Abstract
Background and purpose:
Recent studies on blood vessels have provided evidence that testosterone may exert direct effects on smooth muscle. However, an acute effect on airway reactivity has not been shown yet. The aim of this study was to assess the direct effect of testosterone on the responsiveness of male adult rabbit airway smooth muscle (ASM), precontracted with 10 μM acetylcholine, 10μM carbachol or 80 mM KCl.
Experimental approach:
Contractility studies of rabbit tracheal smooth muscle were performed.
Key results:
Testosterone at concentrations of or above 1 nM had a significant relaxant effect on ASM precontracted with acetylcholine or carbachol, but did not affect ASM precontracted with KCl. The mechanical removal of airway epithelium as well as the inhibition of NO synthetase (by 100μM L-NAME) reduced the relaxation caused by testosterone. The effect of testosterone was not altered by impairing prostanoid synthesis (by 10μM indomethacin). The nitric oxide donor, sodium nitroprusside, had the same relaxant effect on ASM precontracted with either carbachol or KCl. Inhibitors of androgen receptors (10μM flutamide) or DNA transcription (100μM actinomycin D) did not alter the effect of testosterone. Prolonged incubation of ASM with 100 nM or 100 μM testosterone for 24 or 48 h did not alter their responsiveness to acetylcholine. BSA-testosterone (1pM to 100nM) relaxed significantly ASM precontracted with carbachol. The mechanical removal of airway epithelium abolished the relaxant effect of BSA-testosterone.
Conclusions and implications:
Testosterone relaxes precontracted ASM via an epithelium and NO-mediated way. This effect is mediated via a non-genomic pathway.
Keywords: airway smooth muscle, testosterone, epithelium, non genomic pathway, rabbit
Introduction
Epidemiological data suggest a role for sex hormones in the aetiology and/or evolution of some airway diseases, in particular, asthma. Gender differences in the incidence of asthma are attributed mainly to differences in the immune response as testosterone is considered to be immunosuppressant while female sex steroids are proinflammatory (Baizano et al., 2001; Osman 2003). Nevertheless, it is possible that sex hormones may have a direct effect on airway function and development. Recent studies have shown that the treatment of ovariectomized rats with low doses of oestrogens decreases airway reactivity to acetylcholine through an increase in epithelial acetylcholinesterase activity (Degano et al., 2001, 2003). Oestrogens have also been suggested to influence airway responsiveness to acetylcholine acutely in an epithelium-independent way in rabbits (Pang et al., 2002) and through stimulation of endothelial nitric oxide (NO) synthetase in humans (Kirsch et al., 1999). Although androgens may regulate lung development at early gestation (Hume et al., 1996; Wilson and McPhaul, 1996; Levesque et al., 2000) and growth of airway smooth muscle (ASM) (Dashtaki et al., 1998), an acute effect on airway reactivity has not been shown yet.
Studies on blood vessels demonstrate that sex hormones affect the contractility of smooth muscle both in endothelium dependent (Chou et al., 1996; Geary et al., 2000a; Tep-areenan et al., 2002; Thompson and Khalil, 2003) and independent ways (Yue et al., 1995; Perusquia and Villalon, 1999; Teoh et al., 2000), depending on the vessel type and/or species studied. The endothelium-dependent effect of sex hormones may be mediated by NO and/or prostaglandin production (Geary et al., 2000b), while the endothelium independent relaxant effect of sex hormones may be due to various mechanisms, which include potassium channel opening (Yue et al., 1995; Deenadayalu et al., 2001; Ding and Stallone, 2001) and/or inhibition of calcium entry (Crews and Khalil, 1999; Murphy and Khalil, 1999).
The purpose of this study was to assess the direct effect of testosterone on ASM precontracted with KCl, ACh or carbachol (CCh). Our results demonstrate a relaxant effect of testosterone on precontracted ASM. We further show that this effect depends on the presence of an intact epithelium, the production of NO and appears to be mediated by a non-genomic pathway.
Methods
Test systems used
The direct effect of testosterone and BSA-testosterone was investigated with contractility studies of tracheal strips obtained from overall 65 6-week-old male rabbits. Rabbits were maintained in individual cages under a controlled environment consisting of a 12-h light–dark cycle and ambient temperature of 22°C and were provided with food and water before use for the study. Animals were treated in compliance with ethical and institutional guidelines.
In nine animals, blood was collected by cardiac puncture, immediately after killing for immunoassaying the serum levels of testosterone.
Measurements made
Animals and isolated tissues
Animals were killed by an overdose of intravenously (i.v.) administered sodium pentobarbitone (Vétoquinol, France). The extrathoracic tracheal tissue was removed and placed in baths containing Krebs solution bubbled with 95% O2 and 5% CO2. Temperature was maintained at 37°C. After the removal of connective tissue, tracheal rings, 2 mm in width, were dissected from tracheas under a stereoscope (SZ30 Olympus, Japan) and cut longitudinally through the cartilage opposite the smooth muscle layer. The thickness of smooth muscle layer was measured with the assistance of an inverted microscope (DIAPHOT 300 Nikon), a colour video camera (TK-1281, JVC) and monitor (TM-290ZE, JVC) as well as by using a calliper (0.0025 mm2 resolution), as previously described (Hatziefthimiou et al., 2005).
