Abstract
Background and purpose:
Carbon monoxide (CO) generated by the enzyme haeme oxygenase-1 (HO-1) during the breakdown of haeme is known to mediate a number of biological effects. Here, we investigated whether CO liberated from two water soluble carbon monoxide-releasing molecules (CO-RMs) exerts inotropic effects on the myocardium.
Experimental approach:
Rat isolated hearts perfused either at constant flow or constant pressure were used to test the effect of CO-RMs.
Key results:
CORM-3, a fast CO releaser, produced a direct positive inotropic effect when cumulative doses (3, 10 and 30 μg min−1) or a single dose (5 μM) were infused at either constant coronary pressure (CCP) or constant coronary flow (CCF). The inotropic effect mediated by CORM-3 was abolished by blockade of soluble guanylate cyclase or Na+/H+ exchanger, but not by inhibitors of L-type Ca2+ channels and protein kinase C. CORM-3 also caused a slight reduction in heart rate but did not alter coronary flow. In contrast, the slow CO releaser CORM-A1 produced significant coronary vasodilatation when given at the highest concentration (30 μg min−1) but exerted no effect on myocardial contractility or heart rate.
Conclusion and implications:
A rapid CO release from CORM-3 exerts a direct positive inotropic effect on rat isolated perfused hearts, whereas CO slowly released by CORM-A1 had no effect on myocardial contractility but caused significant coronary vasodilatation. Both cGMP and Na+/H+ exchange appear to be involved in this effect but further work is needed to determine the relative contribution of each pathway in CO-mediated inotropic effect.
Keywords: carbon monoxide-releasing molecules (CO-RMs), inotropic effect, guanylate cyclase, Na+/H+ exchanger
Introduction
Carbon monoxide (CO) is a chemically stable gas that is generated endogenously during haeme degradation by the enzyme haeme oxygenase (HO). This reaction also produces biliverdin and free ferrous iron (Maines, 1988, 1997). Of the three HO isozymes known to date, two (HO-2 and HO-3) are constitutively expressed, whereas the third (haeme oxygenase-1 (HO-1)) is induced by an array of noxious stimuli (Otterbein and Choi, 2000; Maines and Panahian, 2001). HO-1 can be expressed in almost any cell, with hypoxia (Motterlini et al., 2000), heavy metals (Elbirt et al., 1998) and endotoxin (Carraway et al., 1998) being among the inducers. Several studies have demonstrated that this enzyme possesses cytoprotective properties such as anti-inflammatory (Lee and Chau, 2002), antiapoptotic (Petrache et al., 2000) and antioxidative (Foresti et al., 2004a) effects. CO and the other products of HO have been demonstrated to contribute effectively to the multiple cytoprotective activities conferred by HO-1 expression (Dore et al., 1999; Ferris et al., 1999; Otterbein et al., 2000; Szabo et al., 2004). The cardiovascular system is no exception, since HO and its products have been found to protect the heart against various types of injury. Akamatsu et al. (2004) have shown HO-1 and CO to attenuate the damage caused by ischaemia reperfusion associated with heart transplantation. In another study, downregulation of HO-1 was linked to the generation of ventricular fibrillation in rat hearts exposed to warm ischaemia reperfusion (Csonka et al., 1999; Bak et al., 2003). Our group has shown in a previous study (Clark et al., 2000) that bilirubin produced by HO-1 protected hearts against reperfusion injury. CO has also been reported to be protective against myocardial ischaemia-reperfusion injury (Fujimoto et al., 2004), transplantation associated injury (Akamatsu et al., 2004) as well as endotoxaemia (Mayr et al., 2005) and haemorrhagic shock (Zuckerbraun et al., 2005).
The pleiotropic actions exerted by CO renders this gaseous mediator a good candidate for therapeutic purposes. Until recently, exploring the potential beneficial effects of CO gas was limited by the fact that the only available delivery methods of CO were either direct administration by inhalation or the use of prodrugs (e.g. methylene chloride), which liberate CO as they undergo catabolism in the liver (Chauveau et al., 2002). The high affinity of haemoglobin and other haeme proteins for CO makes it difficult to achieve desired tissue concentrations without causing serious toxicity when given by inhalation. The development of carbon monoxide-releasing molecules (CO-RMs) as a practical tool to deliver CO with promising clinical prospects has facilitated more precise examination of the biological roles of CO in disease models. The physiological actions of CO both in vitro and in vivo have been reproduced elegantly by the use of CO-RMs (Motterlini et al., 2002). After the identification of early CO-RMs (CORM-1 and CORM-2), which were soluble in organic solvents and required chemical or physical stimuli to release CO (Motterlini et al., 2002), our group has synthesized water soluble CO-RMs (CORM-3 and CORM-A1) demonstrating their biological importance as they are capable of liberating CO in biological systems (Clark et al., 2003; Motterlini et al., 2005b) and exert beneficial effects including protection against myocardial ischaemia-reperfusion injury in a rat model ex vivo (Clark et al., 2003) and reduced infarct size in vivo (Guo et al., 2004).
Both CORM-3 and CORM-A1 have been shown to promote a concentration-dependent vasodilatation in isolated aortae (Foresti et al., 2004b; Motterlini et al., 2005b). However, while CORM-3 induced rapid endothelium-dependent vasorelaxation involving both ATP-dependent potassium channels and cyclic guanosine-3′,5′-monophopshate (cGMP) activation (Foresti et al., 2004b), CORM-A1 caused a much slower but still profound endothelium-independent vasodilatation, with cGMP being the major mediator of this effect (Motterlini et al., 2005b). Some of the differences can be explained on the basis that CORM-3 rapidly releases CO in aqueous solutions, whereas CORM-A1 exhibits a slow and sustained CO liberation that is temperature- and pH-dependent (Motterlini et al., 2005a). In addition, CORM-3 contains ruthenium, while CORM-A1 contains no transition metals (Motterlini et al., 2005a); thus, the different chemical behaviour of CO-RMs dictates a differential biological activity and pharmacological action (Motterlini et al., 2005a).
