Abstract
Background and purpose:
This study was carried out to elucidate which α2-adrenoceptor subtypes mediated the inhibition of noradrenaline and adrenaline release from the adrenal medulla of mice.
Experimental approach:
Isolated adrenal medullae from wild-type and α2A, α2B and α2C-adrenoceptor knockout (KO) mice were placed in superfusion chambers. Catecholamine overflow was evoked by 1,1-dimethyl-4-phenylpiperazinium (500 μM) in absence or in presence of the α2-adrenoceptor agonist medetomidine. The effect of medetomidine was tested in presence of the α-adrenoceptor antagonists rauwolscine, WB 4101, spiroxatrine, phentolamine and prazosin.
Key results:
In wild-type mice, medetomidine reduced noradrenaline and adrenaline overflow in a concentration-dependent manner (EC50 in nM: 1.54 and 1.92; Emax in % of inhibition: 91 and 94, for noradrenaline and adrenaline, respectively). The pK D values of the antagonists for noradrenaline overflow did not correlate with pKD values at α2A, α2B, or α2C binding sites. The pK D values of the antagonists for adrenaline overflow correlated positively with pKD values at α2C binding sites (opossum kidney cells). The effect of medetomidine (100 nM) on noradrenaline overflow was significantly reduced in all three α2KO mice (57, 54, 44 % inhibition, for α2A, α2B, and α2C, respectively), whereas the effect of medetomidine on adrenaline overflow was greatly reduced in α2CKO mice (14 % inhibition).
Conclusions and implications:
In the adrenal medulla of mice, all three α2-adrenoceptor subtypes (α2A, α2B, and α2C) play an equal role in the inhibition of noradrenaline overflow, whereas the α2C-adrenoceptor is the predominant α2-adrenoceptor subtype involved in the inhibitory mechanism controlling adrenaline overflow.
Keywords: adrenal medulla, adrenaline, noradrenaline, α2-adrenoceptors, knockout mice, medetomidine
Introduction
In terms of physiology and pharmacology, three main divisions of the adrenergic system should be considered: adrenergic neurons in the central nervous system, sympathetic neurons that innervate peripheral organs and tissues and chromaffin cells from the adrenal medulla (Hoffman and Taylor, 2001). Chromaffin cells are in effect modified post-ganglionic sympathetic neurons of the autonomic nervous system that have lost axons, dendrites and other neuron-specific traits. Chromaffin cells synthesize, store and secrete the endogenous catecholamines, noradrenaline and adrenaline, that upon stimulation are directly released into the bloodstream through regulated exocytosis (Young and Landsberg, 1998).
The adrenal medulla is composed principally of groups of adrenergic and noradrenergic chromaffin cells, with minor populations of small intensely fluorescent cells and ganglionic neurones. Different molecular stimuli evoke distinct secretory events in the gland, involving the release of either adrenaline or noradrenaline together with various active neuropeptides (Aunis and Langley, 1999). In these groups, regulation of catecholamine biosynthesis, composition of secretory granules and secretory activity are controlled by a particular pattern of cell surface receptor expression and by separate innervation. This heterogeneous morphology allows chromaffin cells to respond to physiological and pathophysiological molecular stimuli by evoking distinct secretory events, releasing either noradrenaline or adrenaline (Goldstein et al., 2003). Catecholamine exocytosis from chromaffin cells must be strictly regulated to avoid excessive release that could be harmful or even fatal. In view of this, negative feedback control systems that regulate the amount and nature of catecholamine synthesis and release are essential for chromaffin cell function in vivo.
Neuroendocrine chromaffin cells of the adrenal medulla share a common neural crest precursor with post-ganglionic sympathetic neurons (Anderson and Axel, 1986). In sympathetic neurons, α2-adrenoceptors mediate an inhibitory feedback mechanism for noradrenaline release (Starke, 2001). Although all three α2-adrenoceptor subtypes, α2A, α2B and α2C, may contribute to the presynaptic control of catecholamine release from adrenergic nerves in the central and sympathetic nervous system (Trendelenburg et al., 2003), the physiologically predominant subtype controlling noradrenaline release is the α2A adrenoceptor (Altman et al., 1999; Hein et al., 1999). Until recently it was unclear if catecholamine release from chromaffin cells of the adrenal medulla was subject to a similar mechanism of feedback regulation (Starke, 1987). Studies on isolated adrenal chromaffin or pheochromocytoma cells yielded conflicting results (Powis and Baker, 1986; Gollasch et al., 1991; Kleppisch et al., 1992). However, studies with cultured chromaffin cells obtained from the adrenal medulla of mice lacking α2-adrenoceptor subtypes revealed that α2-adrenoceptors control adrenaline release by a negative feedback loop similar to the one present in sympathetic neurons (Brede et al., 2003). If a similar mechanism is indeed responsible for the inhibition of catecholamine release from the adrenal medulla in vivo, predominant expression of one of the α2-adrenoceptor subtypes over the other could be physiologically relevant. Furthermore, the difference in the groups of chromaffin cells (noradrenergic and adrenergic) could represent different α2-adrenoceptor subtypes controlling noradrenaline and adrenaline release. Therefore we decided to characterize pharmacologically the α2-adrenoceptor subtype involved in the regulation of noradrenaline and adrenaline release from adrenal medulla of mice. For this purpose, we investigated the inhibition of noradrenaline and adrenaline release from the adrenal medulla by activation of α2-adrenoceptors in wild-type mice and in mice with genetic deletion of individual α2-adrenoceptor subtypes (knockout strains).
