Abstract
Prostaglandin E2 (PGE2) is one of the most important biologically active prostanoids found throughout the gastrointestinal tract. Despite the fact that PGE2 regulates many physiological functions of the gut including mucosal protection, gastrointestinal secretion and motility, it is implicated in the pathophysiology of inflammatory bowel diseases (IBD) and colorectal neoplasia. The varied biological functions exerted by PGE2 are through the pharmacologically distinct, G-protein coupled plasma membrane receptors termed EP receptors. Disruptions of various prostanoid receptor genes have helped in unravelling the physiological functions of these receptors. To date, all four subtypes of EP receptors have been individually knocked out in mice and various phenotypes have been reported for each subtype. Similarly, in vitro and in vivo studies using EP receptor agonists and antagonists have helped in uncoupling the diverse functions of PGE2 signalling involving distinct EP receptors in the gut. In this review, we will summarize and conceptualize the salient features of EP receptor subtypes, their regional functions in the gut and how expressions of EP receptors are altered during disease states.
Keywords: prostaglandin E2, gastrointestinal tract, EP receptors, distribution, functions, inflammation, differential expression
Introduction
The interests in understanding prostaglandin (PG)-mediated functions of the gastrointestinal (GI) tract have grown steadily since the discovery of PGs in 1957. In recent years, technological advances in the field of molecular biology have helped researchers make substantial progress towards unraveling the complex functions of PGs in the gut. It is only now that the functional roles of the various PGs in the GI tract are established. Apparently, each of the bioactive PGs exhibits versatility and diversity in functions in various segments of the gut. However, the mechanisms by which they exert their biological functions are not clearly known. To discern the variable roles of PGs, it is imperative to understand thoroughly the mode of action of these mediators, including the pharmacology, expression and distribution of their respective receptors in the gut. Nevertheless, insights into PG receptor biology in the GI tract will be of fundamental importance in developing strategies for pharmacological intervention in various GI ailments.
PG biosynthesis
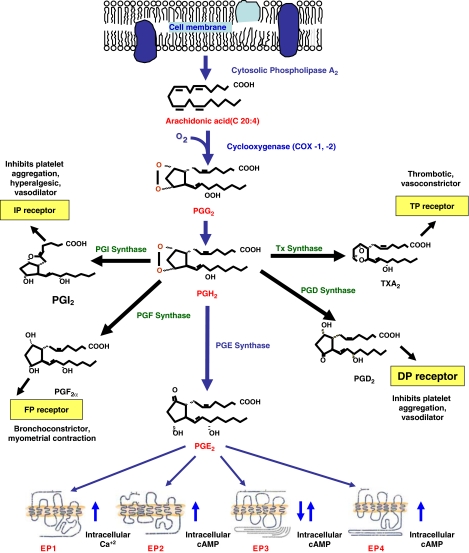
PGs are 20-carbon fatty acid derivatives that are found ubiquitously in all tissues and organs and mediate variety of physiological and pathological functions. They are synthesized in the cell from different essential fatty acid precursors, including arachidonic acid (AA). PGs derived from AA are termed series-2 PGs, which consist of prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostaglandin I2 (PGI2), prostaglandin F2α (PGF2α) and thromboxane A2 (TXA2) (Calder, 2001). All of these PGs share a common initial biosynthetic pathway that begins with the hydrolysis of cell-membrane phospholipids, mediated by the enzyme phospholipase A2 (PLA2), which is found mostly in cellular membranes including the plasma membrane (Murakami et al., 1997). Diverse physiological and pathological stimuli can result in the activation of PLA2 to liberate AA from membrane phospholipids into the cytoplasm (Murakami and Kudo, 2002). Upon release, AA is converted into unstable endoperoxide intermediates, PGG2 and PGH2 (Hamberg et al., 1974) by the action of cyclooxygenase (COX) in a rate limiting enzymatic reaction. Three isoforms of COX have been identified to date. The constitutively expressed COX-1 and the inducible COX-2 are the most important isoforms (Smith et al., 1994, 1996). COX-3 is a splice variant of COX-1 and is mostly expressed in the brain and the heart (Chandrasekharan et al., 2002). The oxygenated intermediate PGH2 is in turn metabolized by cell-specific synthases and isomerases into PGD2, PGE2, PGF2α, PGI2, and TXA2 (Vane et al., 1998; Figure 1). Prostanoids are released outside the cell immediately after their synthesis where they exert their biological functions through their interaction with the cell surface prostanoid receptors in an autocrine or paracrine fashion (Narumiya, 1994). Alternatively, the action of prostanoids are terminated when they are transported across the cell membrane into the cytoplasmic compartment with the help of PG transporters (Kanai et al., 1995) where they are acted upon by oxidizing and reducing enzymes, 15 hydroxy PG dehydrogenases and delta 13–15-ketoprostaglandin reductase, respectively (Tai et al., 2002).
Figure 1.
Biosynthesis of prostaglandins. Note that arachidonic acid liberated by the action of membrane bound phospholipase A2 and cyclooxygenase are the key rate-limiting steps for prostaglandin biosynthesis. Shown are the prostanoids, their respective receptors and their related pathophysiological functions.
Prostanoid receptors
Prostanoid receptors belong to the family of Rhodopsin-type receptors characterized by their seven transmembrane domains that are intracellularly coupled to different subunits of G proteins (Breyer et al., 2001). Five major types of prostanoid receptors, namely; D prostanoid (DP), E prostanoid (EP), F prostanoid (FP), I prostanoid (IP) and T prostanoid (TP), which include six subtypes namely; DP1, DP2, EP1, EP2, EP3 and EP4, have been described for the prostanoids PGD2, PGE2, PGF2α, PGI2 and TXA2. The structures, properties and functions of most of these receptors have previously been reviewed (Narumiya et al., 1999). Investigation of the functional role of these receptors in gut physiology as well as pathophysiology is presently an important pursuit. For example, the role of DP, IP, EP and TP receptors in the propulsive peristaltic movement of the guinea-pig small intestine has been studied and the receptors responsible for differential peristaltic motor effect identified (Shahbazian et al., 2002). Similarly, the role of DP, FP, IP, TP and the EP receptor subtypes in the development of carcinogen-induced aberrant crypt foci in colon has been studied extensively (Watanabe et al., 1999; Mutoh et al., 2002).
It is clear that the different prostanoids synthesized and their interactions with respective cellular receptors are responsible for the varied biological actions of the host. As the cellular components of the GI tract are known to possess the machinery for the biosynthesis of PG, it is not surprising that PGs are produced throughout the gut. Among the different PGs that are produced, PGE2 is considered to be the most important for normal physiological functions of the GI tract including gastric mucosal protection and motility and is also implicated in the pathology of various disease conditions such as inflammatory bowel disease (IBD) (Ahrenstedt et al., 1994), entero-invasive bacterial diseases (Reseta and Barrett, 2002) and colorectal cancers (Eberhart et al., 1994). Diversity in the cellular functions of PGE2 is attributed to its binding to four different subtypes of EP receptors that in turn propagates signals through alteration in the intracellular calcium (Ca2+) or cyclic adenosine monophosphate (cAMP) levels. This results in the activation of an array of kinases modulating diverse cellular functions (Figure 2). Although, signalling through different EP receptor determines the various effects of PGE2, regional and differential expression of EP receptors in the GI tract is critical for determining its biological functions. Based on this premise, we will summarize in this review the salient features of EP receptor subtypes, their regional functions in the gut and their differential expression during normal and disease states.
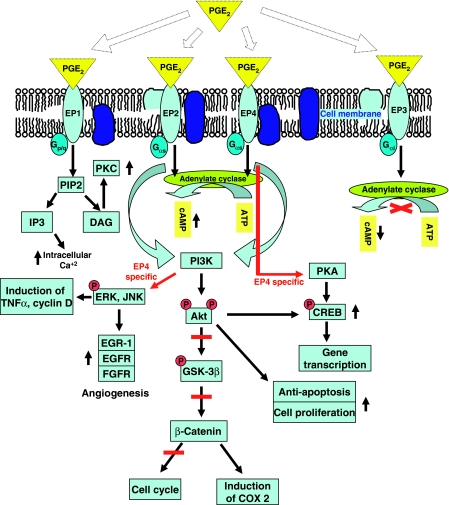
Figure 2.