In the present study, we investigated the physiological effects of CORM-3 and CORM-A1 in the isolated perfused heart of the rat and examined how a ‘fast' and ‘slow' CO release affects positive inotropism and the possible mechanism(s) involved in this effect.
Methods
Animals
All animals received humane care in accordance with UK Home Office guidelines on the Animals (Scientific Procedures) Act 1986. Male Lewis rats weighing 250–320 g were treated with 200 IU heparin, i.p. Following cervical dislocation and exsanguination, hearts were immediately excised and placed in ice-cold Krebs–Henseleit buffer (KHB) to cause cardiac arrest. Each heart was then rapidly mounted on a cannula attached to a Langendorff apparatus and perfused retrogradely at a constant perfusion pressure of 100 cm water with KHB consisting of (in mM): NaCl, 118.5; KCl, 4.7; CaCl2, 1.4; MgSO4, 1.2; NaHCO3, 25; KH2PO4, 1.2; glucose, 11.1. The buffer was filtered through a 5 μM filter and continuously gassed with 95% O2 and 5% CO2. The whole system was water jacketed and maintained at 37°C.
Rat isolated heart preparation
Two methods of heart perfusion were used in the study: constant coronary flow (CCF) and constant coronary pressure (CCP). In some circumstances the physiological responses of the isolated perfused hearts differ under conditions of either CCF or CCP (Sutherland and Hearse, 2000), and thus it was deemed important to investigate any identified inotropic effects under both conditions. In addition, CCF experiments were selected to ensure accurate delivery of a single dose of the drug. Experiments performed with CCP were used to study different concentrations of the drugs in progressive dosing studies and exposure to inhibitors, as well as a set of single dose experiments, since this mode of perfusion better maintains stable long-term cardiac perfusion and permits physiological autoregulation. For measurement of ventricular systolic and end diastolic pressures (EDP), balloons were inserted into the left ventricle of the heart through the mitral valve and connected to a Grass PT300 pressure transducer. In constant flow experiments a latex balloon was used and EDP was set to around 10 mm Hg during stabilization. In constant pressure studies, the balloon was made of house-hold cling film and was inflated with water to maintain the EDP between 1 and 5 mm Hg during the stabilization period, and then the balloon volume was kept constant throughout the experiment. Heart rate, systolic and diastolic pressures were measured by the balloon. The coronary flow was determined by weight for timed collections of the effluent perfusate from the hearts. CO-RMs were injected through a side cannula immediately above the heart at a constant rate to ensure delivery of the required concentrations in each case. The inhibitors were administered by switching between the main buffer reservoir and another reservoir in which drug-containing buffer was held. The drug-containing reservoir was protected from light since some of the inhibitors are light sensitive. Left ventricular developed pressure (LVDP) was calculated as: LVDP=systolic pressure–diastolic pressure. The rate-pressure product (RPP) was calculated according to the following equation: LVDP × heart rate/1000.
Experimental protocols
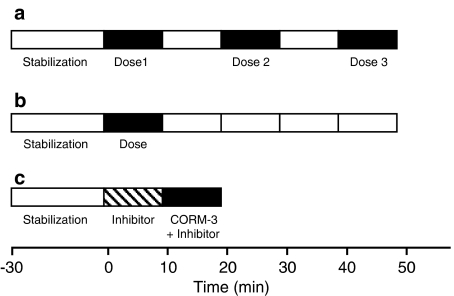
Fourteen groups of hearts were used in this study. These comprised three main sets of experiments: (1) groups with cumulative doses of CO-RMs or their respective inactive counterparts; (2) groups treated with single doses of CO-RM or their inactive counterparts for kinetic studies; and (3) groups treated with both CO-RM and inhibitors of signalling pathways. A scheme of the protocol used showing the different steps taken during perfusion of the hearts undergoing the different treatments is represented in Figure 1.
Figure 1.
Schematic illustration of the experimental protocols. (a) Represents the protocol in which the drugs were added in a sequential mode (administration for 10 min followed by 10 min drug-free perfusion). (b) Shows the experimental protocol of the single dose experiments in which drugs were infused for 10 min followed by 40 min with drug-free buffer. (c) Represents the protocol use for addition of the inhibitors of the signalling pathways where the inhibitors were administered before infusion of CORM-3.
Dose–response experiments
Hearts were allowed to equilibrate for 20–30 min and then three different doses (3, 10 or 30 μg min−1) of either CORM-3, CORM-A1 or their inactive analogues (iCORM-3 or iCORM-A1), were administered under conditions of constant pressure. Each dose was infused over a 10 min period followed by a drug-free interval of 10 min. Heart rate, coronary flow, systolic and diastolic pressures were measured throughout the experiment.
