Abstract
Background and purpose:
Sulphatides are sulphated glycosphingolipids expressed on the surface of many cell types, particularly neurones. Changes in sulphatide species or content have been associated with epilepsy and Alzheimer's disease. As the large conductance, calcium sensitive K+ channel (BKCa) are modulated by membrane lipids, the aim of the study was to explore possible effects of sulphatides on BKCa channels.
Experimental approach:
Using patch-clamp techniques, we studied effects of exogenous sulphatides on BKCa channels expressed in Chinese hamster ovary cells.
Key results:
Sulphatides reversibly increased the whole-cell current and the single channel open probability of BKCa channels dose-dependently. The EC50 value on the channel at +10 mV was 1.6 μM and the Hill coefficient was 2.5. In inside-out patches, sulphatides increased the single channel open probability from both intra- and extra-cellular faces of the membrane, but more effectively with external application. Furthermore, activation of the channels by sulphatides was independent of intracellular Ca2+ concentration. Sulphatides also shifted the activation curve of the channels to less positive membrane potentials. Mutant BKCa channels lacking a 59 aminoacid region important for amphipath activation (STREX) were less activated by the sulphatides.
Conclusions and Implications:
Sulphatides are novel activators of BKCa channels, independent of intracellular Ca2+ or other signalling molecules but partly dependent on the STREX sequence of the channel protein. As changes of sulphatide content are associated with neuronal dysfunction, as in epilepsy and Alzheimer's disease, our results imply that these effects of sulphatides may play important pathophysiological roles in regulation of BKCa channels.
Keywords: sulphatides, BKCa channel, cell membrane, glycosphingolipid, STREX
Introduction
Sulphatides, consisting primarily of galactocerebroside sulphate, are a major species of glycosphingolipids in the central and peripheral nervous systems (Ishizuka, 1997; Isaac et al., 2006). Sulphatides are critical to the formation and maintenance of myelin and axon structure in the central nervous system (Dupree et al., 1998; Marcus et al., 2006). Besides, sulphatides can alter cytokine production in mononuclear cells (Bovin et al., 1999), facilitate ionic transportation in kidney tubule cells (Vos et al., 1994), influence cytokine and chemokine production in whole-blood cells (Roeske-Nielsen et al., 2004) and moderate insulin secretion in pancreatic beta cells (Buschard et al., 2005). Sulphatides have been shown to be located in the plasma membrane and enriched in detergent-insoluble microdomains (Blomqvist et al., 2003), suggesting that sulphatides participate in regulating the function of membrane proteins, such as ion channels. Indeed, sulphatides are critical to the proper localization and maintenance of ion channel clusters on myelinated axons (Ishibashi et al., 2002). Sulphatides have also been shown to control insulin secretion by modulation of ATP-sensitive K+ channel activity in rat pancreatic beta cells (Buschard et al., 2002). Mass spectrometric analysis has revealed changes in sulphatide species in progressive epilepsy with mental retardation (Hermansson et al., 2005). A marked decrease in sulphatides has been reported to be associated with pathology of Alzheimer's disease (Han et al., 2002; Irizarry, 2003). As integral membrane proteins lodged in the lipid environment of the plasma membrane, ion channels are modulated by dynamic alterations in the microenvironment of the membrane (Ordway et al., 1989; Barrantes, 2002; Tillman and Cascio, 2003). Therefore, perturbations of the membrane microenvironment caused by changes in membrane sulphatides are likely to affect function of some ion channels.
The large conductance, calcium-sensitive, K+ channel (BKCa channel) is a voltage-activated ion channel in which direct calcium binding shifts gating to more negative cellular membrane potentials. It has been reported that some types of the BKCa channel can also be modulated by their lipid membrane environments (Kirber et al., 1992; Chang et al., 1995; Denson et al., 2000; Clarke et al., 2002; Park et al., 2003; Lam et al., 2004; Wolfram Kuhlmann et al., 2004; Qi et al., 2005). The aim of this study was to explore a possible influence of sulphatides on BKCa channels. To this end, we applied patch-clamp techniques to study the effect of sulphatides on the BKCa channels that were expressed in Chinese hamster ovary (CHO) cells. We have demonstrated that sulphatides were potent openers of BKCa channels that activated the channel by increasing single-channel open probability (Po) in a dose-dependent manner.