PGE2-EP receptor signaling pathways. Four major types of EP receptors are involved in the signaling pathway mediated via different G proteins (p/q, αs and αi) using different second messenger. PGE2 induces intracellular Ca2+ or cAMP when it couples and signals through EP1 or EP2/4 receptors respectively. However, it reduces intracellular cAMP when it signals through EP3 receptors. Several different kinases are involved in PGE2 induced signaling pathways. PGE2-EP4 receptor coupling induces TNFα, cyclin D and angiogenesis using a specific pathway that is mediated via different MAP kinases. PGE2 also promotes cancer cell proliferation and inhibits apoptosis. It also induces COX-2 gene transcription and induces cell proliferation. On the other hand, it could also inhibit cell cycle through phosphorylation of Akt, GSK-3β and β-catenin. Abbreviations: AC, adenylate cyclase; DAG, diacylglycerol; IP3, inositol triphosphate; PIP2, phosphatidylinositol diphosphate; PKA, protein kinase A; PKC, protein kinase C; PI3K, phosphoinositide-3-kinase; Akt, protein kinase B; ERK, extracellular signal-regulated protein kinase; JNK, c-Jun N-terminal kinase; EGR-1, early growth response 1; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GSK-3, glycogen synthase kinase-3; ®, phosphorylation; ↑, up regulated; ↓, down regulated; X, inhibition.
EP receptors
The cellular membrane receptors for PGE2 are termed the EP receptors that consist of four different subtypes namely EP1, EP2, EP3 and EP4. They are encoded by different genes and are well conserved throughout the mammalian system from mouse to human. Phylogenetic analysis of the amino-acid sequence of all the EP receptors indicates that they were all sequentially (EP2, EP4, EP3 and EP1) derived from a primitive PGE receptor by gene duplication (Toh et al., 1995). Isoforms of EP receptor subtypes generated by alternative splicing of their mRNA have been reported for EP3 and EP1 (Irie et al., 1993; Namba et al., 1993; An et al., 1994; Breyer et al., 1994; Regan et al., 1994b; Schmid et al., 1995; Okuda et al., 1996; Pierce and Regan, 1998; Oldfield et al., 2001). All EP receptor subtypes are expressed on the plasma membrane; additionally EP3 and EP4 also exhibit nuclear membrane localization (Bhattacharya et al., 1998, 1999).
EP1 receptor
EP1 is a 42-kDa protein that has been cloned and expressed from humans and rodents (Funk et al., 1993; Watabe et al., 1993; Okuda et al., 1996). Two splice variants of EP1 receptor have been reported (Okuda et al., 1996). Among the four subtypes of EP receptors, EP1 has the least affinity for PGE2 (dissociation constant (KD) of 16–25 nM, (Table 2)). It binds PGs in the rank order PGE2>sulprostone, iloprost>PGE1>misoprostol, M&B-28767>PGF2α>PGD2 (Abramovitz et al., 2000; Table 3). A few selective agonists for EP1 like ONO-DI-004, 17-phenyl trinor PGE2 are available, 17-phenyl trinor PGE2 being the most well known of them. Other agonists including sulprostone, carbacyclin and enprostil exhibit the highest affinity for EP1, but are also very potent EP3 agonists. ONO-AE-829, ONO-8711, ONO-8713, SC-19220 and AH6809 are used as antagonists for the EP1 receptor but AH6809 is a weak antagonist (Woodward et al., 1995, 2005).
Table 2.
Dissociation constants (Kd) of PGE2 for EP receptor subtypes in various species
| Receptor | Kd (nM) (human) | Kd (nM) (rat) | Kd (nM) (mouse) |
|---|---|---|---|
| EP1 | 25 (Abramovitz et al., 2000) | 24 (Boie et al., 1997) | 21 (Watabe et al., 1993) |
| 16 (Sharif and Davis 2002) | |||
| EP2 | 13 (Abramovitz et al., 2000) | 5 (Boie et al., 1997) | 116 (Nishigaki et al., 1996) |
| 12.9 (Stillman et al., 1998) | |||
| EP3 | 0.33 (Abramovitz et al., 2000) | 1 (Boie et al., 1997) | 2.9 (Sugimoto et al., 1992) |
| EP4 | 0.59 (Abramovitz et al., 2000) | 1 (Boie et al., 1997) | 1.27 (Nishigaki et al., 1996) |
| 0.72 (Davis and Sharif, 2000) | |||
| 1.12 (Marshall et al., 1997) |
Abbreviations: EP, E prostanoid; PGE2, prostaglandin E2.
Table 3.
Competition for radioligand binding to HEK 293(EBNA) cell membranes expressing recombinant prostanoid receptors
| Ligands | EP1 | EP2 | EP3 | EP4 |
|---|---|---|---|---|
| PGE2 | 9.1 | 4.9 | 0.33 | 0.79 |
| Butaprost | 27 721 | 91 | 1643 | 19 104 |
| AH-6809 | 1217 | 1150 | 1597 | 100 000 |
| Sulprostone | 107 | 100 000 | 0.35 | 7740 |
| M&B-28767 | 419 | 988 | 0.14 | 10 |
| Iloprost | 11 | 1870 | 56 | 284 |
| Misoprostol | 11 935 | 34 | 7.9 | 23 |
| PGD2 | 5820 | 2973 | 421 | 1483 |
| PGF2α | 547 | 964 | 38 | 288 |
| SC-51322 | 13.8 | >100 000 | 698 | 14 032 |
| Enprostil | 82 | >10 000 | 12 | >10 000 |
| Carbacyclin | 23 | 942 | 14 | 352 |
| Cicaprost | >1340 | >1340 | 255 | 44 |
Abbreviations: EP, E prostanoid; PGD2, prostaglandin D2; PGE2, prostaglandin; PGF2α, prostaglandin F2α.
Inhibitory constant (Ki) values are expressed in nM.
Adapted from Abramovitz et al. (2000).
EP2 receptor
Before the discovery of the EP2 receptor in 1994, the cloned EP4 receptor was referred to as EP2 (Bastien et al., 1994; Regan et al., 1994a). The EP2 receptor is a 53 kDa protein that has been cloned and expressed from humans, bovine, rabbits, rodents and several other species (An et al., 1993; Katsuyama et al., 1995; Guan et al., 1996; Nemoto et al., 1997; Arosh et al., 2003). The affinity of PGE2 for EP2 receptor differs greatly across species. The rat EP2 receptor has a significantly higher affinity for PGE2 than human or mouse. The mouse EP2 receptor shows the least affinity (Table 2). The relative affinity of binding of various EP ligands for the mouse EP2 receptor was PGE1, PGE2>16, 16-dimethyl-PGE2>11-deoxy-PGE1>butaprost>AH13205, misoprostol> AH-6809. Similarly, the human EP2 receptor binds PGE2 and PGE analogues with a rank order of PGE2>PGE1>16, 16-dimethyl-PGE2>11-deoxy-PGE1>butaprost, 1-OH-PGE1, M&B-28767> sulprostone (Table 3). Butaprost and ONO-AE1-259-01 are highly selective EP2 receptor agonists, whereas AH-6809 is a well-known EP2 antagonist.
EP3 receptor
The EP3 receptor of humans and rodents has been successfully cloned and expressed (Sugimoto et al., 1992; Yang et al., 1994). The EP3 is unique among the prostanoid family of receptors in the sense that multiple alternatively spliced variants of EP3 exist that can activate contrasting second messenger signalling (Pierce and Regan, 1998). To date, eight different isoforms for human EP3 have been reported. In general, the EP3 receptor has relatively higher affinity for PGE2 reflected by a very low KD value of 0.33–2.9 (Table 2). The rank order of affinity of PGE2 to the mouse EP3 receptor is sulprostone, M&B-28767, PGE2, PGE1, 11-deoxy-PGE1, 16,16-dimethyl-PGE2, misoprostol>1-OH-PGE1. Different PGE analogs, SC-46275, ONO-AE-248, sulprostone, GR 63799X and 11-deoxy-PGE1 are used as agonists for EP3 with SC-46275 and ONO-AE-248 being highly selective. L826266 is an EP3 antagonist.
