Abstract
Classical biogenic amines (adrenaline, noradrenaline, dopamine, serotonin and histamine) interact with specific families of G protein-coupled receptors (GPCRs). The term ‘trace amines' is used when referring to p-tyramine, β-phenylethylamine, tryptamine and octopamine, compounds that are present in mammalian tissues at very low (nanomolar) concentrations. The pharmacological effects of trace amines are usually attributed to their interference with the aminergic pathways, but in 2001 a new gene was identified, that codes for a GPCR responding to p-tyramine and β-phenylethylamine but not to classical biogenic amines. Several closely related genes were subsequently identified and designated as the trace amine-associated receptors (TAARs). Pharmacological investigations in vitro show that many TAAR subtypes may not respond to p-tyramine, β-phenylethylamine, tryptamine or octopamine, suggesting the existence of additional endogenous ligands. A novel endogenous thyroid hormone derivative, 3-iodothyronamine, has been found to interact with TAAR1 and possibly other TAAR subtypes. In vivo, micromolar concentrations of 3-iodothyronamine determine functional effects which are opposite to those produced on a longer time scale by thyroid hormones, including reduction in body temperature and decrease in cardiac contractility. Expression of all TAAR subtypes except TAAR1 has been reported in mouse olfactory epithelium, and several volatile amines were shown to interact with specific TAAR subtypes. In addition, there is evidence that TAAR1 is targeted by amphetamines and other psychotropic agents, while genetic linkage studies show a significant association between the TAAR gene family locus and susceptibility to schizophrenia or bipolar affective disorder.
Keywords: G-protein coupled receptors, trace amines, thyronamines, amphetamine, tyramine, thyroid hormone, signal transduction
Introduction
In the last 5 years, important discoveries have been made in the field of aminergic signaling. Beginning in 2001, a new family of G protein-coupled receptors (GPCRs) was reported (Borowsky et al., 2001; Bunzow et al., 2001). Members of this large family of GPCRs are now referred to as trace amine-associated receptors or TAARs based on the pharmacological profile of its prototypical member, TAAR1 (Lindemann et al., 2005). In 2004, some amines derived from thyroid hormone decarboxylation and deiodination, known as thyronamines, were shown to be endogenous compounds that stimulate cAMP production via activation of heterologously expressed mouse and rat TAAR1 (Scanlan et al., 2004). Recently, it has been suggested that TAARs may represent a class of chemosensory receptors in the olfactory epithelium (Liberles and Buck, 2006). The discovery of a novel family of receptors and a novel complement of endogenously produced ligands throws open completely new perspectives, from biochemistry to cell biology, impacting physiology, pharmacology and pathophysiology of the thyroid gland, brain, olfactory sensory neurons and likely other organ systems, as well. The present review summarizes the key new developments in this field. For additional background information, the reader is referred to recent reviews on aminergic receptors (Shi and Javitch, 2002; Hill, 2006), trace amines (Premont et al., 2001; Davenport, 2003; Berry, 2004), and thyroid hormone metabolism (Wu et al., 2005). Reviews focussed on trace amine receptors have also appeared (Branchek and Blackburn, 2003; Lindemann and Hoener, 2005; Lindemann et al., 2005; Lewin, 2006).
Biological amines and trace amines
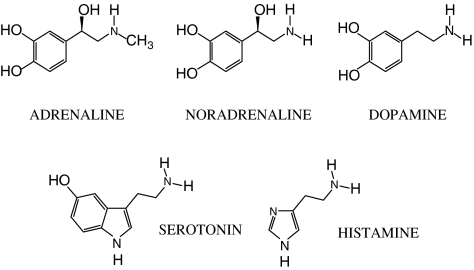
The term ‘biogenic amines' was introduced to designate a collection of amines that exert important biological effects as chemical messengers, that is, as hormones, local hormones, neuromodulators or neurotransmitters (Figure 1). They are all produced from aromatic amino acids and their synthetic pathways include a decarboxylation step that is catalyzed by one of several aromatic amino acid decarboxylases. The first biogenic amines to be discovered were the catecholamines, that is adrenaline (epinephrine), noradrenaline (norepinephrine) and dopamine, which derive from tyrosine. Other well-known biogenic amines are histamine and serotonin (5-hydroxytryptamine), which are derived from histidine and tryptophan, respectively.
Figure 1.
Structures of the classical biogenic amines.
The catecholamines noradrenaline and adrenaline were soon recognized to interact with cell surface membrane proteins known collectively as GPCRs (Dixon et al., 1986; Lefkowitz, 2004). Other metabotropic, aminergic GPCRs have subsequently been identified for dopamine, histamine and serotonin, although with the latter interacting also with an ionotropic receptor, the 5-HT3.
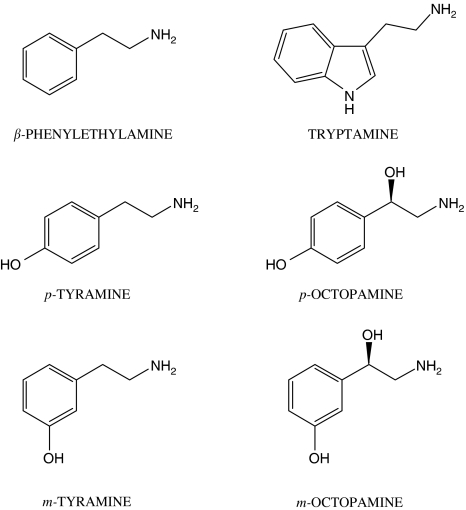
Other biogenic amines are present in the central nervous system at very low concentrations in the order of 0.1–10 nM, representing <1% of total biogenic amines (Berry, 2004). For these compounds, the term ‘trace amines' was introduced. Although somewhat loosely defined, the molecules generally considered to be trace amines include para-tyramine, meta-tyramine, tryptamine, β-phenylethylamine, para-octopamine and meta-octopamine (Berry, 2004) (Figure 2).
Figure 2.
Structures of the compounds usually referred to as ‘trace amines'.
Contrary to the situation in vertebrates, some trace amines are the chief amines found in many invertebrate species (Axelrod and Saavedra, 1977; David and Coulon, 1985; Evans and Robb, 1993; Roeder, 1999; Saraswati et al., 2004); for instance, octopamine and tyramine are thought to be the chief insect neurotransmitters, and octopamine is regarded as the sympathetic nervous system counterpart to noradrenaline in invertebrates. Trace amines are also produced in bacteria, fungi, plant cells, and can be found in some foods, most notably chocolate, cheese and red wine (Branchek and Blackburn, 2003).
The functional effects of trace amines have been traditionally referred to as ‘sympathomimetic' (Brunton et al., 2005). Some of these sympathomimetic symptoms appear when sensitive subjects treated with inhibitors of mono-amine oxidases (the enzymes primarily responsible for trace amine degradation: see below) consume foods that contain high concentrations of trace amines. In the central nervous system, trace amines can produce amphetamine-like responses, for example increased alertness, euphoria, irritability, decreased appetite, insomnia and tremor. Cardiovascular responses include tachycardia and vasomotor effects, which may lead to either hypertension or hypotension, depending on the dosage, experimental/clinical conditions and on the organism being studied. Nausea, emesis, decreased or increased bronchial resistance, sweating, hyperthermia and headache have also been reported. In the kidney, tyramine can increase chloride permeability (Blumenthal, 2003) and urinary flow (Rahman et al., 1995), even when the blood flow is unchanged. In contrast, octopamine decreases sodium and water excretion (Levy, 1988). Endocrine effects have also been reported: p-tyramine, β-phenylethylamine and octopamine can each inhibit prolactin secretion in anterior pituitary cells grown in vitro and in vivo (Becu-Villalobos et al., 1992); β-phenylethylamine increases ACTH and glucocorticoid production in rats (Kosa et al., 2000); octopamine produces an insulin-like effect on glucose uptake in adipocytes, skeletal muscle and myocardium (Visentin et al. 2001; Morin et al., 2002).