Materials and methods
Animals
Experiments were carried out in male wild-type (C57BL/6) and α2A, α2B and α2C knockout mice aged 4–6 months. The generation of the mouse lines lacking α2-adrenoceptor subtypes has been described previously (Link et al., 1996, 1995; Altman et al., 1999). Animals were anaesthetized with sodium pentobarbital (60 mg/kg, i.p.). In the experiments carried out to evaluate the release of catecholamine from the adrenal medulla we proceeded as follows. The right and left adrenal glands were rapidly removed through an abdominal midline incision and immediately placed in a modified Krebs–Henseleit solution (Guimaraes and Osswald, 1969) of the following composition (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11, ascorbic acid 0.57, disodium ethylenediaminetetraacetic acid 0.03, oxygenated with a mixture of 95% O2 and 5% CO2 in the presence of a monoamine oxidase inhibitor (pargyline, 100 μM) and a catechol-O-methyltransferase inhibitor (tolcapone, 1 μM). In experiments carried out to evaluate enzyme activity and catecholamine levels in the adrenal medulla, the right and left adrenal gland were rapidly removed through an abdominal midline incision: the right gland was placed in a vial containing 1 ml of perchloric acid (0.2 M) for the catecholamine assay and the left gland was immediately frozen at −80°C for evaluation of enzyme activity.
Noradrenaline and adrenaline release
The adrenal medullae were isolated from the gland and then placed in superfusion chambers, one per chamber, where they were superfused with Krebs–Henseleit solution at a rate of 0.5 ml/min, at 37°C. After a 90-min period of stabilization, successive 5 min samples of the superfusate were collected into tubes containing 0.3 ml of perchloric acid (2 M), from t=90 min to t=150 min (t=0 min being the start of superfusion). At the end of the experiments, the adrenal medullae were placed in 1 ml of perchloric acid (0.2 M) and catecholamines determined in superfusates and tissues. Spontaneous outflow of noradrenaline and adrenaline was measured in the presence and in the absence of the α2-adrenoceptor agonist clonidine (30 μM).
A concentration–response curve for the effect of the nicotinic receptor agonist 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) on catecholamine release was determined by addition of either 30, 100, 300, 500, 1000 or 3000 μM in a single 5-min period delivered at t=125 min. In the subsequent experiments nicotinic stimulation consisted of a single concentration of DMPP (500 μM) delivered at t=125 min. Concentration–response curves for the inhibitory effect of the α2-adrenoceptor agonist medetomidine on catecholamine release were determined by addition of either 0.1, 0.3, 1, 3, 10, 30, 100 or 300 nM 15 min before nicotinic stimulation (from t=110 until the end of nicotinic stimulation). When used, the α-adrenoceptor antagonists rauwolscine, phentolamine, spiroxatrine, prazosin or WB4101, were present from t=90 min until the end of nicotinic stimulation. The spontaneous outflow of noradrenaline and adrenaline was calculated as a fraction of the noradrenaline or adrenaline content of the tissue at the onset of the respective collection period (fractional rate; min−1). The overflow elicited by nicotinic stimulation was calculated as the difference ‘total noradrenaline or adrenaline outflow during and after stimulation' minus ‘basal outflow', and was then expressed as a percentage of the noradrenaline or adrenaline content of the tissue at the onset of stimulation (Trendelenburg et al., 1997). Overflow ratios obtained after addition of a drug were also calculated as a percentage of the corresponding ratio in controls in which no drug was added. Effects of drugs on spontaneous outflow of noradrenaline or adrenaline were evaluated similarly.
Catecholamine assay
The assay of the catecholamines (noradrenaline, adrenaline and dopamine) and L-3,4-dihydroxyphenylanine (L-DOPA), in plasma, tissues and superfusate samples was performed by high performance liquid chromatography with electrochemical detection (HPLC-ED) as described previously (Soares-da-Silva et al., 1995). In brief, aliquots of 500 μl of the superfusate samples or 50 μl of the perchloric acid extract of tissues were placed in 5 ml conical base glass vials containing 50 mg of alumina, and the pH of the samples was adjusted to 8.6 by addition of Tris buffer. 3,4-Dihydroxybenzylamine hydrobromide was used as internal standard. The adsorbed catecholamines were then eluted from the alumina with 200 μl of 0.2 M perchloric acid on Costar Spin-X microfilter tubes; 50 μl of the eluate was injected into an HPLC-ED system (Gilson Model 141, Gilson Medical Electronics, Villiers, Le Bel, France). The lower limit of detection of catecholamines and L-DOPA ranged from 350 to 1000 fmol.
Tyrosine hydroxylase activity
Tyrosine hydroxylase activity was measured as described previously (Moura et al., 2005). In brief, the left adrenal gland was homogenized in 500 μl of a 0.25 M sucrose solution using a glass homogenizer (Heidolph); an aliquot of 40 μl was used for each tyrosine hydroxylase assay. Protein concentration was measured as described previously (Bradford, 1976). The reaction mixture for the tyrosine hydroxylase assay contained, sodium acetate buffer (0.1 M, pH=6.0), 6-methyl-5,6,7,8-tetrahydropterine (6-BH4) in a solution of 2-mercaptoethanol (1 M) and ferrous sulphate heptahydrate (0.50 mM). In the standard assay, 10 min incubation at 37°C was performed either with a saturating concentration of the substrate (L-tyrosine, 1000 μM) and varying concentrations of the cofactor (6-BH4, 10–1000 μM), or with a saturating concentration of the cofactor (6-BH4, 1000 μM) and varying concentrations of substrate (L-tyrosine, 10–1000 μM). The reaction was stopped with 300 μl of perchloric acid (0.5 M). In the blank incubation, 3-iodo-L-tyrosine (100 μM), a tyrosine hydroxylase inhibitor, was present and L-tyrosine was replaced by D-tyrosine (10–1000 μM). The L-DOPA formed in the reaction was measured by HPLC-ED. Tyrosine hydroxylase activity was expressed as the amount of L-DOPA formed per milligram protein per hour.