EP4 receptor
The EP4 receptor cDNA encodes a 487–513 amino-acid polypeptide that has been cloned and expressed in vitro from human, rodents, rabbit, bovine and several other species (An et al., 1993; Honda et al., 1993; Breyer et al., 1996; Boie et al., 1997). They have very high affinity for PGE2 with a KD value of 0.59–1.27 (Table 2). The rank order of affinity of PGE ligands for the mouse EP4 receptor was PGE2, PGE1>11-deoxy-PGE1, 16, 16-dimethyl-PGE2, misoprostol>1-OH-PGE1 and M&B-28767 (Table 3). At present, there are few selective agonists or antagonists available for EP4. ONO-AE1-329 is a selective EP4 agonist whereas L161982 and ONO-AE3-208 are selective antagonists.
Diversity in EP receptor signalling and functions
Diverse factors govern the outcome of EP receptor signalling. Basically, structural, pharmacological and functional differences that exist between the subtypes of EP receptors determine the biological effects of PGE2. Structurally, differences observed in the length and composition of amino acids of the N and the C-termini, the second and the third extracellular as well as the intracellular domains are associated with variations in receptor signalling. In fact, differences in the length of C-termini of EP3 isoforms are attributed for their differential activation of varied second messenger pathways (Pierce and Regan, 1998). Likewise, the extracellular sequence of the EP2 receptor is a critical determinant of its structure and function (Stillman et al., 1999). Similarly, a cluster of hydrophobic aromatic amino acids in the second intracellular loop of EP2 but not EP3 isoform β is absolutely essential for activation of Gαs subunit (Sugimoto et al., 2003, 2004). Pharmacologically, EP receptors exhibit differences in binding to various ligands (Table 1) and surprisingly, the affinity of these receptors to its principal ligand, PGE2, greatly varies between receptor subtypes (Tables 2 and 3). In fact, affinity of PGE2 to its receptor may depend on the state of coupling of G protein subunits. A G-protein coupled receptor displays more affinity for PGE2 than a free uncoupled receptor. Apart from differences in the ligand affinity to EP receptors, the phenomena of receptor desensitization and internalization may regulate preferential signalling through certain EP receptor subtypes. Agonist-induced desensitization is a common phenomenon in G-protein coupled receptor that is characterized by the loss of receptor signalling. The EP receptors have been shown to undergo PGE2-induced desensitization. Interestingly, a differential desensitization of EP receptor subtypes has been observed wherein, the EP4 receptor is highly desensitized in contrast to the EP2 receptor which is not (Nishigaki et al., 1996). Similarly, differences in ligand-induced internalization of EP receptors have been reported. EP4 and EP3 isoform-I are readily internalized when activated by PGE2 whereas EP2 as well as EP3 isoform-III and IV are not. Interestingly, the C-terminus of EP4 has been implicated in its ligand-induced sequestration (Desai et al., 2000; Bilson et al., 2004). Functionally, differences among EP receptors directly correlate with the type of signal that it transduces. For example, receptor activation leading to intracellular Ca2+ mobilization is associated with the contraction of smooth muscle cells, whereas increase in cytoplasmic cAMP levels is associated with its relaxation. Evidently, signalling through EP1 receptors increases intracellular Ca2+ levels whereas, EP2 and EP4 have been shown to increase cytoplasmic cAMP. Signalling via EP3 receptors is unique wherein cAMP levels are decreased (Narumiya et al., 1999). Clearly, diverse factors govern the fate of signalling through EP receptors and all these may play a critical role in determining the differential biological effect of PGE2 in the GI tract.
Table 1.
Ki values of PGE2 for heterologously expressed human and mouse EP receptors
| Species | Heterologous system | EP1 | EP2 | EP3 | EP4 | References |
|---|---|---|---|---|---|---|
| Humans | Human embryonic kidney cells | 9.1 | 4.9 | 0.33 | 0.79 | Abramovitz et al. (2000) |
| Mouse | Chinese hamster ovary cells | 20 | 12 | 0.85 | 1.9 | Kiriyama et al. (1997) |
Abbreviations: EP, E prostanoid; PGE2, prostaglandin.
Inhibitory constant (Ki) values are expressed in nM.
Downstream signalling pathways affected by EP receptors
Coupling of PGE2 or the specific receptor agonists to EP receptors results in their activation and induces signalling cascade inside the cell (Figure 2). Interestingly, signalling through different subtypes of EP receptors seems to alternate and sometimes overlap; yet they are unique in terms of their signalling outcomes. EP1 receptors mediate signalling events by activation of phospholipase C and elevation of cytoplasmic signalling intermediates including inositol triphosphate, diacylglycerol and Ca2+. Coupling of PGE2 to EP1 activates protein kinase Cα and c-Src. In addition to directly activating downstream kinases, signalling through EP1 can also transactivate HER's-2/Neu tyrosine kinase receptor, which is mediated by c-Src resulting in upregulation of vegetative endothelial growth factor-C (Su et al., 2004). Recently, it was reported (Han and Wu, 2005) that c-Src mediated the transactivation of epidermal growth factor receptor by the EP1 receptors through activation of protein kinase B (Akt) that promotes cell proliferation and invasion. These data corroborate with those obtained from studies in EP1 receptor knockout mice, thus implicating a role of EP1 receptors in colon carcinogenesis (Watanabe et al., 1999).
The EP2 and EP4 receptors are linked to the stimulation of cAMP/protein kinase A (PKA) signalling through the sequential activation of Gαs and adenylate cyclase. Contradictory reports associate this signalling pathway to growth and proliferation. Increased cAMP production, upon activation of EP2 and EP4 has been shown to have an antiproliferative effect in human gastric carcinoma cell lines (Okuyama et al., 2002). In contrast, activation of PKA is increasingly being linked to proliferation of various epithelial cell types. Phosphorylation of PKA is coupled to the regulation of glycogen synthase kinase-3 (GSK-3) and Akt (Filippa et al. 1999; Li et al., 2000). PKA phosphorylates and activates Akt kinase, which indirectly inhibits GSK-3. Inhibition of GSK-3 decreases the inhibitory phosphorylation of cytosolic β-catenin that promotes the translocation of β-catenin to the nucleus resulting in cellular proliferation (Cadigan and Nusse, 1997). A recent study has directly linked EP2 receptor activation to cellular proliferation. Upon activation the Gαs subunit of EP2 receptors can directly associate with the regulator of G protein signalling domain of Axin, which inactivates and releases GSK-3β from the Axin complex causing β-catenin activation and nuclear translocation (Castellone et al., 2005). Until recently, it was believed that the phosphoinositide-3-kinase (PI3K) signaling pathway was activated only by the EP4 receptor. However, recent studies have shown that PGE2 signalling through EP2 receptors can activate both PI3K and Akt by using the free βγ subunit of G-protein (Castellone et al., 2005).
Although EP2 and EP4 receptors are capable of stimulating both PKA and PI3K signalling pathway, a receptor-specific signalling outcome has always been observed. For example, a study showed that PGE2 stimulation of EP4, but not EP2 receptor that are stably expressed in HEK cells lead to the phosphorylation of the extracellular signal-regulated kinases (ERKs) by a PI3K-dependent mechanism and induced the expression of early growth response factor-1 (Fujino et al., 2003). The differences in the signalling potential of both EP2 and EP4 receptors are corroborated by the fact that both receptors mediate the phosphorylation of cAMP responsive element binding protein (CREB) by different signalling pathways (Fujino et al., 2005). Signalling through EP2 receptors can decrease the inhibitory tyrosine phosphorylation of PTEN, a phosphatase that can act as a PI3K pathway inhibitor (White et al., 2005). A novel EP2 receptor-signalling pathway, which can transactivate the EGF receptor leading to increased migration and invasion of colon cancer cells, has also been reported (Pai et al., 2002; Buchanan et al., 2003).