Methods
Cell culture and gene transfection
CHO-K1 cells were grown in Ham's F-12 nutrient mixture (Invitrogen Co., Grand Island, NY, USA) supplemented with 10% foetal bovine serum. Cells were grown in a 37°C incubator with 5% CO2 humidified environment and passaged twice weekly through exposure to 0.05% trypsin, 0.5 mM ethylenediaminetetraacetic acid in phosphate-buffered saline(−) solution. For gene transfection, CHO-K1 cells were transferred to poly-L-lysine- (Sigma, St Louis, MO, USA) coated glass coverslips. After cell density reached 50–70% confluence, phosphorylated enhanced green fluorescent protein (GFP) (Clontech, Palo Alto, CA, USA) was transiently coexpressed with the BKCa channel gene (a generous gift from Dr Sokabe and Dr Naruse, Nagoya University) that is in mammalian expression vectors (pTarget, Promega, Madison, WI, USA) at a ratio of 5:1 (weight/weight) using LipofectAMINE Plus reagent (Invitrogen). Cells were used for electrophysiological studies for 1–3 days after the transfection.
Electrophysiology
The whole-cell currents were measured using a conventional tight seal whole-cell recording technique at room temperature. Only GFP-positive cells were selected for the patch-clamp experiments. Virtually no detectable endogenous BKCa channel activity was observed in non-transfected CHO cells. Pipettes pulled by a List Medical vertical pipette puller (L/M-3P-A) were fire-polished to give a resistance in a range of 3–7 MΩ in recording solution containing (in mM): 145 KCl, 10 ethyleneglycoltetraacetate EGTA and 10 HEPES (pH 7.3 with KOH), 1 CaCl2. The extracellular solution was Hanks' balanced salts solution (HBSS, Sigma): 1.3 CaCl2, 0.8 MgSO4, 5.4 KCl, 0.4 KH2PO4, 136.9 NaCl, 0.3 Na2PO4, 10 D-glucose and 4.2 NaHCO3.
Compensation for cell capacitance and series resistance was made automatically by an EPC-10 amplifier; only recordings with stable series resistances ⩽25MΩ were included in the study. Currents were amplified using the EPC-10 patch-clamp amplifier (HEKA), sampled at 2∼5 kHz and filtered at 1.5∼2.9 kHz via a four-pole low-pass Bessel filter. The programme package Patchmaster 2.1 and TAC 4.1 (HEKA, Germany) were used for data acquisition and analysis. The probability of a single channel being open (Po) was simply calculated from the total time spent in the open state divided by the total time of the recording for the patches containing a single channel. Continuous recordings of 2000–4000 ms were used to estimate Po. When multiple channels were present in a patch, the Po was calculated from the amplitude histogram as Po=(1−PC1/N), where PC is the fraction of area under the closed state, and N is the number of channels. To avoid underestimation of the number of channels in a patch, the number of active single channels in the patch was counted at the condition where maximum number of channels was observed. Data were obtained from at least three experiments and presented as mean±s.e.m. Intracellular calcium concentration ([Ca2+]i) was buffered to be at nanomolar level with 10 mM EGTA on the basis of calculations using a free software CALCON3 (http://user.ecc.u-tokyo.ac.jp/~ckam/calcon.html, Takeshi Tojo and Go Nakayama, 1995), unless otherwise noted. To avoid changes in [Ca2+]i, a more efficient Ca2+ buffer, BAPTA (10 mM), was used in some experiments. All experiments of single-channel recordings performed in this study were conducted with the inside-out configuration. The Origin programme (OriginLab) using non-linear regression methods was used to obtain the best-fitted curve to the experimental results.
Statistical analysis
The difference between each set of original data was analysed with one-way analysis of variance and significance was set at P<0.01.
Materials
Bovine sulphatides (cerebroside sulphate from bovine brain, Sigma) were dissolved in dimethyl sulphoxide (DMSO) as a stock solution and dissolved into the intra- or extracellular solutions to different concentrations as required. The amount of DMSO was less than 0.5% per assay. This amount of DMSO did not have noticeable effects on the channel behaviour. Other chemicals and the HBSS were also obtained from Sigma.