Statistics
Concentration–response curves for the nicotinic agonist DMPP and the α2-adrenoceptor agonist medetomidine were evaluated by sigmoid curve fitting (Trendelenburg et al., 1997). The calculation yielded the agonist EC50 and Emax values. Antagonist pKD values were calculated from the antagonist-induced EC50 increase (equation 4 of (Furchgott, 1972)); it should be noticed that the values are apparent pKD values because the competitive character of the antagonist was not verified. Results are expressed as arithmetic mean±s.e.m. The significance of differences between means was evaluated using Student's t-test or two-way analysis of variance. Values were considered statistically different when P<0.05. n is the number of adrenal medullae used.
Drugs
L-DOPA, dopamine hydrochloride, (−)-adrenaline (+)-bitartrate salt bitartrate, L-(−)-noradrenaline (+)-bitartrate salt monohydrate, 3,4-dihydroxybenzylamine hydrobromide, L-tyrosine, D-tyrosine, 3-iodo-L-tyrosine, ferrous sulphate heptahydrate, 6-BH4 dihydrochloride, phentolamine HCl and DMPP were from Sigma Chemical Company (St Louis, MO, USA). Medetomidine HCl, (±)-2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane HCl (WB 4101), prazosin HCl, rauwolscine HCl and spiroxatrine were from Tocris (Ellisville, MO63021, USA). All compounds were dissolved in water except spiroxatrine (0.1 M HCl).
Results
Spontaneous outflow of catecholamines in wild-type mice
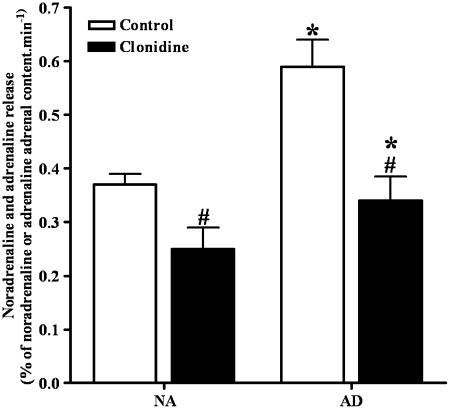
In the adrenal medulla of wild-type mice, the spontaneous outflow of noradrenaline was about 30% lower than that of adrenaline. The α2-adrenoceptor agonist clonidine (30 μM) produced a significant reduction in the spontaneous outflow of noradrenaline (25% reduction) and adrenaline (37% reduction) (Figure 1).
Figure 1.
Effect of clonidine (30 μM) on the spontaneous outflow of noradrenaline (NA) and adrenaline (AD) from the adrenal medulla of wild-type mice. Clonidine was present from the beginning of superfusion. Results are presented as percentage of NA or AD adrenal content per minute. Columns and vertical lines represent mean±s.e.m. of six assays. *Significantly different from the values of the spontaneous outflow of NA; P<0.05. #Significantly different from corresponding control values P<0.05.
Effect of DMPP on catecholamine release in wild-type mice
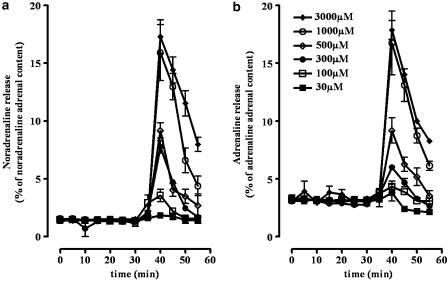
The nicotinic receptor agonist DMPP increased the outflow of noradrenaline and adrenaline in a concentration-dependent manner, with EC50 values (in μM) of 578±8 and 555±9 and with Emax values (in % of adrenal noradrenaline or adrenaline content) of 17.5±0.17 and 18.4±0.19, for noradrenaline and adrenaline, respectively (Figure 2). Based on these results and on previous studies (Borges, 1997), 500 μM of DMPP was established as the concentration to be used to stimulate noradrenaline and adrenaline overflow in the subsequent experiments.
Figure 2.
Effect of DMPP (30–3000 μM) on the spontaneous outflow of noradrenaline (a) and adrenaline (b) from the adrenal medulla of wild-type mice. Results are presented as percentage of noradrenaline or adrenaline adrenal content. Symbols and vertical lines represent mean±s.e.m. (n=5).
Effect of medetomidine on catecholamine release in wild-type mice
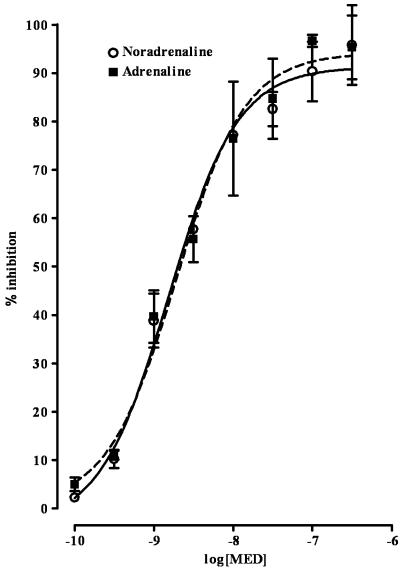
The α2-adrenoceptor agonist medetomidine reduced, in a concentration-dependent manner, the DMPP-evoked overflow of noradrenaline and adrenaline. The EC50 values (in nM) were 1.5±0.06 and 1.9±0.06 and the Emax values (in percentage of inhibition) were 91.3±0.89 and 94.1±1.06, for noradrenaline and adrenaline, respectively (Figure 3).
Figure 3.