The EP3 receptors are unique in their ability to couple to multiple G proteins. They couple and activate the Gi subunits, which results in the inhibition of adenylyl cyclase. Apart from activation of Gi subunits, signalling through EP3 receptors can also activate Gs resulting in cAMP production. Evidence also indicates that the EP3 receptor can activate the small G protein Rho and its target p160 Rho-A binding kinase ROK α (Katoh et al., 1998; Tamma et al., 2003). EP3 receptors have also been shown to activate the Ras signalling pathway leading to cancer (Yano et al., 2002).
Distribution of EP receptors in the gut
Although PGE2 is produced by a variety of cells in the GI mucosa and is found throughout the gut, its cellular targets in the mucosa and the resulting physiological changes in the GI tract are predominantly determined by the presence and the distribution of EP receptors. Pharmacological as well as cellular localization studies have been aimed towards identifying the type and pattern of EP receptor distribution and their density in major cell types in the GI mucosa. However, very little is known of the regional distribution or the temporal changes in the pattern of EP receptor expression and function under normal and disease states in the gut.
EP1 receptor distribution
EP1 receptor expression has been reported in the GI tract of various species. In rat, EP1 mRNA expression is detected at the gastric, small intestinal and colonic muscle layers (Ding et al., 1997). A non-radioactive in situ hybridization technique used to study the pattern of localization of EP receptors in the rat GI tract revealed the presence of EP1 receptors in gastric chief cells that secrete pepsinogen, parietal cells that secrete hydrochloric acid and in mucus secreting gastric epithelial cells. In the small intestine, EP1 receptor expression was noticed only in goblet cells whereas in the large intestine, EP1 receptor expression was seen in goblet cells and in other epithelial cell types. Expression of EP1 receptors has been reported in enteric glial cells (Northey et al., 2000). In mice, EP1 mRNA expression was noticed in the muscularis mucosa (Morimoto et al., 1997). In rabbits, EP1 receptors are highly expressed on the intestinal brush border membranes of intestinal villi. Goblet cells of the jejunum and ileum also show considerable expression. In contrast, goblet cells of the duodenum show a lack of expression of EP1 receptors. Interestingly, EP1 receptors are also highly expressed in the neurons of the myenteric and submucosal ganglia throughout the rabbit intestinal tract (Grasa et al., 2006).
EP2 receptor distribution
EP2 receptors are normally expressed in rat gastric mucous cells, in the goblet cells of the small intestine and also in various epithelial cells of the large intestine (Northey et al., 2000). In mice, an abundant expression of EP2 receptors in the stomach and ileum has been detected by Northern blot analysis (Katsuyama et al., 1995). A recent report suggested a relatively uniform expression of EP2 receptors throughout the mouse GI tract except for the ileum and caecum, where it is weakly expressed. Interestingly, expression of EP2 receptors depends on the state of differentiation of the epithelial cells. Undifferentiated crypt epithelial cells predominantly express EP2 receptors on their nuclear membranes whereas the highly differentiated epithelial cells at the apex of the villi express these receptors on their plasma membrane (Houchen et al., 2003). In rabbits, EP2 receptors are localized on the intestinal brush border membrane of the villi and are also strongly expressed in goblet cells throughout the small intestine. EP2 receptors are weakly expressed in neurons but are completely absent in the smooth muscle cells of the small intestine (Grasa et al., 2006). In humans, EP2 receptor expression is restricted to the luminal surface of the gastric epithelium (Takafuji et al., 2002) and at the apex of the colonic mucosa (Takafuji et al., 2000). In guinea-pigs, the presence of EP2 receptors in the neurons of the enteric nerve plexus in the ileum was detected through pharmacological studies (Lawrence et al., 1992).
EP3 receptor distribution
EP3 receptor gene expression appears to be localized to the muscular parts of the rat intestine whereas in the stomach EP3 mRNA expression is seen in the mucosal layer especially in parietal cells (Ding et al., 1997). In most rodents, EP3 receptor expression is predominantly found in gastric parietal and small intestinal goblet cells. They are also highly expressed in enteric glial and myenteric neuronal cells (Morimoto et al., 1997; Takahashi et al., 1999; Northey et al., 2000). EP3 receptors are highly expressed in rabbit intestinal circular and longitudinal smooth muscles of the duodenum and in the circular muscles of the ileum (Grasa et al., 2006). In humans, EP3 receptor expressions are observed throughout the gastric epithelium (Takafuji et al., 2002) and in the apex of colonic mucosa (Takafuji et al., 2000).
EP4 receptor distribution
EP4 receptor mRNA are mainly expressed in the rodent intestinal mucosal layers and in the parietal as well as the mucosal epithelial cells of the gastric mucosa (Ding et al., 1997; Northey et al., 2000). In the ileum, EP4 receptor expression was demonstrated on mature enterocytes of the villi (Morimoto et al., 1997). Normal EP4 receptor mRNA expression was observed in rabbit gastric epithelial cells (Takahashi et al., 1999). However, EP4 receptor proteins were not detected in any of the segments (Grasa et al., 2006). In humans, EP4 expression was modest in the gastric epithelium but intense expression of EP4 was detected in lamina propria mononuclear cells (Takafuji et al., 2002). In the colon EP4 receptors are strongly expressed in the lateral crypt epithelia (Takafuji et al., 2000) and lamina propria mononuclear cells of CD+ T lymphocytes (Cosme et al., 2000).
Physiological role of EP receptors in the GI tract
The role of PGE2 in GI physiology has been distinctly proved in various animal models. Presently, agonist/antagonist-based approaches along with EP receptor knockout strategies are used to determine the role that each of the EP receptors plays in GI physiology. Using these approaches, the role that EP receptor subtype(s) play in gastric acid secretion, GI motility and mucosal barrier functions including bicarbonate secretion, mucus secretion and epithelial cytoprotection have been elucidated.
Gastric acid secretion
Gastric acid is the main secretion of the stomach. A dual action of PGE2 on gastric acid secretion was observed in rats, wherein, lower concentrations inhibited and higher concentration stimulated secretion (Ding et al., 1997). The inhibitory action of PGE2 on acid secretion in rats was mediated by the EP3 receptor whereas the stimulatory effect was due to the EP4 receptor. It is interesting to note that both EP3 and EP4 receptors are present in parietal cells and acid-producing chief cells in the gastric mucosa. Parietal cells can respond to histamine in releasing acid into the gastric lumen. An EP4 agonist, ONO-AE1-329, was shown to stimulate gastric acid secretion through histamine released from entero chromaffin cells (Kato et al., 2005).
GI motility
The peristaltic movement of the GI tract is maintained through coordinated contraction and relaxation of GI smooth muscle and PGE2 has been reported to play a major role in GI motility. In rabbits, a complex mechanism involving activation of both EP3 and EP1 receptors was shown to be responsible for the contraction of the small intestine. A direct effect of EP3-mediated activation of intestinal smooth muscles and an indirect effect of EP1-mediated stimulation of myenteric neurons acts together during PGE2-induced intestinal contractions (Grasa et al., 2006). In rats, inhibition of small intestinal hypermotility was mediated through EP4 receptors (Kunikata et al., 2002). Studies conducted in EP receptor knockout mice clearly underscore the importance of EP1 and EP3 receptors in the contraction of longitudinal smooth muscles of gastric fundus and ileum and EP4 receptors in the relaxation (Okada et al., 2000). In guinea-pig ileum, pharmacological investigation revealed that EP3 receptor stimulation mediates contraction of circular smooth muscles and increases peristalisis, whereas EP2 mediates relaxation and decreases peristalisis (Botella et al., 1993; Shahbazian et al., 2002).
