has been shown by many groups (Patmore et al., 2000; Gintant et al., 2001; Chen et al., 2005; Fish et al., 2005; Milberg et al., 2005). In intact control dogs, moxifloxacin-induced QT prolongation has been demonstrated in both conscious (Chen et al., 2005) and in anaesthetized animals (Chiba et al., 2004; Mittelstadt and Hart, 2005). The present study confirms the repolarization-prolonging properties of moxifloxacin after a clinically relevant dose and after a higher challenging dose (Figure 2). Furthermore, significant bradycardic effects after 8 mg kg−1 moxifloxacin were seen at both atrial and ventricular levels (Table 1).
The proarrhythmic properties of moxifloxacin have been assessed in both rabbit and canine arterially perfused wedges, in isolated retrogradely perfused rabbit hearts, in methoxamine-sensitized anaesthetized rabbits and in conscious dogs with CAVB (Anderson et al., 2001; Chiba et al., 2004; Chen et al., 2005; Fish et al., 2005; Milberg et al., 2005; Wu et al., 2006). The results from these sensitive proarrhythmia models concur that moxifloxacin lacks that potential to cause TdP at concentrations encountered clinically (2.5±1.3–4.3±4.6 mg ml−1 (Stass et al., 1998; Lubasch et al., 2000)), whereas much higher concentrations possess the ability to induce arrhythmias. The only exception is a study in conscious dogs with CAVB, where a total plasma concentration of 12.6±2.0 mg ml−1 after oral administration of 100 mg kg−1 moxifloxacin, more than 10 times higher than the therapeutic dose, produced TdP in the absence of QT prolongation (Chiba et al., 2004). Nevertheless, the ECG trace in Figure 4b in Chiba et al. (2004) shows a QT interval in excess of 500ms immediately before the onset of TdP. Reanalysis of these data revealed a consistent increase in both RR (1788±140–2259±390 ms; P<0.05) and QT (325±28–411±55 ms; P<0.05) intervals immediately before moxifloxacin-induced TdP. Estimation of STVQT in the conscious dog experiments, showed an increase from 4.5±0.8–5.9±0.6 ms (P<0.05), whereas moxifloxacin caused no change in STVQT in the anaesthetized dogs of the present study (2.4±1.7 versus 2.6±1.0 ms; P=NS).
Thus, there are essential differences in proarrhythmic outcome between the conscious and the anaesthetized CAVB dog. The conscious CAVB dogs possess a control QT of 349±36 ms (Chiba et al., 2004), significantly less than the anaesthetized CAVB dogs (P<0.05; Table 1). On the other hand, the baseline STVQT is largest in the conscious dogs (P<0.05). This reinforces the observation of independence between duration and variability of repolarization.
Moxifloxacin did not increase either STVLV or STVQT in the present anaesthetized dog experiments, whereas an increased STVQT in conscious dogs was associated with proarrhythmia. This suggests the presence of additional undetermined proarrhythmic predisposing factors in the conscious CAVB dogs. Such factors could include adrenergic and/or vagal influences on cardiac activity, which is likely to change over the course of the day. The presence of additional predisposing factors for a limited time could explain how the moxifloxacin-induced QT prolongation was missed in the first study (Chiba et al., 2004). Further studies are needed to determine whether the altered autonomic drive or the anaesthesia alone is decreasing BVR in the anaesthetized animals. Importantly, oral administration of 100 mg kg−1 moxifloxacin provides a larger area under the time-versus plasma concentration curve than does i.v. administration of 8 mg kg−1, which provides the largest peak in the plasma concentration. Thus, oral administration possibly leads to greater chance of tissue-drug accumulation, so myocardial concentrations of moxifloxacin after oral and i.v. administration should be established before conclusions are reached on any differences in proarrhythmic characteristics between the two situations. In the present study, total plasma concentrations (protein bound and freely dissolved) are presented, despite differences in free fractions: moxifloxacin, 50% (Stass et al., 1998); azithromycin, >93% (Pfizer, 2003) and dofetilide, 30–40% (Pfizer, 1999).