Single dose experiments
Based on the results obtained from the above dose–response experiments, an appropriate dose of CO-RM (5 μM, equivalent to a delivery of about 20 μg min−1) was chosen for further investigations. Two sets of experiments were performed to test the kinetic effects of this single dose of CORM-3 on the hearts. The first set of hearts was perfused at constant flow to allow more accurate dosing of CORM-3 at a rate of 11 ml min−1 with a concentration of 5 μM CORM-3. The drug was infused using a syringe pump connected to a port just above the aortic cannula at a rate which resulted in a concentration of 5 μM CORM-3 at the heart. All the hearts were followed for 40 min after the end of drug administration. Coronary pressure in the constant flow experiments was used to reflect changes in coronary vascular tone and was measured using a pressure transducer connected to a side port just above the aortic cannula. CORM-3 was only compared with iCORM-3 in this set. The second set of hearts was perfused at constant perfusion pressure (100 cmH2O) to allow possible autoregulation by the heart in response to the drug. After a steady state had been reached for the measured parameters, a single dose (20 μg min−1) of either CORM-3 (group 1, n=9) or iCORM-3 (group 2, n=4) was administered for 10 min. The haemodynamic parameters were recorded for 40 min from the end of dose. A third group (control, n=6) received buffer alone to determine any possible variation in cardiac physiology during the longer (50 min) perfusion period.
Effects of inhibitors of signalling pathways on positive inotropism produced by CO-RM
Five groups were studied to investigate the signalling pathways. Inhibitors and drugs were administered as shown in Figure 1c. The first group (n=9) was perfused with KHB alone during the 10 min before CORM-3 administration. The second group of hearts (n=6) received 10 μM 1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one (ODQ) for 10 min before and continued during CORM-3 infusion. Nifedipine (10 nM) was given to the third group (n=8). Chelerythrine (3 μM) was given to the fourth group (n=6) while 5-(N-ethyl-N-isopropyl)amiloride (EIPA) (1 μM) was used in the fifth group (n=5). All experiments in this set were performed under constant perfusion pressure conditions (100 cm H2O). Hearts were allowed to stabilize for 20 to 30 min, and then all the inhibitors, apart from chelerythrine, were administered for a 10 min predosing period before the addition of CORM-3. Then the inhibitors and CORM-3 were administered together for the chosen 10 min period of CO-RM dosing. Chelerythrine was started 2 min before CORM-3 and continued thereafter as described in a study by Mielke et al. (2003). The data for this set were calculated as percentage of pre-CORM-3 values.
Reagents
All reagents used in this study (except CO-RMs) were purchased from Sigma (Poole, Dorset, UK). CORM-3 and CORM-A1 were synthesized as described previously (Alberto et al., 2001; Clark et al., 2003). Stock solutions of CORM-3 and CORM-A1 were prepared on the day of the experiment by dissolving the compounds in distilled water. To prepare inactive CORM-3 (iCORM-3), the required amount of compound was dissolved in KHB and left at room temperature for 24 h to liberate its entire CO content. The residual CO present was finally removed by bubbling the solution with N2 for 10 min. Depletion of CO from CORM-A1 to obtain the inactive counterpart (iCORM-A1) was achieved by initially dissolving the compound in 0.1 M HCl followed by bubbling the solution with N2 for 10 min. The pH of iCORM-A1 stock solution was then adjusted to 7.4 before its use. The concentration of nifedipine used (10 nM) was chosen on the basis of previous studies (Karmazyn and Moffat, 1990). Similarly, the concentrations of ODQ (10 μM) and EIPA (1 μM) were based on those used by others (Kleyman and Cragoe, 1988; Bugge and Ytrehus, 1995a). The IC50 of chelerythrine for protein kinase C (<0.7 μM) was indicative for choosing the 3 μM dose applied in this study (Bugge and Ytrehus, 1995b). Stock solutions of all inhibitors except chelerythrine were prepared in ethanol and then diluted to the final concentration in KHB. The final concentration of ethanol was 0.1%. Chelerythrine stock solution was prepared in distilled water.
Statistical analysis
Statistical significance was determined by analysis of variance (ANOVA) for repeated measures followed by Bonferroni post hoc test. To compare mean percentages of baseline values with 100%, one sample t-tests were used. One way ANOVA was carried out to compare the groups treated with the inhibitors and the group treated with CORM-3 alone. P<0.05 was considered to be an indication of statistical significance in all cases.
Results
Dose–response experiments
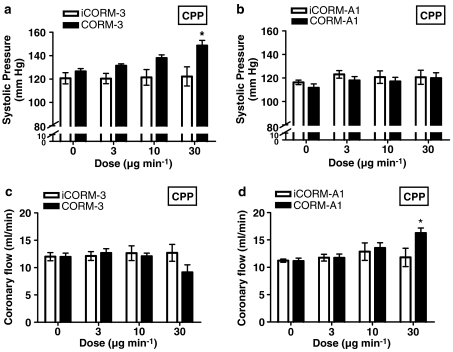
Preliminary experiments were conducted to select the effective dose ranges for CORM-3 and CORM-A1 to be used in this study (data not shown). Figure 2 depicts the changes in systolic pressure of isolated hearts exposed to sequential challenges with increasing doses of CORM-3, CORM-A1 or their inactive counterparts (iCORM-3 and iCORM-A1). CORM-3 elicited a positive inotropic effect in a dose-dependent manner with the maximum response seen at the highest dose tested (30 μg min−1). However, no difference in systolic pressure was observed when equivalent doses of iCORM-3 were administered (Figure 2a), strongly suggesting that the liberated CO caused the enhanced myocardial contractility. A dose of 30 μg min−1 CORM-3 was infused for 10 min resulting in an approximately 20–30 mm Hg rise in systolic pressure. In contrast, neither CORM-A1 nor iCORM-A1 had any significant effect on the systolic pressure of the treated hearts over the dose range applied (Figure 2b). EDP did not differ statistically between CORM-3 and iCORM-3 groups at any given dose (data not shown). Similar results were seen when comparing the CORM-A1 versus iCORM-A1 groups. In addition, no change in heart rate was observed with the 3 or 10 μg min−1 CORM-3 doses, while a tendency towards a decrease in heart rate was noted with the highest dose, which did not reach statistical significance (data not shown). No statistically significant changes in heart rate were observed with CORM-A1 or iCORM-A1. Coronary flow did not change in response to CORM-3 doses of 3 or 10 μg min−1 (Figure 2c). However, a tendency for the coronary flow to decline was evident, although not statistically significant, when 30 μg min−1 CORM-3 was infused. In contrast, the highest dose of CORM-A1 significantly increased the coronary flow, while neither of the other two doses exerted any effect (Figure 2d). The inactive drugs (iCORM-3 or iCORM-A1) were without effect.