In experiments with epithelium-denuded tracheal strips the epithelial layer was removed with a cotton-tipped applicator. Each strip was placed with the superfused luminal side up in a water-jacketed horizontal organ bath. One end of the tracheal strip was fixed on the bottom of the organ bath and the other to the transducer tip. The entire strip was continuously perfused with oxygenated Krebs solution at 37°C. The volume of the organ bath was approximately 5 ml and the perfusion rate was 4 ml min−1. Changes in tension were recorded on a Grass FT03C force displacement transducer (Astro Med Inc., West Warwick, USA) and displayed via an oscillograph recorder (Grass 7400 Physiological Recorder).
Organ culture
Tracheal rings were washed in sterile Krebs solution, placed in DMEM/F12 (Ham) medium containing L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, and incubated at 37°C in a humidified incubator under 5% CO2.
Immunoassay for testosterone in serum
The collected blood was placed in plain tubes and centrifuged at 1000 r.p.m. for 5 min. The supernatant was decanted. Serum testosterone levels were determined by immunoassay with commercially available kits (VIDAS Assays, BIOMERIEUX).
Experimental design
Tracheal strips were stretched manually to 1 g resting tension and were allowed to equilibrate in the organ bath for at least 60 min. Following the equilibration period, the strips were contracted by addition of 10 μM ACh two or three times until a constant and reproducible contraction was achieved. Then tracheal strips were contracted again with 10 μM ACh or 10 μM CCh or 80 mM KCl to achieve a stable plateau tension and then testosterone (1 pM–100 μM) or testosterone-3-(O-carboxymethyl)-oxime: BSA (BSA-testosterone conjugate, 1 pM–100 nM) was added to the baths. Tracheal strips were perfused with solution containing the hormone for 10 min until a maximal effect was obtained and then were washed with ACh or CCh solution. In experiments where the NO donor, sodium nitroprusside (SNP) was used, tracheal strips were precontracted with either 10 μM CCh or 80 mM KCl to achieve a stable plateau tension and then SNP in concentrations 100 nM–1 mM was added cumulatively. Nω-nitro-L-arginine methyl ester (L-NAME) (100 μM), indomethacin (10 μM), flutamide (10 μM) or actinomycin D (100 μM) was added to the perfusion medium 30 min before the addition of ACh or CCh.
In organ cultures, 10 nM or 100 μM testosterone was added to the culture medium, where indicated. After 24 or 48 h, the tracheal rings where prepared for organ bath experiments. Contractions were obtained by the cumulative addition of ACh (1 nM–1 mM).
Solutions
Krebs solution was used for dissecting the tissue and contractility studies; it contained (in mM): NaCl 110.9, KCl 5.9, MgCl2 1.1, CaCl2 2, NaH2PO4 1.2, NaHCO3 25 and glucose 9.6. The solutions with increasing concentration of KCl had the same composition as normal Krebs solution with equimolar substitution of NaCl with KCl.
Data analysis and statistical procedures
Values are expressed as tension at the time of testosterone administration minus tension at peak relaxation over maximal force generated by each contractile agent or as tension in g mm−2. Data are expressed as means±standard error of the mean (s.e.m.) and the number of animals in each group is represented by n. Comparisons between two groups of experiments have been done by Mann–Whitney U unrelated test and for more than two groups by one way analysis of variance (ANOVA) with statistically significant differences between groups being determined by Bonferroni's post hoc test. For all tests used a comparison is considered significant when P<0.05. The statistical analysis was performed using SPSS v11. The curve-fitting and graph drawing were carried out using the graphical package Sigma Plot 2001.
Drugs, chemicals reagents and other materials
ACh, CCh, SNP, testosterone, L-NAME, indomethacin, flutamide, actinomycin D, BSA, BSA-testosterone were purchased from Sigma Chemical (Germany). DMEM/F12 (Ham) medium with L-glutamine, penicillin/streptomycin were from Gibco BRL.
ACh, BSA, CCh, SNP and L-NAME were dissolved in distilled water. BSA-testosterone was dissolved in Krebs solution. Solutions containing actinomycin D were prepared from stock solutions of 1 mg ml−1 in DMSO. Stock solutions of testosterone and flutamide as 100 mM in 100% ethanol and indomethacin as 1 mM in 100 mM sodium carbonate solution were prepared before each experiment. The equivalent ethanol or sodium carbonate solvent had no effect on precontracted tracheal strips.
