Abstract
Background and purpose:
The functional roles of M2 and M3 muscarinic receptors in neurogenic cholinergic contractions in gastrointestinal tracts remain to be elucidated. To address this issue, we studied cholinergic nerve-induced contractions in the ileum using mutant mice lacking M2 or M3 receptor subtypes.
Experimental approach:
Contractile responses to transmural electrical (TE) stimulation were isometrically recorded in ileal segments from M2-knockout (KO), M3-KO, M2/M3-double KO, and wild-type mice.
Key results:
TE stimulation at 2-50 Hz frequency-dependently evoked a fast, brief contraction followed by a slower, longer one in wild-type, M2-KO or M3-KO mouse preparations. Tetrodotoxin blocked both the initial and later contractions, while atropine only inhibited the initial contractions. The initial cholinergic contractions were significantly greater in wild-type than M2-KO or M3-KO mice; the respective mean amplitudes at 50 Hz were 91, 74 and 68 % of 70mM K+-induced contraction. Pretreatment with pertussis toxin blocked the cholinergic contractions in M3-KO but not in M2-KO mice. Cholinergic contractions also remained in wild-type preparations, but their sizes were reduced by 20-30 % at 10-50 Hz. In M2/M3-double KO mice, TE stimulation evoked only slow, noncholinergic contractions, which were significantly greater in sizes than in any of the other three mouse strains.
Conclusion and Implications:
These results demonstrate that M2 and M3 receptors participate in mediating cholinergic contractions in mouse ileum with the latter receptors assuming a greater role. Our data also suggest that the lack of both M2 and M3 receptors causes upregulation of noncholinergic excitatory innervation of the gut smooth muscle.
Keywords: M2 receptors, M3 receptors, knockout mice, intestinal smooth muscle, electrical field stimulation, cholinergic contraction, noncholinergic contraction, pertussis toxin
Introduction
Gastrointestinal smooth muscles receive a variety of excitatory and inhibitory inputs from the enteric nervous system. In most species, cholinergic nerves play a crucial role in stimulatory regulation of smooth muscle activity and peristaltic movement. As cholinergic nerves are activated, the transmitter ACh is released from their terminals. ACh then acts on smooth muscle cells to activate cell surface muscarinic receptors, thus activating various intracellular signaling pathways, which lead to a rise in cytosolic Ca2+ concentrations and, eventually, smooth muscle contraction (Caulfield, 1993; Bolton et al., 1999; Unno et al., 2003a). Also, recent evidence indicates the possibility that interstitial cells of Cajal (ICCs), which exist in the myenteric and submucosal regions, are involved as intermediating cells in the neurogenic ACh-induced contractions by intervening between the cholinergic nerves and smooth muscles (Ward et al., 2000; Ward and Sanders, 2001; Hirst and Ward, 2003).
In gastrointestinal smooth muscles, the M2 and M3 muscarinic receptor subtypes are preferentially expressed with the preponderance of the former subtype (Eglen et al., 1996). However, a recent reverse transcriptase–polymerase chain reaction study has reported the possible expression of all five subtypes (M1–M5) in gastric smooth muscles (So et al., 2003). To elucidate the functional roles of each muscarinic receptor subtype, the contractile responses to muscarinic agonists including carbachol have been extensively studied using various muscarinic receptor antagonists. Most, but not all, of the studies indicate that the contractile responses are mediated exclusively by M3 receptors, and that M2 receptors appear non-functional or may act only indirectly (e.g., Parekh and Brading, 1991; Hishinuma et al., 1997; Unno et al., 2003b; also see Eglen et al., 1996). Nonetheless, the recent use of mutant mice lacking certain muscarinic receptor subtypes has revealed that not only the M3 but also M2 receptors may have a direct role in inducing contraction in gastric and ileal smooth muscles (Stengel et al., 2000, 2002; Matsui et al., 2002; Stengel and Cohen, 2003; Unno et al., 2005). It is therefore possible that both M2 and M3 receptors take part in mediating contractions induced by stimulation of cholinergic nerves. However, this issue has not been addressed experimentally so far.
Studies with ileal smooth muscle preparations prepared from M2-knockout (KO) and M3-KO mice (Matsui et al., 2002; Unno et al., 2005) have led to the following conclusions: (1) M2 receptors are less active than M3 receptors in mediating carbachol-induced contractions, (2) the M2 activity considerably varies according to agonist application protocols used for receptor activation, (3) the mechanism via which Ca2+ required for contraction is mobilized differs between M2 and M3 receptors and (4) M3-linked multiple mechanisms for Ca2+ mobilization vary in their contribution depending on different phases of the contractile response and/or different agonist concentrations. The ACh released upon nerve stimulation differs from bath-applied agonists in its action on target receptors, as it is degraded rapidly by cholinesterases and its release is modulated by presynaptic autoinhibition (Bennett, 1997; Nishiwaki et al., 1998; Takeuchi et al., 2005). The roles of M2 and M3 receptors in mediating contractions evoked by ACh released from cholinergic nerve endings therefore remain to be elucidated.