The above-described effects are typically produced by micromolar concentrations of trace amines, and their physiological relevance has therefore been questioned. At submicromolar concentrations, trace amines have been reported to produce neuromodulatory actions; that is, they either increase or decrease a cell's response to different neurotransmitters. As reviewed elsewhere (Berry, 2004), β-phenylethylamine and p-tyramine potentiate the responses elicited by noradrenaline and dopamine, whereas octopamine selectively increases neuronal responses to noradrenaline, while tryptamine potentiates the inhibitory response of cells to serotonin. In the neocortex of reserpinized rats atropine-resistant, low-voltage fast activity was restored by β-phenylethylamine (Vanderwolf et al., 1980). Inhibitory actions of trace amines have also been observed: in rat midbrain slices, β-phenylethylamine and p-tyramine depressed the inhibitory postsynaptic response mediated by GABA (B) receptors, possibly by interfering with the coupling of the receptor to inward rectifying potassium channels (Federici et al., 2005). In addition, β-phenylethylamine, p-tyramine and octopamine inhibited the electrically evoked release of acetylcholine in rat striatum, an effect that required the integrity of the striato-nigral dopaminergic system (Baud et al., 1985). In contrast, intraperitoneal administration of β-phenylethylamine increased striatal acetylcholine release, possibly through a glutamatergic pathway (Ishida et al., 2005). Whether these neuromodulatory actions have any physiological role remains to be determined.
Over the years, a pathophysiological role for trace amines in the genesis of several neurological or mental disorders has been advocated. Deficiency in β-phenylethylamine has been correlated with depression, and decreased β-phenylethylamine catabolism has been associated with schizophrenia. Individuals suffering from Parkinson's disease or those diagnosed with attention-deficit hyperactivity disorder may benefit from β-phenylethylamine administration (Schildkraut, 1976; Usdin and Sandler, 1976; Reynolds, 1979; Boulton, 1980; Davis and Boulton, 1994). Interestingly, it has been suggested that p-tyramine is essential for sensitization to cocaine in Drosophila (McClung and Hirsh, 1999). Traditionally, it has been taught that p-tyramine plays a role in the development of migraine headache; however, the veracity of this connection remains controversial. Foods rich in tyramine, such as chocolate, have been thought for a long time to trigger migraine attacks, but direct clinical studies have failed to demonstrate a firm link between migraines and tyramine exposure to date (Branchek and Blackburn, 2003; D'Andrea et al., 2004). A pathophysiological role for trace amines in hepatic encephalopathy has also been proposed (Mousseau and Butterworth, 1995b).
At the molecular level, the pharmacological effects of the trace amines have traditionally been attributed to their ability to induce noradrenaline release from sympathetic nerve endings, and, in general, to compete for catecholamine or serotonin-binding sites on their cognate receptors, transporters or storage sites (Raiteri et al., 1977; Jones, 1983; Locock et al., 1984; McCormack et al., 1986; Parker and Cubeddu, 1988; Dyck, 1989; Paterson et al., 1990). The biogenic monoamine transporters in particular have received the most attention in recent years, since concrete evidence for the existence of cell surface receptors specific for trace amines has been lacking in spite of the fact that the existence of high-affinity trace amine-binding sites was documented in the mammalian brain and other tissues (particularly the liver) over 20 years ago (Hauger et al., 1982; Cascio and Kellar, 1983; Bruning and Rommelspacher, 1984; Vaccari, 1986; Nguyen and Juorio, 1989; Mousseau and Butterworth, 1995a). However, this simple picture of how trace amines manifest their physiological and behavioral effects must be substantially modified with the recent discovery of a new family of GPCRs that are reported to be specific for trace amines.
Trace amine-associated receptors
The discovery of the first trace amine-associated receptor (TAAR1) was made independently by two groups of investigators, Borowsky et al. (2001) and Bunzow et al. (2001), each using a similar approach. Complex mixtures of oligonucleotides whose sequences were chosen based on GPCRs for serotonin (Borowsky et al., 2001) or dopamine (Bunzow et al., 2001) were used to amplify novel DNA sequences by PCR using rat cDNA and genomic DNA as templates. Both groups independently reported the cloning of several novel and unique sequences not present in any of the publicly accessible databases. The cloned sequences predicted a 332 amino-acid protein that had all of the hallmarks an aminergic GPCR. Expression of the putative orphan receptor in either Xenopus oocytes or human embryonic kidney (HEK) cells revealed that it can couple to the stimulation of cAMP production upon exposure to p-tyramine or β-phenylethylamine. Classical biogenic amines, including the catecholamines dopamine, noradrenaline and adrenaline, as well as serotonin and histamine are either ineffective or much less potent with EC50 values two orders of magnitude higher than the trace amines. Based on its pharmacological profile and functional coupling to cAMP production, Borowsky et al. and Bunzow et al. concluded that their orphan GPCR was a bone fide receptor for trace amines.
Bunzow et al.'s study (2001) focused on the cloning of both rat and human trace amine receptor sequences and on extensive pharmacological characterization of the cloned rat trace amine receptor stably expressed heterologously in HEK cells. Borowsky et al. (2001) also performed numerous degenerate oligonucleotide-primed PCRs using rat and human genomic DNAs as templates and identified 15 sequences with high homology to each other. Seventy-four amino-acid residues are completely conserved in all 15 genes, and 52 of these are unique to this GPCR family. The latter are scattered over the whole molecule and in particular they are present in all the seven transmembrane segments, but it is still unclear as to which of these are included into the ligand-binding pocket.
The availability of nearly complete genomic sequences for several vertebrate and invertebrate species recently enabled two groups working independently to assemble what is likely to be a complete catalog of all trace amine receptor genes including how many there are in each species, their chromosomal localization, their orientation relative to one another and the presence or absence of introns (Lindemann et al., 2005; Gloriam et al., 2005b). From the deduced amino-acid sequences of the human, mouse, rat and chimpanzee TAARs, Lindemann et al. extracted a polypeptide sequence whose motif is characteristic of every member of this large receptor family and 100% specific when used to query all current SwissProt entries. This motif overlaps with what is considered to be the receptor's putative seventh transmembrane domain and is defined as NSXXNPXX[YH]XXX[YF]XWF.
Collectively, 53 genes have been reported by genome-scanning efforts with nine of them in humans (including three pseudogenes); nine in chimpanzees (including six pseudogenes); 19 in rats (including two pseudogenes); and 16 in mice (including one pseudogene). As only one member of this family of GPCRs clearly responds to trace amines, at least in vitro (see below), Lindemann et al. (2005) proposed that all members of the family be referred to as ‘trace amine-associated receptors' (TAARs). In addition, Lindemann et al. (2005) proposed that each receptor gene be given a unique identifier that reflects where it is physically located on the chromosome relative to TAAR1, what other TAAR sequences it is most closely related to and if it is likely to be a pseudogene or not (Table 1). The human and chimpanzee genomes include nine TAAR genes, and the labels TAAR1 to TAAR9. The rat and mouse genomes contain additional genes that on the basis of sequence homologies, appear to have been generated by duplication events within the lineage of each species. Such genes are called paralogue genes and are identified by a letter suffix, while genes with the same number, which were likely generated by speciation events, are called orthologous genes. For instance, the rat genome contains three genes that are orthologues of the human TAAR8 and are designated: TAAR8a, TAAR8b and TAAR8c; and nine genes that are orthologues of human TAAR7 and are designated: TAAR7a to TAAR7i (Lindemann et al., 2005).
Table 1.
TAAR classification and nomenclature
| Human | Chimpanzee | Rat | Mouse | |||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | TAAR1 | TA1 | TAAR1 | TAAR1 | TA1 | TAAR1 | TA1 | |
| TAAR2 | GPR58 | (TAAR2) | TAAR2 | TAAR2 | ||||
| (TAAR3) | (GPR57) | (TAAR3) | TAAR3 | TAAR3 | ||||
| (TAAR4) | (TA2) | (TAAR4) | TAAR4 | TA2 | TAAR4 | |||
| Group 2 | TAAR 5 | PNR | TAAR5 | TAAR5 | TAAR5 | |||
| Group 3 | TAAR6 | TA4 | TAAR6 | TA4 | TAAR6 | TA4 | TAAR6 | |
| (TAAR7) | (TAAR7) | TAAR7a | TAAR7a | |||||
| TAAR7b | TA12 | (TAAR7b) | ||||||
| TAAR7c | TAAR7c | |||||||
| TAAR7d | TA15 | TAAR7d | ||||||
| TAAR7e | TA14 | TAAR7e | ||||||
| (TAAR7f) | (TA13) | TAAR7f | ||||||
| TAAR7g | TA9 | |||||||
| TAAR7h | TA6 | |||||||
| (TAAR7i) | ||||||||
| TAAR8 | TA5 | (TAAR8) | TAAR8a | TA11 | TAAR8a | |||
| TAAR8b | TA7 | TAAR8b | ||||||
| TAAR8c | TA10 | TAAR8c | ||||||
| TAAR9 | TA3 | (TAAR9) | TAAR9 | TA3 | TAAR9 | |||
List of TAAR genes in human, chimpanzee, rat and mouse. The table is derived from Lindemann et al. (2005). Pseudogenes are shown in brackets. Old names used in previous papers are reported on the right of the new standard name. TA stands for ‘trace amine receptor'. Other abbreviations were sometimes used in the place of TA, namely TAR or TRAR. GPR stands for G-protein receptor. PNR stands for putative neurotransmitter receptor. Human TA2 was also named 5-HT4, since it was initially interpreted as a serotonin receptor pseudogene. Human TA5 was also named GPR102.