Effect of medetomidine (MED) on DMPP-evoked overflow of noradrenaline and adrenaline from the adrenal medulla of wild-type mice. Concentration–inhibition curves were obtained by adding MED (0.1–300 nM) 15 min before DMPP (500 μM). Results are presented as percentage inhibition. Symbols and vertical lines represent mean±s.e.m. (n=5).
Effect of α-adrenoceptor antagonists on catecholamine release in wild-type mice
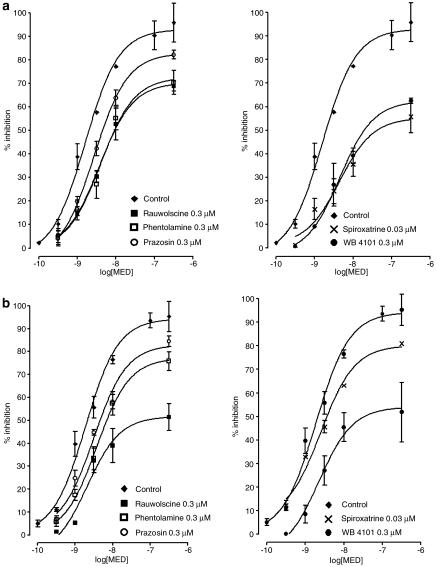
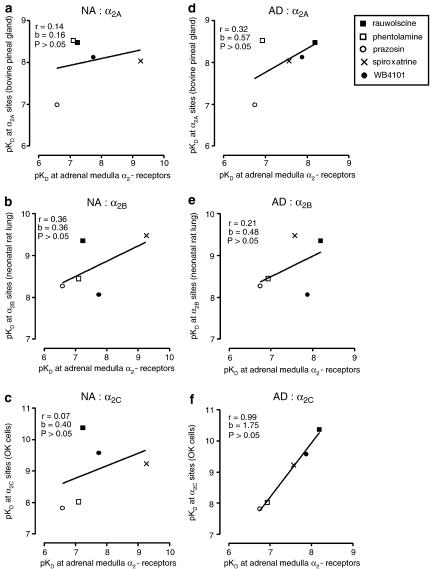
In the adrenal medulla of wild-type mice, the five α-adrenoceptor antagonists shifted the concentration–inhibition curve of medetomidine to the right. Results with rauwolscine (0.3 μM), phentolamine (0.3 μM), prazosin (0.3 μM), spiroxatrine (0.03 μM) and WB 4101 (0.3 μM) are shown in Figure 4. For the evoked overflow of noradrenaline, the pKD values of rauwolscine, phentolamine, prazosin, spiroxatrine and WB 4101, were: 7.24, 7.11, 6.59, 9.26 and 7.75, respectively. In respect to the evoked overflow of adrenaline, the pKD values of rauwolscine, phentolamine, prazosin, spiroxatrine and WB 4101, were: 8.19, 6.94, 6.75, 7.57 and 7.88, respectively. These findings support an α2-adrenoceptor mediated mechanism. To identify the subtype of α2-receptor we correlated our receptor pKD values with published pKD values for the five antagonists at prototypical α2A, α2B and α2C radioligand binding sites (Blaxall et al., 1991; Simonneaux et al., 1991). The α2A binding sites were those of the bovine pineal gland; the α2B binding sites were those of the neonatal rat lung and the α2C sites were those of opossum kidney cells. The correlations are shown in Figure 5. The autoreceptor pKD values for noradrenaline release did not correlate (P>0.05) with pKD values at α2A, α2B or α2C binding sites. The autoreceptor pKD values for adrenaline release did not correlate with pKD values at α2A or α2B binding sites, but correlated (P<0.05) with the pKD values at the α2C binding sites in opossum kidney cells. The antagonists per se had no effect on noradrenaline or adrenaline outflow (data not shown).
Figure 4.
Concentration–inhibition curves of medetomidine, in the absence (control) and in the presence of α-antagonists, for the DMPP-evoked overflow of noradrenaline (a) and adrenaline (b) from the adrenal medulla of wild-type mice. Results are presented as percentage inhibition. Symbols and vertical lines represent mean±s.e.m. (n=6).
Figure 5.
Correlation between (abscissae) pKD values of antagonists at α2-autoreceptors in the adrenal medulla of wild-type mice, and (ordinates) pKD values at α2 binding sites in bovine pineal gland (a, d), neonatal rat lung (b, e) and opossum kidney (OK) cells (c, f), for noradrenaline (NA) (a, b, c) or adrenaline (AD) (d, e, f). Autoreceptor pKD values are from present experiments. Binding site pKD values are from Simonneaux et al. (1991) (a, d) and Blaxall et al. (1991) (b, c, e, f).
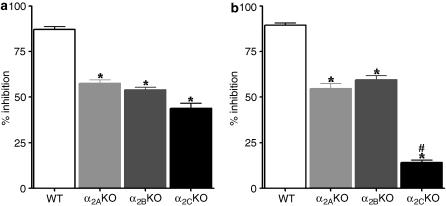
Effect of DMPP and medetomidine on catecholamine release in knockout mice
As shown in Table 1, the spontaneous outflow of noradrenaline and the spontaneous outflow adrenaline from the adrenal medulla of the knockout mice, were similar between the three knockout mice strains and significantly higher than that in wild-type mice. The increase in spontaneous outflow of noradrenaline and adrenaline from the adrenal medulla, evoked by DMPP (500 μM), was similar between the knockout and the wild-type mice strains (Table 1). Medetomidine (100 nM) reduced the evoked overflow of noradrenaline from the adrenal medulla of all three α2-adrenoceptor knockout mice (Figure 6a). This effect was significantly lower when compared to the effect of medetomidine on the evoked overflow noradrenaline from adrenal medulla of wild-type mice. Medetomidine (100 nM) also reduced the evoked overflow of adrenaline from the adrenal medulla of α2A- and α2B-adrenoceptor knockout mice, but had almost no effect on the evoked overflow of adrenaline from the adrenal medulla of the α2C-adrenoceptor knockout mice (Figure 6b).