In the present work, we have studied cholinergic contractions evoked by transmural electrical (TE) stimulation in ileal segments from M2-KO, M3-KO and M2/M3-double KO mice and their corresponding wild-type strains. As pharmacological tools, we used the Na+ channel blocker tetrodotoxin, the muscarinic receptor antagonist atropine and pertussis toxin (PTX) that has been shown to abolish M2-mediated contractions in mouse ileum (Unno et al., 2005). We also investigated the effects of disrupting the M2 or M3 receptor genes on noncholinergic excitatory innervation of ileal tissues.
Methods
All procedures described below were performed according to the guidelines approved by the Animal Ethics Committee at the Faculty of Applied Biological Sciences, Gifu University.
Animals and preparations
The generation of M2-KO, M3-KO and M2/M3-double KO mice has been described previously (Gomeza et al., 1999; Yamada et al., 2001; Struckmann et al., 2003). The genetic background of mice used in the present study was 129J1 (50%) × CF1 (50%) for the M2-KO, 129SvEv (50%) × CF1 (50%) for the M3-KO, 129J1 (25%) × 129SvEv (25%) × CF1 (50%) for the M2/M3-double KO mice and their respective corresponding wild types. Animals were housed under the same conditions as described previously (Unno et al., 2005).
Mice of either sex, aged 3–10 months and weighing 25–40 g, were killed by cervical dislocation. The whole intestine was quickly excised and placed in a Petri dish filled with Tyrode solution (NaCl 137 mM, KCl 2.9 mM, CaCl2 1.8 mM, MgCl2 2.1 mM, NaH2PO4 0.4 mM, NaHCO3 11.9 mM, glucose 5.6 mM, pH 7.2), and gut segments of ∼1 cm in length were dissected from the ileal region at a distance greater than 2 cm from the ileocaecal junction. The intestinal content was flushed away by injection of Tyrode solution and adhering tissues cutoff. One end of the segment was closed with a piece of thread, and the other end was fitted over a J-shaped rigid tissue holder (3 mm in tip diameter) by a few millimeters and then firmly tied to it.
Isometric tension recording and TE stimulation
The ileal segment prepared was vertically mounted in an organ bath of 20 ml filled with Tyrode solution aerated and kept at 30°C; the J-shaped holder was appropriately fixed and the piece of thread from the other end of the tissue attached to a transducer. The tissue was subjected to a load of 0.3–0.4 g and incubated for 20 min, followed by further 60-min incubation in fresh Tyrode solution but containing the adrenergic neuron blocker guanethidine (1 μM) and the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 100 μM). Hereafter, these drugs were allowed to be present throughout in the bathing solution so that possible inhibitory effects due to adrenergic nerves or nitric oxide could be minimized. During the second incubation, a 1-min application of hypertonic 70 mM KCl was repeated at intervals of 10–15 min. Changes in tension of the tissue along the longitudinal axis were isometrically recorded with a force-displacement transducer, as described previously (Unno et al., 2005). The tension changes recorded might be somewhat affected by contractions or relaxations of circular smooth muscles concomitantly occurring.
Electrical stimuli, pulse width 0.5 ms and strength 50 V, were generated with a stimulator (SEN-3301, Nihon Kohden, Japan) and delivered using a pair of platinum electrodes, one positioned in the organ bath and the other set previously on the tip of the J-shaped holder. A 5-s TE stimulation was applied at different frequencies of 2–50 Hz. The time interval between successive trials varied from ∼5 to 10 min, as more time was required for the responses at higher stimulus frequencies to subside. As preparations exhibited spontaneous activity throughout the experiments, the mean peak level of the spontaneous contractions ∼2 min before each TE stimulation was taken as a base line for measurement of contractions evoked by TE. TE-induced contraction amplitudes were expressed as percentage of a reproducible 70-mM K+ contraction similarly measured in the same preparation.
PTX treatment
PTX was injected intraperitoneally at a dose of 100 μg kg−1 body weight, and 70–74 h later, ileal segments were prepared from the mice, as described previously (Unno et al., 2005).
Data analysis
Values in the text are given as means±s.e.m. (n=the number of preparations used). Student's unpaired t-test was used to determine the statistical significance of differences between two group means. For statistical comparison between multiple group means, one-way analysis of variance followed by a post hoc Bonferroni test to compare between two of the multiple groups was used (Unno et al., 2003a). Differences were considered statistically significant when P<0.05. Averaged curves representing the relationships between stimulus frequency and contraction size (Figures 4, 5 and 6) were constructed by direct curve fitting using the computer software Delta Graph 4.0 (SPSS Inc., Chicago, IL, USA), providing a stimulus frequency required to produce a half maximum response (50% effective frequency).
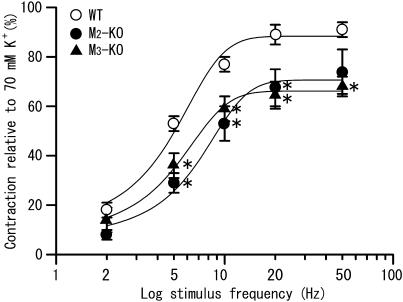
Figure 4.
Relationships between the stimulus frequency and the size of initial fast contractions to TE stimulation in ileal segments from wild-type (open circle), M2-KO (closed circle) and M3-KO mice (closed triangle). The contraction size was expressed as percentage of the 70-mM K+-evoked contraction in the same segment. Each point represents the mean±s.e.m. of measurements in 10–20 tissues. *Significantly different (P<0.05) from the corresponding wild-type value.
