Abstract
Background and purpose:
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are frequently used lipid-lowering drugs. Moreover, they exert pleiotropic effects on cellular stress responses and death. Here, we analysed whether lovastatin affects the sensitivity of primary human endothelial cells (HUVEC) to the anticancer drug doxorubicin.
Experimental approach:
We investigated whether pretreatment of HUVEC with low dose of lovastatin influences the cellular sensitivity to doxorubicin. To this end, cell viability, proliferation and apoptosis as well as DNA damage-triggered stress response were analysed.
Key results:
Lovastatin reduced the cytotoxic potency of doxorubicin in HUVEC. Lovastatin attenuated the doxorubicin-induced increase in p53 as well as activation of checkpoint kinase (Chk-1) and stress-activated protein kinase/c-Jun-N-terminal kinase (SAPK/JNK). Acquired doxorubicin resistance was independent of alterations in doxorubicin efflux and cell cycle progression. Also, doxorubicin-triggered production of reactive oxygen species (ROS) and formation of oxidative DNA lesions remained unaffected by lovastatin. However, lovastatin impaired DNA strand break formation induced by doxorubicin. Notably, lovastatin also conferred cross-resistance to the cytotoxic and genotoxic effects of etoposide, indicating that lovastatin shields topoisomerase II against poisons.
Conclusions and implications:
Based on these data, we suggest that lovastatin-mediated resistance to topoisomerase II inhibitors is due to a reduction in DNA damage and, hence, it attenuates stress responses leading to cell death that are triggered by DNA damage. Therefore, lovastatin might be useful clinically for alleviating side-effects of anticancer therapies that include topoisomerase II inhibitors.
Keywords: genotoxic stress response, endothelial cells, HMG-CoA reductase inhibitors, lovastatin, doxorubicin, etoposide
Introduction
Cellular susceptibilty to anticancer drugs is influenced by numerous factors. Most important are DNA repair (Friedberg et al., 1995), drug transport (Faneyte et al., 2004) and stress-induced signalling mechanisms (Canman and Kastan, 1996; Cortez et al., 2001). The latter cause changes in gene expression, cell cycle progression and, eventually, in apoptosis. A pharmacological approach for intervening with anticancer drug-induced stress responses is based on the fact that Ras and Ras-homologous (Rho) GTPases, which play a pivotal role in the regulation of genotoxic stress responses (Coso et al., 1995; Canman and Kastan, 1996; Gnad et al., 2000), are subject to C-terminal prenylation (Adamson et al., 1992). Attachment of a C15 or C20 lipid moiety to the cystein of the C-terminal located CAAX-box is required for correct intracellular localization of Ras/Rho on the cell membrane and hence is essential for their physiological function (Adamson et al., 1992). The clinically highly relevant group of 3-hydroxy-3-methylglutaryl--coenzyme A (HMG-CoA) reductase inhibitors (statins), which are widely used to reduce lipids, cause depletion of the cellular pool of isopren precursor molecules. Thereby, statins eventually lead to a downmodulation of Ras/Rho-regulated signal mechanisms (Liao and Laufs, 2005; Walker and Olson, 2005) and, furthermore, affect proliferation and cell death (Graaf et al., 2004; Fritz, 2005). Preclincal in vitro studies showed that statins impair the G1–S transition (Rao et al., 1998) and trigger apoptosis in tumour cells (Dimitroulakos et al., 2001) as well as in normal endothelial cells of rodent (Kaneta et al., 2003) and human origin (Li et al., 2002b). Furthermore, they are reported to promote cell killing by anticancer drugs (Dimitroulakos et al., 1999; Cafforio et al., 2005). These effects require a high dose of statin. This is important to note as dose-dependent opposite effects of statins have been reported, for example, for angiogenesis (Urbich et al., 2002). Bearing this in mind, the impact of physiological relevant low doses of statins on the susceptibility of malignant and non-malignant human cells exposed to anticancer drugs remains unclear. This, however, is of outmost importance in view of the question of whether intake of statins, for example, for cardiovascular protection, adversely affects a patient's prognosis in the case of tumour therapy. Recently, we found that a low concentration of lovastatin confers radioresistance to human endothelial cells (Nuebel et al., 2006), indicating that statins might be able to attenuate the side effects of radiotherapy by protecting normal tissue.
Apart from attenuating radiation-induced stress responses (Nuebel et al., 2006), lovastatin also impairs doxorubicin-stimulated stress responses, such as activation of stress-activated protein kinases/c-Jun-N-terminal kinases (SAPK/JNK) and nuclear factor kappaB (NF-κB) (Gnad et al., 2001; von Bardeleben et al., 2002). As both factors are important for the regulation of cell death (Chen et al., 1996; Wang et al., 1996; Panaretakis et al., 2005), we addressed the question of whether the HMG-CoA-reductase inhibitor lovastatin modulates the sensitivity of primary human endothelial cells to the potent and frequently used anticancer drug doxorubicin. We present evidence that lovastatin shields primary human umbilical endothelial cells (HUVEC) from the cytotoxicity of topoisomerase II inhibitors by reducing their genotoxic potency.
Methods
Cell culture conditions and determination of cell viability
Primary HUVEC and human fibroblasts normal human dermal fibroblasts (NHDF) as well as the corresponding growth media (endothelial growth media medium and fibroblast growth medium supplemented with 2% foetal calf serum) were obtained from Cambrex Bio Science (Verviers, Belgium). Cell viability was quantified by use of the water-soluble tetrazolium salt (WST) assay according to the manufacturers protocol (Roche Diagnostics, Mannheim, Germany).
Analysis of DNA replication
To assay the effect of lovastatin on doxorubicin-induced block of DNA replication, cells were pulse-labelled with bromodeoxyuridine (BrdU) for 2–3 h. Afterwards, BrdU incorporation was determined by enzyme-linked immunosorbent assay (ELISA)-based method (Roche Diagnostics GmbH, Mannheim, Germany). DNA replication in doxorubicin-treated cells was related to that of the corresponding control (untreated or lovastatin only pretreated cells), which was set to 100%.
Analysis of cell cycle progression and apoptotic cell death
Cell cycle analysis was achieved by fluorescence activated cell sorting (FACS). The frequency of apoptotic cell death was determined by Annexin V/propidium iodide double staining followed by FACS analysis. Activation of caspases was assayed by Western blot analysis using antibodies that specifically detected the cleaved (activated) form of caspase-3, -7, -8 or -9 or, alternatively by immunofluorescence using antibody directed against activated caspase-3 followed by incubation with fluorescein isothiocyanate (FITC)-coupled secondary antibody (Alexa Fluor 488, Molecular Probes, Karlsruhe, Germany).