Results
Effect of sulphatides on the whole-cell BKCa channel currents
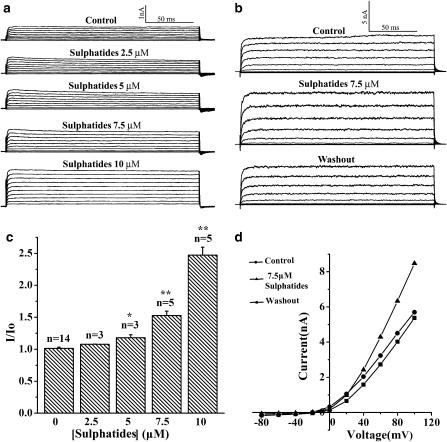
The whole-cell configuration was used to investigate the effect of sulphatides on macroscopic ionic currents of BKCa channels expressed in CHO cells. Figure 1a clearly shows graded increases in the whole-cell BKCa channel currents from the same cell in response to increases in the concentration of sulphatides from 2.5 to 10 μM. In this cell, the current at +100 mV was 0.75 nA in control, then it was gradually increased to 0.82, 0.97, 1.2 and 2.0 nA in response to addition of 2.5, 5, 7.5 and 10 μM sulphatide, indicating a dose-dependent activation of the channels by the sulphatides. A dose-dependent effect of sulphatides on the channels was also shown by the statistics of the data in Figure 1c. At a concentration of 10 μM, sulphatides increased the current as much as 2.5-fold the control value. Furthermore, Figure 1b and d shows a reversible effect of sulphatides on the regulation of the channel. At a voltage of +100 mV, the current was increased by 7.5 μM sulphatide from 5.9 nA (the control) to 8.5 nA and returned to 5.4 nA after washout.
Figure 1.
Effect of sulphatide on the whole-cell BKCa current. (a) Current traces showing effect of different sulphatide concentrations on the whole-cell BKCa channel current from the same cell (representative for three cells). The current was activated by rectangular voltage steps from −80 to +100 mV for 200 ms with 10 mV increment. (b) Current traces before, during and after washout of sulphatide (representative for three cells). (c) Ratio values of the whole-cell current before (Io) and after (I) application of sulphatides (2.5–10 μM) are summarized. Asterisk indicates significant differences between control and different sulphatide concentrations on the current (*P<0.005, **P<0.001, one-way AVOVA). (d) Current–voltage relationship for the traces in (b).
Effect of sulphatides on the probability of a single BKCa channel being open (Po)
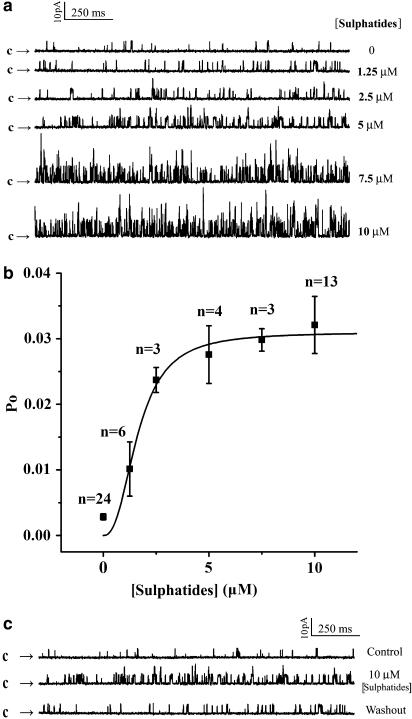
To examine whether the increase in the whole-cell currents of the BKCa channels by sulphatides was due to some diffusible intracellular signal molecules, the relationship between sulphatide concentration and Po of the single BKCa channels was further examined at a holding potential of +10 mV at various sulphatide concentrations in excised inside-out patches. As shown in Figure 2a, application of different sulphatide concentrations from 1.25 to 10 μM to the cytosolic surface of an inside-out patch greatly enhanced Po. In this patch, the Po of BKCa channels at +10 mV was 1.0% in control and increased to 2.1, 3.0, 7.3, 7.7 and 8.8% in the presence of 1.25, 2.5, 5, 7.5 and 10 μM sulphatides, respectively. As shown in Figure 2b, sulphatides (1.25–10 μM) effectively increased the Po of the channel in a dose-dependent manner (n=3–24). The increase in Po of the channel with increasing sulphatide concentration was fitted by the Hill equation: Po=Pmax*[sulphatide]N/(KN+[sulphatide]N), where Pmax is the maximum Po of the channel at the experimental condition, K is the sulphatide concentration producing the half-maximal activity and N is the Hill coefficient. The best fit to the mean data was obtained with values of 0.031 for Pmax, 1.6 μM for K and 2.5 for N, suggesting that there was positive cooperation for the stimulation of the BKCa channels by sulphatides. This effect was reversible; the Po of the channel was 1.2% under control conditions, increased to 5.5% by 10 μM sulphatides and then returned back to 1.5% after washout (Figure 2c, n=6).
Figure 2.