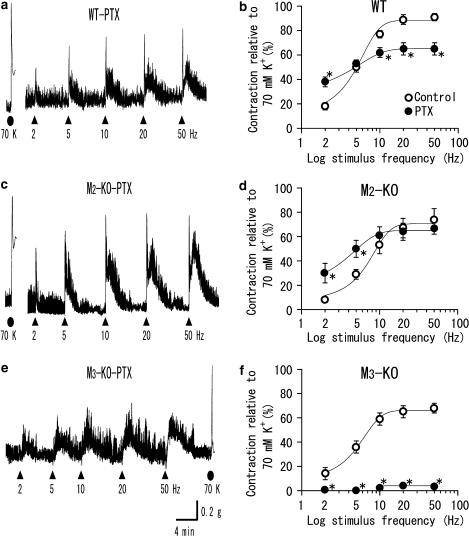
Figure 5.
Contractions to TE stimulation in ileal segments from PTX-treated mice of the wild-type (a), M2-KO (c) and M3-KO strains (e). Note that the initial fast contractions upon TE stimulation are not seen in (c). The later slow contractions are seen in all three traces. (b, d, f) show summary of the effects of PTX treatment on the initial fast contractions to TE stimulation in the wild-type, M2-KO and M3-KO strains, respectively. Each point for PTX treatment (closed circle) represents the mean±s.e.m. (n=11–16 for (a), n=7–10 for (c) and n=6–8 for (e)). The open circles for the control are all taken from Figure 4. *Significantly different from the corresponding control value.
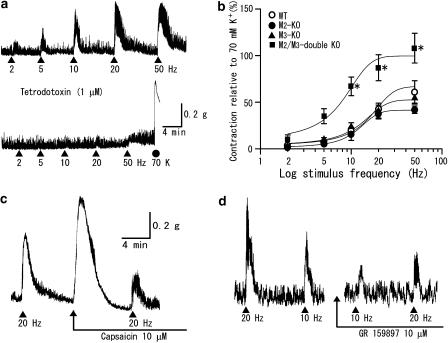
Figure 6.
Noncholinergic contractions to TE stimulation and effects of capsaicin and GR 159897. (a) Atropine-resistant contractions to TE stimulation in an ileal segment from an M2/M3-double KO mouse before and after application of tetrodotoxin (1 μM). (b) Relationships between the stimulus frequency and the size of the atropine-resistant (noncholinergic) contractions relative to a 70-mM K+ contraction. Each point represents the mean±s.e.m. of measurements (n=7–9 for the wild-type, n=4–7 for the M2-KO, n=5–6 for the M3-KO and n=8–17 for the M2/M3-double KO strains). *Significantly different from the corresponding value for any of the three other strains. (c) Ileal contractions to TE stimulation in the M2-KO strain before and after prolonged application of capsaicin (10 μM), which prevents stimulus-dependent release of NKs from sensory nerves. (d) Ileal contractions to TE stimulation in the M2/M3-double KO strain before and 15 min after application of GR159897 (10 μM), an NK2 receptor antagonist. Experiments in (c, d) were carried out in the presence of atropine (2 μM).
K+ (70 mM) or TE stimulation caused similar contraction responses in the three wild-type strains (see above), as described previously for carbachol-induced contractions (Unno et al., 2005). Therefore, data obtained with the three wild-type strains were pooled.
Drugs
PTX, guanethidine and L-NAME were from Sigma (St Louis, MO, USA), atropine and tetrodotoxin from Wako (Osaka, Japan), capsaicin and 5-fluoro-3-[2-[4-methoxy-4-[[(R)-phenylsulphinyl]-1-piperidinyl]ethyl]-1H-indole (GR159897) from Tocris Cookson Inc. (Missouri, USA) and spantide from Peptide Institute Inc. (Osaka, Japan). GR159897 was dissolved in dimethyl sulfoxide (DMSO) and the other compounds in distilled water, to give concentrations more than 100 times higher than the final concentrations in the bathing solution. The final concentration of DMSO was no more than 0.1%, which did not affect spontaneous activity or high-K+ contractions.
Results
Ileal segments prepared from M2-KO, M3-KO, M2/M3-double KO or wild-type mice were bathed in Tyrode solution containing guanethidine (1 μM) and L-NAME (100 μM) (see Methods), and tension changes in their longitudinal direction were recorded. All preparations showed spontaneous contractions and application of 70 mM KCl evoked phasic contractions (Figures 1, 2 and 3). The magnitude of contractions, as measured in grams, amounted to 0.73±0.06 g (n=19) in wild-type mice and 0.89±0.10 g (n=14), 0.63±0.09 g (n=11) and 0.63±0.05 g (n=20) in M2-KO, M3-KO, and M2/M3-KO mice, respectively. These four mean values did not statistically differ from one another.
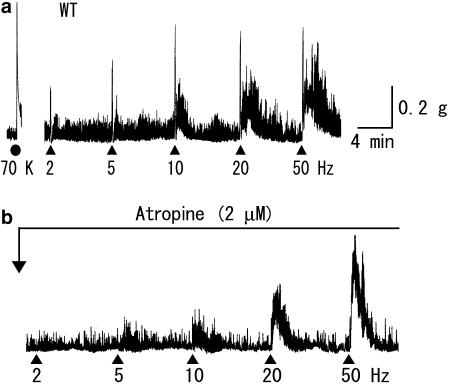
Figure 1.