Results
Effect of testosterone on precontracted ASM
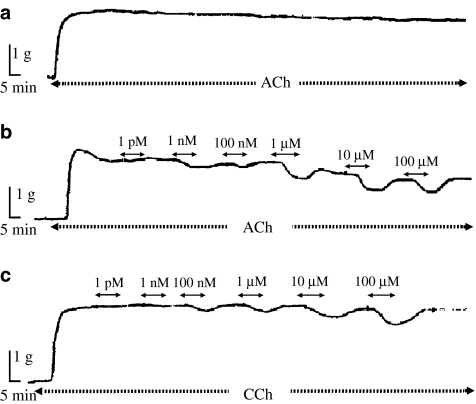
As shown in Figure 1, testosterone relaxed epithelium intact tracheal strips precontracted with 10 μM ACh (Figure 1b) or 10 μM CCh (Figure 1c). This relaxant effect of testosterone was achieved 5 min after its addition and reached a maximum within 10 min. In ASM precontracted with ACh, when testosterone was added at concentrations from 100 nM to 100 μM, a concentration-dependent decrease in tension remained at least 10 min after the removal of the hormone (Figure 1b and Table 1). In control experiments (Figure 1a and Table 1), relaxation of ACh-induced tension was also observed in tracheal strips precontracted with 10 μM ACh for 3 h and not exposed to testosterone. The relaxation after the removal of testosterone was significantly different from the control only after the removal of 100 μM testosterone (Table 1).
Figure 1.
Isometric contractile force recordings from epithelium intact tracheal strips contracted with 10 μM ACh without addition of testosterone (a) and precontracted with 10 μM ACh (b) or 10 μM CCh (c), showing the effect of testosterone. Exposure of the tracheal strips to testosterone is indicated by the timeline above the traces.
Table 1.
Values of tension drop to 10 μM ACh in the absence of testosterone and after the removal of testosterone at each concentration (1 pM–100 μM)
| Residual relaxation (% of initial contraction) | ||||||
|---|---|---|---|---|---|---|
| ACh 10 μM (n=7) | 0±0 | 0.6±0.6 | 3.7±1.8 | 3.1±1.6 | 4.5±1.9 | 4.4±1.9 |
| Concentration of testosterone | ||||||
| ACh 10 μM | 1 pM | 1 nM | 100 nM | 1 μM | 10 μM | 100 μM |
| +Testosterone (n=18) | 0±0 | 0±0 | 6.7±1.6 | 11.3±2.5 | 15.3±3.1 | 29.4±3.5*** |
Values are presented as percentage of initial contraction.
In experiments without the addition of testosterone, tension was measured at time points corresponding to those used for testosterone administration.
Data are presented as mean±s.e.m. and n indicates the number of animals studied.
Significant difference between values with and without testosterone at P<0.001 (***), Mann–Whitney U unrelated samples test.
In ASM precontracted with CCh no relaxation was observed after the removal of testosterone (Figure 1c). The relaxation of ASM precontracted with CCh caused by a single addition of 100 μM testosterone (10.7±1.2% of initial tension (n=10)) was similar to that obtained after cumulative addition of 100 μM testosterone (i.e. following the repeated addition of increasing concentrations, 1 pM–100 μM, of testosterone), 11.2±2.0% of initial tension (n=13). However, the effect of 100 nM and 1 μM testosterone on ASM precontracted with CCh varied when different concentrations of testosterone were applied in a random sequence (Table 2).
Table 2.
The effect of testosterone depends on the order of its addition
| Mode of testosterone addition | Concentration of testosterone | |||
|---|---|---|---|---|
| 1 pM | 100 nM | 1 μM | 100 μM | |
| Relaxation (% of initial contraction) | ||||
| Increasing concentrations | 1.9±0.6 | 2.1±0.6 | 5.4±1.5 | 10.6±3.7 |
| Random sequence | 0.9±0.8 | 4.5±0.8* | 1.4±0.7* | 8.4±0.4 |
Relaxation of airway smooth muscle precontracted with 10 μM CCh after addition of testosterone in increasing concentrations or after addition of different concentrations of testosterone in a random sequence (100 μM, 100 nM, 1 pM, 1 μM). Values are presented as percentage of initial contraction.
Data are presented as mean values of 5 experiments±s.e.m.
Significant differences between groups at P<0.05 (*), Mann–Whitney U unrelated samples test.
The relaxant effect of testosterone on ASM precontracted with ACh or CCh was significant even at low concentrations of testosterone (1 nM; P<0.05, Figure 2). The relaxant effect was more potent with ASM precontracted with ACh compared to ASM precontracted with CCh (P<0.05, Figure 2).
Figure 2.
The relaxant effect of testosterone on ASM precontracted with 10 μM ACh or 10 μM CCh. Data points are mean±s.e.m. and N indicates the number of animals studied. Significant difference of the effect of different concentrations of testosterone between ASM precontracted with ACh or CCh and the respective controls at P<0.05 (*), P<0.01 (**) and P<0.001 (***). Significant difference between the effect of different concentrations of testosterone on ASM precontracted with ACh and ASM precontracted with CCh at P<0.05 (#), P<0.01 (##) and P<0.001 (###). Comparisons between groups have been done by ANOVA, Bonferroni's post hoc test.