Increase in open probability (Po) of the single BKCa channels by intracellular application of sulphatide at inside-out patches. (a) Current traces before (control) and after successive applications of sulphatide (1.25–10 μM) to the intracellular face of channels on the same patch. (b) Dose–response curve for the effect of sulphatide on the Po of the BKCa channels. Solid line is the best fit to the Hill equation (see text). (c) Current traces before, during application and washout of 10 μM sulphatide (representative for six cells). Channel openings are shown as an upward deflection. The arrows indicate the level at which all the channels are at their closed state.
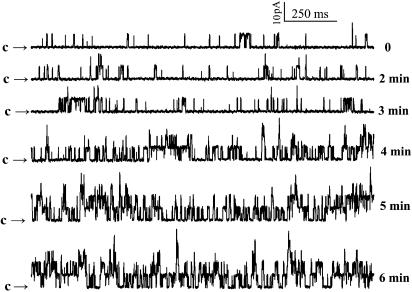
Next, by back-filling the pipette with a sulphatide-containing (10 μM) solution after filling the pipette tip with sulphatide-free solution, we examined the effect of extracellular application of sulphatides on the single-channel properties at inside-out patches. Owing to a slow diffusion of sulphatides towards the patch membrane at the pipette tip, the values of Po were increased slowly from 0.9 to 5.3, 7.7, 24.7, 31.1 and 33.2% with time of 0 to 2, 3, 4, 5 and 6 min after a seal was made in inside-out configuration (Figure 3). During this process, no change in the current amplitude was observed. This result indicates that the increases in the whole-cell currents by sulphatides were due to increases in the Po of the channels and not due to an increase in the single-channel conductance. The increase in Po by both extra- and intracellular sulphatides in patches excised from the cell membrane strongly suggests that their actions were not mediated by diffusible intracellular signal molecules. It is noteworthy that the stable value of Po that corresponded to 10 μM sulphatide was 33.2%, much higher than that obtained with the same concentration of sulphatide given intracellularly, suggesting that the sulphatides applied externally were much more effective than those applied internally.
Figure 3.
Effect of extracellularly applied sulphatide on the Po. The pipette is back-filled with a sulphatide-containing (10 μM) solution after filling the pipette tip with sulphatide-free solution. The recordings started when the seal was made (0 min) in inside-out patches at a voltage of +10 mV (representative for three patches). Arrows indicate the level at which all the channels are in their closed state.
Effect of [Ca2+]i on activity of BKCa channels stimulated by sulphatides
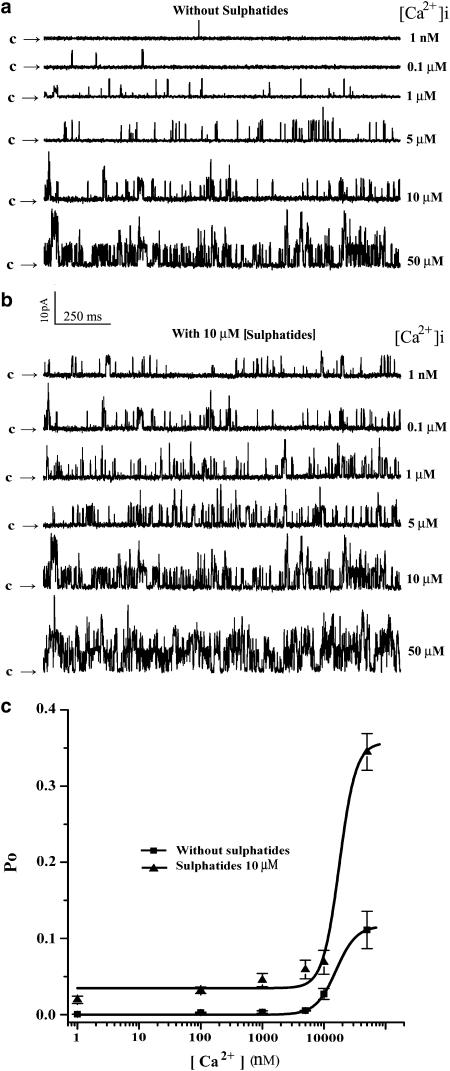
To determine whether the sulphatide-induced increase in Po of the channel is dependent on [Ca2+]i, the single-channel currents were recorded at +10 mV, and membrane patches were exposed to internal solutions containing 1 nM, 0.1, 1, 5, 10 and 50 μM concentrations of free Ca2+. Figure 4a and b shows single-channel recordings in control and at 10 μM sulphatide, respectively. In control conditions, the Po of the single BKCa channels gradually increased from 0.05 to 12.7% with increase in [Ca2+]i from 1 nM to 50 μM. In contrast, application of sulphatides induced an increase in the channel openings from Po of 1.8 to 30.4% corresponding to the same [Ca2+]i as that of the control. Comparing Figure 4a and b, it is clear that the