Determination of cellular uptake/efflux of doxorubicin
Uptake of fluorescent doxorubicin into cells as well as efflux were analysed by FACS as described by Garcia-Escarp et al. (2004).
FITC phalloidin staining
In order to investigate the effects of lovastatin and doxorubicin treatment on cell morphology and shape of the actin cytoskeleton, F-actin was stained with FITC-coupled phalloidin as described previously (Koch et al., 1997). Cell morphology was analysed by microscopy.
Western blot analysis
Protein (10–30 μg), from total or nuclear extract was separated by sodium dodecyl sulphate gel electrophoresis. After wet blotting to nitrocellulose and blocking of nonspecific binding (5% dry milk in phosphate-buffered saline (PBS)/0.1% Tween 20; overnight at 4°C) filters were incubated for 2 h at room temperature (or alternatively overnight at 4°C) with the corresponding primary antibody (diluted 1:100–1:1000 in PBS/5% bovine serum albumin/0.1% Tween 20). After being washed and incubated with secondary peroxidase-coupled anti-rabbit or anti-mouse antibody (1:5000), proteins were visualized by autoradiography using the Renaissance enhanced luminol reagent (Du Pont NEN, Belgium).
RT–PCR analysis
To determine the expression of mdr-1, mrp-1, Fas receptor (FAS-R; CD95R) and Fas ligand (FAS-L; CD95L) on messenger RNA (mRNA) level, reverse transcriptase polymerase chain reaction (RT–PCR) analysis was performed. Upon isolation of total RNA using Qiagen total RNA isolation kit, first strand complementary DNA (cDNA) synthesis was performed (Qiagen cDNA synthesis kit). For standard PCR reaction (30 cycles, annealing temperature 58°C) the following primer pairs were used:
For Fas-R: 5′-AAGGGATTGGAATTGAGGAAGACTG-3′ and 5′-GTGGAATTGGCAAAAGAAGAAGAC-3′; Fas-L: 5′-CCCCTCCAGGCACAGTTCTTCC-3′ and 5′-CTTGTGGCTCAGGGGCAGGTTGT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′; multidrug resistance (Mdr)-1: 5′-ATATCAGCAGCCCACACAT-3′ and 5′-GAAGCACTGGGATGTCCGGT-3′; Mrp-1: 5′-GTCTTACTCATTGCAGG-3′ and 5′-CTTCTGCACATTCATGGTC-3′. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
Analysis of DNA strand break induction
DNA strand break induction was assayed by the comet assay as described (von Bardeleben et al., 2002). Briefly, at different time points after exposure, ∼105 cells were embedded in low melting-point agarose and transfered onto agarose-covered microscope slides. Cells were lysed for 1 h in lysis buffer (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid (EDTA), 10 mM Tris, 1% Na-laurylsarcosinate, 1% Triton X-100, 10% dimethyl sulfoxide, pH 10 (alkaline) or pH 7.5 (neutral)). For denaturation of DNA, cells were incubated in alkaline electrophoresis buffer (300 mM NaOH, 1 mM EDTA, pH 13) for 25 min before electrophoresis (25 V, 300 mA for 15 min). Afterwards, cells were washed with 0.4 M Tris (pH 7.5), distilled H2O and finally with ethanol. DNA was stained with ethidium bromide. Comets were visualized by microscopy and quantified by determination of the ‘Olive tail moment' (OTM) using computer-based software (Komet 4.02, Kinetics Imaging, UK). Fifty cells were analysed per measurement for calculation of the mean value.
Analysis of formation of ROS and oxidative DNA damage
Formation of reactive oxygen species (ROS) was quantitated by oxidation-based fluorescence assay using 2′, 7′-dichlorofluorescein diacetate (DCFDA) (Li et al., 2002a). Briefly, logarithmically growing HUVEC were incubated in the absence and presence of lovastatin (1 μM) overnight and subsequently exposed to doxorubicin (5 μg ml−1). Afterwards, cells were incubated with DCFDA (10 μM) for different periods of time (up to 90 min) before fluorescence was determined. To this end, excitation wavelength of 485 nm was used and emission of fluorescence was detected at 530 nm in a 96-well fluorometer. Oxidative DNA damage was measured by analysis of formamidopyrimidine glycosylase-sensitive sites (FPG-sites) as described previously (Osterod et al., 2002).
Materials
The HMG-CoA-reductase inhibitor lovastatin was purchased from Calbiochem (Bad Soden, Germany). Extracellular regulated kinase 2 (ERK2), p53, p21, Mdr-1 and topoisomerase II alpha antibodies were obtained from Santa Cruz (San Diego, USA). p-Chk-1 (p-Ser345), checkpoint kinase-1 (Chk-1), p-Akt (p-Ser473), Akt kinase/protein kinase B (Akt), p-JNK (p-Thr183/p-Tyr185) and caspase antibodies originate from New England Biolabs GmbH (Frankfurt, Germany), phosphorylated histone H2AX (γ-H2AX) antibody from Upstate (Hamburg, Germany). FITC phalloidin was purchased from Sigma Aldrich (Taufkirchen, Germany).
Results
Lovastatin protects HUVEC from doxorubicin-induced cell killing
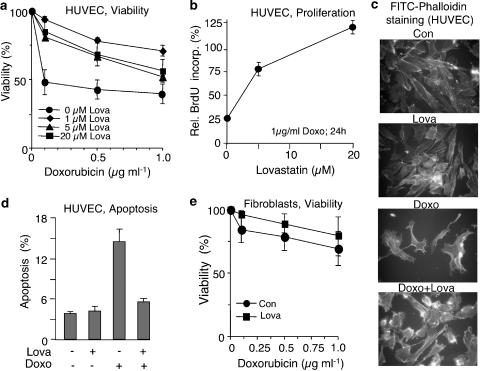
To examine the influence of lovastatin on the susceptibility of primary human endothelial cells (HUVEC) to doxorubicin, cells were pretreated overnight with different concentrations of lovastatin before doxorubicin was added. Measuring cell viability using the WST assay, we observed that even a low dose of lovastatin, 1 μM, largely reduced the cytotoxic effects of doxorubicin (Figure 1a). Higher doses of lovastatin did not further increase doxorubicin resistance (Figure 1a) but, instead, were less effective at reducing the cytotoxic effects. This phenomenon is probably due to the fact that, if used at higher concentration, lovastatin starts to provoke cytotoxicity on its own (Nuebel et al., 2006). Lovastatin had no significant protective effect on primary human fibroblasts (Figure 1e). Lovastatin-mediated increase in the viability of HUVEC was paralleled by an enhanced recovery from doxorubicin-induced DNA replication block. As shown in Figure 1b, in the absence of lovastatin, exposure of HUVEC to doxorubicin reduced BrdU-incorporation by about 75%, as measured 24 h after exposure (Figure 1b). Presumably, this effect is mainly owing to doxorubicin-induced cell cycle arrest. Pretreatment with lovastatin attenuated the doxorubicin-induced reduction in BrdU incorporation in a dose-dependent manner (Figure 1b). Apparently, lovastatin attenuates doxorubicin-provoked cell cycle arrest and restores DNA synthesis in exposed cells. Figure 1c illustrates the protective effect of lovastatin and shows that under our experimental conditions (i.e. low-dose treatment) lovastatin does not cause breakdown of the actin cytoskeleton as reported previously (Koch et al., 1997). Notably, apoptotic death induced by doxorubicin was also reduced upon pretreatment with the statin (Figure 1d).