Figure 2.
Effect of CORM-3 and CORM-A1 on systolic pressure (a and b, respectively) and coronary flow (c and d, respectively). Rat hearts were exposed to increasing doses of CO-RMs or their inactive counterparts (iCORM-3 and iCORM-A1) and the parameters measured as described in Methods. Vertical lines represent the mean±s.e.m. of 5–6 independent experiments (*P<0.05 vs control).
Kinetic studies on positive inotropism following single doses
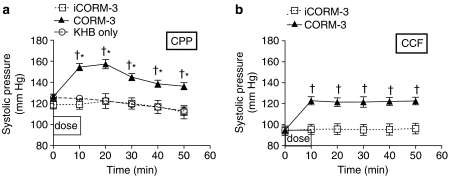
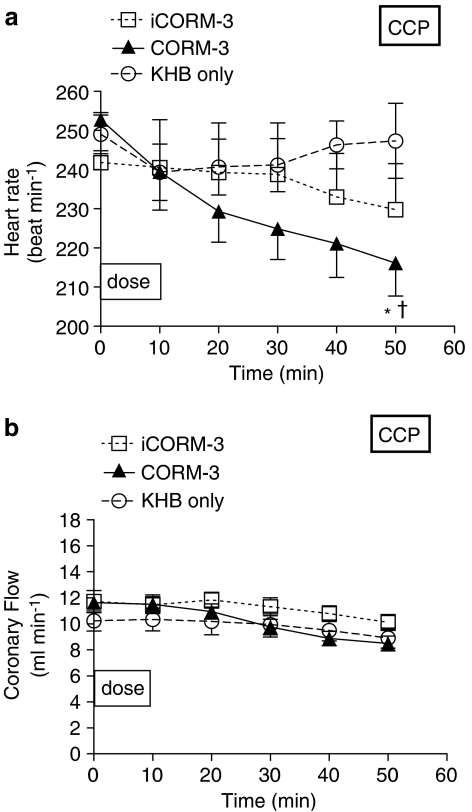
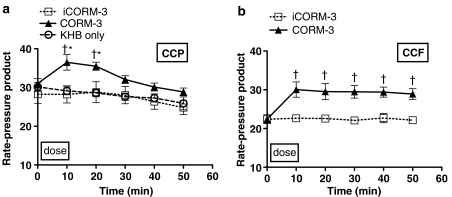
Having identified a clear positive inotropic effect using CORM-3, we investigated the kinetics of action following a single dose regime. We decided to examine the effects of CORM-3 using a single dose over 10 min followed by an observation period of 40 min using constant perfusion pressure preparation. Infusion of 20 μg min−1 CORM-3 for 10 min (Figure 3a) caused a significant positive inotropic effect (as noted previously in Figure 2a), with the systolic pressure increased by 25–30 mm Hg. After that, the systolic pressure started declining but remained significantly higher than baseline throughout the perfusion period. In contrast, hearts given iCORM-3 or Krebs–Henseleit alone did not show any change in systolic pressure. Similarly, in the CCF perfusion experiments, an immediate rise in systolic pressure was seen when 5 μM CORM-3 was infused (Figure 3b). This effect peaked by the end of CORM-3 infusion and showed a plateau throughout the rest of the experiment. No change was noted after infusion of iCORM-3 in the constant flow preparation. When compared to the Krebs alone group, hearts that had received iCORM-3 in the CCF set showed very similar systolic pressures, indicating the lack of effect of iCORM-3 per se on the myocardial contractility. EDP was not altered by any of the treatments given in both perfusion sets (data not shown). In hearts perfused at constant pressure, a statistically significant reduction in heart rate was evident with CORM-3 when compared to hearts treated with Krebs alone but not compared to the iCORM-3 group (Figure 4a). Moreover, infusion of iCORM-3 resulted in a slight reduction in heart rate compared to the Krebs group but this was not statistically significant. The heart rate did not change with CORM-3 treatment in hearts perfused according to constant flow preparation (data not shown). Figure 4b illustrates the changes in coronary circulation in response to CORM-3. We found that administration of 20 μg min−1 CORM-3 for 10 min produced no change in coronary flow compared to hearts receiving iCORM-3 or Krebs alone in the CCP experiments. However, in the CCF experiments CORM-3-treated hearts tended to display higher coronary perfusion pressures than iCORM-3-treated hearts as the perfusion time increased. However, the difference was not statistically significant at any time point studied (data not shown). In the CCP experiments, we also observed a significant increase in the RPP at 10 and 20 min after CORM-3 infusion, but this change started to wane and became nonsignificant at 30 min (Figure 5a). In the CCF experiments, the RPP rose significantly towards the end of the CORM-3 infusion and was maintained over the entire perfusion period (Figure 5b).
Figure 3.