sulphatides could enhance the single-channel openings even when the [Ca2+]i was buffered to levels as low as 1 nM. Figure 4c shows the relationship between the Po of the BKCa channel and [Ca2+]i in the absence and presence of 10 μM sulphatide in the cytoplasmic side of the channel (n=3–24). Clearly, the activation of BKCa channels by sulphatides was much effective at physiological Ca2+ concentration (less than 10 μM). The increase in Po of the channel with [Ca2+]i in both conditions was fitted by the Hill equation: Po=[Ca2+]N/(KN+[Ca2+]N)+Psul, where K is the [Ca2+]i producing the half-maximal activity, N is the Hill coefficient and Psul is the Po in the presence of sulphatide without Ca2+. The best fit to the mean data was obtained with values of 15.7 μM for K and 2.6 for N in the absence of sulphatides and with values of 18.0 μM for K, 3.2 for N and 3% for Psul in the presence of 10 μM sulphatide. It is worth noting that this effect of sulphatides was also observed when 0.1 μM intracellular Ca2+ was buffered with 10 mM BAPTA, implying that the channel activation induced by sulphatides is independent of [Ca2+]i.
Figure 4.
Ca2+ dependence of sulphatide induced increase in Po of the channel. Current traces showing effect of sulphatide on the BKCa channels at various concentrations of [Ca2+]i in the bath before (a) and during exposure to 10 μM sulphatide (b) at a holding potential of +10 mV. (c) Statistics of the data (n=3–24). Solid line is the best fit to the Hill equation (see text).
Effect of sulphatides on voltage-dependent activation of BKCa channels
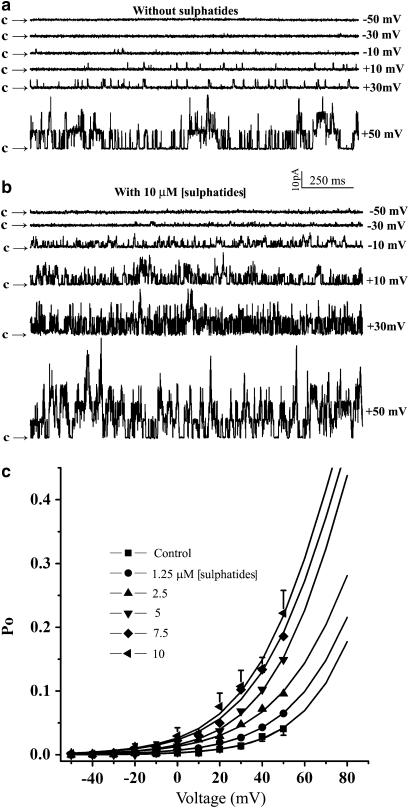
The effect of sulphatides on the activity of the BKCa channels at 0.1. μM [Ca2+]i corresponding to the resting [Ca2+]i level for many types of cells and various membrane potentials was also examined (Figure 5). Figure 5a shows current traces from an excised patch at different membrane potentials before addition of sulphatides. A low activity of BKCa channels was observed at a membrane potential of −10 mV (Po=0.3%), and the Po of the channel was progressively increased to 7.5% at a depolarized membrane potential of +50 mV. In contrast, in the presence of 10 μM sulphatide, the Po of the channel was 6.1% at −10 mV and 47% at +50 mV (Figure 5b), indicating that sulphatides strongly increased the voltage dependence of the channel. Figure 5c shows the Po of the BKCa channels as a function of the membrane potential in the absence and presence of 1.25, 2.5, 5, 7.5 and 10 μM sulphatides (n=6–24). The data were fitted to the Boltzmann equation: where V is voltage and V1/2 is the voltage producing the half-maximal activity. Values of +109 mV for V1/2 and +19 mV for k were obtained in the absence of sulphatides. In contrast, +77 mV for V1/2 and 21 mV for k were obtained at 10 μM sulphatide. Thus, application of sulphatides not only produced an increase in the maximal opening probability of the BKCa channels, but also significantly shifted the activation curve to the less positive potential. It is clear that sulphatides activated the channel as indicated by a parallel leftward shift of Po–V relations on the voltage axis without affecting the slope of the curve, suggesting that the voltage sensitivity of the channel was not altered by sulphatides.