Table 1.
Spontaneous outflow (in % of NA or AD adrenal content per minute) and DMPP-evoked overflow (in fraction of increase of spontaneous outflow) of NA and AD from the adrenal medulla WT and KO mice for each of the three α2 adrenoceptor subtypes (α2A, α2B and α2C)
| WT | α2AKO | α2BKO | α2CKO | |
|---|---|---|---|---|
| Spontaneous outflow | ||||
| NA | 0.37±0.02 | 0.70±0.04* | 0.79±0.06* | 0.75±0.02* |
| AD | 0.59±0.05 | 0.99±0.06* | 0.95±0.02* | 0.97±0.05* |
| DMPP-evoked overflow | ||||
| NA | 4.59±0.39 | 5.03±0.57 | 4.10±0.50 | 4.86±0.29 |
| AD | 2.56±0.27 | 2.28±0.18 | 2.36±0.19 | 2.28±0.10 |
Abbreviations: AD, adrenaline; DMPP, 1,1-dimethyl-4-phenylpiperazinium; KO, knockout; NA, noradrenaline; WT, wild type.
Values are presented as mean±s.e.m. (n=6).
Significantly different from corresponding values in WT mice P<0.05.
Figure 6.
Effect of medetomidine (100 nM) on DMPP-evoked overflow of noradrenaline (a) and adrenaline (b) from the adrenal medulla of wild-type (WT) and knockout (KO) mice for each of the three α2-adrenoceptor subtypes (α2A, α2B and α2C). Medetomidine was added 15 min before DMPP 500 μM. Results are presented as percentage inhibition. Columns and vertical lines represent mean±s.e.m. (n=5). *Significantly different from corresponding values for the effect of medetomidine in WT; P<0.05. #Significantly different from corresponding values for the effect of medetomidine in α2A and α2B KO mice P<0.05.
Catecholamine levels in the adrenal gland
The absolute and relative adrenal content of the catecholamines dopamine, noradrenaline and adrenaline and L-DOPA for all three α2-adrenoceptor knockout mice and the wild-type mice are presented in Table 2. There were no significant differences in the adrenal catecholamine content of the three knockout mice compared to the wild-type mice. However, the adrenal content of L-DOPA of the α2C-adrenoceptor knockout mice was significantly higher than in wild-type mice. The noradrenaline/dopamine and adrenaline/noradrenaline ratios were similar between the α2-adrenoceptor knockout mice and the wild-type mice, whereas the dopamine /L-DOPA ratio was significantly lower in the α2C-adrenoceptor knockout mice.
Table 2.
Absolute and relative tissue levels (in pmol/mg tissue) of L-DOPA and DA, NA and AD in the adrenals from WT and KO mice for each of the three α2-adrenoceptor subtypes (α2A, α2B and α2C)
| WT | α2AKO | α2BKO | α2CKO | |
|---|---|---|---|---|
| L-DOPA | 5±2 | 5±1 | 3±1 | 16±3* |
| DA | 191±56 | 198±67 | 200±94 | 177±51 |
| NA | 5921±866 | 5372±698 | 5678±1055 | 6274±529 |
| AD | 15113±2887 | 15107±2321 | 14678±2195 | 15322±1278 |
| DA/L-DOPA | 42±3 | 42±18 | 54±10 | 11±2* |
| NA/DA | 33±5 | 29±3 | 26±5 | 34±4 |
| AD/NA | 2.9±0.6 | 3.0±0.7 | 2.5±0.5 | 2.6±0.3 |
Abbreviations: AD, adrenaline; DA, dopamine; DMPP, 1,1-dimethyl-4-phenylpiperazinium; KO, knockout; L-DOPA, L-3,4-dihydroxyphenylalanine; NA, noradrenaline; WT, wild type.
Values are presented as mean±s.e.m. (n=5–6). Significantly different from corresponding values in WT (*P<0.05).
Tyrosine hydroxylase activity
In order to determine the kinetic parameters of the enzyme, saturation curves using the substrate (L-tyrosine) and the cofactor (6-BH4) were performed. Incubation of the tyrosine hydroxylase assay mixture prepared from adrenals of wild-type and each of the α2-adrenoceptor knockout mice in the presence of increasing concentrations of either L-tyrosine or 6-BH4 resulted in a concentration-dependent formation of L-DOPA. The values of the kinetic parameters, Vmax and KM, obtained from the corresponding saturation curves are given in Table 3. As shown in this table, the Vmax and KM values for tyrosine hydroxylase activity in the adrenal medulla were similar between wild-type and all three α2-adrenoceptor knockout mice strains.
Table 3.
Kinetic parameters, Vmax and KM, of tyrosine hydroxylase activity for the substrate (L-tyrosine) and the cofactor (6-BH4)
|
L-tyrosine |
6-BH4 |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT | α2AKO | α2BKO | α2CKO | WT | α2AKO | α2BKO | α2CKO | |
| Vmax (nmol/mg protein/h) | 28±1 | 31±2 | 27±1 | 28±1 | 25±1 | 24±3 | 26±1 | 26±2 |
| KM (μM) | 106±20 | 102±25 | 97±12 | 134±19 | 124±15 | 137±13 | 101±12 | 126±9 |
Abbreviations: KM, Michaelis constant; Vmax, maximum velocity; 6-BH4, 6-methyl-5,6,7,8-tetrahydropterine.
Enzyme assay was performed with adrenal homogenates obtained from WT mice and KO mice for each of the three α2-adrenoceptor subtypes (α2A, α2B and α2C). Values are presented as mean±s.e.m. (n=4).