Bicarbonate secretion
GI bicarbonate secretion is one of the first lines of defense of the host epithelial cells against the harsh acidic environment of the stomach and the duodenum. Bicarbonates help in maintaining a narrow zone of neutral pH just above the mucosal lining that offers a protective barrier against diffusing acid (Takeuchi et al., 1997). PGE2 has a stimulatory effect on the GI bicarbonate secretion. In fact, low physiological concentrations of PGE2 in the duodenum would induce an acid-provoked bicarbonate secretion (Hirokawa et al., 2004). An agonist–antagonist-based approach in rats, as well as studies involving EP receptor knockout mice have proved the involvement of different EP receptor subtypes in bicarbonate secretion in different segments of the gut. In the stomach of rats and mice, EP1 receptors are responsible for bicarbonate secretion whereas, the same effect was mediated through EP3 receptors in the duodenum (Takeuchi et al., 1999a). In fact, the presence of EP3 receptors in the duodenum is essential for duodenal bicarbonate secretion to counter luminal acid-induced mucosal damage (Takeuchi et al., 1999b). In addition to EP3 receptors, EP4 receptors have also been proved to be involved in duodenal bicarbonate secretion (Aoi et al., 2004). In humans, EP4 receptors are solely responsible for duodenal bicarbonate secretion, which is in contrast to that seen in rats and mice, where EP3 receptors mediate duodenal bicarbonate secretion (Larsen et al., 2005). This study suggests that species differences occur with regard to EP receptor function(s) and therefore caution should be taken in ascribing a particular function to a particular EP receptor.
Mucus secretion
Mucins are polymers made of glycoproteins that are secreted by the mucous cells of the stomach and the goblet cells of the intestine that form a protective covering over the lining of the mucosa. They form the major component of the innate immune response of the GI mucosa. PGE2 is reported to be a potent mucin secretagogue. It strongly stimulates mucin secretion from rat gastric epithelial cells (Tani et al., 1997). More recently, the molecular mechanism of PGE2-induced-mucin gene transcription via the ERK MAPK/RSK1/CREB pathway has been elucidated (Cho et al., 2005). PGE2-induced mucus secretion from the gastric epithelial cells of rabbit are mainly mediated through EP4 receptors in a cAMP/PKA-dependent manner (Takahashi et al., 1999). Mucin exocytosis from the antral mucous cells of guinea-pigs is also mediated through EP4 but activated EP1 receptors are shown to potentiate the effect of mucus secretion mediated by EP4 (Ohnishi et al., 2001). An agonist-based approach in identifying the EP receptors responsible for mucin exocytosis in the rat colon and in a human colonic epithelial cell line LS174T revealed that coupling of PGE2 with EP4 receptors was essential for mucin secretion (Belley and Chadee, 1999).
Cytoprotection
PGE2 plays an important role in protecting the GI mucosa from injuries caused by the harsh environment of the lumen. The mechanism by which PGE2 exerts its protective effects on GI mucosa from noxious agents is termed cytoprotection. For example, PGE2 exerts a potent cytoprotective effect on gastric glandular cells against indomethacin-induced injury, which is independent of neural, vascular and hormonal factors (Brzozowski et al., 2005). Current studies are underway to identify EP receptor subtype that mediates this protective action. It has been reported that, in an acute rat esophagitis model, low doses of PGE2 confer a protective effect that is mediated through EP1 receptors (Yamato et al., 2005). Similarly, in the stomach of rat, EP1 receptors are essential for offering protection against indomethacin or ethanol-induced injury (Araki et al., 2000; Suzuki et al., 2001). In rodents, EP1 receptors also mediate adaptive gastric cytoprotection, under conditions in which initial exposure to mild irritants prevents subsequent damage by more severe irritants (Takeuchi et al., 2001). Activation of the EP1 receptors helps in maintaining gastric mucosal integrity (Takeuchi et al., 2002). In the small intestine of rats, EP3 and EP4 receptors offer cytoprotection against indomethacin-induced injury possibly through increased mucus secretion, enteropooling and through inhibition of hypermotility mediated by EP4 alone (Kunikata et al., 2002). Activation of both EP2 and EP4 receptors in the guinea-pig gastric mucosal cells offers cytoprotection against ethanol-induced apoptosis (Hoshino et al., 2003). In mice, EP2 receptors are essential for preventing radiation-induced apoptosis of crypt epithelial cells at the jejunum (Houchen et al., 2003).
Differential expression of EP receptors during pathological conditions in the gut
This area is perhaps the least studied aspect of EP receptor biology in the gut. To complicate the issue, the limited data that are available does not always discriminate whether EP receptor expressions are altered in individual cells, mucosa or submucosa or the full thickness of the gut. Nevertheless, these data on differential expression of EP receptors during various pathological conditions such as radiation injury, tumorigenesis and inflammation may explain the modus operandi of PGE2 in exerting its pathological functions as against its well-known physiological roles.
Radiation-induced injury
A regional and temporal difference in the expression of EP receptors following radiation-induced injury in mice has been observed. Significant changes in EP2 and EP4 mRNA and protein levels were noticed in the jejunum and colon after radiation-induced injury. EP2 receptor expression increased steeply whereas a decrease in EP4 expression was observed. Interestingly, EP2 receptor expression corresponded with epithelial restitution and early crypt morphogenesis of the injured tissues (Houchen et al., 2003).
GI tumorigenesis
A differential expression of EP receptors in the colon during early stages of tumorigenesis is observed in rodents and humans. The most notable changes reflect an increase in the expression of EP1 and EP2 receptors and a marked decrease in EP3 receptors. However, EP4 receptor levels remained constant (Shoji et al., 2004). Upregulation of EP1 receptors is clearly observed during early tumorigenesis that helps in cellular proliferation and in the antiapoptotic effects observed in cancer cells, indicating a major role played by EP1 receptors in cancer (Kawamori et al., 2005). A few studies involving RT–PCR analysis have revealed that as well as EP1, EP2 and EP4 receptor mRNAs expression are increased in azoxymethane-induced colorectal cancer tissues (Mutoh et al., 2002; Kawamori et al., 2003). Similarly, EP2 and EP4 mRNA levels are also increased in ApcΔ716 mouse small intestinal and colonic polyps (Sonoshita et al., 2001). These studies implicate EP2 and EP4 receptors in tumorigenesis. The signalling mediated by EP1/2/4 receptors that lead to cellular proliferation and tumorigenesis was briefly discussed under the previous section ‘Downstream signalling pathways affected by EP receptors.'' Apart from the upregulation and activation of certain EP receptors during cancer, it is interesting to note that EP3 receptor is downregulated. Downregulation of the EP3 receptor is linked to the hypermethylation of the EP3 receptor gene, thus inhibiting the suppressive role of EP3 in colon cancer development. Increased expression of EP4 receptors on CD3+ lymphocytes adjacent to the tumour was also evident in human gastric mucosal cancers but the role of T lymphocyte EP4 signalling in cancer is not clear (Takafuji et al., 2002). An increased expression of EP2, EP3 and EP4 receptor mRNA levels was observed in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux; however, no changes in the mRNA levels of the EP1 receptor was observed (Jang et al., 2004). This study suggests that regional differences may exist for the role of EP receptor subtypes in GI tumorigenesis.
GI inflammation
A differential expression of EP receptors on the GI mucosa may occur during inflammation. One interesting study showed defined patterns and identified striking differences of mucosal expression of the EP4 receptors between cells in normal human colonic lamina propria and those from the inflamed human colon (Cosme et al., 2000). In particular, mucosal EP4 receptor expression was upregulated in T lymphocytes. In ulcerative colitis, apart from an increase in EP4 receptor expression in lamina propria T lymphocytes, an increase in the levels of EP2 and EP3 receptors was apparent in epithelial cells (Takafuji et al., 2000). At present, there are no reports on EP receptor expression levels in the gut during microbial or parasitic infections.