Figure 1.
Lovastatin protects primary human endothelial cells from doxorubicin-induced cytotoxicity. (a) Primary human endothelial cells (HUVEC) were pretreated overnight with the indicated concentration of lovastatin. Afterwards, cells were exposed to different doses of doxorubicin. After incubation period of 48 h in the absence of lovastatin, cell viability was assayed using the WST assay as described in Methods. Data shown are the mean, and vertical lines show s.d., from three independent experiments each performed in triplicate. (b) HUVEC were pretreated overnight with different concentration of lovastatin. Afterwards, cells were treated with doxorubicin (1 μg ml−1). After incubation period of 24 h in the absence of lovastatin, cells were pulse-labelled with BrdU for 2 h. Incorporation of BrdU was assayed as described in Methods. BrdU labelling of doxorubicin-treated cells was related to that of the corresponding untreated control which was set to 100%. Data shown are the mean and s.d. from a representative experiment performed in quadruplicate. (c) HUVEC were pretreated overnight with lovastatin (1 μM) before they were exposed to doxorubicin (5 μg ml−1, 1 h treatment). After an incubation period of 48 h, morphological alterations of the actin cytoskeleton were analysed by FITC phalloidin staining followed by microscopic analysis. (d) After overnight pretreatment of HUVEC with lovastatin (Lova, 20 μM), doxorubicin (1 μg ml−1) was added and cells were post-incubated for 72 h in the absence of lovastatin, before the frequency of apoptotic death was determined by FACS-based annexin V method. Data shown are the mean and s.d. from three independent experiments. (e) Primary human fibroblasts were pretreated overnight with lovastatin (20 μM). Doxorubicin exposure and determination of cell viability were performed as described in (a).
Lovastatin reduces the genotoxic effects of doxorubicin
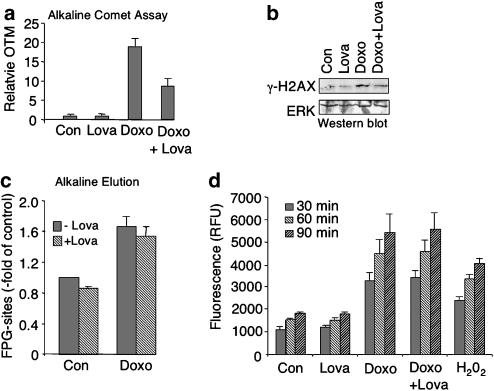
The cytotoxic and apoptotic effect of the anthracycline doxorubicin is based on the induction of DNA damage, which mainly results from the production of ROS (Mizutani et al., 2005; Wu and Hasinoff, 2005) or inhibition of topoisomerase II (Tewey et al., 1984). To examine whether lovastatin interferes with the genotoxic potency of doxorubicin, DNA strand break formation was analysed using the comet assay. As shown in Figure 2, lovastatin reduced doxorubicin-induced DNA strand break formation (Figure 2a) and H2AX phosphorylation (Figure 2b). Protection from doxorubicin-induced DNA strand breakage appears to be independent of the induction of oxidative DNA damage (Figure 2c) and ROS formation (Figure 2d).
Figure 2.
Lovastatin lowers doxorubicin-induced genotoxicity in HUVEC. (a) After overnight pretreatment with lovastatin (1 μM), HUVEC were exposed to doxorubicin (5 μg ml−1). After an incubation period of 1 h, cells were harvested and DNA strand break formation was investigated by the comet assay as described in Methods. Shown are the mean and s.d. (vertical lines) from at least two independent experiments. (b) HUVEC were pretreated with lovastatin as described in (a). One hour after doxorubicin exposure (5 μg ml−1), phosphorylated histone (γ-H2AX) was determined in nuclear extracts. As a loading control, the filter was reprobed with ERK2-specific antibody (ERK). (c) Oxidative DNA damage was assayed by measuring FPG-sensitive sites in the genomic DNA. After lovastatin pretreatment (1 μM, overnight) cells were exposed to doxorubicin (10 μg ml−1) and the level of oxidative DNA damage was quantified one hour later as described in Methods. Data shown are the mean and s.d. (vertical lines) from three independent experiments each performed in duplicate. (d) Cells were left untreated (Con) or were pretreated overnight with lovastatin (Lova; 1 μM) before doxorubicin was added (5 μg ml−1). ROS formation was quantitated upon addition of DCF in 30 min time intervals (up to 90 min) as described in Methods. As positive control, cells were treated for 1 h with hydrogen peroxide (H2O2, 0.01%). Data shown are the mean and s.d. from a representative experiment performed in quadruplicate.