Contractile responses to TE stimulation of an ileal segment from a wild-type mouse, before (a) and after (b) application of atropine. TE stimulation (50 V in strength, 0.5 ms in pulse duration) was applied for 5 s at the different frequencies and at the different time points, as indicated by closed triangles. Guanethidine (1 μM) and L-NAME (100 μM) were added in the bathing solution during all experiments (as in all other figures). As a standard, a K+-evoked contraction was obtained by the addition of 70 mM KCl (70 K, closed circle). Note that the initial fast contraction to TE stimulation was blocked by atropine (2 μM).
Figure 2.
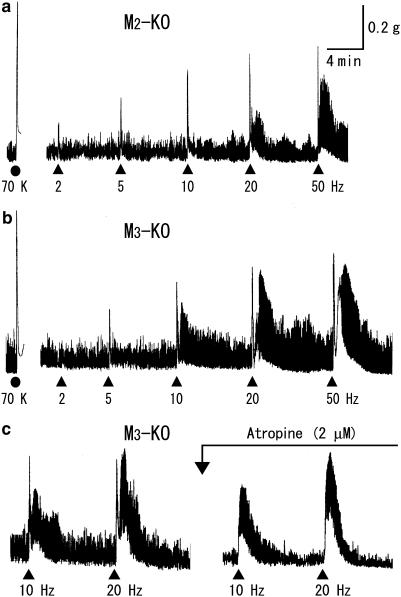
Contractions to TE stimulation in ileal segments from M2-KO (a) and M3-KO mice (b, c). Again, atropine blocked the initial fast contractions to TE stimulation (c). For more details, see the legend to Figure 1.
Figure 3.
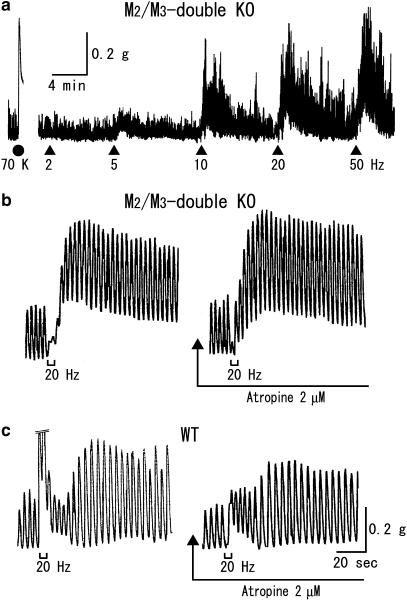
TE stimulation failed to evoke an initial fast contraction in ileal segments from M2/M3-double KO mice (a). (b, c) show the responses to TE stimulation recorded at a fast speed in tissues from M2/M3-double KO and wild-type mice, respectively. In (c), the initial fast contractile response is cutoff at its top by a size corresponding to 0.3 g. See text and the legend to Figure 1 for details.
Cholinergic contractions
TE stimuli, 50 V in strength and 0.5 ms in duration, were applied at an ascending series of frequencies of 2, 5, 10, 20 and 50 Hz (each for 5 s). In wild-type preparations, as shown in Figure 1, TE stimuli initiated a rapid, brief contraction followed by a slower, longer one on which spontaneous contractions were superimposed. Both the initial fast and later slow contractions increased as stimulus frequency was increased, and a maximal response was attained at 10–50 Hz for the initial contraction and at 20 or 50 Hz for the latter. In five out of twenty preparations, no appreciable contractile response was elicited at 2 Hz, but at higher frequencies, an initial fast contraction was invariably observed. Occasionally, a later slow contraction did not follow until the frequency was increased to 10 or 20 Hz. The initial fast contractions appeared immediately after the beginning of TE stimulation, reached a peak within ∼5 s and then rapidly declined. At relatively high frequencies (10 Hz or higher), during the declining phase of the initial contractions, a slow contraction often appeared. The later slow contraction did not only vary in amplitude but also in duration with different stimulus frequencies or among different preparations; at 20 Hz, it reached a peak after 30–90 s and then disappeared within 2–8 min. Tetrodotoxin (1 μM) totally blocked the contractile responses to TE stimulation (data not shown), whereas atropine (2 μM) inhibited only the initial fast contractions (Figure 1). Thus, although the initial fast and the later slow contractions were both neurogenic, only the initial contractions resulted from the activation of cholinergic nerves, involving the activation of muscarinic receptors in ileal smooth muscle by ACh released from these nerves.