Role of EP4 receptors in colitis
Even though PGE2 levels are significantly increased during IBD, the functional role PGE2 and individual EP receptors play in the pathogenesis of IBD remains undefined. PGE2–EP receptor coupling may have a critical role in homeostasis or in the onset of GI inflammation and/or tissue repair. The limited data to date suggest that signalling via EP receptors can predetermine whether PGE2 exerts a proinflammatory or anti-inflammatory effect in the gut. For example, studies in vitro, to address early responses of PGE2 in a variety of colonic epithelial cell lines clearly demonstrate that PGE2 couples via high-affinity EP4 receptors to upregulate IL-8 mRNA expression and protein secretion confirming a proinflammatory role for PGE2 (Yu and Chadee, 1999). IL-8 is a potent chemokine that can attract and activate neutrophils to cause nonspecific tissue damage important in the onset of colonic inflammation. In contrast, in EP4 receptor knockout mice, a 7-day regime of dextran sodium sulphate-induced colitis was reported to be more severe than that in wild-type controls. This suggests that signalling via EP4 receptors may play a critical role in maintaining normal mucosal integrity and/or to promote healing (Kabashima et al., 2002). Unfortunately, the role of EP4 signaling in either epithelial cells or the submucosal cells that induce an anti-inflammatory effect has not been elucidated. Moreover, it is not clear whether epithelial barrier functions or cytokine productions are altered in EP4 knockout animals. Similarly, studies in rats using an EP4 receptor agonist reported the suppression of colitis caused by extended DSS treatment through upregulation of an anti-inflammatory cytokine, IL-10 (Nitta et al., 2002). Suppression of Th1-type response, coupled with evidence of increased expression of EP4 receptors on the mucosal T lymphocytes in inflamed colonic mucosa (Cosme et al., 2000), suggests a fundamental role for PGE2-EP4 signalling in T lymphocytes in repair and restitution of damaged tissues. It appears that EP4 signalling outcomes differ between the early onset (pro-inflammatory on mucosal epithelial cells) and the late progressive stages of colitis (anti-inflammatory on immune cells in the lamina propria). Collectively, these studies emphasize the importance of PGE2-EP4 receptor signalling events in the cell types present in colonic mucosa as a fundamental determinant of colitis. More studies are needed to define the diverse functions of PGE2-EP receptor coupling on various cell types before we can model their role in normal and disease states.
Concluding remarks
Recent evidence clearly suggests that PGE2-EP receptor signalling plays an important role in the GI tract. Importantly, PGE2-EP receptor coupling on different cell types may exert either a pro-inflammatory or anti-inflammatory response. In general, it appears that EP1, EP2 and EP4 receptors are major determinants in the early stages of intestinal tumorigenesis and inflammation, whereas EP4 receptors on immune cells may promote the restitution of colitis/inflammation. There maybe redundancy of EP receptor signalling and EP receptor functions may vary from onset to different stages of disease (initiation versus progression). However, it appears that all receptors are needed for various normal functions of the GI tract. As regional, temporal and species differences exist in EP receptor expression and distribution in the GI tract, future research should aim at identifying EP receptor expression in various cellular components of the mucosa across the length and the breath of the gut. As regional and species differences are apparent with regards to EP receptor functions, it is dangerous to ascribe specific functions for EP receptors in the gut. A priority is to define the distinct EP receptor signalling events in various cell types in the gut. Knowledge of receptor expression coupled with insights into specific signalling events in specific cell types may help the cause of understanding distinct roles that EP receptors play in the GI tract. More research is also needed to define the activity and the toxicity of single/combination EP receptor functions under normal and inflamed/diseased conditions. The long-term goal of future research in EP receptor biology should aim at identifying the Achilles' heel at the receptor level as well as at the signalling that it propagates. This would help in effective pharmacological intervention of various GI ailments whose pathologies are mediated by PGE2.
Acknowledgments
Research in Dr Chadee's laboratory is supported by grants from the Crohn's and Colitis Foundation of Canada, the Canadian Institute for Health Research, the Canadian Foundation for Innovation, the Natural Sciences and Engineering Research Council of Canada and the Canadian Association of Gastroenterology-Industry-CIHR Research and Fellowship Awards. Dr Chadee holds a Canada Research Chair in Gastrointestinal Inflammation.
Abbreviations
- AA
arachidonic acid
- Akt
protein kinase B
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- COX
cyclooxygenase
- CREB
cAMP responsive element binding protein
- DP
D prostanoid
- EP
E prostanoid
- EP1, EP2, EP3 and EP4
E prostanoid receptor subtypes 1, 2, 3 and 4
- ERK
extracellular signal-regulated protein kinase
- FP
F prostanoid
- G (p/q, αs and αi)
G protein subunits
- GI
gastrointestinal
- GSK-3
glycogen synthase kinase-3
- IBD
inflammatory bowel disease
- IP
I prostanoid
- KD
dissociation constant
- PG
prostaglandin
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- PGI2
prostaglandin I2
- PI3K
phosphoinositide-3-kinase
- PKA
protein kinase A
- PLA2
phospholipase A2
- TP
T prostanoid
- TXA2
thromboxane A2
Conflict of interest
The authors state no conflict of interest.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochem Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Ahrenstedt O, Hallgren R, Knutson L. Jejunal release of prostaglandin E2 in Crohn's disease: relation to disease activity and first-degree relatives. J Gastroenterol Hepatol. 1994;9:539–543. doi: 10.1111/j.1440-1746.1994.tb01557.x. [DOI] [PubMed] [Google Scholar]
- An S, Yang J, So S, Zeng L, Goetzl E. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- An S, Yang J, Xia M, Goetzl E. Cloning and expression of the EP2 subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1993;197:263–270. doi: 10.1006/bbrc.1993.2470. [DOI] [PubMed] [Google Scholar]
- Aoi M, Aihara E, Nakashima M, Takeuchi K. Participation of prostaglandin E2 receptor EP4 subtype in duodenal bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2004;287:G96–G103. doi: 10.1152/ajpgi.00038.2004. [DOI] [PubMed] [Google Scholar]
- Araki H, Ukawa H, Sugawa Y, Yagi K, Suzuki K, Takeuchi K. The roles of prostaglandin E receptor subtypes in the cytoprotective action of prostaglandin E2 in rat stomach. Aliment Pharmacol Ther. 2000;14:116–124. doi: 10.1046/j.1365-2036.2000.014s1116.x. [DOI] [PubMed] [Google Scholar]
- Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, Maclaren LA, et al. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology. 2003;144:3076–3091. doi: 10.1210/en.2002-0088. [DOI] [PubMed] [Google Scholar]
- Bastien L, Sawyer N, Grygorczyk R, Metters K, Adam M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994;269:11873–11877. [PubMed] [Google Scholar]
- Belley A, Chadee K. Prostaglandin E2 stimulates rat and human colonic mucin exocytosis via the EP4 receptor. Gastroenterology. 1999;117:1352–1362. doi: 10.1016/s0016-5085(99)70285-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Peri K, Almazan G, Ribeiro-da-silva A, Shichi H, Durocher Y, et al. Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci USA. 1998;95:15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Peri K, Ribeiro-da-silva A, Almazan G, Shichi H, Hou X, et al. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- Bilson HA, Mitchell DL, Ashby B. Human prostaglandin EP3 receptor isoforms show different agonist-induced internalization patterns. FEBS Lett. 2004;572:271–275. doi: 10.1016/j.febslet.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Boie Y, Stocco R, Sawywe N, Slipetz DM, Ungrin MD, Neuschafer-rube F, et al. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- Botella A, Delvaux M, Fioramonti J, Frexinos J, Bueno L. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in isolated ileal smooth muscle cells. Eur J Pharmacol. 1993;237:131–137. doi: 10.1016/0014-2999(93)90102-n. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Ann Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Breyer R, Davis L, Nian C, Redha R, Stillman B, Jacobson HR, et al. Cloning and expression of the rabbit prostaglandin EP4 receptor. Am J Physiol Renal Physiol. 1996;270:F485–F493. doi: 10.1152/ajprenal.1996.270.3.F485. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Emeson RB, Breyer MD, Abromson RM, Davis LS, Ferrenbach SM. Alternative splicing generates multiple isoforms of a rabbit prostaglandin E2 receptor. J Biol Chem. 1994;269:6163–6169. [PubMed] [Google Scholar]