All members of the trace amine receptor gene family produce relatively short transcripts with characteristically short coding regions (∼1000 base pairs in length) derived from a single exon. The exception to this ‘rule' is TAAR2, a receptor whose gene consists of two coding exons. Furthermore, in all mammalian species analyzed to date members of this gene family are linked along a stretch of a single chromosome. In humans, all of the trace amine receptor genes are located on chromosome 6 at band q23.1; in rat, the family is clustered on chromosome 1p12; and, in mouse, the family resides on chromosome 10 (Lindemann et al., 2005).
Other vertebrate genera in which TAARs have been identified include the fish species Takifugu rupbripres (fugu) and Danio rerio (zebrafish). In contrast to mammals, the zebra fish repertoire of 57 trace amine GPCR genes is scattered among at least six chromosomes (Gloriam et al., 2005b). In addition to their extensive analysis of the Danio genome, Gloriam et al. (2005a, 2005b) also made a surprising observation; contrary to expectations, invertebrate genomes do not contain sequences belonging to the TAAR family of GPCRs in spite of the fact that trace amine binding has been demonstrated in several invertebrate species including Drosophila melanogaster (Saudou et al., 1990), honeybee (Apis mellifera) (Blenau et al., 2000; Grohmann et al., 2003) and molluscs (Gerhardt et al., 1997). This is in contrast to the extensive inter-species homology reported for adrenoceptors and for muscarinic cholinergic receptors (Venter et al., 1984; Pelacios et al., 1989).
The extensive analysis of vertebrate and invertebrate genomes (Gloriam et al., 2005a, 2005b) leads to the conclusion that all vertebrate TAARs likely are derived from a common ancestor that existed prior to the separaton of fish and mammalian lineages. Additional analysis of their deduced amino-acid sequences allowed Lindemann et al. (2005) to separate each species's trace amine receptor gene family into three subdivisions consisting of TAAR1-4, TAAR5 and TAAR6-8. In contrast, invertebrate trace amine-activated receptors are not closely related to vertebrate TAARs but rather more similar to receptors activated by serotonin, in particular 5HT1 receptors. Therefore, it appears that, during speciation, the capability to preferentially bind trace amines has developed at least twice, and that vertebrate TAARs have not evolved from the invertebrate receptors for trace amines.
Subsequent to the reports of Borowsky et al. (2001) and Bunzow et al. (2001), for a long time, all attempts to heterologously express other putative trace amine receptors proved to be unsuccessful except possibly for TAAR4. Although there have been reports made at scientific meetings claiming expression and characterization of various rat and human trace amine receptor clones, none have been published until the very recent description of the results obtained in HEK293 cells transfected with human or mouse TAARs (Liberles and Buck, 2006). An explanation for the widespread difficulty to heterologously express most TAARs in vitro remains elusive.
Several authors reported TAAR transcripts to have a broad tissue distribution. In situ hybridization histochemistry revealed the presence of TAAR1 mRNA in many regions of the mouse brain (Borowsky et al., 2001). Using quantitative RT-PCR to amplify mRNA isolated from various human tissues, Borowsky et al. (2001) found that TAAR1 is expressed at moderate levels (100 copies/ng cDNA) in the stomach and at low levels (15–100 copies/ng cDNA) in the amygdala, kidney, lung and small intestine. Trace amounts were detected in liver, pancreas, prostate, skeletal muscle, spleen, as well as in many central nervous system locations. TAAR6 and TAAR8 were expressed in kidney and amygdala (Borowsky et al., 2001), while TAAR9 was expressed in kidney, pituitary and skeletal muscle (Vanti et al., 2003). Human leukocytes express TAAR1, TAAR6, TAAR8 and TAAR9 (D'Andrea et al., 2003), whereas rat hearts contain transcripts for TAAR8a, and, at a lower level, TAAR1, TAAR2, TAAR3 and TAAR4 (Chiellini G et al., unpublished observations).
Recently, Liberles and Buck (2006) reported that all TAAR subtypes, except for TAAR1, are expressed in the mouse olfactory epithelium. Double-labeling experiments suggested that different TAAR subtypes are expressed in different cells, and that TAARs are not coexpressed with odorant receptors. Notably, the authors obtained no evidence for TAAR gene expression in the brain and in other mouse tissues, with a detection threshold of about 100 copies of mRNA per cell. It remains to be determined whether the discrepancy with previous reports is due to species differences or rather due to differences in the sensitivity of the detection technique.
Endogenous TAAR ligands: (a) derivatives of standard amino acids
The metabolites considered by most to be trace amines derive from standard aromatic amino acids (i.e. phenylalanine, tyrosine, tryptophan) through a single enzymatic step: decarboxylation catalyzed by either aromatic L-amino acid decarboxylase (AADC; E.C. 4.1.1.28) or L-histidine decarboxylase (E.C. 4.1.1.22), the former known to be a rather non-selective enzyme since it requires only an aromatic group linked to alanine C(β) as the key feature for substrate recognition (Berry et al., 1996). The action of AADC directly yields p-tyramine from L-tyrosine, β-phenylethylamine from L-phenylalanine and tryptamine from L-tryptophan. Amino-acid decarboxylase is a single enzyme with one catalytic site but with different locations for attachment of the substrates. The enzyme is widely distributed in the brain and in the peripheral tissues. Recent investigations have shown that the enzyme is regulated by short-term mechanisms that may involve activation of adenyl cyclase or protein kinase C. In addition, a long-term mechanism of activation by altered gene expression has been suggested (Zhu and Juorio, 1995). Interestingly, a specific allele of the human dopamine D2 receptor is associated with increased activity of AADC in the striatum (Laakso et al., 2005).
The synthesis of some trace amines requires additional steps. The major biosynthetic route for m-tyramine formation is by the hydroxylation of phenylalanine, probably by the enzyme tyrosine hydroxylase to produce m-tyrosine, followed by decarboxylation catalyzed by AADC (Dyck et al., 1983). Octopamine can be produced from p-tyramine by the enzyme dopamine β-hydroxylase. Trace amines can also be synthesized from the conversion of the corresponding secondary amines (N-methyltyramine, N-methylphenylethylamine, N-methyltryptamine and synephrine) by the enzyme phenylethanolamine N-methyltransferase or by the nonspecifc enzyme N-methyltransferase, which are widely expressed in brain and in peripheral tissues (Saavedra et al., 1973, 1974).
Trace amine inactivation primarily involves the oxidation of the amino group, a reaction that is catalyzed by monoamine oxidase (MAO; E.C. 1.4.3.4). There are two MAO isoforms: MAO-A and MAO-B. Each isoform displays a characteristic preference for each trace amine; for example, β-phenylethylamine is primarily oxidized by MAO-B (Yang and Neff, 1973), while the other trace amines are metabolized by both MAO-A and MAO-B (Philips and Boulton, 1979; Durden and Philips, 1980). The occurrence and physiological importance of tryptamine oxidation by polymorphic cytochrome P450 isoenzymes is still a matter of debate (Paterson et al., 1990; Yu et al., 2003). The rate at which trace amines are metabolized is quite high with half-lives in the order of 30 s (Durden and Philips, 1980). Cellular membranes do not represent a significant barrier to trace amine diffusion and no substantial vesicular storage has been documented in the literature for any of these substances, with the exception of p-tyramine which appears to be stored in synaptic vesicles (Berry, 2004 and references therein).
Endogenous trace amine levels have been determined in the central nervous systems of some species of vertebrates and significant differences have been reported to exist between brain regions. In rat brain, the estimated concentration of trace amines in whole tissue ranges: 11–44 nM for β-phenylethylamine, 1–102 nM for p-tyramine, 0.4–73 nM for m-tyramine, 0.4–8 nM for tryptamine and 7–59 nM for octopamine (Berry, 2004 and references therein). Similar concentrations have been estimated in peripheral tissues (Durden et al., 1973; Saavedra and Axelrod, 1973; Philips et al., 1974; Kinniburgh and Boyd, 1979; Ibrahim et al., 1985; Durden and Boulton, 1988; D'Andrea et al., 2003). In terms of cellular distribution, each radiolabeled trace amine appears to have a rather uniform distribution, with its concentration determined principally by the relative rate of synthesis and degradation.