Electrophysiological and proarrhythmic properties of azithromycin
Only very high concentrations of azithromycin cause action potential prolongation in vitro (Gintant et al., 2001; Milberg et al., 2002). However, no proarrhythmia effects have been reported in other experimental studies. The present study identified an IC50 for azithromycin that is more than three orders of magnitude larger than the plasma concentration obtained after therapeutic doses (Rapp, 1998). As only 22% of IhERG was blocked by azithromycin and the IC50 was estimated from this (Figure 1), caution should be exercised when comparing this value to IC50s of other drugs.
This is the first time azithromycin has been evaluated in an integrated in vivo proarrhythmia model. Although Table 1 indicates a nonsignificant trend towards a decrease in repolarization duration (QT, QTc and LV MAPD), this was an inconsistent and dose-independent effect. The clinical case reports of proarrhythmia associated with azithromycin were either in a hypokalemic patient and occurred in the absence of QT prolongation (Kim et al., 2005) or during co-administration of amiodarone (Samarendra et al., 2001). The Adverse Event Reporting System of the American Food and Drug Administration contains 12 spontaneous reports of TdP associated with azithromycin (Shaffer et al., 2002). Such a spontaneous reporting system is valuable in generating hypotheses concerning possible adverse effects of marketed drugs. However, they are not sufficiently powered to verify or falsify the suggestion. The presumed proarrhythmic characteristic of azithromycin was not confirmed in the present study in a susceptible TdP model.
Temporal stability of BVR
In Figure 2, a trend towards an increase in STVLV after moxifloxacin is observable. Although this was not statistically significant, it calls for caution in the interpretation of the safety of moxifloxacin. Apart from the direct effects of moxifloxacin, other explanations for this trend are available. Temporal instability of the measure over prolonged time or influence of heart-rate fluctuations could theoretically increase STVLV. From the data in Figure 6a and b, the possibility that prolonged recording of the LV MAPD causes an increase in the calculated STVLV can be excluded. Figure 2 shows a moxifloxacin-induced momentary increase in the RR interval, which was not seen with either azithromycin or dofetilide (Table 1). Figure 6c shows that a rapid prolongation of the ventricular rate does not lead to a protractedly increased STVLV. Furthermore, we have previously shown that proarrhythmic agents increase STVLV independent of the RR interval (Thomsen et al., 2006b) and that STVLV remains unchanged after amiodarone treatment (Thomsen et al., 2004) despite considerable prolongation of both the RR and QT intervals (van Opstal et al., 2001b). Furthermore, there is no significant correlation between the RR and STVLV in the individual moxifloxacin experiments (P=0.42). Thus, it is unlikely that the trend towards an increase in STVLV by moxifloxacin could be secondary to acute changes in RR interval.
Repolarization variability in drug research
BVR has been used as a proarrhythmia-predictive parameter in both repolarization assays (Detre et al., 2005; Schneider et al., 2005; van der Linde et al., 2005; Lu et al., 2006) and proarrhythmia models (Thomsen et al., 2004; Detre et al., 2005; Lu et al., 2006; Takahara et al., 2006). BVR provides data assisting the identification of safe drugs and is thus a useful addition for the assessment of proarrhythmic actions of drugs.
Recently, moxifloxacin has been tested in a novel model based on action potential alternans during fast cardiac pacing in anaesthetized guinea-pigs (Wisialowski et al., 2006). Administration of moxifloxacin lowered the pacing-frequency threshold for induction of alternans and was therefore characterized as a torsadogenic compound. Pacing-induced alternans is a consequence of insufficient calcium cycling appearing at fast heart-rates, whereas BVR occurs at physiological-to-slow ventricular rates and is not likely to be due to calcium deficiencies (Antoons et al., 2005).