Effect of CORM-3 on systolic pressure in rat hearts perfused at CCP (a) or CCF (b). Hearts were perfused with either KHB alone or Krebs buffer supplemented with CORM-3 or iCORM-3 as described in Methods. *P<0.05 vs control (KHB); †P<0.05 vs iCORM-3.
Figure 4.
Effects of CORM-3 on heart rate (a) and coronary flow (b) in rat hearts perfused at CCP. Hearts were perfused with either KHB alone or Krebs buffer supplemented with CORM-3 or iCORM-3 as described in Methods. *P<0.05 vs control (KHB); †P<0.05 vs iCORM-3.
Figure 5.
Effects of CORM-3 on the RPP in rat hearts perfused at CCP (a) and CCF (b). Hearts were perfused with either KHB alone or Krebs buffer supplemented with CORM-3 or iCORM-3 as described in Methods. *P<0.05 vs control (KHB); †P<0.05 vs iCORM-3.
Effects of inhibitors of signalling pathways on positive inotropism from CORM-3
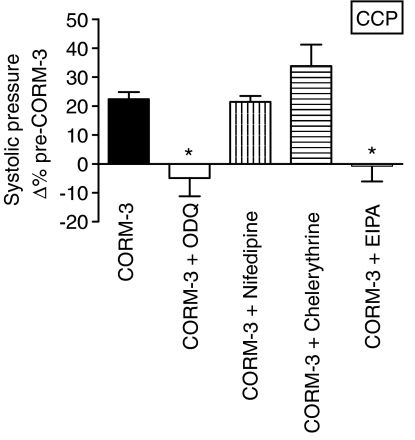
To assess whether CORM-3 increased the systolic pressure by activating soluble guanylyl cyclase to produce cGMP, hearts were perfused with ODQ (10 μM) before and during CORM-3 infusion. ODQ alone significantly decreased peak systolic pressure and coronary flow by 20% over the baseline value but did not have any effect on heart rate. Therefore, the peak systolic pressure reached after CORM-3 administration was calculated as a percentage of the new steady-state value reached after ODQ infusion. ODQ completely abolished the positive inotropic effect of CORM-3 (Figure 6). The role of L-type Ca2+ in CORM-3-mediated inotropic action was examined by administering nifedipine (10 nM) before and during CORM-3 infusion. Nifedipine alone did not affect the peak systolic pressure and heart rate but increased coronary flow by around 80%. In the presence of nifedipine, CORM-3 increased the systolic pressure by 20% (similar to the increase seen in those hearts treated with CORM-3 alone) (Figure 6). To determine the contribution of Na+/H+ exchange to the enhanced myocardial contractility caused by CORM-3, hearts received EIPA before and during CORM-3 infusion. Coronary flow dropped by 20% following infusion with EIPA alone, whereas peak systolic pressure and heart rate remained unchanged. Treatment with EIPA completely inhibited the rise in systolic pressure mediated by CORM-3 (Figure 6). Chelerythrine (3 μM) was also used to determine whether protein kinase C (PKC) activation is involved in the positive inotropic effects of CORM-3. When given alone, chelerythrine increased the coronary flow by 30% and the systolic pressure by 10%, while heart rate remained unchanged. Chelerythrine augmented the rise in systolic pressure caused by CORM-3 by 30% compared to a 20% increase observed in hearts treated with CORM-3 alone (Figure 6).
Figure 6.
Effect of inhibitors of the signalling pathways, ODQ, nifedipine, chelerythrine and EIPA, on the changes in myocardial contractility mediated by CORM-3. The graph depicts the change in peak systolic pressure as a percentage of baseline values. The drugs were infused at the concentrations and times described in detail in Methods *P<0.05 vs CORM-3 alone.
Discussion
In this study, we utilized the rat isolated perfused heart model to elucidate the effects of two CO releasers, CORM-3 and CORM-A1, on myocardial contractility and haemodynamic parameters. Rates of CO release from CORM-3 and CORM-A1 in KHB have been studied previously by our group. CORM-3 liberated most of its CO within 1–5 min depending on the type of solution (Clark et al., 2003), whereas CORM-A1 required 21 min to release half of its CO under physiological conditions (Motterlini et al., 2005b). We show here for the first time that CO released by CORM-3 elicits a profound positive inotropic effect in a dose-dependent fashion, or when given as a single dose either at constant perfusion pressure (20 μg min−1) or at constant flow (5 μM). The fact that the single dose (20 μg min−1) was not associated with changes in coronary flow or heart rate clearly suggests a direct positive inotropic effect. This effect has not been reported previously with CO gas, probably due to the relatively small number of studies looking at the influence of CO on the myocardium. Most of the previous studies have focused on CO toxicity which causes contractile depression (McGrath, 1984; Chen and McGrath, 1985). Interestingly, none of the CORM-A1 doses used in this study affected myocardial contractility, despite indications that it was the CO liberated by CORM-3 which appeared to cause the positive inotropic effect, as suggested by the lack of contractile response to the inactive form of the drug (iCORM-3). The disparity in contractile responses observed using the two CO-RMs can partially be explained by the different rates of CO release by the two agents which could lead to different concentrations of CO being achieved during heart perfusion. In fact, as stated above, CORM-3 is a ‘fast' CO releaser whereas CORM-A1 liberates CO ‘slowly'. Hence, as the positive inotropic effect observed with CO liberated from CORM-3 was dose-dependent, the higher doses of CORM-A1 should be able to elicit the positive inotropic effect seen with CORM-3. This was not the case since the enhanced contractile response started to appear at a dose of 3 μg min−1 CORM-3 and became stronger with the higher doses, but no changes in systolic pressure could be seen even at 30 μg min−1 CORM-A1. This indicates the involvement of other factors, apart from CO release, in this response.