ASM contracted with 10 μM CCh developed increased tension compared to ASM contracted with 10 μM ACh (P<0.05, Table 3).
Table 3.
Tension induced by 10 μM ACh or 10 μM CCh in the presence or absence of epithelium, L-NAME, indomethacin, actinomycin and flutamide
| Contractile agent | Tension g mm−2 | |||||
|---|---|---|---|---|---|---|
| Control | Epithelium denuded | (+) L-NAME | (+) Indomethacin | (+) Actinomycin | (+) Flutamide | |
| 10 μM ACh | 43.1±6.2 (n=18) | 35.8±6.3 (n=6) | 28.8±0.2 (n=8) | 33.5±5.5 (n=6) | 47.6±8.5 (n=5) | 33.4±6.9 (n=7) |
| 10 μM CCh | 61.3±4.3* (n=17) | 61.02±8.7 (n=9) | 67.8±14.5 (n=6) | 55.6±7.5 (n=5) | 77.4±2.3 (n=5) | 84.9±1.2 (n=5) |
Data are means±s.e.m. from five to 18 experiments.
Significant differences between strips contracted with 10 μM ACh or 10 μM CCh at P<0.05 (*), Mann–Whitney U unrelated samples test.
Finally, testosterone had no effect on strips precontracted with 80 mM KCl (n=5, data not shown).
Involvement of the epithelium in the testosterone effect
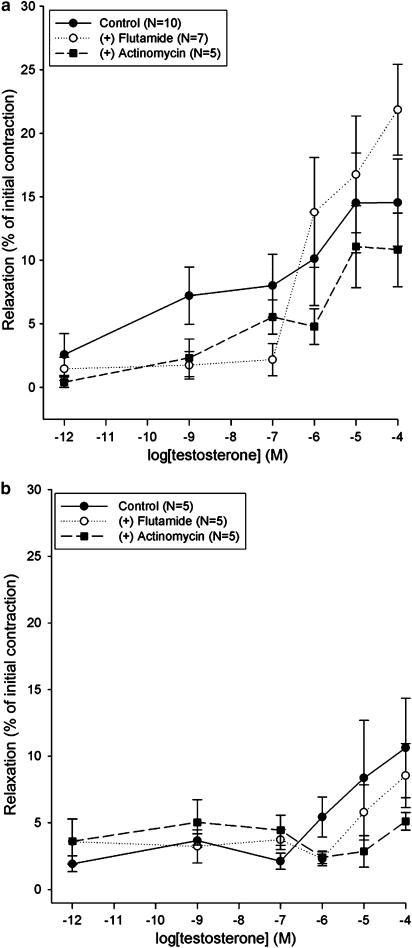
The mechanical removal of tracheal epithelium reduced the relaxant effect of testosterone in ASM precontracted with either ACh or CCh (Figure 3). Testosterone (100 μM) relaxed the epithelium intact tracheal strips precontracted with ACh by up to 16.6±3.2% of the initial contraction, while the relaxation of the epithelium denuded strips was 0% (P<0.01, Figure 3a). The relaxant effect of 100 μM testosterone in tracheal strips precontracted with CCh was 10.9±2.3% of the initial contraction in epithelium intact preparations, while epithelium denuded tracheae were not affected at all (P<0.001, Figure 3b).
Figure 3.
The effect of testosterone on epithelium intact and epithelium denuded preparations precontracted with 10 μM ACh (a) or 10 μM CCh (b). Data points are mean±s.e.m. and N indicates the number of animals studied. Significant difference between epithelium intact and epithelium denuded tracheal strips at P<0.05 (*), P<0.01 (**) and P<0.001 (***), Mann–Whitney U unrelated test.
The epithelium removal did not alter the tension of the contraction induced by ACh or CCh (Table 3).
The role of endogenous NO and prostanoids in the testosterone effect
The pretreatment of epithelium intact tracheal strips with the inhibitor of NO synthetase L-NAME (100 μM) reduced significantly the relaxant effect of testosterone on ASM precontracted with ACh (Figure 4a) or CCh (Figure 4b). Testosterone (100 μM) in the presence of L-NAME relaxed ASM precontracted with ACh up to 1.5±1.5% of the initial contraction, while it relaxed ASM up to 14.1±2.7% in the absence of L-NAME (P<0.05, Figure 4a). L-NAME also reduced the relaxant effect of testosterone on ASM precontracted with CCh, but this effect was statistically significant only at the response to 100 μM testosterone (Figure 4b). The pretreatment of epithelium intact tracheal strips with the cyclooxygenase inhibitor indomethacin (10 μM) did not alter significantly the relaxant effect of testosterone on ASM precontracted with either ACh (Figure 4a) or CCh (Figure 4b). Treatment of ASM with L-NAME or indomethacin did not alter the tension of the contraction induced by ACh or CCh (Table 3).
Figure 4.