Pharmacological implications
Besides antibiotic treatment, moxifloxacin is widely used in the Thorough QT/QTc study, which tests the influence of a drug on human repolarization before approval is given to market the drug. As moxifloxacin has very predictable QT prolonging effects and pharmacokinetics in humans, it is often used as a positive control in these studies to test for sensitivity, as the treatment reproducibly induces 5–10 ms QT prolongation (Demolis et al., 2000). The present study demonstrates that neither therapeutic nor investigational use of moxifloxacin is likely to cause proarrhythmia, even in subjects with diminished repolarization reserve. However, the bradycardic effects of the drug may have clinical implications. The postulated incidence of azithromycin-induced proarrhythmia in the clinical setting is not supported by the present experimental findings.
In the present study, we opted to focus on the cardiac safety at clinically relevant plasma concentrations, rather than determining the dose required for proarrhythmia. Still, the total peak-plasma concentrations obtained in the present study slightly exceed the reported mean clinically encountered concentrations, thus allowing for individual variation in patient pharmacokinetics. Previously, a safety margin more than 30-fold higher has been advocated between clinical maximal plasma concentrations and hERG IC50 (Redfern et al., 2003), but clearly this margin is not attributable to the integrated in vivo pathological models of TdP. Rather, the high proarrhythmic susceptibility and clinical-predictive value of these models suggest that clinically encountered plasma concentrations should be re-evaluated (Thomsen et al., 2006a).
Limitations
Analysis of the STVQT in CAVB dogs is hampered by P waves in the terminating part of the T wave, but LV MAP recordings are not possible in conscious dogs. The STVQT measurements reported here are thus nonconsecutive as some complexes were excluded from analysis. Steady-state plasma concentrations were not obtained in this study, as opposed to some clinical and experimental studies, and the pharmacokinetic difference between acute i.v. and oral dosing should be considered when extrapolating data from our study. Differences in accumulated tissue concentrations are also likely to exist between the two routes of administrations.
Conclusions
In these serial experiments in anaesthetized dogs with CAVB, high i.v. doses of moxifloxacin and dofetilide caused prolongation of repolarization. However, only dofetilide elevated BVR and initiated TdP. At clinically relevant doses, neither moxifloxacin nor azithromycin seem to possess proarrhythmic properties even in the remodelled heart. Nevertheless, caution should be taken with moxifloxacin where significant tissue accumulation can occur in the susceptible patient.
Acknowledgments
The experimental assistance of Stephan Winckels and Avram Oros is greatly appreciated. We thank Dr M Tachibana for the determination of plasma-drug concentrations.
Abbreviations
- BVR
beat-to-beat variability of repolarization
- CAVB
chronic atrioventricular block
- hERG
human ether-a-go-go-related gene
- LV
left ventricle
- MAPD
monophasic action potential duration to 90% repolarization
- RV
right ventricle
- STV
short-term variability
- TdP
torsades de pointes
Conflict of interest
The authors state no conflict of interest.
References
- Anderson ME, Mazur A, Yang T, Roden DM. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther. 2001;296:806–810. [PubMed] [Google Scholar]
- Andriole VT, Haverstock DC, Choudhri SH. Retrospective analysis of the safety profile of oral moxifloxacin in elderly patients enrolled in clinical trials. Drug Safety. 2005;28:443–452. doi: 10.2165/00002018-200528050-00007. [DOI] [PubMed] [Google Scholar]
- Antoons G, Stengl M, Thomsen MB, Beekman JD, Vos MA, Sipido KR. Sarcoplasmic reticulum Ca2+ release and repolarization lability in myocytes from the dog with chronic atrioventricular block (cAVB) Biophys J. 2005;88:298A. [Google Scholar]