In trying to identify the mechanisms of the CORM-3-derived inotropic effect, we noted that the inhibitor of soluble guanylyl cyclase (sGC) (ODQ) completely blocked the rise in systolic pressure produced by CORM-3, suggesting that this action may be mediated by cGMP.
Although ODQ was shown to be not entirely specific as an inhibitor of sGC and to interfere with other systems such as NOS and cytochrome P450 (Feelisch et al., 1999), these effects occurred only at concentrations of 30–300 μM, which are much higher compared to the ones (10 μM) needed to block sGC activity completely. In addition, there is strong evidence from the literature that, at the concentration and time used in this study, ODQ can potently and selectively inhibit sGC without affecting NOS activity (Moro et al., 1996; Olson et al., 1997). A limitation of this study is that cGMP levels were not determined; this should be performed to confirm that the positive inotropic effect is a direct result of sGC activation. Previous studies have shown cGMP to mediate a positive inotropic effect by at least two different mechanisms: (1) Wang et al. (1998) have shown that by inhibiting cyclic adenosine monophosphate (cAMP) phosphodiesterase and thus allowing cAMP to accumulate, cGMP induced a positive inotropic effect in cat atrial myocytes; (2) Langer et al. (2003) have suggested that nitric oxide (NO) produced a positive inotropic response through cGMP-dependent coupling of Gsα proteins to adenylyl cyclase. CO was demonstrated to act through sGC to produce vasodilatation in rat isolated aortic rings (Foresti et al., 2004b) and bronchodilatation in vivo (Cardell et al., 1998). Interestingly, Bak et al. (2005) demonstrated that exogenous CO improved myocardial recovery following 30 min of ischaemia by increasing cGMP levels and part of the LVDP recovery may have been caused by a positive inotropic effect. This supports our hypothesis that CORM-3 mediates its positive inotropic action via a cGMP-dependent mechanism.
Nifedipine failed to prevent the increase in systolic pressure by CORM-3, suggesting that the positive inotropic effect was not mediated through the stimulation of Ca2+ currents via L-type Ca2+ channels. This finding does not accord with the above-mentioned mechanisms of the cGMP-mediated inotropic effect, since they both suggest that cGMP will result in higher levels of cAMP which will in turn increase Ca2+ through L-type Ca2+ channels. Further elucidation of the role of cAMP will be required before a definitive conclusion can be reached on how cGMP mediates the inotropic effect. It is worth pointing out that CO has been shown to activate L-type Ca2+ channels in human intestinal smooth muscles via an NO-dependent pathway (Lim et al., 2005). We also determined whether PKC activation contributed to the inotropic effect observed with CORM-3. Previous findings have revealed that stimulating PKC could result in a positive inotropic effect by increasing calcium entry via L-type Ca2+channels (Puri et al., 1997), as well as by stimulating Na+/H+ and hence making the cytosol more alkaline and sensitizing the myofilaments to the locally available Ca2+ (Ito et al., 1997). Chelerythrine, a PKC inhibitor, augmented the positive inotropic effect caused by CORM-3. The reason behind this observation is unclear. It is unlikely to be due to any increase in coronary flow, since this parameter stabilized before CORM-3 was infused and did not change thereafter. Moreover, in our conditions, nifedipine increased the coronary flow by 80% compared to 30% by chelerythrine, yet nifedipine was without effect on the myocardial contractility.
Our findings suggest that the CORM-3-mediated positive inotropic action also involves the stimulation of Na+/H+ exchange, since blocking this exchange by EIPA resulted in complete inhibition of the increase in systolic pressure seen with the CO releaser. The stimulation of Na+/H+ exchange can mediate positive inotropic effect through two mechanisms: (1) by making the cytosol more alkaline and hence sensitizing the myofilaments to whatever Ca2+ ions are available (Matsui et al., 1995); and (2) by building up intracellular Na+ which is exchanged for Ca2+ via Na+/Ca2+ exchange (Iwakura et al., 1990). This latter mechanism is interesting in relation to our observations on the role of the Na+/H+ exchanger shown in our present study and the possible effects of CO from CO-RMs on mitochondrial respiration in previous work from our group (Sandouka et al., 2005). The latter findings suggested that CO-RMs could modulate substrate-coupled respiration, and by inference, the balance between ATP production and consumption. In the present study, if CO from CO-RMs could influence a reversible shift between aerobic metabolism and anaerobic glycolysis in the heart, a transient increase in intracellular H+ may ensue, which could drive the accumulation of intracellular Na+ via the exchange mechanism. This, in turn, could enhance intracellular Ca2+ levels (and myocardial contractility) via Na+/Ca2+ exchange. The conclusion from this study about the role of Na+/H+ exchange in CO induced positive inotropic effect is limited by the lack of measurements of intracellular pH and ion transits across cell membranes. Further work using more specific Na+/H+ inhibitors (cariporide) and the simultaneous use of Na+/H+ exchange and Na+/Ca2+ exchange alongside direct measurements of ion transits and intracellular pH is necessary to explore this mechanism more fully.