The effect of testosterone on epithelium intact preparations precontracted with 10 μM ACh (a) or 10 μM CCh (b) and pretreated with 100 μM L-NAME or 10 μM indomethacin. Data points are mean±s.e.m. and N indicates the number of animals studied. Significant difference between tracheal strips precontracted with ACh or CCh in the absence and presence of L-NAME at P<0.05 (*) and P<0.01 (**), ANOVA with statistically significant differences between groups being determined by Bonferroni's post hoc test.
As testosterone had no effect on ASM precontracted with 80 mM KCl, we investigated the effect of an exogenous NO donor, SNP, on ASM precontracted with KCl. SNP had a significant relaxant effect on ASM precontracted with KCl from the concentration of 100 nM (P<0.05, Figure 5). Furthermore, the relaxant effect of SNP on ASM precontracted with KCl was not significantly different from its effect on ASM precontracted with 10 μM CCh (Figure 5).
Figure 5.
The effect of increasing concentrations of the NO-donor, SNP on epithelium intact preparations precontracted with either 80 mM KCl or 10 μM CCh. Data points are mean±s.e.m. and N indicates the number of animals studied.
The effect of inhibitors of androgen receptors and transcription on the responses to testosterone
The relaxant effects of testosterone on epithelium intact tracheal strips precontracted with ACh (Figure 6a) or CCh (Figure 6b) were not affected significantly by pretreatment of preparations with the classical testosterone receptor antagonist flutamide (10 μM). Similarly, the pretreatment of tracheal strips with the DNA transcription inhibitor, actinomycin D (100 μM), for 30 min before the contraction experiment, had no influence on the relaxant effect of testosterone (Figure 6).
Figure 6.
The effect of testosterone on epithelium intact preparations precontracted with 10 μM ACh (a) or 10 μM CCh (b) and pretreated with 100 μM flutamide or 10 μM actinomycin D. Data points are mean±s.e.m. and N indicates the number of animals studied.
Flutamide or actinomycin D did not alter the tension of the contraction induced by ACh or CCh (Table 3).
The effect of BSA-testosterone on precontracted ASM
To examine whether the effect of testosterone on tracheal smooth muscle is mediated, at least partly, by non-classical cell-surface androgen receptors, we studied the effect of BSA-testosterone on ASM precontracted with CCh. Albumin alone (BSA) in concentrations from 1 pM to 100 nM had no effect on ASM precontracted with CCh (data not shown). However, as shown in Figure 7, BSA-testosterone relaxed significantly ASM precontracted with CCh (P<0.05). Thus, 1 pM of BSA-testosterone relaxed ASM precontracted with CCh by up to 11.9±3.6% of the initial contraction. The mechanical removal of airway epithelium abolished the relaxant effect of BSA-testosterone (Figure 7, P<0.01).
Figure 7.
The effect of BSA-testosterone on epithelium intact and epithelium denuded preparations precontracted with 10 μM CCh. Data points are mean±s.e.m. of eight experiments. Significant difference between epithelium intact and epithelium denuded preparations at P<0.05 (*) and P<0.01 (**), Mann–Whitney U unrelated test.
Long-term effect of testosterone on ASM responsiveness to acetylcholine
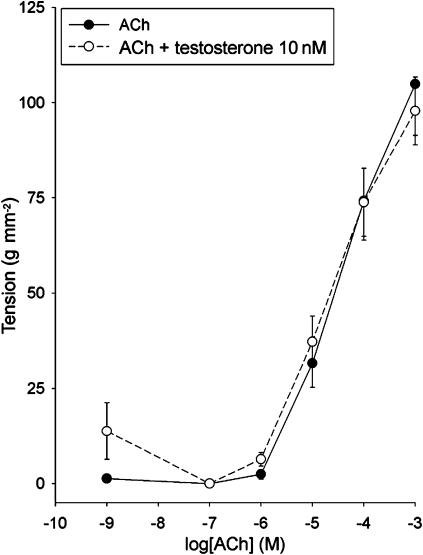
We next investigated the effect of prolonged testosterone treatment on ASM contractility. Incubation of tracheal strips from male rabbits at 37°C in the presence of 10 nM testosterone (Figure 8) or 100 μM testosterone (n=5, data not shown) for 24 h did not alter their responsiveness to increasing concentrations of ACh, as compared to controls strips incubated in medium without testosterone. The same results were obtained when tracheal strips were incubated with testosterone for 48 h before exposure to ACh (n=5, data not shown).
Figure 8.
Effect of testosterone on the responsiveness of tracheal strips to acetylcholine. Strips were pre-incubated for 24 h with 100 nM testosterone. Data points are mean±s.e.m. of five experiments.
Determination of total testosterone concentration in rabbit serum
The concentration of testosterone was determined in the sera of nine animals in order to estimate the normal range of the hormone in rabbit serum. The mean level of testosterone was 4.54±1.85 ng ml−1 (range 0.7–9.85 ng ml−1) that is equivalent to the concentration of 1–10 nM.