Discussion
In the present study, we have characterized pharmacologically the predominant α2-adrenoceptor subtype involved in the feedback inhibitory mechanism for the evoked overflow of noradrenaline and adrenaline from the adrenal medulla of mice. The results show that the α2C-adrenoceptor is the predominant α2-adrenoceptor subtype mediating the feedback inhibition of the evoked overflow of adrenaline from the adrenal medulla. This finding was supported by data from two different sets of experiments: the rank potency of α-adrenoceptor antagonists in wild-type mice and studies with knockout mice for each of the three α2-adrenoceptor subtypes. In the first set of experiments, we found that the only α2-adrenoceptor subtype for which there was a positive correlation between the pKD values obtained experimentally and those supplied by radioligand binding studies was the α2C-adrenoceptor. Furthermore, the inhibitory effect of the α2-adrenoceptor agonist medetomidine over the DMPP-evoked overflow of adrenaline was drastically decreased in the adrenal medulla of mice lacking the α2C-adrenoceptor. Nevertheless, in the adrenal medulla of α2C-adrenoceptor knockout mice there was still 14% inhibition (Figure 6). This is an indication that α2A- and α2B-adrenoceptor subtypes also play a role, albeit minor, in this inhibitory mechanism. These results are contrary to those from a study with isolated cultured chromaffin cells, in which the α2C-adrenoceptor subtype was shown to be the only subtype responsible for the inhibitory feedback of adrenaline and noradrenaline release (Brede et al., 2003).
Cultured chromaffin cells do not fully reproduce the physiological properties of the intact adrenal medulla. In the intact adrenal medulla, regulation of adrenergic and noradrenargic chromaffin cell groups occurs through different mechanisms and release of noradrenaline or adrenaline occurs in response to different stimuli (Cahill et al., 1996; Aunis and Langley, 1999). This may also be true for the inhibitory mechanism mediated by α2-adrenoceptors: there may be different α2-adrenoceptors subtypes operating for each catecholamine. Our data shows that, contrary to what was found in isolated chromaffin cells, α2-adrenoceptor control over noradrenaline release from the adrenal medulla of mice in vivo is in fact different from adrenaline release: there is no predominant α2-adrenoceptor subtype regulating the evoked release of noradrenaline. The rank potency of antagonists was the first evidence for this conclusion: there was no significant correlation between pKD values obtained experimentally and those from radioligand binding assays for any of the three α2-adrenoceptor subtypes. Supporting evidence came from experiments with knockout mice for each of the α2-adrenoceptor subtypes: lack of any of the three α2-adrenoceptor subtypes resulted in a reduced effect of medetomidine, which was similar between α2-adrenoceptor subtypes. Therefore, in the adrenal medulla of mice, all three α2-adrenoceptor subtypes, α2A, α2B and α2C, must be present in order that α2-adrenoceptor mediated inhibitory feedback mechanism operates fully over the evoked overflow of noradrenaline release.
The concept that endocrine function of the adrenal medulla can be adequately interpreted by studying the functions of individual chromaffin cells in isolation is useful, but does not always reflect in vivo function (Bornstein et al., 2000). Chromaffin cells are altered by enzyme digestion steps in their isolation and, once in culture, they are away from the cyclic influence of cortical hormones. Furthermore, they are denervated and cell-to-cell communication is lacking (Borges, 1997). Within the intact adrenal gland, the adrenomedullary and adrenocortical systems are linked functionally and anatomically. Evidence obtained from in vitro studies has led to the suggestion that intact intra-adrenal cellular communication is crucial for the functioning of both endocrine systems in the gland (Schinner and Bornstein, 2005). Glucocorticoids, a class of steroid hormones produced by the adrenal cortex, exert a variety of effects on medullary chromaffin cells (Hodel, 2001). Recent gene knockout experiments suggest that glucocorticoid signalling is required only for the acquisition of the adrenergic, but not the noradrenergic, phenotype (Finotto et al., 1999). Compatible with this finding is the distribution of phenylethanolamine N-methyltransferase (PNMT) in chromaffin cells of the intact adrenal medulla. This enzyme is required for the formation of adrenaline from noradrenaline and PNMT-containing cells are often found adjacent to the adrenal cortex, where the concentration of steroids is likely to be higher than in the centre of the medulla, where cells lacking PMNT tend to be located (Schinner and Bornstein, 2005). In cultured chromaffin cells, contact with glucocorticoids is continuous, as the presence of glucocorticoids in the culture medium is required to sustain PNMT activity over several days (Muller and Unsicker, 1986). In the intact adrenal medulla, the adrenergic chromaffin cell group is more exposed to glucocorticoids, although even then exposure would be brief as blood flow through the gland is massive. If glucocorticoids are able to promote selectively expression of the α2C-adrenoceptor subtype, they may be responsible for the differences found between the intact gland and the cultured chromaffin cells, as well as for the difference found between the control of noradrenaline and adrenaline release by α2-adrenoceptor subtypes, from noradrenergic and adrenergic chromaffin cells. In support of this possibility, it has been shown that exposure to glucocorticoids promotes a differential expression of α2A-and α2C-adrenoceptors in the brain under stress situations (Flugge et al., 2003).
Thus far the inhibitory mechanisms mediated by α2-adrenoceptors had only been considered in relation to the induced release. Our study shows that the presence of α2-adrenoceptors is also important in regard to the spontaneous outflow of both noradrenaline and adrenaline from the adrenal medulla. The α2-adrenoceptor agonist clonidine (30 μM) reduced by about 30% the spontaneous outflow of catecholamines from the adrenal medulla of wild-type mice. Furthermore, the spontaneous outflow of catecholamines from the adrenal medulla of knockout mice was significantly higher, regardless of the α2-adrenoceptor subtype that had been deleted. In this system the lack of any one of the adrenoceptors resulted in an increased spontaneous outflow of the two catecholamines.