- Brzozowski T, Tarnawski A, Hollander D, Sekhon S, Krause WJ, Gergely H. Comparison of prostaglandin and cimetidine in protection of isolated gastric glands against indomethacin injury. J Physiol Pharmacol. 2005;56:75–88. [PubMed] [Google Scholar]
- Buchanan FG, Wang D, Bargiacchi F, Dubois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1023. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KN, Choi JY, Kim CH, Baek SJ, Chung KC, Moon UY, et al. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/CREB activation in human airway epithelial cells. J Biol Chem. 2005;280:6676–6681. doi: 10.1074/jbc.M412722200. [DOI] [PubMed] [Google Scholar]
- Cosme R, Lublin D, Takafuji V, Lynch K, Roche JK. Prostanoids in human colonic mucosa: effects of inflammation on PGE2 receptor expression. Hum Immunol. 2000;61:684–696. doi: 10.1016/s0198-8859(00)00131-2. [DOI] [PubMed] [Google Scholar]
- Davis TL, Sharif NA. Pharmacological characterization of [(3) H]-prostaglandin E2 binding to the cloned human EP4 prostanoid receptor. Br J Pharmacol. 2000;130:1919–1926. doi: 10.1038/sj.bjp.0703525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol. 2000;58:1279–1286. doi: 10.1124/mol.58.6.1279. [DOI] [PubMed] [Google Scholar]
- Ding M, Kinoshita Y, Kishi K, Nakata H, Hassan S, Kawanami C, et al. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins. 1997;53:199–216. doi: 10.1016/s0090-6980(97)00015-4. [DOI] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Filippa N, Sable CL, Filloux C, Hemmings B, Van obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- Funk C, Furchi L, FitzGerald G, Grygorczyk R, Rochette C, Bayne MA, et al. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- Grasa L, Arruebo MP, Plaza MA, Murillo MD. PGE2 receptors and their intracellular mechanisms in rabbit small intestine. Prostaglandins Other Lipid Mediat. 2006;79:206–217. doi: 10.1016/j.prostaglandins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Guan Y, Zhang YH, Stillman BA, Schneider A, Saito O, Davis LS, et al. Cloning and functional expression of the rabbit prostaglandin EP2 receptor. J Am Soc Nephrol. 1996;7:1646. doi: 10.1186/1471-2210-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Svensson J, Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci USA. 1974;71:3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–24063. doi: 10.1074/jbc.M500562200. [DOI] [PubMed] [Google Scholar]
- Hirokawa M, Furukawa O, Guth PH, Engel E, Kaunitz JD. Low-dose PGE2 mimics the duodenal secretory response to luminal acid in mice. Am J Physiol. 2004;286:G891–G898. doi: 10.1152/ajpgi.00458.2003. [DOI] [PubMed] [Google Scholar]
- Honda A, Sugimoto Y, Namba T, Watabe A, Irie A, Negishi M, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem. 1993;268:7759–7762. [PubMed] [Google Scholar]
- Hoshino T, Tsutsumi S, Tomisato W, Hwang HJ, Tsuchiya T, Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J Biol Chem. 2003;278:12752–12758. doi: 10.1074/jbc.M212097200. [DOI] [PubMed] [Google Scholar]
- Houchen CW, Sturmoski MA, Anant S, Breyer RM, Stenson WF. Prosurvival and antiapoptotic effects of PGE2 in radiation injury are mediated by EP2 receptor in intestine. Am J Physiol Gastrointest Liver Physiol. 2003;284:490–498. doi: 10.1152/ajpgi.00240.2002. [DOI] [PubMed] [Google Scholar]
- Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, et al. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem. 1993;217:313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- Jang TJ, Min SK, Bae JD, Jung KH, Lee JI, Kim JR, et al. Expression of cyclooxygenase 2, microsomal prostaglandin E synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004;53:27–33. doi: 10.1136/gut.53.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science (Washington DC) 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- Kato S, Aihara E, Yoshii K, Takeuchi K. Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol. 2005;289:G64–G69. doi: 10.1152/ajpgi.00397.2004. [DOI] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A, Negishi M. P160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Katsuyama M, Nishigaki N, Sugimoto Y, Morimoto K, Negishi M, Narumiya S, et al. The mouse prostaglandin E receptor EP2 subtype: cloning, expression and Northern blot analysis. FEBS Lett. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Kitamura T, Watanabe K, Uchiya N, Maruyama T, Narumiya S, et al. Prostaglandin E receptor subtype EP (1) deficiency inhibits colon cancer development. Carcinogenesis. 2005;26:353–357. doi: 10.1093/carcin/bgh322. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunikata T, Tanaka A, Miyazawa T, Kato S, Takeuchi K. 16, 16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47:894–904. doi: 10.1023/a:1014725024519. [DOI] [PubMed] [Google Scholar]
- Larsen R, Hansen MB, Bindslev N. Duodenal secretion in humans mediated by the EP4 receptor subtype. Acta Physiol Scand. 2005;185:133–140. doi: 10.1111/j.1365-201X.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Jones RL, Wilson NH. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FH, Patel K, Lundstrom K, Camacho J, Foord SM, Lee MG. Characterization of [3H]-prostaglandin E2 binding to prostaglandin EP4 receptors expressed with Semliki Forest virus. Br J Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Sugimoto Y, Katsuyama M, Oida H, Tsuboi K, Kishi K, et al. Cellular localization of mRNAs for prostaglandin E receptor subtypes in mouse gastrointestinal tract. Am J Physiol. 1997;272:G681–G687. doi: 10.1152/ajpgi.1997.272.3.G681. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Phospholipase A2. J Biochem (Tokyo) 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, et al. Involvement of prostaglandin E receptor subtype EP (4) in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, et al. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Narumiya S. Prostanoid receptors. Structure, function and distribution. Ann N Y Acad Sci. 1994;744:126–138. doi: 10.1111/j.1749-6632.1994.tb52729.x. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Pilbeam CC, Bilak S, Raisz L. Molecular cloning and expression of the rat prostaglandin E2 receptor of the EP2 subtype. Prostaglandins. 1997;54:713–725. doi: 10.1016/s0090-6980(97)00145-7. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–1037. [PubMed] [Google Scholar]
- Nitta M, Hirata I, Toshina K, Murano M, Maemura K, Hamamoto N, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002;56:66–75. doi: 10.1046/j.1365-3083.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- Northey A, Denis D, Cirino M, Metters KM, Nantel F. Cellular distribution of prostanoid EP receptors mRNA in the rat gastrointestinal tract. Prostaglandins Other Lipid Mediat. 2000;62:145–156. doi: 10.1016/s0090-6980(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Ohnishi A, Shimamoto C, Katsu K, Ito S, Imai Y, Nakahari T. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E2 in guinea-pig antral mucous cells. Exp Physiol. 2001;86:451–460. doi: 10.1113/eph8602160. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hara A, Ma H, Xiao CY, Takahata O, Kohgo Y, et al. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: studies using mice deficient in prostanoid receptors. Br J Pharmacol. 2000;131:745–755. doi: 10.1038/sj.bjp.0703627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda AE, Sakamoto K, Ezashi T, Miwa K, Ito S, Hayaishi O. Suppression of prostaglandin E receptor signaling by the variant form of EP1 subtype. J Biol Chem. 1996;271:31255–31261. doi: 10.1074/jbc.271.49.31255. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Ishihara S, Sato H, Rumi MA, Kawashima K, Miyaoka Y, et al. Activation of prostaglandin E2-receptor EP2 and EP4 pathways induces growth inhibition in human gastric carcinoma cell lines. J Lab Clin Med. 2002;140:92–102. doi: 10.1067/mlc.2002.125784. [DOI] [PubMed] [Google Scholar]