Discussion and conclusions
The results of the present study demonstrate that testosterone has a relaxant effect on tracheal strips precontracted with ACh or CCh. The integrity of airway epithelium is necessary for the relaxing effect of testosterone as its mechanical removal reduced this effect (Figure 3). The relaxing effect of testosterone was also reduced in the presence of the NO synthetase inhibitor L-NAME (Figure 4). These observations suggest that testosterone acts, at least in part, via an epithelium and NO-dependent pathway in rabbit trachea. In contrast, the presence of the cyclooxygenase inhibitor indomethacin in the perfusing medium did not alter the relaxing effect of testosterone (Figure 4), indicating that prostaglandin production is not implicated in the testosterone effect.
We have additionally shown that the testosterone relaxant effect was rapid. It was observed only 5 min after the exposure of the tracheal strip to the steroid and peaked within 10 min. Furthermore, a single administration of testosterone (100 μM) had a relaxing effect on ASM precontracted with CCh similar to that obtained after the repeated addition of increasing concentrations (1 pM–100 μM) of testosterone. The effect of testosterone, as shown in Figure 6, was not influenced by the presence of a specific androgen receptor antagonist, flutamide, or an inhibitor of DNA transcription, actinomycin D, in the perfusing medium. On the other hand, the non-permeable analogue of testosterone, BSA-testosterone, relaxed ASM precontracted with CCh (Figure 7). Taken together, these results suggest that the relaxing effect induced by testosterone on precontracted tracheal strips is mediated via a non-genomic pathway.
There is evidence suggesting that androgens may modulate the density of cholinereceptors (Bleisch et al., 1984; Bleisch and Harrelson, 1989; Souccar et al., 1991). To test this, we treated tracheal strips from male animals with low or high concentrations of testosterone for 24 or 48 h before the addition of ACh. The finding that exposure of ASM to testosterone did not alter their responsiveness to ACh (Figure 8) indicates that testosterone does not affect the density of cholinereceptors under the conditions used in the present study. This further supports our hypothesis that the effect of testosterone is not mediated via a genomic pathway.
Our results suggest that the relaxant effect of testosterone depends on the magnitude of the cholinereceptor-mediated contraction (Figure 2 and Table 3). Also, the total tone and amplitude of ACh-induced contraction did not completely recover after removal of the testosterone, as a decrease in tension was observed after the tissue had been washed for 10 min (Figure 1 and Table 1). In contrast, the effect of testosterone on ASM precontracted with CCh was completely reversible. Although not directly addressed in this study, this difference could be attributed to an increase in acetylcholinesterase activity caused by testosterone, which would affect ASM precontracted with ACh but not CCh. Indeed, it has been shown that testosterone increases acetylcholinesterase activity in the neuromuscular junction (Vercelli and Cracco, 1989; Gondinho et al., 1994). Moreover, low doses of another sex steroid hormone have been recently shown to decrease airway reactivity to acetylcholine through an increase in epithelial acetylcholinesterase activity (Degano et al., 2001, 2003).
According to our results (Table 2), the effects of 100 nM and 1 μM testosterone on precontracted ASM depends on the order of their addition, that is if they are given in a random order or in a cumulative manner. When 100 nM testosterone was added after 100 μM testosterone, its effect was increased two-fold compared to that observed with 100 nM applied after the addition of 1 pM. It has been shown that in rat Sertoli cells, human prostatic cells (Lyng et al., 2000) and T cells (Benten et al., 1999) testosterone increases the intracellular calcium levels in a dose-dependent way and this increase could persist for more than 5 min. On the other hand, alterations of intracellular calcium levels may affect the activity of epithelial NO synthase (Michel and Feron, 1997). Based on the above, we suggest that in rabbit airways testosterone also activates an intracellular messenger in a dose-dependent way and that this messenger probably does not return to its basal levels immediately after the removal of the hormone.
Our results show that the effect of testosterone depends on the contractile agent used to precontract the tissues. Thus, testosterone has no effect on ASM precontracted with 80 mM KCl. This lack of effect could not be attributed to the different sensitivity of precontracted ASM to NO, as our results demonstrate that the NO donor, SNP relaxed ASM precontracted with either CCh or KCl similarly (Figure 5). Also this lack of effect does not seem to be related to the magnitude of KCl-induced contraction, as this is similar to the ACh-induced contraction (37.3 vs 43 gm m−2, respectively). A probable explanation is that the insensitivity of KCl-induced contractions to testosterone may involve alterations in subsequent signal transduction mechanisms. In accordance with our findings, studies on blood vessels have also shown that the relaxant effect of testosterone was inhibited when 80 mM KCl was used (Deenadayalu et al., 2001; Ding and Stallone, 2001) and contractions in porcine coronary arteries elicited by KCl were unaffected by 17b-oestradiol and testosterone (Teoh et al., 2000).