It should be noted that the major origin for plasma noradrenaline is spill-over from peripheral sympathetic nerve terminals and whereas that for adrenaline is the adrenal medulla (Goldstein et al., 2003). Mice lacking the α2C-adrenoceptor have circulating levels of adrenaline almost twice as high as the levels in wild-type, whereas mice lacking the α2A subtype present higher plasma levels of noradrenaline compared to wild-type (Brede et al., 2003). The higher levels of adrenaline in the α2C-adrenoceptor knockout mice are further evidence for the physiological importance of this receptor in the control of adrenaline release from the adrenal medulla. The difference in noradrenaline levels could be attributed to the lack of control of noradrenaline release from sympathetic nerve terminals, where the α2A-adrenoceptor is predominant (Trendelenburg et al., 2003).
Given that α2-adrenoceptors are involved in the regulation of synthesis and release of noradrenaline from sympathetic nerves, lack of these receptors could also influence catecholamine synthesis in the adrenal medulla of mice. In agreement with the study of Brede et al. (2003) α2C knockout mice have a higher content of L-DOPA in their adrenal glands. A possible explanation for this could be a higher activity of the rate-limiting step enzyme for catecholamine synthesis tyrosine hydroxylase (Kumer and Vrana, 1996). However, in the present study, the lack of any one of the α2-adrenoceptor subtypes in mice did not change the levels of dopamine, noradrenaline or adrenaline as catecholamine levels were similar between the knockout and the wild-type mice. Moreover, the activity of tyrosine hydroxylase was similar between the knockout and the wild-type mice. In fact, the kinetic parameters (Vmax and KM) determined for tyrosine hydroxylase, using either the substrate or the cofactor, were similar between all three α2-adrenoceptor knockout and the wild-type mice. Therefore, a higher activity of tyrosine hydroxylase cannot account for the higher levels of L-DOPA in the α2C knockout mice. The results from relative catecholamine tissue content in the adrenals, namely dopamine/L-DOPA, noradrenaline/dopamine and adrenaline/noradrenaline ratios, may give an indirect measurement for aromatic L-aminoacid decarboxylase, dopamine β-hydroxylase and PNMT, respectively. These ratios showed that in α2C knockout mice the ratio dopamine/L-DOPA was significantly lower compared to the other strains, with no significant differences between the other ratios. Two possible explanations for the increase in L-DOPA adrenal levels and the lower dopamine/L-DOPA ratio may be a lower activity of the enzyme aromatic L-aminoacid decarboxylase or a higher L-DOPA cellular uptake, both leading to higher intracellular levels of L-DOPA.
Evidence for the physiological relevance of α2-adrenoreceptors role in adrenal catecholamine release, both in animals and in humans, is growing. Mice deficient in the α2C-adrenoceptor show enhanced cardiac hypertrophy and heart failure in response to cardiac pressure overload (Brede et al., 2002) and humans carrying a signalling-deficient variant of the α2C-adrenoceptor (α2C-Del322–325) (Small et al., 2000) suffer from a more severe heart failure and decreased cardiac function than patients with intact α2-adrenoceptors (Brede et al., 2002). In healthy humans, α2C-Del322–325 polymorphism is associated with increased sympathetic nervous and adrenomedullary hormonal activity, during supine rest and with increased pharmacologically evoked catecholamine release. Polymorphisms of the α2C-adrenoreceptor may help explain individual differences in predisposition to a variety of disorders of catecholaminergic function, such as cardiovascular disorders, depression or anxiety disorders (Neumeister et al., 2005, 2006).
In conclusion, the present study supports the proposal that α2-adrenoceptors mediate inhibition of the spontaneous outflow and of the evoked overflow of noradrenaline and adrenaline from the adrenal medulla of mice. Although all three α2-adrenoceptor subtypes play a role in this inhibitory feedback mechanism, the α2C-adrenoceptor is the predominant subtype regulating adrenaline release.
Acknowledgments
This work was supported by grants from FCT, Programa Ciência, Tecnologia e Inovação do Quadro Comunitário de Apoio and FEDER (POCTI/FCB/40832/2001) and by grant from Univesity of Porto INVESTIGAÇÃO CIENTÍFICA na pré-graduação Projectos pluridisciplinares Ano lectivo 2005/2006 supported by Caixa Geral de Depósitos.
Abbreviations
- 6-BH4
6-methyl-5,6,7,8-tetrahydropterine
- DMPP
1,1-dimethyl-4-phenylpiperazinium
- L-DOPA
L-3,4-dihydroxyphenylalanine
- WB 4101
(±)-2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane HCl
Conflict of interest
The authors state no conflict of interest.