Upon TE stimulation, preparations from either M2-KO or M3-KO mice behaved similar to wild-type preparations, as exemplified in Figure 2a and b. Briefly, all preparations responded with an initial fast and a later slow contraction, and only the initial contraction was abolished by atropine (Figure 2c). In preparations from M2/M3-double KO mice, TE stimulation produced frequency-dependent contractile responses, but these did not involve any initial fast contractions (Figure 3a). This was confirmed by faster recording of the responses to TE stimulation. Figure 3b and c show such responses at 20 Hz in M2/M3-double KO and wild-type preparations. It can be seen (left panels) that the M2/M3-double KO preparation did not display an initial fast response, unlike the wild-type response, but instead exhibited an initial inhibition of spontaneous activity. To rule out the possibility that an initial cholinergic contraction was masked by the observed initial inhibition, we examined whether atropine potentiated the inhibitory response by blocking the (potential) masked contraction. However, we found that atropine reduced the initial inhibitory response, while leaving the following contraction almost intact (see Figure 3b). Therefore, no appreciable cholinergic contraction occurred in the absence of both M2 and M3 receptors, strongly suggesting that the cholinergic contractions in M2-KO and M3-KO mice were mediated by M3 and M2 receptors, respectively.
Figure 4 shows averaged relationships between stimulus frequency and cholinergic contraction size. The contraction size, expressed as percentage of the contraction induced by 70 mM K+, was greater in wild-type than in M2-KO or M3-KO mice; differences were statistically significant at all frequencies except 2 Hz. The two KO strains showed similar responses at all frequencies. At 50 Hz, the averaged contraction sizes in the wild type, M2-KO and M3-KO strains were 91±3% (n=20), 74±9% (n=12) and 68±4% (n=11), respectively. The 50% effective frequencies determined by curve fitting of the data points in Figure 4 (see Methods) were 4.5, 6.6 and 4.8 Hz for the three mouse strains.
PTX treatment
Injection of PTX to mice has been shown to prevent the muscarinic agonist carbachol from producing M2-mediated contractions in ileal muscle strips (Unno et al., 2005). We therefore investigated the effect of PTX on cholinergic contractions in ileal segments. Treatment with PTX (100 μg kg−1) did not affect the spontaneous activity and 70-mM K+-induced contractions in any mouse strain. The high-K+-induced contractions in preparations from PTX-treated wild-type, M2-KO and M3-KO mice were 0.66±0.07 g (n=12), 0.68±0.07 g (n=10) and 0.66±0.10 g (n=6), respectively. These values were comparable to the respective control values (0.73±0.06 g for wild type, 0.89±0.10 g for M2-KO and 0.63±0.09 g for M3-KO).
Figure 5 shows typical responses produced by TE stimulation in preparations from PTX-treated wild-type, M2-KO and M3-KO mice. In PTX-treated M3-KO mice, TE stimulation failed to initiate an appreciable fast contraction at any frequency, but did produce slow contractions normally (Figure 5e), indicating that M2-mediated cholinergic contractions were blocked by PTX treatment. In PTX-treated M2-KO or wild-type mice, TE stimulation continued to evoke the initial fast contraction as well as the later slow contraction (Figure 5a and c). In preparations from PTX-treated M2-KO mice, the sizes of the initial fast contractions closely resembled the corresponding control values at 10–50 Hz, but were significantly greater at 2 or 5 Hz (Figure 5d). In preparations from PTX-treated wild-type mice, the contraction sizes were significantly reduced at 10–50 Hz, little altered at 5 Hz, and significantly increased at 2 Hz compared to the corresponding control values (Figure 5b). Consequently, following PTX treatment, the averaged relationships between stimulus frequency and contraction size were very similar for wild-type and M2-KO preparations (cf. the curves with closed circles in Figure 5b and d). The contraction sizes at 50 Hz for the PTX-treated wild-type and M2-KO groups were 65±5% (n=11) and 67±5% (n=7), respectively. The 50% effective frequencies were ∼1 and 2 Hz, respectively.
Noncholinergic contractions
As mentioned earlier, TE stimulation evoked atropine-resistant contractile responses in ileal segments from all mouse strains studied. The contractile responses were completely blocked by tetrodotoxin (1 μM) (Figure 6a), indicating that they resulted from the activation of noncholinergic nerves. To characterize the noncholinergic contractions, we carried out TE stimulation experiments in the presence of atropine (2 μM).
Averaged relationships between stimulus frequency and noncholinergic contraction size for the individual mouse strains are shown in Figure 6b. Contraction sizes, normalized by 70-mM K+-induced contractions, increased as the stimulus frequency was increased. Responses were greater in M2/M3-double KO than in wild-type, M2-KO and M3-KO mice; differences were statistically significant at 10 Hz and higher frequencies. The latter three strains showed similar responses at all frequencies. The mean contraction size at 50 Hz in the M2/M3-double KO strain (108±16%, n=16) was about twice as great as the corresponding values for the wild-type (67±17%, n=6), M2-KO (42±4%, n=5) and M3-KO strains (54±6%, n=6). The 50% effective frequencies estimated from the data in Figure 6b were 7.7 Hz for the M2/M3-double KO mice and 15.7, 12.5 and 11.8 Hz for the wild-type, M2-KO and M3-KO strains, respectively. The latter three values were greater than the corresponding frequencies for cholinergic contractions (4.5, 6.6 and 4.8 Hz).
The noncholinergic contractions in all mouse strains studied were insensitive to PTX. Their amplitudes at 20 Hz in PTX-treated M2/M3-double KO mice (101±15%, n=4) did not significantly differ from the corresponding control value (87±14%; see 20 Hz in Figure 6b), and neither did the responses at 20 Hz in PTX-treated wild-type preparations (48±9%, n=4) differ from the control value (41±11%; Figure 6b).