- Antzelevitch C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm. 2005;2:S9–S15. doi: 10.1016/j.hrthm.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406:341–343. doi: 10.1016/s0014-2999(00)00693-2. [DOI] [PubMed] [Google Scholar]
- Chen X, Cass JD, Bradley JA, Dahm CM, Sun Z, Kadyszewski E, et al. QT prolongation and proarrhythmia by moxifloxacin: concordance of preclinical models in relation to clinical outcome. Br J Pharmacol. 2005;146:792–799. doi: 10.1038/sj.bjp.0706389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Sugiyama A, Hagiwara T, Takahashi S-i, Takasuna K, Hashimoto K. In vivo experimental approach for the risk assessment of fluoroquinolone antibacterial agents-induced long QT syndrome. Eur J Pharmacol. 2004;486:189–200. doi: 10.1016/j.ejphar.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Demolis JL, Kubitza D, Tenneze L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658–666. doi: 10.1067/mcp.2000.111482. [DOI] [PubMed] [Google Scholar]
- Detre E, Thomsen MB, Beekman JD, Petersen KU, Vos MA. Decreasing the infusion rate reduces the proarrhythmic risk of NS-7: confirming the relevance of short-term variability of repolarisation in predicting drug-induced torsades de pointes. Br J Pharmacol. 2005;145:397–404. doi: 10.1038/sj.bjp.0706203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faich GA, Morganroth J, Whitehouse AB, Brar JS, Arcuri P, Kowalsky SF, et al. Clinical experience with moxifloxacin in patients with respiratory tract infections. Ann Pharmacother. 2004;38:749–754. doi: 10.1345/aph.1C066. [DOI] [PubMed] [Google Scholar]
- Fish JM, Di Diego JM, Belardinelli L, Antzelevitch C. Moxifloxacin-induced torsade de pointes in an experimental model of long QT syndrome. Heart Rhythm. 2005;2:S108. [Google Scholar]
- Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy. 2001;21:1468–1472. doi: 10.1592/phco.21.20.1468.34482. [DOI] [PubMed] [Google Scholar]
- Gintant GA, Limberis JT, McDermott JS, Wegner CD, Cox BF. The canine Purkinje fiber: an in vitro model system for acquired long QT syndrome and drug-induced arrhythmogenesis. J Cardiovasc Pharmacol. 2001;37:607–618. doi: 10.1097/00005344-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol. 2001;59:122–126. doi: 10.1124/mol.59.1.122. [DOI] [PubMed] [Google Scholar]
- Kim MH, Berkowitz C, Trohman RG. Polymorphic ventricular tachycardia with a normal QT interval following azithromycin. Pac Clin Electrophysiol. 2005;28:1221–1222. doi: 10.1111/j.1540-8159.2005.50146.x. [DOI] [PubMed] [Google Scholar]
- Kim OK, Ohemeng K, Barrett JF. Advances in DNA gyrase inhibitors. Expert Opin Investig Drugs. 2001;10:199–212. doi: 10.1517/13543784.10.2.199. [DOI] [PubMed] [Google Scholar]
- Lacroix P, Crumb WJ, Durando L, Ciottoli GB. Prulifloxacin: in vitro (HERG current) and in vivo (conscious dog) assessment of cardiac risk. Eur J Pharmacol. 2003;477:69–72. doi: 10.1016/s0014-2999(03)02180-0. [DOI] [PubMed] [Google Scholar]
- Lu HR, Vlaminckx E, Van de Water A, Gallacher DJ. Calmodulin antagonist W-7 prevents sparfloxacin-induced early afterdepolarizations (EADs) in isolated rabbit purkinje fibers: importance of beat-to-beat instability of the repolarization. J Cardiovasc Electrophysiol. 2006;17:415–422. doi: 10.1111/j.1540-8167.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother. 2000;44:2600–2603. doi: 10.1128/aac.44.10.2600-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RL, McDermott JS, Salmen HJ, Palmatier J, Cox BF, Gintant GA. The utility of hERG and repolarization assays in evaluating delayed cardiac repolarization: influence of multi-channel block. J Cardiovasc Pharmacol. 2004;43:369–379. doi: 10.1097/00005344-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002;303:218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- Milberg P, Haverkamp W, Monnig G, Talebpour F, Breithardt G, Eckardt L. Quinolones and proarrhythmia: differgent torsadogenic potential despite similar effects in myocardial repolarization. Heart Rhythm. 2005;2:S137. [Google Scholar]