Although evidence in support of there being specific trace amine-binding sites in vertebrate tissues has been accumulating over the years (Hauger et al., 1982; Cascio and Kellar, 1983; Bruning and Rommelspacher, 1984; Vaccari, 1986; Nguyen and Juorio, 1989), the demonstration of specific receptors was only recently forthcoming. Now with the molecular cloning of rat, mouse, rhesus monkey and human TAAR sequences, they should be amenable to characterization by heterologously and stably expressing them in tissue culture (Borowsky et al., 2001; Bunzow et al., 2001; Miller et al., 2005). In COS-7 cells expressing recombinant human TAAR1, cAMP production can be induced by several biogenic amines in the following order of potency: p-tyramine>β-phenylethylamine>octopamine>dopamine>>tryptamine, histamine, serotonin, noerpinephrine. The EC50 for cAMP production averages 214 and 324 nM for p-tyramine and β-phenylethylamine, respectively, while it is over one order of magnitude higher for octopamine. A similar order of potency is observed in binding studies with regard to [3H]tyramine displacement. Similarly, in HEK293 cells stably expressing that the rat TAAR1 cloned independently by Bunzow et al. (2001), the rank order of potency for cAMP production is p-tyramine>β-phenylethylamine>tryptamine>octopamine>m-tyramine>>dopamine. In this cell line, the EC50 for p-tyramine and β-phenylethylamine averages 69 and 240 nM, respectively.
In general, TAAR1 orthologues obtained from different species show EC50 values for p-tyramine and β-phenylethylamine in the range of 0.1–1.4 μM (Lindemann et al., 2005; Liberles and Buck, 2006), while EC50 values for octopamine and tryptamine fall in the range of 2–10 μM and 1.5–45 μM, respectively (Lindemann et al., 2005). Interestingly, secondary amines such as N-ethyl-p-tyramine, N-ethyl-β-phenylethylamine and synephrine are slightly more potent than the corresponding primary amines. In contrast, dopamine and serotonin are 5- to 25-fold less potent than p-tyramine and β-phenylethylamine, respectively, based on EC50 values and maximum cAMP levels achieved, which are about half of what the trace amines produce (Lindemann et al., 2005), suggesting that they may act as partial agonists. At the rat TAAR1 histamine, adrenaline and noradrenaline at 1 μM concentrations are totally ineffective in stimulating cAMP production in tissue culture cells (Bunzow et al., 2001).
Studies performed with COS-7 cells expressing the rat TAAR4, previously known as TA2 (see Table 1), also show stimulation of cAMP production by trace amines, but the effect is limited to β-phenylethylamine and p-tyramine with EC50's in the order of 1.9 and 17 μM, respectively (Borowsky et al., 2001). The mouse TAAR4 shows EC50's>50 μM for all trace amines (Lindemann et al., 2005). However, an EC50 ∼1 μM for β-phenylethylamine was obtained in HEK293 cells transfected with the mouse TAAR4, if the latter was modified with an amino-terminal addition that facilitates cell-surface expression (Liberles and Buck, 2006).
In contrast, so far no response to p-tyramine, β-phenylethylamine, tryptamine, octopamine or other biogenic amines has been observed in transfected heterologous cells expressing TAAR subtypes other than TAAR1 or TAAR4 (Borowsky et al., 2001; Lindemann et al., 2005), and it should be pointed out that TAAR4 is a pseudogene in human. It is, however, worth noting that the apparent refractory nature of other members of this receptor family to trace amine agonism might just as well be an artefact of the heterologous expression systems being employed or second messengers being monitored. Coexpression of rhesus monkey TAAR1 and human dopamine transporter was associated with increased cAMP production by β-phenylethylamine and decreased cAMP production by tyramine (Miller et al, 2005). It is not clear whether these findings are due to the modulation of amine transport or rather due to the direct interaction between TAAR1 and dopamine transporter.
In conclusion, the identification of physiologically and behaviorally relevant interactions between p-tyramine or β-phenylethylamine (or other trace amines) and members of the TAAR family remains elusive. So far pharmacological effects were reported only for two receptor subtypes, using trace amines at concentrations, which are substantially higher than their endogenous levels. These considerations have fueled the search for other endogenous ligands.
Endogenous TAAR ligands: (b) thyronamines
An interesting line of research grew out of the extensive structure activity functional profiling of various species of TAAR1 carried out in the Grandy laboratory. The insight these investigators had was to realize that the β-phenylethylamine skeleton is an essential molecular feature that all TAAR1 agonists share and that this structural element is present in the decarboxylated skeleton of thyroid hormone molecular derivatives known as thyronamines (Scanlan et al., 2004).
Thyroid hormone metabolism involves different types of reactions, namely inner and outer ring deiodinations, deamination/transamination, decarboxylation and esterification (Wu et al., 2005). Decarboxylation is usually thought to occur after the amino group has been removed by MAOs or amino transferases, yielding tyroacetic acid derivatives. However, it cannot be excluded that decarboxylation may precede deamination, for example, as it does in the biosynthesis of the catecholamine series of neurotransmitters, and hence thyroxine, 3,5,3′-triiodothyronine, as well as their lower iodination state metabolites are converted to the corresponding arylethylamine compounds, also known as iodothyronamines (Figure 3).
Figure 3.
Structures of thyroid hormones and of thyronamines. T0AM, thyronamine; T1AM, 3-iodothyronamine; T3, 3,5,3′-triiodothyronine; T4, thyroxine.
Given the structural similarity between iodothyronamines and other biogenic amines it followed that iodothyronamines could be agonists of TAAR1. So, to test this hypothesis, nine different thyronamines were chemically synthesized (Scanlan et al., 2004; Hart et al., 2006) and then evaluated in transfected HEK293 cells stably expressing either the rat or mouse TAAR1. This approach revealed that several thyronamine derivatives induced a concentration-dependent increase in cAMP concentration. 3-Iodothyronamine (T1AM) appeared to be the most effective compound, with EC50 values in the range 10 to 100 nM for the rat and mouse receptors, respectively. 3,5-Diiodothyronamine, 3,5,3′-triiodothyronamine and the deiodinated derivative thyronamine were also effective, with decreasing EC50. Importantly, T1AM at concentrations <10 μM does not modify cAMP production in HEK293 cells expressing either the D1 dopamine receptor or the β2 adrenoceptor, and it has no affinity for the traditional nuclear thyroid hormone receptors (Scanlan et al., 2004) that belong to the nuclear receptor superfamily of hormone-activated transcription factors (Yen, 2001), and conversely 3,5,3′-triiodothyronine does not activate heterologously expressed TAAR1.
The combined use of liquid chromatography and tandem mass spectrometry allowed the identification of thyronamines (particularly, T1AM) in tissue homogenates derived from the brain and other tissues, confirming that these molecules can be correctly referred to as endogenous compounds. Although the assay was not quantitative, the use of internal standards showed that whole tissue T1AM content is in the order of 100 pmol/g (Scanlan et al., 2004; Chiellini et al., unpublished observations). It should be emphasized that the subcellular distribution of thyronamines is unknown. Therefore, their effective concentrations in specific compartments might be significantly different from the average concentrations.
While most effects of thyroid hormone are mediated by changes in gene transcription, non-genomic effects have also been described, which occur within a matter of seconds to minutes and are insensitive to inhibitors of protein synthesis such as cyclohexamide (Davis and Davis, 1996), although the underlying transduction pathways remain obscure (Falkenstein et al., 2000). Such effects concern sugar and calcium uptake (Davis and Blas, 1983; Segal and Ingbar, 1989), oxygen consumption (Ikemoto et al., 1967), ion channel activation (Yalcin et al., 1999) and cardiac function (Davis and Davis, 2002).
Therefore, it was interesting to evaluate the functional consequence of T1AM exposure, both in vivo and in vitro, on isolated organs. To date, several effects have been described and, surprisingly, they are opposite in direction to those produced by thyroid hormone, suggesting that T1AM could employ GPCRs as mediators of short-term modulation. In adult C57BL/6J mice, intraperitoneal injection of either T1AM or thyronamine (20–50 mg/kg) produced a rapid, dose-dependent decrease in body temperature (Scanlan et al., 2004). In isolated rat hearts, infusion of either T1AM or thyronamine (20–60 μM) produced a rapid, dose-dependent decrease in cardiac output and heart rate, providing evidence of a negative inotropic and chronotropic action (Chiellini et al., 2004; Scanlan et al., 2004). The decrease in heart rate was also confirmed in the in vivo mouse model, after intraperitoneal administration. In all cases, T1AM appeared to be more potent than thyronamine, and the effectiveness ratio was comparable to that observed in the heterologous cell model.