Tachykinin (NK)-releasing nerves are known to be involved in the generation of neurogenic atropine-resistant contractions in various gut smooth muscles including the mouse ileal circular muscle (De Schepper et al., 2005). We therefore wanted to examine whether TE stimulation-evoked noncholinergic contractions in ileal segments involved the activation of tachykininergic nerves. Experiments were carried out in the presence of atropine (2 μM), unless otherwise stated. Data were pooled without distinction of the mouse strain, because atropine was continued to be present throughout the experiments and there was no notable difference in data obtained among the different strains used. As a tool, we used capsaicin, desensitization to which is known to block the effect of a subsequent stimulus in releasing NKs from enteric sensory nerves (Maggi, 2000; Barthó et al., 2004). Application of capsaicin (10 μM) to wild-type or M2-KO preparations produced a contraction, and 8–12 min later, the contractile response disappeared owing to desensitization (Figure 6c). Under these conditions, TE stimulation evoked a reduced contraction response (by 47±15% (n=3) at 10 Hz and by 43±7% (n=4) at 20 Hz), compared with the respective control responses. When similar experiments were carried out in the absence of atropine, desensitization to capsaicin did not affect the initial fast contraction but did reduce the following slow contraction in wild-type preparations (n=2, data not shown).
We carried out additional studies with NK receptor antagonists in atropine (2 μM)-treated, M2/M3-double KO preparations. The NK1 receptor-preferring antagonist spantide (1 or 5 μM; Beaujouan et al., 1993) failed to reduce the contractions to TE stimulation at 10 or 20 Hz but rather increased contraction amplitudes by 10–40% (n=4). In contrast, the NK2 receptor-preferring antagonist GR159897 (10 μM; Beresford et al., 1995) significantly reduced the contraction sizes at 10, 20 and 50 Hz by 47±8% (n=4), 42±6% (n=6) and 45±10 (n=3), respectively (Figure 6d). The percent reduction in contraction amplitudes at 20 and 50 Hz in M2/M3-double KO preparations was similar to that in wild-type preparations (44±12% at 20 Hz and 47±5% at 50 Hz, n=4 each), suggesting that the proportion of NK2 antagonist-sensitive component to the whole noncholinergic contraction was similar between the two mouse strains. The different effects of spantide and GR159897 on the noncholinergic contractions would be consistent with the view that NK1 receptors are located mainly on myenteric nerves, whereas NK2 receptors are largely confined to smooth muscle cells (Grady et al., 1996; Portbury et al., 1996a, 1996b).
Discussion
In the present study, we examined the functional roles of M2 and M3 muscarinic receptors in ileal smooth muscle contractions produced by cholinergic nerve stimulation, using M2-KO, M3-KO or M2/M3-double KO mice as tools. Previous studies using these mutant mice revealed that the M2 and M3 receptors, but not any other muscarinic receptor subtypes, participate in mediating the contractions to exogenously applied agonists such as carbachol in gastrointestinal smooth muscles and that the contractile responses in M2-KO and M3-KO mice are mediated by M3 and M2 receptors, respectively (Stengel et al., 2000, 2002; Matsui et al., 2002; Stengel and Cohen, 2003; Unno et al., 2005). These concepts also hold true for the cholinergic nerve-induced contractions in mouse ileum.
M2 and M3 receptor activities in inducing contraction
Although activation of M2 receptors by exogenously applied agonists can directly induce contractions of gut smooth muscle, this activity is generally thought to be considerably less pronounced compared with that of M3 receptors (Matsui et al., 2002; Unno et al., 2005). However, M2 receptors were highly efficacious in mediating smooth muscle contractions when ileal M2 receptors were stimulated by endogenous ACh released upon nerve stimulation (present study; Figure 4). It is unlikely that this high activity of M2 receptors in M3-KO mice is caused by compensatory overexpression of M2 receptors, as disruption of one muscarinic receptor gene does not seem to have major effects on the expression levels of the remaining receptor subtypes (Gomeza et al., 1999; Yamada et al., 2001; Wess, 2004). Moreover, the amount of ACh released from ileal enteric neurons by TE stimulation does not significantly differ among M2-KO, M3-KO and wild-type mice (Takeuchi et al., 2005). Thus, M2 receptors seem to be equipotent to M3 receptors in triggering ACh-mediated neurogenic ileal contractions, especially in the absence of M3 receptors (see next section).
Ileal muscle contractions to carbachol in M3-KO mice are blocked by PTX treatment, whereas those in M2-KO mice are PTX-resistant, suggesting that M2 and M3 receptors induce smooth muscle contractions via activation of G proteins of the Gi and Gq family, respectively (Unno et al., 2005). The present results obtained from experiments with PTX-treated M2-KO or M3-KO mice (Figure 5) strongly support this view. The reason why PTX treatment increased cholinergic contractions at 2 and 5 Hz in M2-KO mice remains unclear at present. One possible explanation is that ACh release is inhibited by activation of various receptors present on cholinergic nerve terminals including M2 and M4 muscarinic receptors and opioid receptors (Nishiwaki et al., 1998; Wess, 2004; Takeuchi et al., 2005). As these receptors are preferentially coupled to Gi family G proteins, the release of ACh upon TE stimulation is expected to be increased by PTX treatment, resulting in increased cholinergic contractions.