Protection from doxorubicin by lovastatin is independent of drug influx/efflux and changes in cell cycle progression
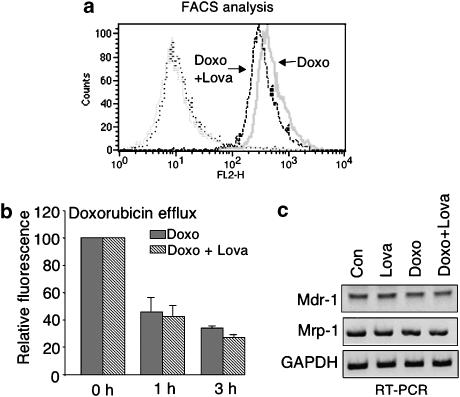
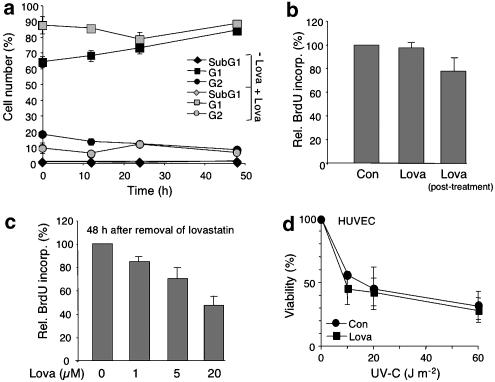
To further address the question of what molecular mechanisms are involved in lovastatin-mediated protection from the genotoxic effect of doxorubicin, uptake and efflux of the drug were investigated. As shown in Figure 3, lovastatin caused only a very faint (i.e. ⩽20%) reduction in the uptake of doxorubicin (Figure 3a). Efflux of doxorubicin was not affected by the statin at all (Figure 3b). In line with this finding, mRNA expression of the main doxorubicin transporters mdr-1 and mrp-1 remained unchanged (Figure 3c). Mdr-1 and topoisomerase IIα protein expression was also not altered by lovastatin pretreatment (data not shown), which is in line with data obtained for a rodent cell line (von Bardeleben et al., 2002). Based on these data, it appears that the mechanisms leading to doxorubicin resistance are independent of changes in doxorubicin transport. As statins are known to affect cell cycle progression (Rao et al., 1998; Cooper, 2002), we investigated whether low-dose pretreatment with lovastatin impacts the cell cycle distribution of HUVEC. Overnight pretreatment with 1 μM of lovastatin caused accumulation of HUVEC in G1 phase, which was paralleled by a decrease in the percentage of cells present in G2, as expected (Figure 4a). Increases in subG1 fraction, which represents the fraction of apoptotic cells, were not observed (Figure 4a). Upon removal of lovastatin, the cells rapidly resumed normal DNA synthesis (Figure 4b) and re-entered the cell cycle (Figure 4a). On assaying DNA synthesis 48 h after removal of lovastatin, we observed that the effect of the low lovastatin dose (i.e. 1 μM) on BrdU incorporation is very weak (Figure 4c). Hence, low dose (i.e. 1 μM) of lovastatin causes only moderate and reversible changes on cell cycle progression that are unlikely to be relevant to its doxorubicin-resistant effects. The view that lovastatin-mediated doxorubicin resistance is not a nonspecific cell cycle (i.e. S phase)-related phenomenon is supported by the observation that lovastatin did not affect the sensitivity of HUVEC towards the S-phase-dependent clastogen ultraviolet (UV) light (Figure 4d) and renders human fibroblasts even more sensitive to UV-induced cell killing (data not shown). Furthermore, doxorubicin was observed to damage about 70% of the cells under our experimental conditions, independent of whether HUVEC were pretreated or not with lovastatin (comet analysis-based data; not shown). Hence, a selective protective effect of lovastatin on doxorubicin-damaged S-phase cells is unlikely.
Figure 3.
Lovastatin mediated protection is independent from uptake and efflux of doxorubicin. (a) HUVEC were left untreated or were pretreated overnight with lovastatin (1 μM) before addition of doxorubicin (5 μg ml−1). One hour after treatment, doxorubicin uptake was determined by FACS-based analysis of doxorubicin fluorescence. Shown is the result of one representative experiment out of three. (b) Lovastatin pretreated (1 μM, overnight)- or untreated HUVEC were exposed to doxorubicin as described in (a). Afterwards, doxorubicin was removed and doxorubicin fluorescence was determined 1 and 3 h later by FACS. Data shown are the mean and s.d. (vertical lines) from at least two independent experiments each performed in duplicate. (c) Analysis of mRNA expression of two main drug transporters, namely mdr-1 and mrp-1. GAPDH mRNA expression was analysed as an internal control.
Figure 4.
Effect of lovastatin on cell cycle progression of HUVEC. (a) HUVEC were pretreated overnight with lovastatin (1 μM)(+Lova) or were left untreated (−Lova). Afterwards, fresh medium was added. After a further incubation period of up to 48 h, cells were harvested and cell cycle distribution was analysed by FACS. Data shown are the mean and s.d. from at least two independent experiments. (b) After overnight pretreatment of HUVEC with lovastatin (1 μM) fresh medium containing BrdU was added and cells were further incubated for 3 h in the absence (Lova) or presence (Lova, post-treatment) of lovastatin. BrdU incorporation was quantified by ELISA as described in Methods. Data shown are mean and s.d. from one representative experiment performed in quadruplicate. Con, untreated cells. (c) HUVEC were pretreated overnight with different concentration of lovastatin as indicated. Afterwards, medium was replaced by fresh medium. After a postincubation period of 48 h, cells were pulse-labelled with BrdU for 2 h. BrdU incorporation was quantified by ELISA as described in Methods. Data show are the mean and s.d. from at least two independent experiments each performed in triplicate. (d) HUVEC were left untreated (Con) or were pretreated overnight with lovastatin (Lova). Afterwards, cells were irradiated with different doses of UV-C light. After incubation period of 48 h, cell viability was determined as described in Methods. Data shown are the mean and s.d. from at least two independent experiments each performed in triplicate. Under identical experimental conditions, lovastatin pretreatment rendered human fibroblasts more sensitive to UV-C irradiation (data not shown).