Further investigations are needed to clarify the receptor subtypes responsible for mediating the effects of T1AM as well as their physiological relevance. Decreases in body temperature and cardiac function are not consistent with increased cAMP production at the cellular level, raising the possibility that, in some tissues, either TAAR1 activation is not coupled to Gs proteins or T1AM may interact with other receptor subtypes. In rat, the cardiac effects of T1AM are remarkably accentuated by the tyrosine kinase inhibitor genistein, while they are dampened by the tyrosine phosphatase inhibitor vanadate (Chiellini et al., 2005), suggesting that a crucial step in signal transduction involves changes in the phosphorylation state of tyrosine residues. Evidence of tyrosine de-phosphorylation in cytosolic and microsomal proteins has been observed in rat hearts perfused with T1AM (Chiellini et al., unpublished observations).
Although T1AM is a novel compound only recently discovered, a few studies with other thyronamine congeners were performed many years ago. In the anaesthetized dog, thyronamine administration produced an increase in heart rate and cardiac inotropic state (Buu-Hoi et al., 1966; Buu-Hoi et al., 1969; Boissier et al., 1973; Cote et al., 1974). These effects appeared after a lag of about 10 min, and were remarkably blunted or abolished by adrenergic blockade, suggesting at the time that thyronamine induced catecholamine release. In contrast, after catecholamine depletion and/or adrenergic blockade, thyronamine infusion produced an immediate negative inotropic effect (Cote et al., 1974). On the basis of our recent findings (Scanlan et al., 2004), the latter might represent a TAAR-mediated response. Other investigations were performed with 3,5,3′-triiodothyronamine, which was reported to inhibit prolactin secretion in cultured pituitary cells (Cody et al., 1984). While this effect was attributed to interference with the adrenergic system (Meyer and Hesch, 1983), the involvement of TAARs cannot be excluded.
Recently, many thyronamine derivatives have been synthesized, and they have been comparatively evaluated with regard to their ability to induced hypothermia in mice and cAMP production in HEK cells stably expressing either the mouse or the rat TAAR1s (Hart et al., 2006). Some derivatives turn out to be more active than T1AM; namely, 3-methyl-thyronamine, N-methyl-O-(p-triflouromethyl)benzyl-tyramine, O-phenyl-3-iodotyramine and O-(p-fluoro)phenyl-3-iodotyramine. In general, structural–activity relationship studies have led to the following conclusions: a basic amino group at Cα is required for activity, and monomethylation of the amine can be beneficial; an iodide or methyl substituent at the 3-position of the thyronamine scaffold is optimal for activity; the 4′-OH of thyronamine is not necessary for activity but its removal may render the remaining compound difficult to metabolize and possibly result in impaired clearance.
In summary, there is evidence that T1AM and possibly other thyronamines interact with heterologously expressed TAAR1 and produce functional effects in vivo. However, there is still no direct evidence that the functional effects of exogenous T1AM are mediated by TAAR1 (or other TAAR subtypes), nor is there conclusive evidence that endogenous T1AM concentrations are sufficient to determine a physiological response in vivo. Therefore, the thesis that thyronamines are endogenous TAAR agonists should still be regarded as an interesting working hypothesis.
Odorous amines as TAAR ligands
The observation that most TAAR subtypes are expressed in the mouse olfactory epithelium induced to investigate whether TAARs may interact with odorants. For this purpose, HEK cells were transfected with vectors encoding for individual mouse or human TAARs, and cAMP production was determined after exposure to a large number (over 300) of odorous compounds (Liberles and Buck, 2006). A positive response was obtained with five mouse TAAR subtypes and 13 specific odorants, all of which were amines. In particular, TAAR3 responded to isoamylamine (EC50=10 μM), cyclohexylamine (EC50=20–30 μM), 2-methylbutamine (EC50=100 μM), isobutylamine and 3-(methylthio)-propylamine; TAAR4 responded to β-phenylethylamine (EC50=1 μM) and N,N-dimethyl-2-phenylethylamine; TAAR5 responded to trimethylamine (EC50=0.3 μM), dimethylethylamine (EC50=0.7 μM) and N-methylpiperidine; TAAR7f responded to N-methylpiperidine (EC50=20 μM). The mouse and human TAAR1 also responded to several volatile amines, although this subtype is not expressed in the olfactory epithelium.
These results induced to speculate that TAAR subtypes other than TAAR1 may represent a second class of chemosensory receptors, at least in mouse. Interestingly, at least three putative TAAR ligands, that is isoamylamine, trimethylamine and β-phenylethylamine, can be detected in mouse urine. Their concentration is related to gender or stress exposure, and isoamylamine acts as a pherormone in mouse (Liberles and Buck, 2006). Therefore, these compounds may be regarded as endogenous ligands, and it has been hypothesized that TAARs might be involved in the behavioral and physiological responses to social cues present in urine.
The transduction pathway(s) activated by TAAR stimulation in olfactory sensory neurons have not been determined, although coexpression of several TAAR subtypes (TAAR2, TAAR6, TAAR7f and TAAR9) with Gαolf proteins has been observed in these cells (Liberles and Buck, 2006).
Pharmacological agents as TAAR1 ligands
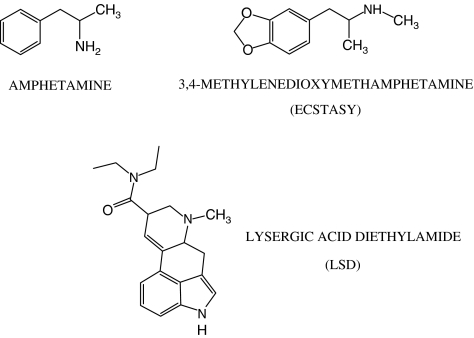
Given the structural similarity of amphetamine's molecular structure to that of β-phenylethylamine and p-tyramine (Figure 4), Bunzow et al. (2001) tested the hypothesis that amphetamine and its congeners would be potent agonists of heterologously expressed TAAR1s. In HEK293 cells stably expressing rat TAAR1, amphetamine stimulated cAMP production with an EC50 comparable to that of β-phenylethylamine (210 nM for R-amphetamine and 440 nM for S-amphetamine). Amphetamine derivatives also increased cAMP production with different potency. Methamphetamine, 3,4-methylenedioxymetamphetamine (MDMA, known as ‘Ecstasy') and the hallucinogenic amphetamine 2-amino,1-[2,5-dimethoxy-4-iodophenyl]-propane (DOI) were only slightly less potent than amphetamine. N-ethylderivatives such as fenfluramine and N-ethylamphetamine were substantially less effective, while 4-hydroxyamphetamine turned out to be the most potent rat TAAR1 agonist with an EC50 of 51 nM. Amphetamine and MDMA also stimulated cAMP production in HEK293 cells stably expressing the cloned rhesus monkey TAAR1 (Miller et al., 2005).
Figure 4.
Structures of some pharmacological modulators of TAARs.
Bunzow et al. (2001) also demonstrated that several widely used ergot alkaloids and ergoline derivatives can potently and efficaciously activate the rat TAAR1 in this heterologous model. Effective compounds included ergometrine, dihydroergotamine, D-lysergic acid diethylamide and the antiparkinsonian agents, bromocriptine and lisuride.
Finally, TAAR1-mediated cAMP production was reported in the presence of nonsubstrate inhibitors of dopamine transporter, namely 1 μM nomifensine and 10 μM 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT) (Bunzow et al, 2001).
All the drugs mentioned previously have other established molecular targets, so the relevance of their interaction with TAAR1 remains to be determined. However, human TAAR genes are all clustered on the long arm of chromosome 6 in one of the few regions (6q23.2), which is consistently associated with schizophrenia (Cao et al., 1997; Levinson et al., 2000; Schwab et al., 2000; Mowry and Nancarrow, 2001) or bipolar affective disorder (Rice et al., 1997; Ewald et al., 2002; Freudenberg-Hua et al., 2003) in linkage studies. Therefore, the finding that psychostimulant and hallucinogenic drugs interact with TAAR1 opens fascinating perspectives about the potential role of this signaling pathway in mental disorders and in drug addiction.