The positive contractile response to CORM-3 observed in the present study started to decline after reaching a peak under normal pressure perfusion, whereas hearts perfused with a constant flow maintained the same levels of contractility until the conclusion of perfusion. The normal decline in coronary flow with time in the preparation at constant pressure may be due to tissue oedema since 20 μg min−1 CORM-3 did not alter the coronary flow compared to control. It may also reflect an autoregulatory response by the hearts to CO-RMs, since autoregulation is possible during constant pressure perfusion. Neither CORM-3 nor CORM-A1 had any effect on EDP at any of the doses or preparations used which is in agreement with previous findings (Chen and McGrath, 1985), where it was shown that there was no difference in EDP of the hearts exposed to CO gas. Although not statistically significant, the tendency of heart rate to drop with higher doses of CORM-3 and also when given as a single dose at constant pressure is consistent with observations by other researchers who reported a reduction in heart rate with exogenous CO administration (Chen and McGrath, 1985; Patel et al., 2004). This decrease has been attributed to CO toxicity in a number of studies. However, the notion of a direct negative chronotropic effect of CO on the excitation-conduction system is supported by the observed positive inotropic effect coinciding with the rate drop, since CO toxicity is associated with depression in myocardial contractility. This also accords with the more rapid decline in heart rate observed by McGrath (1984), in hearts challenged with 95% CO–5% CO2 compared to 95% N2–5% CO2. The ability of CO to modulate heart rate is another aspect with similarity to NO. Kojda et al. (1997) have reported an increase in heart rate associated with endogenous as well as exogenous NO. The mechanism of CO-related chronotropic effect is unclear and could possibly involve either soluble guanylyl cyclase or activation of ATP-dependent potassium channels.
In this study, hearts perfused with 5 μM CORM-3 have a tendency to display a higher coronary pressure when compared with the iCORM-3 group, albeit this did not reach statistical significance. Among the reasonable explanations for the increased resistance to coronary flow associated with CORM-3 is the extravascular compression of coronary vessels by the strongly contracting myocardium. Moreover, the study of Thorup et al. (1999) can provide a possible basis to vindicate the discrepancy in coronary response to CORM-3 and CORM-A1. In their study, it was shown that high CO concentrations inhibited nitric oxide synthase (NOS) and NO generation, whereas enhanced NO release was caused by lower CO levels in renal resistance arteries. Therefore, the higher dose of CORM-3 may have reduced the coronary flow by inhibiting NO synthesis and thus causing coronary vasoconstriction. On the other hand, 30 μg min−1 CORM-A1 may have increased the coronary flow through inducing sGC to generate cGMP, since an earlier study by our group (Motterlini et al., 2005b) established that the vasoactive properties of CORM-A1 in aortic tissue are mediated by guanylate cyclase but not potassium channels, NOS activity or the presence of endothelium. Although no changes in RPP were evident in studies using increasing doses of the CO carrier, CORM-3 increased this parameter significantly in the single dose studies. In contrast, sustained elevation of the RPP was seen in experiments conducted at CCF and a peak followed by a decline was observed at CCP. This indicates that CORM-3 is capable of enhancing the overall cardiac performance in both preparations, and again raises the possibility of an autoregulatory response in the constant pressure preparation.
In conclusion, we have shown that CO released by CORM-3 possesses a direct positive inotropic effect on rat isolated perfused hearts. Both cGMP and Na+/H+ exchange appear to be involved in this effect but further work is needed to determine more fully the relative contribution of each pathway.
Acknowledgments
This study was supported by grants from The Foundation for Al-Quds University Medical School (FQMS) and Karim Rida Said Foundation (KRSF). We thank Dr Richard Morris, Department of Primary Care and Population Science, University College London for his help. We thank Dr Tony Johnson for the synthesis of CORM-3.
Abbreviations
- CCF
constant coronary flow
- CCP
constant coronary pressure
- CO
carbon monoxide
- CO-RMs
carbon monoxide-releasing molecules
- HO-1
haeme oxygenase-1
- LVDP
left ventricular developed pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one
Conflict of interest
Dr Roberto Motterlini and Professor Brian E Mann are consultants of hemoCORM Ltd.
References
- Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- Alberto R, Ortner K, Wheatley N, Schibli R, Schubiger AP. Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [(99m)Tc(OH(2))3(CO)3]+ J Am Chem Soc. 2001;123:3135–3136. doi: 10.1021/ja003932b. [DOI] [PubMed] [Google Scholar]
- Bak I, Szendrei L, Turoczi T, Papp G, Joo F, Das DK, et al. Heme oxygenase-1-related carbon monoxide production and ventricular fibrillation in isolated ischemic/reperfused mouse myocardium. FASEB J. 2003;17:2133–2135. doi: 10.1096/fj.03-0032fje. [DOI] [PubMed] [Google Scholar]
- Bak I, Varadi J, Nagy N, Vecsernyes M, Tosaki A. The role of exogenous carbon monoxide in the recovery of post-ischemic cardiac function in buffer perfused isolated rat hearts. Cell Mol Biol (Noisy-le-grand) 2005;51:453–459. [PubMed] [Google Scholar]
- Bugge E, Ytrehus K. Inhibition of sodium-hydrogen exchange reduces infarct size in the isolated rat heart – a protective additive to ischaemic preconditioning. Cardiovasc Res. 1995a;29:269–274. [PubMed] [Google Scholar]
- Bugge E, Ytrehus K. Ischaemic preconditioning is protein kinase C dependent but not through stimulation of alpha adrenergic or adenosine receptors in the isolated rat heart. Cardiovasc Res. 1995b;29:401–406. [PubMed] [Google Scholar]