The relaxation of ASM precontracted with CCh was reduced when testosterone or BSA-testosterone were added at a concentration of 100 nM (Figures 2 and 7). A similar concentration-dependent non-genomic effect of sex hormones has been described in vascular (Somjen et al., 2004) and endothelial cells (Goetz et al., 1999). In theory, low (1–100 nM) concentrations of the hormone may regulate the levels of the second messenger involved in a bell-shaped mode, like it does in osteoblasts (Lieberherr and Grosse 1994; Zagar et al., 2003). Alternatively, this messenger may affect the release of the agent responsible for the effect of the hormone differently, depending on its concentration. For example, it has been shown that the intracellular concentration of calcium regulates the interaction of epithelial NO synthase with caveolin (Feron et al., 1998; Goetz et al., 1999).
According to our results, 1 pM and 1 nM of BSA-testosterone produced more pronounced relaxation of ASM precontracted with CCh compared to the same concentrations of testosterone. This difference in potency between testosterone and BSA-testosterone may be due to their diverse affinity for a membrane androgen receptor that is not blocked by flutamide, to different binding sites and/or differences in the formation of intracellular messengers. This interpretation is in accordance with observations from studies in human vascular cells (Somjen et al., 2004) and male osteoblasts (Lieberherr and Grosse, 1994).
Epidemiological data suggest that with the onset of puberty, the incidence of asthma becomes higher in females than males and remains higher throughout the reproductive years (Marco et al., 2000). Testosterone serum levels have also been shown to be depressed in patients with respiratory failure (Semple et al., 1980), cystic fibrosis (Leifke et al., 2003), hypoxic pulmonary fibrosis (Semple et al., 1984) and chronic obstructive pulmonary disease (Semple et al., 1981; Kamischke et al., 1998; Creutzberg and Casaburi, 2003). The relaxant effect of testosterone or BSA-testosterone appeared at a concentration of 1 pM indicating the physiological relevance of these results, as serum testosterone levels are in the range 1–10 nM. The direct effect of testosterone on ASM responsiveness might be important for physiological and pathophysiological processes.
In conclusion, testosterone has a relaxant effect on precontracted tracheal smooth muscle. This effect requires the integrity of epithelium and is mediated via a non-genomic pathway and NO production.
Acknowledgments
We thank Dr G Simos for critical reading of the manuscript. This work was supported by a grant from the Greek Ministry of Education and Religion (Irakleitos) to P-AM.
Abbreviations
- ACh
acetylcholine
- ASM
airway smooth muscle
- CCh
carbachol
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Baizano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy. 2001;56:13–20. doi: 10.1034/j.1398-9995.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, et al. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- Bleisch W, Luine VN, Nottebohm F. Modification of synapses in androgensensitive muscle. I. Hormonal regulation of acetylcholine receptor number in the songbird syrinx. J Neurosci. 1984;4:786–792. doi: 10.1523/JNEUROSCI.04-03-00786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson A. Androgens modulate endplate size and ACh receptor density at synapses in rat levator ani muscle. J Neurobiol. 1989;20:189–202. doi: 10.1002/neu.480200403. [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison S, Ko E, Amidon TM, Collins P, et al. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive disease. Eur Respir J Suppl. 2003;46:76s–80s. doi: 10.1183/09031936.03.00004610. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26:707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- Dashtaki R, Whorton AR, Murphy TM, Chitano P, Reed W, Kennedy TP. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J Pharmacol Exp Ther. 1998;285:876–883. [PubMed] [Google Scholar]
- Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol. 2001;281:H1720–H1727. doi: 10.1152/ajpheart.2001.281.4.H1720. [DOI] [PubMed] [Google Scholar]
- Degano B, Mourlanette P, Valmary S, Pontier S, Prevost MC, Escamilla R. Differential effects of low and high-dose estradiol on airway reactivity in ovariectomized rats. Resp Physiol and Neurobi. 2003;138:265–274. doi: 10.1016/j.resp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Degano B, Prevost MC, Berger P, Molimard M, Pontier S, Rami J, et al. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med. 2001;164:1849–1854. doi: 10.1164/ajrccm.164.10.2102009. [DOI] [PubMed] [Google Scholar]
- Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol. 2001;91:2742–2750. doi: 10.1152/jappl.2001.91.6.2742. [DOI] [PubMed] [Google Scholar]
- Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- Geary G, Krause D, Duckles S. Gonadal hormones affect diameter of male rat cerebral arteries through endothelium-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000a;279:H610–H618. doi: 10.1152/ajpheart.2000.279.2.H610. [DOI] [PubMed] [Google Scholar]