- Oldfield S, Grubb BD, Donaldson LF. Identification of a prostaglandin E2 receptor splice variant and its expression in rat tissues. Prostaglandins Other Lipid Mediat. 2001;63:165–173. doi: 10.1016/s0090-6980(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Regan JW. Prostanoid receptor heterogeneity through alternative mRNA splicing. Life Sci. 1998;62:1479–1483. doi: 10.1016/s0024-3205(98)00093-9. [DOI] [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Donello JE, Pierce KL, Pepperl DJ, Zhang D, et al. Molecular cloning and expression of human EP3 receptors: evidence of three variants with differing carboxyl termini. Br J Pharmacol. 1994b;112:377–385. doi: 10.1111/j.1476-5381.1994.tb13082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Pepperl DJ, Pierce KL, Bogardus AM, Donello JE, et al. Cloning of a novel human prostaglandin receptor with characteristics of pharmacologically defined EP2 subtype. Mol Pharmacol. 1994a;46:213–220. [PubMed] [Google Scholar]
- Reseta LS, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX2. Gastroenterology. 2002;122:1070–1087. doi: 10.1053/gast.2002.32372. [DOI] [PubMed] [Google Scholar]
- Schmid A, Thierauch K, Schleuning W, Dinter H. Splice variants of the human EP3 receptor for prostaglandin E2. Eur J Biochem. 1995;15:23–30. doi: 10.1111/j.1432-1033.1995.tb20223.x. [DOI] [PubMed] [Google Scholar]
- Shahbazian A, Heinemann A, Peskar BA, Holzer P. Differential peristaltic motor effects of prostanoid (DP, EP, IP, TP) and leukotriene receptor agonists in the guinea-pig isolated small intestine. Br J Pharmacol. 2002;137:1047–1054. doi: 10.1038/sj.bjp.0704958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Davis TL. Cloned human EP1 prostanoid receptor pharmacology characterized using radioligand binding techniques. J Pharm Pharm. 2002;54:539–547. doi: 10.1211/0022357021778655. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, et al. Down regulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut. 2004;53:1151–1158. doi: 10.1136/gut.2003.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Garavito M, Dewitt D. Prostaglandin endoperoxide H synthase (cyclooxygenases) -1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Smith WL, Meade EA, Dewitt DL. Pharmacology of prostaglandin endoperoxide synthase isozyme 1 and 2. Ann N Y Acad Sci. 1994;714:136–142. doi: 10.1111/j.1749-6632.1994.tb12037.x. [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc (Delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- Stillman BA, Audoly L, Breyer RM. A conserved threonine in the second extracellular loop of the human EP2 and EP4 receptors is required for ligand binding. Eur J Pharmacol. 1998;357:73–82. doi: 10.1016/s0014-2999(98)00522-6. [DOI] [PubMed] [Google Scholar]
- Stillman BA, Breyer MD, Breyer RM. Importance of the extracellular domain for prostaglandin EP (2) receptor function. Mol Pharmacol. 1999;56:545–551. doi: 10.1124/mol.56.3.545. [DOI] [PubMed] [Google Scholar]
- Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymph angiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Nakato T, Kita A, Hatae N, Tabata H, Tanaka S, et al. Functional domains essential for Gs activity in prostaglandin EP2 and EP3 receptors. Life Sci. 2003;74:135–141. doi: 10.1016/j.lfs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Nakato T, Kita A, Takahashi Y, Hatae N, Tabata H, et al. A cluster of aromatic amino acids in the i2 loop plays a key role for Gs coupling in prostaglandin EP2 and EP3 receptors. J Biol Chem. 2004;279:11016–11026. doi: 10.1074/jbc.M307404200. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Honda A, Hayashi Y, Negishi M, Ichikawa A, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992;267:6463–6466. [PubMed] [Google Scholar]
- Suzuki K, Araki H, Mizoguchi H, Furukawa O, Takeuchi K. Prostaglandin E inhibits indomethacin-induced gastric lesions through EP-1 receptors. Digestion. 2001;63:92–101. doi: 10.1159/000051876. [DOI] [PubMed] [Google Scholar]
- Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- Takafuji V, Cosme R, Lublin D, Lynch K, Roche JK. Prostanoid receptors in intestinal epithelium: selective expression, function, and change with inflammation. Prostaglandins Leukot Essent Fatty Acids. 2000;63:223–235. doi: 10.1054/plef.2000.0144. [DOI] [PubMed] [Google Scholar]
- Takafuji VA, Evans A, Lynch KR, Roche JK. PGE2 receptors and synthesis in human gastric mucosa: perturbation in cancer. Prostaglandins Leukot Essent Fatty Acids. 2002;66:71–81. doi: 10.1054/plef.2001.0299. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Takeuchi K, Okabe S. EP4 receptor mediation of prostaglandin E2-stimulated mucus secretion by rabbit gastric epithelial cells. Biochem Pharmacol. 1999;58:1997–2002. doi: 10.1016/s0006-2952(99)00286-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Araki H, Umeda M, Komoike Y, Suzuki K. Adaptive gastric cytoprotection is mediated by prostaglandin EP1 receptors: a study using rats and knockout mice. J Pharmacol Exp Ther. 2001;297:1160–1165. [PubMed] [Google Scholar]
- Takeuchi K, Ogawa Y, Kagawa S, Ukawa H. Gastric ulcerogenic responses following barrier disruption in knockout mice lacking prostaglandin EP1 receptors. Aliment Pharmacol Ther. 2002;2:74–82. doi: 10.1046/j.1365-2036.16.s2.21.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Ukawa H, Furukawa O, Kawauchi S, Araki H, Sugimoto Y, et al. Prostaglandin E receptor subtypes involved in stimulation of gastroduodenal bicarbonate secretion in rats and mice. J Physiol Pharmacol. 1999a;50:155–167. [PubMed] [Google Scholar]
- Takeuchi K, Ukawa H, Kato S, Furukawa O, Araki H, Sugimoto Y, et al. Impaired duodenal bicarbonate secretion and mucosal integrity in mice lacking prostaglandin E-receptor subtype EP3. Gastroenterology. 1999b;117:1128–1135. doi: 10.1016/s0016-5085(99)70398-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Yagi K, Shinichi K, Ukawa H. Roles of prostaglandin E-receptor subtypes in gastric and duodenal bicarbonate secretion in rats. Gastroenterology. 1997;113:1553–1559. doi: 10.1053/gast.1997.v113.pm9352857. [DOI] [PubMed] [Google Scholar]
- Tamma G, Wiesner B, Furkert J, Hahm D, Oksche A, Schaefer M, et al. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J Cell Sci. 2003;116:285–294. doi: 10.1242/jcs.00640. [DOI] [PubMed] [Google Scholar]
- Tani S, Okuda M, Morishige R, Tanaka T. Gastric mucin secretion from cultured rat epithelial cells. Biol Pharm Bull. 1997;20:482–485. doi: 10.1248/bpb.20.482. [DOI] [PubMed] [Google Scholar]
- Toh H, Ichikawa A, Narumiya S. Molecular evolution of receptors for eicosanoids. FEBS Lett. 1995;361:17–21. doi: 10.1016/0014-5793(95)00129-w. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, et al. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993;268:20175–20178. [PubMed] [Google Scholar]
- Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, et al. Prostaglandin E2 inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6- Isopropoxy-9-oxoanthene-2-carboxylic acid (AH6809), a human EP2 receptor antagonist. Biochem Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Woodward T, Inoue H, Nishihara T. SC-19220, antagonist of prostaglandin E2 receptor EP1, inhibits osteoclastogenesis by RANKL. J Bone Miner Res. 2005;1:15–22. doi: 10.1359/JBMR.041011. [DOI] [PubMed] [Google Scholar]
- Yamato M, Nagahama K, Kotani T, Kato S, Takeuchi K. Biphasic effect of prostaglandin E2 in a rat model of esophagitis mediated by EP1 receptors: relation to pepsin secretion. Digestion. 2005;72:109–118. doi: 10.1159/000088365. [DOI] [PubMed] [Google Scholar]
- Yang J, Xia M, Goetzl E, Songzhu A. Cloning and expression of the EP3-subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1994;198:999–1006. doi: 10.1006/bbrc.1994.1142. [DOI] [PubMed] [Google Scholar]
- Yano T, Zissel G, Muller QJ, Jae SS, Satoh H, Ichikawa T. Prostaglandin E2 reinforces the activation of Ras signal pathway in lung adenocarcinoma cells via EP3. FEBS Lett. 2002;518:154–158. doi: 10.1016/s0014-5793(02)02689-3. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J Immunol. 1999;161:3746–3752. [PubMed] [Google Scholar]