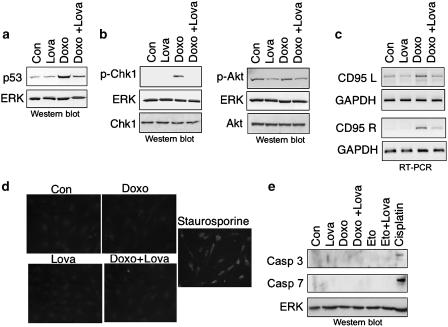
Lovastatin has pleiotropic inhibitory effects on doxorubicin-induced stress responses
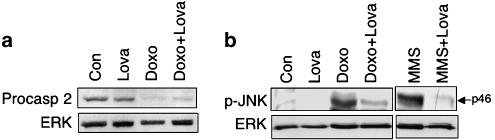
As lovastatin reduces the genotoxic potency of doxorubicin, stress responses dependent on DNA damage would be expected to be attenuated by the statin. Hence, we investigated the effect of lovastatin on doxorubicin-induced stress responses owing to DNA damage. As shown in Figure 5, both the increase in p53 protein level (Figure 5a) and the ataxia telangiectasia mutated ATM and Rad3-related (ATM/ATR)-dependent phosphorylation of Chk-1 (Figure 5b) were abrogated by lovastatin. As regards Akt-kinase, basal phosphorylation was reduced by lovastatin and doxorubicin did not cause activation of Akt (Figure 5b). Furthermore, doxorubicin-stimulated mRNA expression of Fas-ligand (CD95L) and Fas-receptor (CD95R), which are regulated in an activator protein-1 (AP-1) and p53-dependent manner, respectively (Faris et al., 1998a, 1998b; Muller et al., 1998b), became attenuated by lovastatin (Figure 5c). The observed attenuation of p53-driven CD95R expression by lovastatin is thus in line with the observed inhibitory effect of lovastatin on p53 protein induction (see Figure 5a). Despite the increase in CD95L and CD95R mRNA expression, doxorubicin did not provoke activation of capase-3 (Figure 5d and e) and -7 (Figure 5e), as determined by immunofluorescence (Figure 5d) and Western blot analyses (Figure 5e). We also failed to detect activation of caspase-8 and -9 (data not shown). In contrast to doxorubicin, cisplatin was quite efficient at activating caspase-3 and -7 in HUVEC (Figure 5e), showing that HUVEC are not generally compromised in the activation of these caspases. Recently, it was suggested that doxorubicin provokes apoptosis by activation of caspase-2 and SAPK/JNK (Panaretakis et al., 2005). Pro-caspase-2 is known to be constitutively expressed in the nucleus and becomes activated by DNA damage (Zhivotovsky and Orrenius, 2005). As shown in Figure 6a, doxorubicin treatment caused a decrease in the protein level of pro-caspase-2, which is indicative of activation of caspase-2. Yet, lovastatin did not reverse this effect. On the other hand, doxorubicin-triggered activation of SAPK/JNK was partially blocked by lovastatin (Figure 6b). This finding is in line with the observation that the CD95L expression, which is regulated in a SAPK/JNK-dependent manner (Faris et al., 1998a), was reduced by the statin (see Figure 5c).
Figure 5.
Lovastatin has pleiotropic inhibitory effects on doxorubicin-induced stress responses of HUVEC. (a, b) Lovastatin pretreated (1 μM, overnight) or untreated cells (Con) were exposed to doxorubicin (2 μg ml−1; 1 h pulse treatment). After a post-incubation period of 8 h, expression of p53 (a) and of phosphorylated (activated) forms of p-Chk-1 (phosphorylated at Ser345) and Akt kinase (p-Akt) (phosphorylated at Ser473) (b) were assayed by Western blot analysis. Shown are the autoradiograms. Expression levels of EKR2 (ERK) and non-phosphorylated Chk-1 and Akt, respectively, are included as loading controls. (c) Cells were treated with lovastatin and doxorubicin, as described above; 12 h after exposure, mRNA expression of Fas ligand (CD95L) and Fas receptor (CD95R) was analysed by RT–PCR. mRNA expression of GAPDH was used as an internal control. Shown are the ethidium bromide stained gels (inverse presentation). (d) HUVEC were pretreated or not (Con) with lovastatin (1 μM, overnight) before doxorubicin was added (5 μg ml−1, 1 h). After a post-incubation period of 48 h, activation of caspase-3 was analysed by immunohistochemistry as described in Methods. As a positive control, cells were treated with staurosporine (1 μM, 6 h). (e) HUVEC were pretreated with lovastatin and exposed to doxorubicin (5 μg ml−1, 1 h) or etoposide (12 μM, 1 h). Cisplatin (50 μM, 3 h) was used as a positve control. Activated forms of caspase-3 and -7 were analysed 72 h after genotoxin exposure by Western blot analysis. As a loading control, the filter was reprobed with anti-ERK2 (ERK) antibody. The autoradiogram is shown.
Figure 6.
Effect of lovastatin on doxorubicin-triggered activation of caspase-2 and stress kinase (SAPK/JNK). (a) HUVEC, pretreated or not (Con) overnight with lovastatin (1 μM), were exposed to doxorubicin (5 μg ml−1, 1 h). After a post-incubation period of 72 h, expression levels of procaspase-2 were determined by Western blot analysis. Expression of ERK2 (ERK) protein was determined as a loading control. (b) HUVEC were pretreated with lovastatin (1 μM; overnight) and subsequently exposed to doxorubicin (2.5 μg ml−1). Six hours after doxorubicin exposure, activation of the stress kinase p46/JNK1 (p-JNK) (dual phosphorylation at Thr183/Tyr185) was determined by Western blot analysis using phosphospecific JNK antibody. The alkylating agent MMS (1 mM, 2 h) was used as a positive control (Fritz and Kaina (2006)).
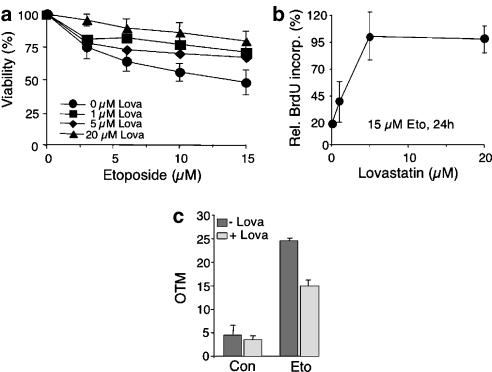
Lovastatin confers cross-resistance to etoposide
As lovastatin reduced doxorubicin-triggered strand break formation without affecting the formation of ROS (see Figure 2d), we speculated that the statin impairs the interaction of doxorubicin with topoisomerase II (Tewey et al., 1984). This hypothesis was substantiated by the finding that lovastatin confered cross-resistance to the highly specific topoisomerase II inhibitor etoposide. As shown in Figure 7, lovastatin increased viability (Figure 7b) and proliferation (Figure 7b) of etoposide-treated HUVEC and, furthermore, reduced DNA strand break formation (Figure 7c).
Figure 7.
Lovastatin confers cross-resistance to the topoisomerase II-specific inhibitor etoposide. (a) HUVEC were pretreated overnight with the indicated concentration of lovastatin before etoposide was added. After a post-incubation period of 48 h in the absence of the statin, cell viability was assessed using the WST assay, as described in Methods. Data shown are the mean and s.d. (vertical lines) from three independent experiments each performed in triplicate. (b) HUVEC were pretreated with different concentration of lovastatin (1–20 μM, overnight). Afterwards, cells were exposed to etoposide (15 μM). After an incubation period of 24 h (in the absence of lovastatin), cells were pulse-labelled with BrdU for 2 h. Incorporation of BrdU was assayed as described in Methods. BrdU labelling of irradiated cells was related to that of the corresponding non-irradiated control, which was set to at 100%. Data shown are the mean and s.d. from one representative experiment performed in quadruplicate. (c) HUVEC were left untreated (−Lova) or were pretreated overnight with 1 μM of lovastatin (+Lova) before addition of etoposide (15 μM). After a further incubation period of 1 h, cells were harvested and the level of DNA strand breaks was quantified by the comet assay, as described in Methods. Shown are the mean and s.d. from two independent experiments. Con, untreated cells.