References
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47:1079–1090. doi: 10.1016/0092-8674(86)90823-8. [DOI] [PubMed] [Google Scholar]
- Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand. 1999;167:89–97. doi: 10.1046/j.1365-201x.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- Blaxall HS, Murphy TJ, Baker JC, Ray C, Bylund DB. Characterization of the alpha-2C adrenergic receptor subtype in the opossum kidney and in the OK cell line. J Pharmacol Exp Ther. 1991;259:323–329. [PubMed] [Google Scholar]
- Borges R. The rat adrenal gland in the study of the control of catecholamine secretion. Semin Cell Dev Biol. 1997;8:113–120. doi: 10.1006/scdb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Tian H, Haidan A, Bottner A, Hiroi N, Eisenhofer G, et al. Deletion of tyrosine hydroxylase gene reveals functional interdependence of adrenocortical and chromaffin cell system in vivo. Proc Natl Acad Sci USA. 2000;97:14742–14747. doi: 10.1073/pnas.97.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by alpha 2-adrenoceptor subtypes. Mol Endocrinol. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- Cahill AL, Eertmoed AL, Mangoura D, Perlman RL. Differential regulation of phenylethanolamine N-methyltransferase expression in two distinct subpopulations of bovine chromaffin cells. J Neurochem. 1996;67:1217–1224. doi: 10.1046/j.1471-4159.1996.67031217.x. [DOI] [PubMed] [Google Scholar]
- Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Bruhl B, et al. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development. 1999;126:2935–2944. doi: 10.1242/dev.126.13.2935. [DOI] [PubMed] [Google Scholar]
- Flugge G, van Kampen M, Meyer H, Fuchs E. Alpha2A and alpha2C-adrenoceptor regulation in the brain: alpha2A changes persist after chronic stress. Eur J Neurosci. 2003;17:917–928. doi: 10.1046/j.1460-9568.2003.02510.x. [DOI] [PubMed] [Google Scholar]
- Furchgott RF.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines. Handook of experimental pharmacology 1972Springer: Berlin; 228–335.In: Blaschko H, Muscholl E (eds) [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Hescheler J, Spicher K, Klinz FJ, Schultz G, Rosenthal W. Inhibition of Ca2+ channels via alpha 2-adrenergic and muscarinic receptors in pheochromocytoma (PC-12) cells. Am J Physiol. 1991;260:C1282–C1289. doi: 10.1152/ajpcell.1991.260.6.C1282. [DOI] [PubMed] [Google Scholar]
- Guimaraes S, Osswald W. Adrenergic receptors in the veins of the dog. Eur J Pharmacol. 1969;5:133–140. doi: 10.1016/0014-2999(69)90021-1. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Hodel A. Effects of glucocorticoids on adrenal chromaffin cells. J Neuroendocrinol. 2001;13:216–220. doi: 10.1046/j.1365-2826.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- Hoffman BB, Taylor P.Neurotransmission: the autonomic and somatic motor nervous system Goodman & Gilmans: The Pharmacological Basis of Therapeutics 2001McGraw-Hill: New York; 115–154.In: Hardman JG, Limbird LE (eds) [Google Scholar]
- Kleppisch T, Ahnert-Hilger G, Gollasch M, Spicher K, Hescheler J, Schultz G, et al. Inhibition of voltage-dependent Ca2+ channels via alpha 2-adrenergic and opioid receptors in cultured bovine adrenal chromaffin cells. Pflugers Arch. 1992;421:131–137. doi: 10.1007/BF00374819. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, et al. Cardiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and c. Science. 1996;273:803–805. doi: 10.1126/science.273.5276.803. [DOI] [PubMed] [Google Scholar]
- Link RE, Stevens MS, Kulatunga M, Scheinin M, Barsh GS, Kobilka BK. Targeted inactivation of the gene encoding the mouse alpha 2c-adrenoceptor homolog. Mol Pharmacol. 1995;48:48–55. [PubMed] [Google Scholar]
- Moura E, Pinho Costa PM, Moura D, Guimaraes S, Vieira-Coelho MA. Decreased tyrosine hydroxylase activity in the adrenals of spontaneously hypertensive rats. Life Sci. 2005;76:2953–2964. doi: 10.1016/j.lfs.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Muller TH, Unsicker K. Nerve growth factor and dexamethasone modulate synthesis and storage of catecholamines in cultured rat adrenal medullary cells: dependence on postnatal age. J Neurochem. 1986;46:516–524. doi: 10.1111/j.1471-4159.1986.tb12998.x. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Charney DS, Belfer I, Geraci M, Holmes C, Sharabi Y, et al. Sympathoneural and adrenomedullary functional effects of alpha2C-adrenoreceptor gene polymorphism in healthy humans. Pharmacogenet Genomics. 2005;15:143–149. doi: 10.1097/01213011-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, et al. Effects of a alpha(2C)-Adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1750–1756. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- Powis DA, Baker PF. Alpha 2-adrenoceptors do not regulate catecholamine secretion by bovine adrenal medullary cells: a study with clonidine. Mol Pharmacol. 1986;29:134–141. [PubMed] [Google Scholar]
- Schinner S, Bornstein SR. Cortical-chromaffin cell interactions in the adrenal gland. Endocr Pathol. 2005;16:91–98. doi: 10.1385/ep:16:2:091. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Ebadi M, Bylund DB. Identification and characterization of alpha 2D-adrenergic receptors in bovine pineal gland. Mol Pharmacol. 1991;40:235–241. [PubMed] [Google Scholar]
- Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva P, Pestana M, Vieira-Coelho MA, Fernandes MH, Albino-Teixeira A. Assessment of renal dopaminergic system activity in the nitric oxide-deprived hypertensive rat model. Br J Pharmacol. 1995;114:1403–1413. doi: 10.1111/j.1476-5381.1995.tb13362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Presynaptic alpha-autoreceptors. Rev Physiol Biochem Pharmacol. 1987;107:73–146. [PubMed] [Google Scholar]
- Starke K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J Neurochem. 2001;78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Philipp M, Meyer A, Klebroff W, Hein L, Starke K. All three alpha2-adrenoceptor types serve as autoreceptors in postganglionic sympathetic neurons. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:504–512. doi: 10.1007/s00210-003-0829-x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Sutej I, Wahl CA, Molderings GJ, Rump LC, Starke K. A re-investigation of questionable subclassifications of presynaptic alpha2-autoreceptors: rat vena cava, rat atria, human kidney and guinea-pig urethra. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:721–737. doi: 10.1007/pl00005111. [DOI] [PubMed] [Google Scholar]
- Young JB, Landsberg L.Catecholamines and the adrenal medulla Williams Textbook of Endocrinology 1998WB Saunders: Philadelphia; 665–728.In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds) [Google Scholar]