Some molecular genetic studies in recent years have focused on TAARs. A single nucleotide polymorphism in the 3′untranscribed region of TAAR6 gene (previously named as TRAR4) has been associated with susceptibility to schizophrenia in some families of African American or European ancestry (Duan et al., 2004), although a statistically significant association was not confirmed in Japanese or Arab Israeli kindreds (Ikeda et al., 2005; Amann et al., 2006). In another study, a nonsense mutation in the TAAR2 gene has been associated with nearly double the incidence of schizophrenia, although only a small number of patients were genotyped, and the difference versus control did not reach statistical significance (Bly, 2005).
A different single nucleotide polymorphism in the sixth transmembrane domain of TAAR 6 (V265I) was related to bipolar affective disorder in a German pedigree (Abou Jamra et al., 2005), although the association was not confirmed in a Swedish population (Venken et al., 2006). In contrast, no association was observed between bipolar disorder and a null mutation in TAAR9 (Vanti et al., 2003).
Conclusions and perspectives
The discovery of TAARs suggests the existence of novel aminergic system(s) in vertebrates. Trace amines such as tyramine, β-phenylethylamine, octopamine and tryptamine are known to play a major role in invertebrate physiology by interacting with specific plasma membrane GPCRs. It appears that in vertebrates TAARs evolved independently from the invertebrate receptors and acquired the ability to interact with different amines, including the decarboxylated thyroid hormone derivatives known as thyronamines, several volatile amines and possibly other as yet unidentified endogenous compounds. The relatively large number of TAAR genes and their allegedly widespread tissue expression suggest that this system has a major physiological importance.
Although the aforementioned picture is attractive, it is worth re-emphasizing that many basic issues are still unresolved. While TAAR gene expression has been observed in several tissues by RT–PCR and in situ hybridization, TAAR protein expression has not been formally demonstrated, owing to technical difficulties in developing adequate experimental tools. Effective subtype-specific anti-TAAR antibodies are not yet available, and even the expression of TAARs in heterologous systems has been difficult to achieve, since consistent success has been accomplished only with TAAR1. As a consequence, the best evidence of TAAR-mediated signaling is represented by the pharmacological responses observed in cells expressing TAAR1. Specific binding sites for trace amines and for T1AM have also been demonstrated, but their molecular identity and subcellular distribution are unknown. The lack of specific TAAR antagonists further complicates the interpretation of pharmacological and radioligand-binding experiments, while transgenic models of TAAR knockout or TAAR overexpression are not available, except for a TAAR1-KO mouse, which was the subject of a preliminary report (Wolinsky et al., 2004).
The downstream events involved in TAAR signaling are also poorly understood. Evidence from several laboratories confirms that heterologously expressed TAAR1 can couple with Gs proteins resulting in the stimulation of adenylate cyclase. However, it is possible that different TAAR subtypes might couple with different G proteins, and/or TAAR1 may show different coupling in different cells. In particular, the cardiac effects of thyronamines do not appear to be consistent with increased cAMP, and may involve changes in tyrosine kinase/phosphatase activity.
In spite of these limitations, the potential importance of the new aminergic system(s) should not be overlooked. Modulators of GPCR signaling represent the largest group of drugs currently available. Preliminary evidence that links TAARs to psychiatric diseases and psychotropic agents has been reported, and so exploring and defining the role of TAARs and their ligands in these and other pathological states seems to be the logical next step. Therefore, once TAAR signaling is unraveled and adequate pharmacological tools become available, important new therapeutical opportunities may result.
Abbreviations
- AADC
aromatic L-amino acid decarboxylase
- DOI
2-amino,1-[2,5-dimethoxy-4-iodophenyl]-propane
- GPCR
G protein-coupled receptor
- MAO
mono amino oxidase
- MDMA
3,4-methylenedioxymetamphetamine
- MTPT
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- T0AM
thyronamine
- T1AM
3-iodothyronamine
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TAAR
trace amine-associated receptor
Conflict of interest
The authors state no conflict of interest.
References
- Abou Jamra R, Sircar I, Becker T, Freudenberg-Hua Y, Ohlraun S, Freudenberg J, et al. A family-based and case–control association study of trace amine receptor genes on chromosome 6q23 in bipolar affective disorder. Mol Psychiatry. 2005;10:618–620. doi: 10.1038/sj.mp.4001665. [DOI] [PubMed] [Google Scholar]
- Amann D, Avidan N, Kanyas K, Kohn Y, Hamdan A, Ben-Hasher E, et al. The trace amine receptor 4 gene is not associated with schizophrenia in a sample linked to chromosome 6q23. Mol Psychiatry. 2006;11:119–121. doi: 10.1038/sj.mp.4001752. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Saavedra JM. Octopamine. Nature. 1977;265:501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Baud P, Arbilla S, Cantrill RC, Scatton B, Langer SZ. Trace amines inhibit the electrically evoked release of [3H]acetylcholine from slices of rat striatum in the presence of pargyline: similarities between beta-phenylethylamine and amphetamine. J Pharmacol Exp Ther. 1985;235:220–229. [PubMed] [Google Scholar]
- Becu-Villalobos D, Thyssen SM, Rey EB, Lux-Lantos V, Libertun C. Octopamine and phenylethylamine inhibit prolactin secretion both in vivo and in vitro. Proc Soc Exp Biol Med. 1992;199:230–235. doi: 10.3181/00379727-199-43352. [DOI] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Berry MD, Juorio AV, Li XM, Boulton AA. Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res. 1996;21:1075–1087. doi: 10.1007/BF02532418. [DOI] [PubMed] [Google Scholar]
- Blenau W, Balfanz S, Baumann A. Amtryr 1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila malpighian tubule. Am J Physiol Cell Physiol. 2003;284:C718–C728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- Bly M. Examination of the trace amine-associated receptor 2 (TAAR2) Schizophr Res. 2005;80:367–368. doi: 10.1016/j.schres.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Boissier JR, Giudicelli JF, Larno S, Advenier C. Differential inotropic-chronotropic action of thyronamine. Eur J Pharmacol. 1973;22:141–149. doi: 10.1016/0014-2999(73)90004-6. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddaz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA. Trace amines and mental disorders. Can J Neurol Sci. 1980;7:261–263. doi: 10.1017/s0317167100023313. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Bruning G, Rommelspacher H. High affinity [3H]-tryptamine binding sites in various organs of the rat. Life Sci. 1984;34:1441–1446. doi: 10.1016/0024-3205(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Brunton L, Lazo J, Parker K. Goodman, Gilman's The Pharmacological Basis of Therapeutics 2005McGraw-Hill: New York; (eds)11th edn [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang GE, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of the rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Buu-Hoi NP, Pham-Huu C, Petit L. Some biological effects of thyronamine. Med Pharmacol Exp Int J Exp Med. 1966;15:17–23. doi: 10.1159/000135844. [DOI] [PubMed] [Google Scholar]
- Buu-Hoi NP, Pham-Huu C, Petit L. Thyronamine, a new substance with long-lasting positive inotropic effect. Pharmacology. 1969;2:281–287. doi: 10.1159/000136030. [DOI] [PubMed] [Google Scholar]
- Cao Q, Martinez M, Zhang J, Sanders AR, Badner JA, Cravchik A, et al. Suggestive evidence for a schizophrenia susceptibility locus on chromosome 6q and a confirmation in an independent series of pedigrees. Genomics. 1997;43:1–8. doi: 10.1006/geno.1997.4815. [DOI] [PubMed] [Google Scholar]
- Cascio CS, Kellar KJ. Characterization of [3H] tryptamine binding sites in brain. Eur J Pharmacol. 1983;95:31–39. doi: 10.1016/0014-2999(83)90264-9. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Frascarelli S, Hart ME, Ronca-Testoni S, Scanlan TS, Zucchi R. 3-Iodothyronamine is a novel endogenous modulator of cardiac inotropic state. Circulation. 2004;110:III-258. [Google Scholar]