Figure 5.
Voltage dependence of sulphatide induced increase in Po of the channel. Representative current traces from an inside-out patch before (a) and during exposure to 10 μM sulphatide (b) at various voltage. (c) Effect of sulphatides (1.25–10 μM) on the activation curve of the BKCa channels (n=6–24). Only the error bars for effect of 1.25 and 10 μM sulphatide on the channels are shown, others are omitted for clarity.
STREX partially contributed to the BKCa channel activation by sulphatides
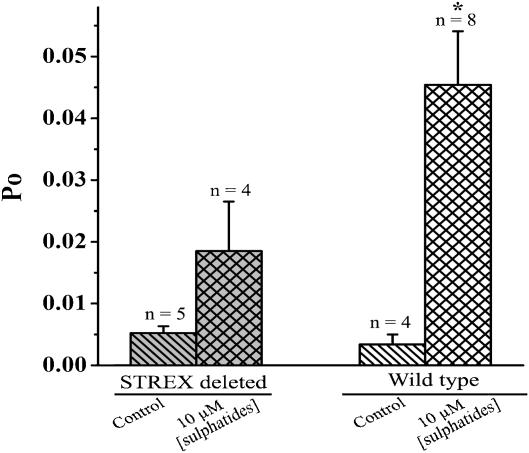
In a previous study, we found that STREX, a 59 amino-acid splice insert located in the cytoplasmic side of the channel, is important for the amphipath activation of the BKCa channels (Qi et al., 2005). Sulphatide is composed of a slightly larger charged hydrophilic head and a hydrophobic tail. Therefore, insertion of sulphatides may cause local membrane stress, which in turn may activate the channel as we found with the amphipaths. To test if STREX contributes to the activation of the BKCa channel by sulphatides, we studied the effect of sulphatides on the STREX-deleted mutant of the channel at the single-channel level. Figure 6 shows that addition of 10 μM sulphatides induced a 13.3-fold increase in Po for wild-type (from 0.34 to 4.5%) and only a 3.5-fold increase for that of STREX-deleted mutant channel (from 0.53 to 1.8%). This result suggests that the STREX region contributed partially to the activation of BKCa channels by sulphatides.
Figure 6.
Comparison of the effect of sulphatide on the activation of the wild-type and the STREX-deleted BKCa channels. Statistics of the data was obtained from recordings at a voltage of +10 mV and 1 μM [Ca2+]i. Asterisk indicates significant difference between control and 10 μM sulphatide on the wild-type BKCa channels (*P<0.01).
Discussion and conclusions
The patch-clamp technique provides a powerful tool to eliminate involvement of intracellular factors in the channel activation by enabling the excision of membrane patches, where little enzymatic machinery is thought to be present. Because inside-out patches are detached from the cell, the persistence of responses to sulphatides in these patches not only suggests a relatively direct action on the channel protein itself or a membrane component closely related to the channel complex, but also rules out forward trafficking of vesicles to recruit new channels as the mechanism underlying the action of sulphatides. Therefore, these results suggest that preservation of the intracellular signalling milieu is not necessary. Increases in the activity of BKCa channels mediated by sulphatides are also unlikely to result from an increase in [Ca2+]i. Our Ca2+ imaging experiments indicated that sulphatides did not induce [Ca2+]i increase in CHO cells (data not shown), suggesting that the sulphatides did not exert their effect on the BKCa channel activity via increased [Ca2+]i. Besides, sulphatides increased channel activity when intracellular solutions contained 10 mM BAPTA, which provided a large buffer against Ca2+ changes, suggesting that the increase in the Po of the channel owing to sulphatides was not likely to be due to changes in [Ca2+]i. In addition, insertion of charged sulphatide may change the surface charge distribution of the membrane, which in turn may influence voltage-dependent gating of the channel (Cukierman et al., 1988). However, we think that the surface charge effect is not the main factor in the effects we have observed, for at least two reasons: firstly, the BKCa channel was activated by both extra- and intracellular application of sulphatides and, secondly, the single-channel amplitude was not changed upon application of sulphatides.
There is a growing body of evidence suggesting that ion channels are regulated by their lipid environment (Barrantes, 2002; Tillman and Cascio, 2003). On the other hand, it has been shown that sulphatide is localized in the plasma membrane (Vos et al., 1994; Bansal et al., 1999) and exogenous sulphatides can incorporate into the plasma membrane (Fantini et al., 1998). Thus, it is reasonable to assume that an increase in the plasma membrane concentration of sulphatides was responsible for the activation of the BKCa channels by sulphatides. This hypothesis is supported by data from the single-channel recordings, where sulphatides could activate the channel from both intra- and extracellular faces of the membrane, suggesting that the plasma membrane might be the site of action of the sulphatides.