Cholinergic contractions in wild-type tissues
Administration of PTX to wild-type mice showed that the cholinergic contractions in ileal muscle are mediated by both M2 and M3 receptors. In our previous study, contractions evoked by exogenously applied carbachol in ileal muscle strips from wild-type mice were significantly reduced by PTX treatment and the reduction was much greater when the agonist concentration was lower (Unno et al., 2005). Therefore, it could be expected that cholinergic contractions evoked by TE stimulation at lower stimulus frequencies causing the release of a small amount of ACh might be significantly reduced by PTX. However, PTX treatment failed to reduce cholinergic contractions at 2 and 5 Hz (Figure 5b). One possible explanation for this phenomenon is that the loss of M2 receptor signaling caused by PTX treatment was overcome or balanced by increased M3 receptor signaling (neurogenic contractions), probably owing to enhanced ACh release following the inactivation of release-inhibitory Gi proteins.
The results obtained with ileal preparations from M2-KO and M3-KO mice suggest that M2 and M3 receptors are about equipotent in inducing cholinergic contractions. In contrast, M2 receptors appear to be clearly less active than M3 receptors in mediating cholinergic contractions in wild-type tissues. Indeed, the proportion of M2-mediated (PTX-sensitive) component to the cholinergic contractions at 10–50 Hz ranged from 20 to 30% (the remaining 70–80% represents the M3-mediated component; Figure 5b). One possible explanation for this observation is that M2 receptor activity is somehow reduced when M2 and M3 receptors are activated simultaneously. M2 receptor-induced contractions of the ileal muscle are proposed to depend largely on Ca2+ entry into the cell associated with accelerated action potential discharges (Unno et al., 2005). On the other hand, it has been suggested that M3 receptor activation leads to intracellular Ca2+ mobilization through multiple mechanisms including voltage-dependent Ca2+ entry, voltage-independent Ca2+ entry and intracellular Ca2+ release, and that the Ca2+ mobilized in these ways not only activates the contractile proteins but also acts in parallel to inactivate Ca2+ channels responsible for the discharge of action potentials (Unno et al., 2005). There is also evidence that M3 receptor activation causes inactivation of Ca2+ channels through mechanisms independent of Ca2+ mobilization (Unno et al., 1995). Therefore, simultaneous activation of M2 and M3 receptors may lead to reduced M2 receptor-linked Ca2+ mobilization, which relies mainly on action potential discharge, as compared to M2 receptor signaling in the absence of M3 receptors.
Recent evidence suggests that gastrointestinal ICCs form synaptic-like structures with enteric motor neurons and gap junctions with smooth muscle cells and that ICCs act as intermediating cells for transducing cholinergic excitatory inputs to smooth muscle cells in various, but not all, regions of the gastrointestinal tract (Ward and Sanders, 2001; Hirst and Ward, 2003). The ICCs seems to express both M2 and M3 receptors, as judged from detection of mRNAs coding for these subtypes (Epperson et al., 2000). If ICCs participate in the cholinergic contractions observed in M2-KO or M3-KO mice as well as wild-type strains, further studies are needed to elucidate the roles of ICC M2 and M3 receptors in cholinergic neuro-muscular transmission.
Noncholinergic excitatory innervation
Apart from cholinergic contractions, TE stimulation evoked neurogenic, noncholinergic contractions in ileal segments, irrespective of the mouse strain studied. The results obtained with capsaicin and GR159897 (Figure 6c and d) suggest that the noncholinergic contractions are caused by NKs and other neurotransmitters, as described for similar contractions in mouse ileal circular muscle (De Schepper et al., 2005). It is of interest that the noncholinergic contractions were most prominent in the M2/M3-double KO strain (Figure 6b). This finding, combined with the observation that the other three strains (wild-type, M2-KO and M3-KO) showed quantitatively similar noncholinergic contractions, suggests that the lack of both M2 and M3 receptors may cause a functional upregulation of noncholinergic excitatory neurotransmission, as previously suggested by Matsui et al. (2002). This functional upregulation seems to involve both tachykininergic and non-tachykininergic nerves, as the extents to which the noncholinergic contractions were reduced by GR159897 were similar in wild-type and M2/M3-double KO mice. Further studies are needed to determine whether this upregulation involves neuronal or postsynaptic mechanisms (or both).
In conclusion, the present results demonstrate that M2 and M3 muscarinic receptors are entirely responsible for mediating neurogenic cholinergic contractions in mouse ileum, with M3 receptors assuming a greater role in these contractions in wild-type tissues. Nonetheless, M2 receptors can exert a potent activity in triggering neurogenic cholinergic contractions in the absence of M3 receptors. Our recent study on single ileal muscle cells from wild-type mice has shown that under voltage-clamp conditions, carbachol initiates inward cationic currents through two distinct mechanisms, which differ in their dependence on Ca2+-store release (Sakamoto et al., 2006). Studies of the role of M2 and M3 receptors in mediating these currents and of the molecular mechanism underlying current generation would be useful to elucidate a possible interaction between M2 and M3 receptors in mediating cholinergic contractions.