- Geary G, Krause D, Duckles S. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000b;279:H511–H519. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondinho RO, Souccar C, Lapa AJ. Testosterone control of endplate and non-endplate acetylcholinesterase in the rat levator ani muscle. Neurochem Res. 1994;19:657–663. doi: 10.1007/BF00967703. [DOI] [PubMed] [Google Scholar]
- Hatziefthimiou A, Karetsi E, Pratzoudis E, Gourgoulianis K, Molyvdas P-A. Resting tension effect on airway smooth muscle: the involvement of epithelium. Respir Physiol Neurobiol. 2005;145:201–208. doi: 10.1016/j.resp.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hume R, Barker E, Coughtrie M. Differential expression and immunohistochemical localisation of the phenol and hydroxysteroid sulphotransferase enzyme families in the developing lung. Histochem Cell Biol. 1996;105:147–152. doi: 10.1007/BF01696154. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Kemper DE, Castel MA, Luthke M, Rolf C, Behre HM, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glycocorticoid therapy. Eur Respir J. 1998;11:41–45. doi: 10.1183/09031936.98.11010041. [DOI] [PubMed] [Google Scholar]
- Kirsch E, Yuhanna I, Chen Z, German Z, Sherman T, Shaul P. Estrogen acutely stimulates endothelial nitric oxide synthase in H441 human epithelial cells. Am J Respir Cell Mol Biol. 1999;20:658–666. doi: 10.1165/ajrcmb.20.4.3241. [DOI] [PubMed] [Google Scholar]
- Leifke E, Friemert M, Heilmann M, Puvogel N, Smaczny C, Von Zur Mühlen A, et al. Sex steroids and body composition in men with cystic fibrosis. Eur J Endocrinol. 2003;148:551–557. doi: 10.1530/eje.0.1480551. [DOI] [PubMed] [Google Scholar]
- Levesque B, Vosatka R, Nielsen H. Dihydrotestosterone stimulates branching morphogenesis, cell proliferation and programmed cell death in mouse embryonic lung explants. Pediatr Res. 2000;47:481–491. doi: 10.1203/00006450-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994;269:7217–7223. [PubMed] [Google Scholar]
- Lyng FM, Jones GR, Rommerts FFG. Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod. 2000;63:736–747. doi: 10.1095/biolreprod63.3.736. [DOI] [PubMed] [Google Scholar]
- Marco R, Locatelli F, Sunyer J, Burney P, The European Community Respiratory Health Survey Study Group Differences in incidence of reported asthma related to age in men and women. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- Michel T, Feron O. Nitric oxide synthases: which, where, how, and why. J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Khalil RA. Decreased [Ca2+] during inhibition of coronary smouth muscle contraction by 17b-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther. 1999;291:44–52. [PubMed] [Google Scholar]
- Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J-J, Xu X-B, Li H-F, Zhang X-Y, Zheng T-Z, Qu S-Y. Inhibition of b-estradiol on trachea smooth muscle contraction in vitro and in vivo. Acta Pharmacol Sin. 2002;23:273–277. [PubMed] [Google Scholar]
- Perusquia M, Villalon CM. Possible role of Ca2+ channels in the vasodilation effect of 5beta-dihydrotestosterone in rat aorta. Eur J Pharmacol. 1999;371:169–178. doi: 10.1016/s0014-2999(99)00161-2. [DOI] [PubMed] [Google Scholar]
- Semple PD, Beastall GH, Brown TM, Stirling KW, Mills RJ, Watson WS. Sex hormone suppression and sexual impotence in hypoxic pulmonary fibrosis. Thorax. 1984;39:46–51. doi: 10.1136/thx.39.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple PD, Beastall GH, Watson WS, Hume R. Serum testosterone depression associated with hypoxia in respiratory failure. Clin Sci (London) 1980;58:105–106. doi: 10.1042/cs0580105. [DOI] [PubMed] [Google Scholar]
- Semple PD, Beastall GH, Watson WS, Hume R. Hypothalamic-pituitary dysfunction in respiratory hypoxia. Thorax. 1981;36:605–609. doi: 10.1136/thx.36.8.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen D, Kohen F, Gayer B, Kulik T, Knoll E, Stern N. Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol. 2004;180:97–106. doi: 10.1677/joe.0.1800097. [DOI] [PubMed] [Google Scholar]
- Souccar C, Yamamoto LA, Goncalo MC, Lapa AJ. Androgen regulation of the nicotinic acetylcholine receptor-ionic channel in a hormone-dependent skeletal muscle. Braz J Med Biol Res. 1991;24:1051–1054. [PubMed] [Google Scholar]
- Teoh H, Quan A, Man R. Acute impairment of relaxation by low levels of testosterone in porcine coronary arteries. Cardiovasc Res. 2000;45:1010–1018. doi: 10.1016/s0008-6363(99)00398-3. [DOI] [PubMed] [Google Scholar]
- Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat masenteric arterial bed is mediated via potassium channels. Br J Pharmacol. 2002;135:735–740. doi: 10.1038/sj.bjp.0704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Khalil RA. Gender differences in the regulation of vascular tone. Clin Exp Pharmacol Physiol. 2003;30:1–15. doi: 10.1046/j.1440-1681.2003.03790.x. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Cracco C. Influence of testosterone on the development of the ischiocavernosus muscle of the rat. Acta Anat (Basel) 1989;134:177–183. doi: 10.1159/000146684. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- Yue P, Chatterjee K, Beale C, Poole-Wilson P, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]
- Zagar Y, Chaumaz G, Lieberherr M. Signaling cross-talk from Gbeta4 subunit to Elk-1 in the rapid action of androgens. J Biol Chem. 2003;279:2403–2413. doi: 10.1074/jbc.M309132200. [DOI] [PubMed] [Google Scholar]