- Cardell LO, Lou YP, Takeyama K, Ueki IF, Lausier J, Nadel JA. Carbon monoxide, a cyclic GMP-related messenger, involved in hypoxic bronchodilation in vivo. Pulm Pharmacol Ther. 1998;11:309–315. doi: 10.1006/pupt.1998.0152. [DOI] [PubMed] [Google Scholar]
- Carraway MS, Ghio AJ, Taylor JL, Piantadosi CA. Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. Am J Physiol Lung Cell Mol Physiol. 1998;275:L583–L592. doi: 10.1152/ajplung.1998.275.3.L583. [DOI] [PubMed] [Google Scholar]
- Chauveau C, Bouchet D, Roussel JC, Mathieu P, Braudeau C, Renaudin K, et al. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant. 2002;2:581–592. doi: 10.1034/j.1600-6143.2002.20702.x. [DOI] [PubMed] [Google Scholar]
- Chen KC, McGrath JJ. Response of the isolated heart to carbon monoxide and nitrogen anoxia. Toxicol Appl Pharmacol. 1985;81:363–370. doi: 10.1016/0041-008x(85)90408-9. [DOI] [PubMed] [Google Scholar]
- Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- Csonka C, Varga E, Kovacs P, Ferdinandy P, Blasig IE, Szilvassy Z, et al. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic Biol Med. 1999;27:119–126. doi: 10.1016/s0891-5849(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbirt KK, Whitmarsh AJ, Davis RJ, Bonkovsky HL. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1998;273:8922–8931. doi: 10.1074/jbc.273.15.8922. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp. 2004a;71:177–192. doi: 10.1042/bss0710177. [DOI] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, et al. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004b;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, et al. Carbon monoxide protects against cardiac ischemia – reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Kagaya Y, Weinberg EO, Barry WH, Lorell BH. Endothelin and angiotensin II stimulation of Na+-H+ exchange is impaired in cardiac hypertrophy. J Clin Invest. 1997;99:125–135. doi: 10.1172/JCI119123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura K, Hori M, Watanabe Y, Kitabatake A, Cragoe EJ, Yoshida H, et al. Alpha 1-adrenoceptor stimulation increases intracellular pH and Ca2+ in cardiomyocytes through Na+/H+ and Na+/Ca2+ exchange. Eur J Pharmacol. 1990;186:29–40. doi: 10.1016/0014-2999(90)94057-5. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Moffat MP. Positive inotropic effects of low concentrations of leukotrienes C4 and D4 in rat heart. Am J Physiol. 1990;259:H1239–H1246. doi: 10.1152/ajpheart.1990.259.4.H1239. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. J Membrane Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Noack E. Inhibition of nitric oxide synthase and soluble guanylate cyclase induces cardiodepressive effects in normal rat hearts. Eur J Pharmacol. 1997;334:181–190. doi: 10.1016/s0014-2999(97)01168-0. [DOI] [PubMed] [Google Scholar]
- Langer M, Luttecke D, Schluter KD. Mechanism of the positive contractile effect of nitric oxide on rat ventricular cardiomyocytes with positive force/frequency relationship. Pflugers Arch. 2003;447:289–297. doi: 10.1007/s00424-003-1187-8. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, et al. Carbon monoxide activates human intestinal smooth muscle L-type Ca2+ channels through a nitric oxide-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2005;288:G7–G14. doi: 10.1152/ajpgi.00205.2004. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Maines MD, Panahian N. The heme oxygenase system and cellular defense mechanisms. Do HO-1 and HO-2 have different functions. Adv Exp Med Biol. 2001;502:249–272. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- Matsui H, Barry WH, Livsey C, Spitzer KW. Angiotensin II stimulates sodium-hydrogen exchange in adult rabbit ventricular myocytes. Cardiovasc Res. 1995;29:215–221. [PubMed] [Google Scholar]
- Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- McGrath JJ. The effects of carbon monoxide on the heart: an in vitro study. Pharmacol Biochem Behav. 1984;21 Suppl 1:99–102. doi: 10.1016/0091-3057(84)90171-0. [DOI] [PubMed] [Google Scholar]
- Mielke M, Paterson DJ, Sang AE, Radda GK, Clarke K. The mechanism underlying the positive inotropic effect of angiotensin II in the isolated perfused rabbit heart: a 31P NMR study. Int J Biochem Cell Biol. 2003;35:984–991. doi: 10.1016/s1357-2725(02)00304-7. [DOI] [PubMed] [Google Scholar]
- Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. J Biol Chem. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005a;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, et al. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005b;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- Olson LJ, Knych ET, Herzig TC, Drewett JG. Selective guanylyl cyclase inhibitor reverses nitric oxide-induced vasorelaxation. Hypertension. 1997;29:254–261. doi: 10.1161/01.hyp.29.1.254. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- Patel AP, Moody AJ, Sneyd JR, Handy RD. Carbon monoxide exposure in rat heart: evidence for two modes of toxicity. Biochem Biophys Res Commun. 2004;321:241–246. doi: 10.1016/j.bbrc.2004.06.124. [DOI] [PubMed] [Google Scholar]
- Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AMK. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000;278:L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- Sandouka A, Balogun E, Foresti R, Mann BE, Johnson TR, Tayem Y, et al. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol (Noisy-le-grand) 2005;51:425–432. [PubMed] [Google Scholar]
- Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res. 2000;41:613–627. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- Szabo ME, Gallyas E, Bak I, Rakotovao A, Boucher F, de Leiris J, et al. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Inves Ophthalmol Vis Sci. 2004;45:3727–3732. doi: 10.1167/iovs.03-1324. [DOI] [PubMed] [Google Scholar]
- Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. J Gen Physiol. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerbraun BS, McCloskey CA, Gallo D, Liu F, Ifedigbo E, Otterbein LE, et al. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23:527–532. [PubMed] [Google Scholar]