The activation of the BKCa channels would drive membrane potential towards the potassium equilibrium potential, which gives the BKCa channel a significant physiological role in controlling the excitability of nerve (Vergara et al., 1998), muscle (Jaggar et al., 2000), and other cells by stabilizing the cell membrane at negative potentials (Kaczorowski et al., 1996). Therefore, as BKCa channel openers, sulphatides may be effective in protecting neurons from damage after an ischaemic stroke and/or suppressing excess activity of smooth muscles as other BKCa channel openers (Lawson, 2000; Shieh et al., 2000; Du et al., 2005; Ghatta et al., 2006).
In summary, the major findings in our present study are as follows: sulphatides are novel activators of the BKCa channel, stimulating the activity of these channels, independent of intracellular factors. The voltage- and Ca2+-dependent activation of the channel are strongly enhanced by the application of sulphatides. Sulphatides induced increased Po of the channels dose dependently but did not modify the single-channel conductance. Sulphatides increased the Po of the channels from both intra- and extracellular faces of the membrane but more effectively with external sulphatide. Finally, the STREX region of the channel partially contributes to the activation of the channel by sulphatides. These data provide evidence that sulphatides are potent in stimulating activity of BKCa channels. As BKCa channels participate in many pathophysiological processes (Ghatta et al., 2006) and changes in sulphatide content are associated with diseases of neuronal dysfunction such as epilepsy and Alzheimer's disease, our results imply that the sulphatides play important physiological roles in regulation of the BKCa channel.
Acknowledgments
We thank Ms MY Huang for technical assistance. This work was partly supported by NSFC (30470447) and 973 programme (2005CB522804).
Abbreviations
- BKCa
high-conductance Ca2+-activated potassium channel
- Po
single-channel open probability
- [sulphatide]
concentration of sulphatides
- [Ca2+]i
intracellular Ca2+ concentration
- STREX
a 59 amino-acid splice insert of BKCa channel
Conflict of interest
The authors state no conflict of interest.
References
- Bansal R, Winkler S, Bheddah S. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci. 1999;19:7913–7924. doi: 10.1523/JNEUROSCI.19-18-07913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes FJ. Lipid matters: nicotinic acetylcholine receptor-lipid interactions. Mol Membr Biol. 2002;19:277–284. doi: 10.1080/09687680210166226. [DOI] [PubMed] [Google Scholar]
- Blomqvist M, Osterbye T, Mansson JE, Horn T, Buschard K, Fredman P. Sulphatide is associated with insulin granules and located to microdomains of a cultured beta cell line. Glycoconj J. 2003;19:403–413. doi: 10.1023/B:GLYC.0000004012.14438.e6. [DOI] [PubMed] [Google Scholar]
- Bovin LF, Fredman P, Månsson JE, Buschard K, Bendtzen K. In vitro production of cytokines is influenced by sulphatide and its precursor galactosylceramide. FEBS Lett. 1999;455:339–343. doi: 10.1016/s0014-5793(99)00908-4. [DOI] [PubMed] [Google Scholar]
- Buschard K, Blomqvist M, Osterbye T, Fredman P. Involvement of sulphatide in beta cells and type 1 and type 2 diabetes. Diabetologia. 2005;48:1957–1962. doi: 10.1007/s00125-005-1926-9. [DOI] [PubMed] [Google Scholar]
- Buschard K, Hoy M, Bokvist K, Olsen HL, Madsbad S, Fredman P, et al. Sulphatide controls insulin secretion by modulation of ATP-sensitive K(+)-channel activity and Ca(2+)-dependent exocytosis in rat pancreatic beta-cells. Diabetes. 2002;51:2514–2521. doi: 10.2337/diabetes.51.8.2514. [DOI] [PubMed] [Google Scholar]
- Chang HM, Reitstetter R, Gruener R. Lipid-ion channel interactions: increasing phospholipid headgroup size but not ordering acyl chains alters reconstituted channel behavior. J Membr Biol. 1995;145:13–19. doi: 10.1007/BF00233303. [DOI] [PubMed] [Google Scholar]
- Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am J Physiol. 2002;283:C1441–C1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- Cukierman S, Zinkand WC, French RJ, Krueger BK. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. J Gen Physiol. 1988;92:431–447. doi: 10.1085/jgp.92.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson DD, Wang X, Worrell RT, Eaton DC. Effects of fatty acids on BK channels in GH(3) cells. Am J Physiol. 2000;279:C1211–C1219. doi: 10.1152/ajpcell.2000.279.4.C1211. [DOI] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Suzuki K, Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulphatide. J Neurocytol. 1998;27:649–659. doi: 10.1023/a:1006908013972. [DOI] [PubMed] [Google Scholar]