- Chiellini G, Frascarelli S, Hart ME, Ronca-Testoni S, Scanlan TS, Zucchi R. Modulation of cardiac inotropic state by a novel thyroid hormone analog acting via non genomic mechanism. J Mol Cell Cardiol. 2005;38:1086. [Google Scholar]
- Cody V, Meyer T, Dohler KD, Hesch RD, Rokos H, Marko M. Molecular structure and biochemical activity of 3,5,3'-triiodothyronamine. Endocr Res. 1984;10:91–99. doi: 10.3109/07435808409035410. [DOI] [PubMed] [Google Scholar]
- Cote P, Polumbo RA, Harrison DC. Thyronamine, a new inotropic agent: its cardiovascular effects and mechanism of action. Cardiovasc Res. 1974;8:721–730. doi: 10.1093/cvr/8.6.721. [DOI] [PubMed] [Google Scholar]
- D'Andrea G, Perini F, Terrazzino S, Nordera GP. Contribution of biochemistry to the pathogenesis of primary headaches. Neurol Sci. 2004;25:S89–S92. doi: 10.1007/s10072-004-0260-1. [DOI] [PubMed] [Google Scholar]
- D'Andrea G, Terrazzino S, Fortin D, Farruggio A, Rinaldi L, Leon A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci Lett. 2003;346:89–92. doi: 10.1016/s0304-3940(03)00573-1. [DOI] [PubMed] [Google Scholar]
- Davenport AP. Peptide and trace amine orphan receptors: prospects for new therapeutic targets. Curr Opin Pharmacol. 2003;3:127–134. doi: 10.1016/s1471-4892(03)00003-1. [DOI] [PubMed] [Google Scholar]
- David JC, Coulon JF. Octopamine in invertebrates and vertebrates. A review. Prog Neurobiol. 1985;24:141–185. doi: 10.1016/0301-0082(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Davis BA, Boulton AA. The trace amines and their acidic metabolites in depression – an overview. Prog Neuropsycopharmacol Biol Psychiatry. 1994;18:17–45. doi: 10.1016/0278-5846(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Blas SD. Role of calmodulin in thyroid hormone stimulation of in vitro human erythrocyte Ca2+ – ATPase activity. J Clin Invest. 1983;71:579–586. doi: 10.1172/JCI110803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Davis FB. Nongenomic action of thyroid hormone. Thyroid. 1996;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB. Nongenomic action of thyroid hormone on the heart. Thyroid. 2002;12:459–466. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- Dixon RAF, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Duan J, Martinez M, Sanders AR, Hou C, Saitou N, Kitano T, et al. Polymorphisms in the trace amine receptor 4 (TRAR4) gene on chromosome 6q23.2 are associated with susceptibility to schizophrenia. Am J Hum Genet. 2004;75:624–638. doi: 10.1086/424887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden DA, Boulton AA. Analysis of tryptamine at the femtomole level in tissue using negative ion chemical ionization gas chromatography-mass spectrometry. J Chromatogr. 1988;440:253–259. doi: 10.1016/s0021-9673(00)94528-x. [DOI] [PubMed] [Google Scholar]
- Durden DA, Philips SR. Kinetic measurements of the turnover rates of phenylethylamine and tryptamine in vivo in the rat brain. J Neurochem. 1980;34:1725–1732. doi: 10.1111/j.1471-4159.1980.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Durden DA, Philips SR, Boulton AA. Identification and distribution of beta-phenylethylamine in the rat. Can J Biochem. 1973;51:995–1002. doi: 10.1139/o73-129. [DOI] [PubMed] [Google Scholar]
- Dyck LE. Release of some endogenous trace amines from rat striatal slices in the presence and absence of a monoamine oxidase inhibitor. Life Sci. 1989;44:1149–1156. doi: 10.1016/0024-3205(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Dyck LE, Yang CR, Boulton AA. The biosynthesis of p-tyramine, m-tyramine, and beta phenylethylamine by rat striatal slices. J Neurosci Res. 1983;10:211–220. doi: 10.1002/jnr.490100209. [DOI] [PubMed] [Google Scholar]
- Evans PD, Robb S. Octopamine receptor subtypes and their modes of action. Neurochem Res. 1993;18:869–874. doi: 10.1007/BF00998270. [DOI] [PubMed] [Google Scholar]
- Ewald H, Flint T, Kruse TA, Mors O. A genome-wide scan shows significant linkage between bipolar disorder and chromosome 12q24.3 and suggestive linkage to chromosomes 1p22-21, 4p16, 6q14-22, 10q26 and 16p13.3. Mol Psychiatry. 2002;7:734–744. doi: 10.1038/sj.mp.4001074. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormone – a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Federici M, Geracitano R, Tozzi A, Longone P, Di Angelantonio S, Bengtson CP, et al. Trace amines depress GABA B response in dopaminergic neurons by inhibiting G-betagamma-inwardly rectifying potassium channels. Mol Pharmacol. 2005;67:1283–1990. doi: 10.1124/mol.104.007427. [DOI] [PubMed] [Google Scholar]
- Freudenberg-Hua Y, Freudenberg J, Kluck N, Cichon S, Propping P, Nöthen MM. Single nucleotide variation analysis in 65 candidate genes for CNS disorders in a representative sample of the European population. Genome Res. 2003;13:2271–2276. doi: 10.1101/gr.1299703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt CC, Bakker RA, Piek GJ, Planta RJ, Vreugdenhill E, Leysen JE, et al. Molecular cloning and pharmacological characterization of a molluscan octopamine receptor. Mol Pharmacol. 1997;51:293–300. doi: 10.1124/mol.51.2.293. [DOI] [PubMed] [Google Scholar]
- Gloriam DEI, Bjarnadottir TK, Schiöth HB, Fredriksson R. High species variation within the repertoire of trace amine receptors. Ann NY Acad Sci. 2005a;1040:323–327. doi: 10.1196/annals.1327.052. [DOI] [PubMed] [Google Scholar]
- Gloriam DEI, Bjarnadottir TK, Yan YL, Postlethwait JH, Schiöth HB, Fredriksson R. The repertoire of trace amine G-protein-coupled receptors: large expansion in zebrafish. Mol Phylogenesis Evol. 2005b;35:470–482. doi: 10.1016/j.ympev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strunker T, Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues. J Med Chem. 2006;49:1101–1112. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Skolnick P, Paul SM. Specific [3H]beta-phenylethylamine binding sites in rat brain. Eur J Pharmacol. 1982;83:147–148. doi: 10.1016/0014-2999(82)90301-6. [DOI] [PubMed] [Google Scholar]
- Hill SJ. G-protein-coupled receptors: past, present and future. Br J Pharmacol. 2006;147:S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim KE, Couh MW, Williams CW, Fregly MJ, Midgley JM. m-Octopamine: normal occurrence with p-octopamine in mammalian sympathetic nerves. J Neurochem. 1985;44:1862–1867. doi: 10.1111/j.1471-4159.1985.tb07180.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwara N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, et al. No association of haplotype-tagging SNPs in TRAR4 with schizophrenia in Japanese patients. Schizophr Res. 2005;78:127–130. doi: 10.1016/j.schres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Ikemoto H, Hiroshige T, Itoh S. Oxygen consumption of brown adipose tissue in normal and hypothyroid mice. Jpn J Physiol. 1967;17:516–522. doi: 10.2170/jjphysiol.17.516. [DOI] [PubMed] [Google Scholar]
- Ishida K, Murata M, Kato M, Utsunomiya I, Hoshi K, Taguchi K. Beta-phenylethylamine stimulates striatal acetylcholine release through activation of the AMPA glutamatergic pathway. Biol Pharm Bull. 2005;28:1626–1629. doi: 10.1248/bpb.28.1626. [DOI] [PubMed] [Google Scholar]
- Jones RSG. Trace biogenic amines: a possible functional role in the CNS. Trends Pharmacol Sci. 1983;4:426–429. [Google Scholar]
- Kinniburgh DW, Boyd ND. Determination of plasma octopamine and its level in renal disease. Clin Biochem. 1979;12:27–32. doi: 10.1016/s0009-9120(79)90048-1. [DOI] [PubMed] [Google Scholar]
- Kosa E, Marcilhac-Flouriot A, Fache MP, Siaud P. Effects of beta-phenylethylamine on the hypothalamo–pituitary–adrenal axis in the male rat. Pharmacol Biochem Behav. 2000;67:527–535. doi: 10.1016/s0091-3057(00)00383-x. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, et al. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet Genom. 2005;15:387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Holmans P, Straub RE, Owen MJ, Wildenauer DB, Gejman PV, et al. Multicenter linkage study of schizophrenia candidate regions on chromosomes 5q, 6q, 10p, and 13q: schizophrenia linkage collaborative group III. Am J Hum Genet. 2000;67:652–663. doi: 10.1086/303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M. Effects of octopamine on renal function in anaesthetized dogs. Clin Invst Med. 1988;11:396–402. [PubMed] [Google Scholar]