Discussion
Physiologically relevant low doses of lovastatin protect human endothelial cells against the antiproliferative and cell killing effects of doxorubicin, as measured on level of cell viability, DNA synthesis and apoptosis. This protective effect of lovastatin is specific for HUVEC as it was not observed for primary human fibroblasts. Furthermore, UV-resistance of HUVEC was not affected by lovastatin, showing that the observed protective effect is agent specific. It has been demonstrated that statins induce cell death specifically in tumour cells (Dimitroulakos et al., 1999; Cafforio et al., 2005), making statins highly attractive as potential anti-cancer drugs (Graaf et al., 2004). Recent studies have also shown that statins cause apoptosis in primary endothelial cells of rodent and human origin (Li et al., 2002b; Kaneta et al., 2003). However, in all of these studies, clear apoptosis-inducing effects were only observed at high dose of statins (i.e. ⩾10 μM). The serum concentrations of statins, therapeutically relevant for lowering lipids, are in the low micromolar range (Thibault et al., 1996) and these concentrations of statins are not expected to cause cytotoxic side effects on endothelial cells in vivo. This is in line with the finding that statins are well tolerated in man (as known from their broad clinical application) when used to reduce lipid levels. Thus, the proapoptotic effect of statins, as observed in vitro at high concentrations, may not be relevant for the in vivo situation. Rather, the data presented here show that the protective effects of a low concentration of lovastatin might be more relevant in men, in particular if genotoxic stress is applied.
Doxorubicin exerts pleiotropic effects that account for its anticancer potency, including the formation of ROS and inhibition of topoisomerase II (Muller et al., 1998a). Regarding the latter, both topoisomerase II alpha and beta have been suggested as relevant targets for doxorubicin (Brown et al., 1995; Durbecq et al., 2004). Furthermore, doxorubicin has also been found to inhibit topoisomerase I (Foglesong et al., 1992) and helicase (Bachur et al., 1992). Hence, it was rational to assume that the mechanism(s) underlying protection by lovastatin are multifarious. We found that the gain of doxorubicin resistance by lovastatin is not accompanied by a reduction in doxorubicin-induced ROS formation or a decrease in oxidative DNA damage. Alterations in the efflux of doxorubicin by lovastatin have also not been found. Therefore, we suggest that transport mechanisms are not overtly involved in the observed drug resistant phenotype of HUVEC. Notably, the statin protects HUVEC from doxorubicin-induced DNA strand break formation. H2AX phosphorylation, which is indicative of the formation of DNA double strand breaks (Rothkamm and Lobrich, 2003), was also reduced by the statin. Lovastatin confered cross-resistance to the killing effect of the topoisomerase II-specific inhibitor etoposide and reduced the level of etoposide-triggered DNA damage. Thus, overall, it appears that lovastatin-mediated resistance of HUVEC to doxorubicin is at least partially owing to reduced DNA damage formation resulting from inhibition of topoisomerase II. As a consequence, the well known ATM/ATR-regulated DNA damage-dependent stress responses (Shiloh, 2001), such as accumulation of p53 protein and phosphorylation of Chk-1, were largely attenuated by lovastatin. Doxorubicin has been shown to activate ATM-dependent pathways through ROS formation (Kurz et al., 2004). Yet, as we did not observe any change in ROS production in the presence of lovastatin, we suggest that the impaired ATM/ATR-triggered signalling to p53 and Chk-1 is independent of ROS. Rather, it is rational to assume that it is a reduction in DNA strand break formation that eventually results in a diminished ATM/ATR activation and, consequently, in an attenuated signalling to p53 and Chk-1. Topoisomerase II was shown to interact with p53 both in vitro and in vivo (Cowell et al., 2000), indicating the p53 participates in the regulation of topoisomerase II function. Moreover, it has been suggested that the complex between topoisomerase II and p53 is disrupted by antitumour drugs (Hochhauser et al., 1999). As p53 deficient cells exhibit enhanced doxorubicin resistance (Dunkern et al., 2003), we suggest that abrogation of p53 induction by the statin is relevant for the development of a doxorubicin- and etoposide-resistant phenotype.
As similarly observed for ionizing radiation (Nuebel et al., 2006), doxorubicin did not cause activation of executor caspase-3 in HUVEC. Also, caspase-7 was not activated in HUVEC by doxorubicin, whereas cisplatin effectively stimulates both caspase-3 and -7. These findings indicate that the antiapoptotic effect of lovastatin is not related to an inhibition of the executor caspase-3 and -7. Recently, it was suggested that doxorubicin-triggered apoptosis is related to activation of caspase-2 and c-Jun-N-terminal kinase (SAPK/JNK) (Panaretakis et al., 2005). In line with this study, we showed that doxorubicin causes activation of capase-2 and SAPK/JNK in HUVEC. Activation of caspase-2 was not prevented by lovastatin, whereas stimulation of SAPK/JNK was. This is in line with previous data showing that activation of SAPK/JNK by different types of genotoxins is effectively blocked by lovastatin (Gnad et al., 2000; von Bardeleben et al., 2002; von Bardeleben et al., 2003; Fritz and Kaina, 2006). SAPK/JNK-regulated signalling is considered to be proapoptotic upon application of topoisomerase II poisons (Sordet et al., 2003). Hence, abrogation of doxorubicin-induced SAPK/JNK activation by lovastatin, subsequently leading to an inhibition of SAPK/JNK-promoted cytotoxic functions, appears to be an additional mechanism (apart from p53-related mechanisms) contributing to the doxorubicin resistance of HUVEC. As well as receptor and mitochondrial pathway-induced apopotosis, endoplasmic reticulum (ER) stress can also initiate programmed cell death (Rao et al., 2004). ER stress-triggered death is suggested to involve calpain-promoted caspase-12 and SAPK/JNK (Tan et al., 2006). As doxorubicin has also been implicated in apoptosis via induction of ER stress in vitro and in vivo (Jang et al., 2004), it is important to determine whether lovastatin interferes with ER stress and calpain-promoted cell death.