- Mittelstadt SW, Hart SM. Effects of moxifloxacin on QT interval in conscious dogs. J Vet Pharmacol Ther. 2005;28:253–256. doi: 10.1111/j.1365-2885.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Patmore L, Fraser S, Mair D, Templeton A. Effects of sparfloxacin, grepafloxacin, moxifloxacin, and ciprofloxacin on cardiac action potential duration. Eur J Pharmacol. 2000;406:449–452. doi: 10.1016/s0014-2999(00)00694-4. [DOI] [PubMed] [Google Scholar]
- PfizerTikosyn (dofetilide) Capsules 1999. US Prescribing Information
- PfizerZithromax (azithromycin) Capsules 2003. US Prescribing Information
- Rapp RP. Pharmacokinetics and pharmacodynamics of intravenous and oral azithromycin: enhanced tissue activity and minimal drug interactions. Ann Pharmacother. 1998;32:785–793. doi: 10.1345/aph.17299. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Retsema J, Fu W. Macrolides: structures and microbial targets. Int J Antimicrob Agents. 2001;18 Suppl 1:S3–S10. doi: 10.1016/s0924-8579(01)00401-0. [DOI] [PubMed] [Google Scholar]
- Samarendra P, Kumari S, Evans SJ, Sacchi TJ, Navarro V. QT prolongation associated with azithromycin/amiodarone combination. Pacing Clin Electrophysiol. 2001;24:1572–1574. doi: 10.1046/j.1460-9592.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Schneider J, Hauser R, Andreas JO, Linz K, Jahnel U. Differential effects of human ether-a-go-go-related gene (HERG) blocking agents on QT duration variability in conscious dogs. Eur J Pharmacol. 2005;512:53–60. doi: 10.1016/j.ejphar.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis. 2002;35:197–200. doi: 10.1086/340861. [DOI] [PubMed] [Google Scholar]
- Stass H, Dalhoff A, Kubitza D, Schuhly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara A, Sugiyama A, Ishida Y, Satoh Y, Wang K, Nakamura Y, et al. Long-term bradycardia caused by atrioventricular block can remodel the canine heart to detect the histamine H1 blocker terfenadine-induced torsades de pointes arrhythmias. Br J Pharmacol. 2006;147:634–641. doi: 10.1038/sj.bjp.0706493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MB, Matz J, Volders PG, Vos MA. Assessing the proarrhythmic potential of drugs: current status of models and surrogate parameters of torsades de pointes arrhythmias. Pharmacol Ther. 2006a;12:150–170. doi: 10.1016/j.pharmthera.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, Van Opstal JM, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Volders PG, Beekman JD, Matz J, Vos MA. Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J Am Coll Cardiol. 2006b;48:1768–1776. doi: 10.1016/j.jacc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Volders PG, Stengl M, Spätjens RL, Beekman JD, Bischoff U, et al. Electrophysiological safety of sertindole in dogs with normal and remodeled hearts. J Pharmacol Exp Ther. 2003;307:776–784. doi: 10.1124/jpet.103.052753. [DOI] [PubMed] [Google Scholar]
- van der Linde H, Van de Water A, Loots W, Van Deuren B, Lu HR, Van Ammel K, et al. A new method to calculate the beat-to-beat instability of QT duration in drug-induced long QT in anesthetized dogs. J Pharmacol Toxicol Methods. 2005;52:168–177. doi: 10.1016/j.vascn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- van Opstal JM, Leunissen JD, Wellens HJ, Vos MA. Azimilide and dofetilide produce similar electrophysiological and proarrhythmic effects in a canine model of Torsade de Pointes arrhythmias. Eur J Pharmacol. 2001a;412:67–76. doi: 10.1016/s0014-2999(00)00943-2. [DOI] [PubMed] [Google Scholar]
- van Opstal JM, Schoenmakers M, Verduyn SC, de Groot SH, Leunissen JD, Der Hulst FF, et al. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001b;104:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- Wisialowski T, Crimin K, Engtrakul J, O'Donnell J, Fermini B, Fossa AA. Differentiation of arrhythmia risk of the antibacterials moxifloxacin, erythromycin, and telithromycin based on analysis of monophasic action potential duration alternans and cardiac instability. J Pharmacol Exp Ther. 2006;318:352–359. doi: 10.1124/jpet.106.101881. [DOI] [PubMed] [Google Scholar]
- Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]