- Fantini J, Hammache D, Delezay O, Pieroni G, Tamalet C, Yahi N. Sulphatide inhibits HIV-1 entry into CD4−/CXCR4+ cells. Virology. 1998;246:211–220. doi: 10.1006/viro.1998.9216. [DOI] [PubMed] [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O'rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther. 2006;110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Han XM, Holtzman D, Mckeel DW, Jr, Kelley J, Morris JC. Substantial sulphatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hermansson M, Kakela R, Berghall M, Lehesjoki AE, Somerharju P, Lahtinen U. Mass spectrometric analysis reveals changes in phospholipid, neutral sphingolipid and sulphatide molecular species in progressive epilepsy with mental retardation, EPMR, brain: a case study. J Neurochem. 2005;95:609–617. doi: 10.1111/j.1471-4159.2005.03376.x. [DOI] [PubMed] [Google Scholar]
- Irizarry MC. A turn of the sulphatide in Alzheimer's disease. Ann Neurol. 2003;54:7–8. doi: 10.1002/ana.10642. [DOI] [PubMed] [Google Scholar]
- Isaac G, Pernber Z, Gieselmann V, Hansson E, Bergquist J, Mansson JE. Sulphatide with short fatty acid dominates in astrocytes and neurons. FEBS J. 2006;273:1782–1790. doi: 10.1111/j.1742-4658.2006.05195.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, et al. A myelin galactolipid, sulphatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci. 2002;22:6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka I. Chemistry and functional distribution of sulfoglycolipids. Prog Lipid Res. 1997;36:245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, Knaus HG, Leonard RJ, Mcmanus OB, Garcia ML. High conductance calcium-activated potassium channels: structure, pharmacology, and function. J Bioenerg Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- Kirber MT, Ordway RW, Clapp LH, Walsh JV, Jr, Singer JJ. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- Lam RS, Shaw AR, Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim Biophys Acta. 2004;1667:241–248. doi: 10.1016/j.bbamem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Lawson K. Is there a role for potassium channel openers in neuronal ion channel disorders. Expert Opin Investig Drugs. 2000;9:2269–2280. doi: 10.1517/13543784.9.10.2269. [DOI] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulphatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Walsh JV, Jr, Singer JJ. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989;244:1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Park JB, Kim HJ, Ryu PD, Moczydlowski E. Effect of phosphatidylserine on unitary conductance and Ba2+ block of the BK Ca2+-activated K+ channel: re-examination of the surface charge hypothesis. J Gen Physiol. 2003;121:375–397. doi: 10.1085/jgp.200208746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Chi S, Su X, Naruse K, Sokabe M. Activation of a mechanosensitive BK channel by membrane stress created with amphipaths. Mol Membr Biol. 2005;22:519–527. doi: 10.1080/09687860500370703. [DOI] [PubMed] [Google Scholar]
- Roeske-Nielsen A, Fredman P, Mansson JE, Bendtzen K, Buschard K. Beta-galactosylceramide increases and sulphatide decreases cytokine and chemokine production in whole blood cells. Immunol Lett. 2004;91:205–211. doi: 10.1016/j.imlet.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Shieh CC, Coghlan MC, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases and therapeutic opportunities. Pharmacol Rev. 2000;52:557–593. [PubMed] [Google Scholar]
- Tillman TS, Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion N, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Vos JP, Lopes-Cardozo M, Gadella BM. Metabolic and functional aspect of sulfogalactolipids. Biochim Biophys Acta. 1994;1211:125–149. doi: 10.1016/0005-2760(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Wolfram Kuhlmann CR, Wiebke Ludders D, Schaefer CA, Kerstin Most A, Backenkohler U, Neumann T, et al. Lysophosphatidylcholine-induced modulation of Ca2+-activated K+ channels contributes to ROS-dependent proliferation of cultured human endothelial cells. J Mol Cell Cardiol. 2004;36:675–682. doi: 10.1016/j.yjmcc.2004.03.001. [DOI] [PubMed] [Google Scholar]