Acknowledgments
This work was supported by a Grant-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 16380199 and 17580253).
Abbreviations
- ICCs
interstitial cells of Cajal
- KO mice
knockout mice
- L-NAME
Nω-nitro-L-arginine methyl ester
- NK receptor
tachykinin receptor
- PTX
pertussis toxin
- TE stimulation
transmural electrical stimulation
Conflict of interest
The authors state no conflict of interest.
References
- Barthó L, Benkó R, Patacchini R, Pethö G, Holzer-Petsche U, Holzer P, et al. Effects of capsaicin on visceral smooth muscle: a valuable tool for sensory neurotransmitter identification. Eur J Pharmacol. 2004;500:143–157. doi: 10.1016/j.ejphar.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Beaujouan JC, Heuillet E, Petitet F, Saffroy M, Torrens Y, Glowinski J. Higher potency of RP 67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br J Pharmacol. 1993;108:793–800. doi: 10.1111/j.1476-5381.1993.tb12880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Beresford IJ, Sheldrick RL, Ball DI, Turpin MP, Walsh DM, Hawcock AB, et al. GR159897, a potent non-peptide antagonist at tachykinin NK2 receptors. Eur J Pharmacol. 1995;272:241–248. doi: 10.1016/0014-2999(94)00655-q. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors – characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Winter BY, Seerden TC, Herman AG, Pelckmans PA, De Man JG. Functional characterization of tachykinin receptors in the circular muscle layer of the mouse ileum. Regul Peptide. 2005;130:105–115. doi: 10.1016/j.regpep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, et al. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, et al. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in rat gastrointestinal tract. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma S, Hongo I, Matsumoto Y, Narita F, Kurokawa M. Contracting effects of carbachol, McN-A-343 and AHR-602 on Ca2+-mobilization and Ca2+-influx pathways in taenia caeci. Br J Pharmacol. 1997;122:985–992. doi: 10.1038/sj.bjp.0701467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA. Principles of tachykininergic co-transmission in the peripheral and enteric nervous system. Regul Peptide. 2000;93:53–64. doi: 10.1016/s0167-0115(00)00177-4. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, et al. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki H, Saitoh N, Nishio H, Takeuchi T, Hata F. Relationship between inhibitory effect of endogenous opioid via mu-receptors and muscarinic autoinhibition in acetylcholine release from myenteric plexus of guinea pig ileum. Jpn J Pharmacol. 1998;77:279–286. doi: 10.1254/jjp.77.279. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Brading AF. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1991;104:412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portbury AL, Furness JB, Southwell BR, Wong H, Walsh JH, Bunnett NW. Distribution of neurokinin-2 receptors in the guinea-pig gastrointestinal tract. Cell Tissue Res. 1996a;286:281–292. doi: 10.1007/s004410050698. [DOI] [PubMed] [Google Scholar]
- Portbury AL, Furness JB, Young HM, Southwell BR, Vigna SR. Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J Comp Neurol. 1996b;367:342–351. doi: 10.1002/(SICI)1096-9861(19960408)367:3<342::AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Unno T, Matsuyama M, Uchida M, Hattori M, Nishimura M, et al. Characterization of muscarinic receptor-mediated cationic currents in longitudinal smooth muscle cells of mouse small intestine. J Pharmacol Sci. 2006;100:215–226. doi: 10.1254/jphs.fp0050973. [DOI] [PubMed] [Google Scholar]
- So I, Yang DK, Kim HJ, Min KW, Kang TM, Kim SJ, et al. Five subtypes of muscarinic receptors are expressed in gastric smooth muscles of guinea pig. Exp Mol Med. 2003;35:46–52. doi: 10.1038/emm.2003.7. [DOI] [PubMed] [Google Scholar]
- Stengel PW, Cohen ML. M1 receptor-mediated nitric oxide-dependent relaxation unmasked in stomach fundus from M3 receptor knockout mice. J Pharmacol Exp Ther. 2003;304:675–682. doi: 10.1124/jpet.102.042283. [DOI] [PubMed] [Google Scholar]
- Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- Stengel PW, Yamada M, Wess J, Cohen ML. M3 receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder and trachea in vitro. Am J Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, et al. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Fujinami K, Goto H, Fujita A, Taketo MM, Manabe T, et al. Roles of M2 and M4 muscarinic receptors in regulating acetylcholine release from myenteric neurons of mouse ileum. J Neurophysiol. 2005;93:2841–2848. doi: 10.1152/jn.00986.2004. [DOI] [PubMed] [Google Scholar]
- Unno T, Komori S, Ohashi H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J Physiol. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Kwon SC, Okamoto H, Irie Y, Katoh Y, Matsuyama H, et al. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br J Pharmacol. 2003a;139:337–350. doi: 10.1038/sj.bjp.0705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Matsuyama H, Komori S. Muscarinic signal transduction in gastrointestinal smooth muscle. Recent Res Dev Physiol. 2003b;1:577–597. [Google Scholar]
- Unno T, Matsuyama H, Sakamoto T, Uchiyama M, Izumi Y, Okamoto H, et al. M2 and M3 muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol. 2005;146:98–108. doi: 10.1038/sj.bjp.0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Beckett EAH, Wang XY, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]