Overall, the data presented indicate that lovastatin might be clinically effective at antagonizing doxorubicin-induced endothelial dysfunction, which is one of the unwanted side effects of this anticancer drug on normal tissue (Duquaine et al., 2003; Chow et al., 2006). With respect to doxorubicin-induced cardiotoxicity, which is an additional major side-effect of anthracyclines, oxidative stress rather than topoisomerase II inhibition is hypothesized to be the main mechanism involved (Hortobagyi, 1997; Singal et al., 2000). Recently, genetic polymorphisms of nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase have been associated with doxorubicin-induced cardiotoxicity (Wojnowski et al., 2005). As statins are antioxidative (Kowalski et al., 2004; Hayashi et al., 2005) and inhibit Rac-regulated NADPH oxidase function (Delbosc et al., 2002; Gao et al., 2005; Cheng et al., 2006; Miyano et al., 2006), it is tempting to speculate that they might also induce a cardioprotective effect upon doxorubicin exposure. In line with this hypothesis, lovastatin has been shown to reduce doxorubicin-induced cardiotoxicity in mice (Feleszko et al., 2000). Whether or not statins also protect human cardiomyocytes from the deleterious effects of doxorubicin is still uncertain and needs to be examined further.
In summary, we have shown that a low dose of lovastatin reduces the cytotoxic, antiproliferative and apoptotic effects of the anticancer drugs doxorubicin and etoposide in primary human endothelial cells. We suggest that this is due to protection from DNA strand break formation because of a reduced susceptibility of topoisomerase II to its inhibitors. As a result of this reduction in DNA damage, stress responses triggered by DNA damage, including activation of p53, Chks and SAPK/JNK, are attenuated by lovastatin. Thereby, eventually, cell viability is increased. An important question that needs to be answered is whether the therapeutic outcome and/or the severity of side effects of doxorubicin and etoposide-based anticancer therapy are altered in cancer patients who are being treated with statins for cardiovascular protection.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Fr-1241/5-1). We thank C Brachetti for technical assistance and S Schmitt for his support with FACS analyses.
Abbreviations
- Akt
Akt kinase/protein kinase B
- ATM
ataxia telangiectasia mutated
- ATR
ATM and Rad3-related
- BrdU
bromodeoxyuridine
- Chk
checkpoint kinase
- ERK
extracellular regulated kinase
- H2AX
histone H2AX
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- HUVEC
human umbilical vein endothelial cells
- Mdr
multidrug resistance
- Rho
Ras-homologous
- ROS
reactive oxygen species
- SAPK/JNK
stress-activated protein kinases/c-Jun-N-terminal kinases
Conflict of interest
The authors state no conflict of interest.
References
- Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- Bachur NR, Yu F, Johnson R, Hickey R, Wu Y, Malkas L. Helicase inhibition by anthracycline anticancer agents. Mol Pharmacol. 1992;41:993–998. [PubMed] [Google Scholar]
- Brown GA, McPherson JP, Gu L, Hedley DW, Toso R, Deuchars KL, et al. Relationship of DNA topoisomerase II alpha and beta expression to cytotoxicity of antineoplastic agents in human acute lymphoblastic leukemia cell lines. Cancer Res. 1995;55:78–82. [PubMed] [Google Scholar]
- Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26:883–891. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]
- Canman CE, Kastan MB. Signal transduction. Three paths to stress relief. Nature. 1996;384:213–214. doi: 10.1038/384213a0. [DOI] [PubMed] [Google Scholar]
- Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- Chow AY, Chin C, Dahl G, Rosenthal DN. Anthracyclines cause endothelial injury in pediatric cancer patients: a pilot study. J Clin Oncol. 2006;24:925–928. doi: 10.1200/JCO.2005.03.5956. [DOI] [PubMed] [Google Scholar]
- Cooper S. Reappraisal of G1-phase arrest and synchronization by lovastatin. Cell Biol Int. 2002;26:715–727. doi: 10.1006/cbir.2002.0925. [DOI] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cowell IG, Okorokov AL, Cutts SA, Padget K, Bell M, Milner J, et al. Human topoisomerase IIalpha and IIbeta interact with the C-terminal region of p53. Exp Cell Res. 2000;255:86–94. doi: 10.1006/excr.1999.4772. [DOI] [PubMed] [Google Scholar]
- Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol. 2002;40:611–617. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- Dimitroulakos J, Nohynek D, Backway KL, Hedley DW, Yeger H, Freedman MH, et al. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: a potential therapeutic approach. Blood. 1999;93:1308–1318. [PubMed] [Google Scholar]
- Dimitroulakos J, Ye LY, Benzaquen M, Moore MJ, Kamel-Reid S, Freedman MH, et al. Differential sensitivity of various pediatric cancers and squamous cell carcinomas to lovastatin-induced apoptosis: therapeutic implications. Clin Cancer Res. 2001;7:158–167. [PubMed] [Google Scholar]
- Dunkern TR, Wedemeyer I, Baumgartner M, Fritz G, Kaina B. Resistance of p53 knockout cells to doxorubicin is related to reduced formation of DNA strand breaks rather than impaired apoptotic signaling. DNA Repair (Amsterdam) 2003;2:49–60. doi: 10.1016/s1568-7864(02)00185-4. [DOI] [PubMed] [Google Scholar]
- Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, et al. Rapid-onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8:101–107. doi: 10.1191/1358863x03vm476oa. [DOI] [PubMed] [Google Scholar]
- Durbecq V, Paesmans M, Cardoso F, Desmedt C, Di Leo A, Chan S, et al. Topoisomerase-II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol Cancer Ther. 2004;3:1207–1214. [PubMed] [Google Scholar]
- Faneyte IF, Kristel PM, van de Vijver MJ. Multidrug resistance associated genes MRP1, MRP2 and MRP3 in primary and anthracycline exposed breast cancer. Anticancer Res. 2004;24:2931–2939. [PubMed] [Google Scholar]
- Faris M, Kokot N, Latinis K, Kasibhatla S, Green DR, Koretzky GA, et al. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998a;160:134–144. [PubMed] [Google Scholar]
- Faris M, Latinis KM, Kempiak SJ, Koretzky GA, Nel A. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol. 1998b;18:5414–5424. doi: 10.1128/mcb.18.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleszko W, Mlynarczuk I, Balkowiec-Iskra EZ, Czajka A, Switaj T, Stoklosa T, et al. Lovastatin potentiates antitumor activity and attenuates cardiotoxicity of doxorubicin in three tumor models in mice. Clin Cancer Res. 2000;6:2044–2052. [PubMed] [Google Scholar]