- Lewin A. Receptors of mammalian trace amines. AAPS J. 2006;8:E138–E145. doi: 10.1208/aapsj080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Hoener M. A renaissance in trace amines inspired by a novel GPCR family. Trends Pramacol Sci. 2005;26:274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Locock RA, Baker GB, Coutts RT, Dewhurst WG. Displacement of serotonin from binding sites in rat cortex: the effects of biogenic ‘trace' amines. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:701–704. doi: 10.1016/0278-5846(84)90041-1. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- McCormack JK, Beitz AJ, Larson AA. Autoradiographic localization of tryptamine binding sites in the rat and dog central nervous system. J Neurosci. 1986;6:94–101. doi: 10.1523/JNEUROSCI.06-01-00094.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Hesch RD. Triiodothyronamine – a beta adrenergic metabolite of triiodothyronine. Horm Met Res. 1983;15:602–606. doi: 10.1055/s-2007-1018803. [DOI] [PubMed] [Google Scholar]
- Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, et al. Primate trace amine receptor 1 modulation by the dopamine transporter. J Pharmacol Exp Ther. 2005;313:983–995. doi: 10.1124/jpet.105.084459. [DOI] [PubMed] [Google Scholar]
- Morin N, Visentin V, Calise D, Marti L, Zorzano A, Testar X, et al. Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidase. J Pharmacol Exp Ther. 2002;303:1238–1247. doi: 10.1124/jpet.102.040592. [DOI] [PubMed] [Google Scholar]
- Mousseau DD, Butterworth RF. A high-affinity [3H]tryptamine binding site in human brain. Prog Brain Res. 1995a;106:285–291. doi: 10.1016/s0079-6123(08)61225-x. [DOI] [PubMed] [Google Scholar]
- Mousseau DD, Butterworth RF. Trace amines in hepatic encephalopathy. Prog Brain Res. 1995b;106:277–284. doi: 10.1016/s0079-6123(08)61224-8. [DOI] [PubMed] [Google Scholar]
- Mowry BJ, Nancarrow DJ. Molecular genetics of schizophrenia. Clin Exp Pharmacol Physiol. 2001;28:66–69. doi: 10.1046/j.1440-1681.2001.03399.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Juorio AV. Binding sites for brain trace amines. Cell Mol Neurobiol. 1989;9:277–311. doi: 10.1007/BF00711411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EM, Cubeddu LX. Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. J Pharmacol Exp Ther. 1988;245:199–210. [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Boulton M. 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system. J Neurochem. 1990;55:1827–1837. doi: 10.1111/j.1471-4159.1990.tb05764.x. [DOI] [PubMed] [Google Scholar]
- Pelacios JM, O'Dowd BF, Cotecchia S, Hnatowich M, Caron MG, Lefkowitz RJ. Adrenergic receptors homologies in vertebrate and invertebrate species examined by DNA hybridization. Life Sci. 1989;44:2057–2065. doi: 10.1016/0024-3205(89)90352-4. [DOI] [PubMed] [Google Scholar]
- Philips SR, Boulton AA. The effect of monoamine oxidase inhibitors on some arylalkylamines in rat striatum. J Neurochem. 1979;33:159–167. doi: 10.1111/j.1471-4159.1979.tb11718.x. [DOI] [PubMed] [Google Scholar]
- Philips SR, Durden DA, Boulton AA. Identification and distribution of p-tyramine in the rat. Can J Biochem. 1974;52:366–373. doi: 10.1139/o74-055. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AR, Lang CC, Struthers AD. The effect of enalapril on tyramine induced changes in renal function in man. Int J Clin Pharmacol Ther. 1995;33:404–409. [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- Reynolds GP. Phenylethylamine – a role in mental illness. Trends Neurosci. 1979;2:265–268. [Google Scholar]
- Rice JP, Goate A, Williams JT, Bierut L, Dorr D, Wu W, et al. Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 1, 6, 8, 10, and 12. Am J Med Genet. 1997;74:247–253. doi: 10.1002/(sici)1096-8628(19970531)74:3<247::aid-ajmg3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–541. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. Effect of drugs on the tryptamine content of rat tissues. J Pharmacol Exp Ther. 1973;185:523–529. [PubMed] [Google Scholar]
- Saavedra JM, Coyle JT, Axelrod J. The distribution and properties of the nonspecific N-methyltransferase in brain. J Neurochem. 1973;20:743–752. doi: 10.1111/j.1471-4159.1973.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Palkovits M, Brownstein MJ, Axelrod J. Localisation of phenylethanolamine N-methyl transferase in the rat brain nuclei. Nature. 1974;248:695–696. doi: 10.1038/248695a0. [DOI] [PubMed] [Google Scholar]
- Saraswati S, Fox LE, Soll DR, Wu CF. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amlaiki N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders. A review of supporting evidence. Int J Psychiatry. 1976;4:203–217. [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Albus M, Lerer B, Eckstein GN, Borrmann M, et al. A genome-wide autosomal screen for schizophrenia susceptibility loci in 71 families with affected siblings: support for loci on chromosome 10p and 6. Mol Psychiatry. 2000;5:638–649. doi: 10.1038/sj.mp.4000791. [DOI] [PubMed] [Google Scholar]
- Segal J, Ingbar SH. 3,5,3′-Triiodothyronine increases 3′,5′-monophoshate concentration and sugar uptake in rat thymocytes by stimulating adenylate cyclase activity: studies with the adenylate cyclase inhibitor MDL 12330A. Endocrinology. 1989;124:2166–2171. doi: 10.1210/endo-124-5-2166. [DOI] [PubMed] [Google Scholar]
- Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop. Annu Rev Pharmacol Toxicol. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- Usdin E, Sandler M. Trace Amines and the Brain 1976Dekker: New York; (eds) [Google Scholar]
- Vaccari A. High affinity binding of [3H]-tyramine in the central nervous system. Br J Pharmacol. 1986;89:15–25. doi: 10.1111/j.1476-5381.1986.tb11116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH, Robinson TE, Pappas BA. Monoamine replacement after reserpine: catecholaminergic agonists restore motor activity but phenylelthylamine restores atropine-resistant neocortical low voltage fast activity. Brain Res. 1980;202:65–77. [PubMed] [Google Scholar]
- Vanti WB, Muglia P, Nguyen T, Cheng R, Kennedy JL, George SR, et al. Discovery of a null mutation in a human trace amine receptor gene. Genomics. 2003;82:531–536. doi: 10.1016/s0888-7543(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Venken T, Alaerts M, Adolfsson R, Broeckhoven CV, Del Favero J. No association of trace amine-associated receptor 6 with bipolar disorder in a northern Swedish population. Psychiatr Gent. 2006;16:1–2. doi: 10.1097/01.ypg.0000180682.18665.a6. [DOI] [PubMed] [Google Scholar]
- Venter JC, Eddy B, Hall LM, Fraser CM. Monoclonal antibodies detect the conservation of muscarinic cholinergic receptor structure from Drosophila to human brain and detect possible structural homology with alpha 1-adrenergic receptors. Proc Natl Acad Sci USA. 1984;81:272–276. doi: 10.1073/pnas.81.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin V, Morin N, Fontana E, Prevot D, Boucher J, Castan I, et al. Dual action of octopamine on glucose transport into adipocytes: inhibition via beta3-adrenoceptor activation and stimulation via oxidation by amine oxidase. J Pharmacol Exp Ther. 2001;299:96–104. [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Zhong H, Smith KE, Branchek TA, Gerald CP. Deficit in prepulse inhibition and enhanced sensitivity to amphetamine in mice lacking the trace amine-1 receptor. Neuropsychopharmacology. 2004;29:S231. [Google Scholar]
- Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. Alternate pathways of thyroid hormone metabolism. Thyroid. 2005;15:943–958. doi: 10.1089/thy.2005.15.943. [DOI] [PubMed] [Google Scholar]
- Yalcin Y, Carman D, Shao Y, Ismail-Beigi F, Klein I, Ojamaa K. Regulation of Na/K-ATPase gene expression by thyroid hormone and hyperkalemia in the heart. Thyroid. 1999;9:53–59. doi: 10.1089/thy.1999.9.53. [DOI] [PubMed] [Google Scholar]
- Yang HY, Neff NH. Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther. 1973;187:365–371. [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Kupfer A, et al. The relative contribution of monoamine oxidase and cytochrome p450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther. 2003;304:539–546. doi: 10.1124/jpet.102.043786. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV. Aromatic L-amino acid decarboxylase: biological characterization and functional role. Gen Pharmacol. 1995;26:681–696. doi: 10.1016/0306-3623(94)00223-a. [DOI] [PubMed] [Google Scholar]