- Foglesong PD, Reckord C, Swink S. Doxorubicin inhibits human DNA topoisomerase I. Cancer Chemother Pharmacol. 1992;30:123–125. doi: 10.1007/BF00686403. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Wallner GC, Siede W. DNA Repair and Mutagenesis. ASM Press: Washington; 1995. [Google Scholar]
- Fritz G. HMG-CoA reductase inhibitors (statins) as anticancer drugs (review) Int J Oncol. 2005;27:1401–1409. [PubMed] [Google Scholar]
- Fritz G, Kaina B. Late activation of stress kinases (SAPK/JNK) by genotoxins requires the DNA repair proteins DNA-PKcs and CSB. Mol Biol Cell. 2006;17:851–861. doi: 10.1091/mbc.E05-07-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- Garcia-Escarp M, Martinez-Munoz V, Sales-Pardo I, Barquinero J, Domingo JC, Marin P, et al. Flow cytometry-based approach to ABCG2 function suggests that the transporter differentially handles the influx and efflux of drugs. Cytometry A. 2004;62:129–138. doi: 10.1002/cyto.a.20072. [DOI] [PubMed] [Google Scholar]
- Gnad R, Aktories K, Kaina B, Fritz G. Inhibition of protein isoprenylation impairs Rho-regulated early cellular response to genotoxic stress. Mol Pharmacol. 2000;58:1389–1397. doi: 10.1124/mol.58.6.1389. [DOI] [PubMed] [Google Scholar]
- Gnad R, Kaina B, Fritz G. Rho GTPases are involved in the regulation of NF-kB by genotoxic stress. Exp Cell Res. 2001;264:244–249. doi: 10.1006/excr.2001.5165. [DOI] [PubMed] [Google Scholar]
- Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hamakawa K, Nagotani S, Jin G, Li F, Deguchi K, et al. HMG CoA reductase inhibitors reduce ischemic brain injury of Wistar rats through decreasing oxidative stress on neurons. Brain Res. 2005;1037:52–58. doi: 10.1016/j.brainres.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Hochhauser D, Valkov NI, Gump JL, Wei I, O'Hare C, Hartley J, et al. Effects of wild-type p53 expression on the quantity and activity of topoisomerase IIalpha and beta in various human cancer cell lines. J Cell Biochem. 1999;75:245–257. doi: 10.1002/(sici)1097-4644(19991101)75:2<245::aid-jcb7>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54 Suppl 4:1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- Kaneta S, Satoh K, Kano S, Kanda M, Ichihara K. All hydrophobic HMG-CoA reductase inhibitors induce apoptotic death in rat pulmonary vein endothelial cells. Atherosclerosis. 2003;170:237–243. doi: 10.1016/s0021-9150(03)00301-0. [DOI] [PubMed] [Google Scholar]
- Koch G, Benz C, Schmidt G, Olenik C, Aktories K. Role of Rho protein in lovastatin-induced breakdown of actin cytoskeleton. J Pharmacol Exp Ther. 1997;283:901–909. [PubMed] [Google Scholar]
- Kowalski J, Pawlicki L, Grycewicz J, Blaszczyk J, Irzmanski R, Ceglinski T, et al. Plasma antioxidative activity during atorvastatin and fluvastatin therapy used in coronary heart disease primary prevention. Fundam Clin Pharmacol. 2004;18:93–96. doi: 10.1046/j.0767-3981.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem. 2004;279:53272–53281. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002a;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- Li X, Liu L, Tupper JC, Bannerman DD, Winn RK, Sebti SM, et al. Inhibition of protein geranylgeranylation and RhoA/RhoA kinase pathway induces apoptosis in human endothelial cells. J Biol Chem. 2002b;277:15309–15316. doi: 10.1074/jbc.M201253200. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–1453. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Muller I, Niethammer D, Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity (review) Int J Mol Med. 1998a;1:491–494. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998b;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuebel T, Damrot J, Roos WP, Kaina B, Fritz G. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res. 2006;12:933–939. doi: 10.1158/1078-0432.CCR-05-1903. [DOI] [PubMed] [Google Scholar]
- Osterod M, Larsen E, Le Page F, Hengstler JG, Van Der Horst GT, Boiteux S, et al. A global DNA repair mechanism involving the Cockayne syndrome B (CSB) gene product can prevent the in vivo accumulation of endogenous oxidative DNA base damage. Oncogene. 2002;21:8232–8239. doi: 10.1038/sj.onc.1206027. [DOI] [PubMed] [Google Scholar]
- Panaretakis T, Laane E, Pokrovskaja K, Bjorklund AC, Moustakas A, Zhivotovsky B, et al. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16:3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. 1998;17:2393–2402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. ATM and ATR: networking cellular responses to DNA damage. Curr Opin Genet Dev. 2001;11:71–77. doi: 10.1016/s0959-437x(00)00159-3. [DOI] [PubMed] [Google Scholar]
- Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- Sordet O, Khan QA, Kohn KW, Pommier Y. Apoptosis induced by topoisomerase inhibitors. Curr Med Chem Anticancer Agents. 2003;3:271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. Ubiquitous calpains promote caspase-12 and Jnk activation during ER stress-induced apoptosis. J Biol Chem. 2006;281:16016–16024. doi: 10.1074/jbc.M601299200. [DOI] [PubMed] [Google Scholar]
- Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2:483–491. [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- von Bardeleben R, Dunkern T, Kaina B, Fritz G. The HMG-CoA reductase inhibitor lovastatin protects cells from the antineoplastic drugs doxorubicin and etoposide. Int J Mol Med. 2002;10:473–479. [PubMed] [Google Scholar]
- von Bardeleben R, Kaina B, Fritz B. Ultraviolet light-induced apoptotic death is impaired by the HMG-CoA reductase inhibitor lovastatin. Biochem Biophys Res Commun. 2003;307:401–407. doi: 10.1016/s0006-291x(03)01205-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev. 2005;15:62–68. doi: 10.1016/j.gde.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- Wu X, Hasinoff BB. The antitumor anthracyclines doxorubicin and daunorubicin do not inhibit cell growth through the formation of iron-mediated reactive oxygen species. Anticancer Drugs. 2005;16:93–99. doi: 10.1097/00001813-200501000-00014. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Caspase-2 function in response to DNA damage. Biochem Biophys Res Commun. 2005;331:859–867. doi: 10.1016/j.bbrc.2005.03.191. [DOI] [PubMed] [Google Scholar]