Abstract
Background and Purpose:
The aim of these experiments was to evaluate the significance of the chemical reaction between hydrogen sulphide (H2S) and nitric oxide (NO) for the control of vascular tone.
Experimental Approach:
The effect of sodium hydrosulphide (NaHS; H2S donor) and a range of NO donors, such as sodium nitroprusside (SNP), either alone or together, was determined using phenylephrine (PE)-precontracted rat aortic rings and on the blood pressure of anaesthetised rats.
Key Results:
Mixing NaHS with NO donors inhibited the vasorelaxant effect of NO both in vitro and in vivo. Low concentrations of NaHS or H2S gas in solution reversed the relaxant effect of acetylcholine (ACh, 400 nM) and histamine (100 μM) but not isoprenaline (400 nM). The effect of NaHS on the ACh response was antagonized by CuSO4 (200 nM) but was unaffected by glibenclamide (10 μM). In contrast, high concentrations of NaHS (200–1600 μM) relaxed aortic rings directly, an effect reduced by glibenclamide but unaffected by CuSO4. Intravenous infusion of a low concentration of NaHS (10 μmol kg-1 min-1) into the anaesthetized rat significantly increased mean arterial blood pressure. L-NAME (25 mg kg-1, i.v.) pretreatment reduced this effect.
Conclusions and Implications:
These results suggest that H2S and NO react together to form a molecule (possibly a nitrosothiol) which exhibits little or no vasorelaxant activity either in vitro or in vivo. We propose that a crucial, and hitherto unappreciated, role of H2S in the vascular system is the regulation of the availability of NO.
Keywords: hydrogen sulphide, sodium hydrosulphide, nitric oxide, nitrosothiol, phenylephrine, acetylcholine, histamine, glibenclamide, rat aorta vascular reactivity, rat mean arterial blood pressure
Introduction
The gaseous mediators, nitric oxide (NO) and hydrogen sulphide (H2S), play a number of important physiological and pathophysiological roles within the body. Within the vascular system, NO and H2S are synthesized from L-arginine and L-cysteine by nitric oxide synthase (NOS) (Moncada and Higgs, 2006) and cystathionine γ lyase (CSE) or cystathionine β synthetase (CBS) (Moore et al., 2003; Wang, 2003), respectively. Both gaseous mediators are synthesized in blood vessels (Palmer et al., 1988; Zhao et al., 2001, 2003). Functionally, NO is an important regulator of vascular tone and its over- or under-production has been linked to a variety of cardiovascular diseases. In contrast, the physiological significance of H2S is not yet as clear, but like NO, it exhibits vasodilator (Zhao et al., 2001) and pro-apoptotic activity in vascular smooth muscle (Yang et al., 2006). Elevated concentrations of H2S also occur in animal models of both septic (Li et al., 2005) and haemorrhagic (Mok et al., 2004) shock, hypertension (Yan et al., 2004) and in inflammation (Bhatia et al., 2005).
Although the possibility of some form of ‘cross talk' between vascular NO and H2S has been suggested previously (Wang, 2003) the precise nature of such an interaction has proved difficult to define. At the level of vascular smooth muscle cells, for example, H2S has been reported to either enhance (Zhao and Wang, 2002) or to attenuate (Hosoki et al., 1997) the relaxant effect of NO in the rat aorta. ‘Cross talk' may also occur between the two gases at other levels, For example, the NO donor, sodium nitroprusside (SNP) upregulates H2S production in rat vascular tissues by augmenting expression of CSE or CBS (Zhao et al., 2003) suggesting a possible interaction of these gases at the expression of their synthesizing enzymes. In addition, we have shown that H2S is a potent scavenger of peroxynitrite (Whiteman et al., 2004), which is perhaps indicative of a chemical interplay between H2S and NO/reactive nitrogen species.
Recently, we reported that H2S reacts chemically with NO to produce an, as yet, unidentified nitrosothiol in vitro (Whiteman et al., 2006). The significance of this observation in pharmacological or physiological terms has not yet been addressed in detail. With this in mind, we have now examined the effect of sodium hydrosulphide (NaHS, an H2S donor) and H2S gas administered alone or in combination with NO (either provided by a range of donors or released by appropriate drugs from endothelial cells in situ) on vascular responsiveness in the isolated rat aorta and on mean arterial blood pressure (MAP) in the anaesthetized rat.
Methods
All experiments on animals were approved by the animal ethics committee (IACUC) of National University of Singapore.
Measurement of rat aorta contractility
Experiments were conducted essentially as described previously (Moore et al., 1990). Briefly, rats (male, Sprague–Dawley, 250–300 g) were killed by cervical dislocation and exsanguination and aortic rings (approx. 2–4 mm diameter) were mounted under a tension of 1.5–2.0 g in 2.5 ml organ baths containing warmed (37°C), oxygenated (95% O2: 5% CO2) Krebs solution (composition, mM); NaCl 118, KCl 5.4, NaHCO3 25, MgSO4 1.2, CaCl2 2.5, KH2PO4 1.2, glucose 11.1 (pH 7.4). Changes in tension were recorded using force transducers connected to a PowerLab (AD Instruments Inc., Bella Vista (NSW), Australia) running Chart v5.1.
After equilibration (1 h), rings were precontracted with phenylephrine (PE), at a concentration (200 nM) found in preliminary experiments (data not shown) to produce approximately 70% of the maximum contraction. Thereafter, two different types of experiments were carried out on separate rings as follows; (i) PE-precontracted rings were first relaxed by cumulative addition of increasing concentrations of either NaHS (10–1600 μM), sodium nitroprusside (SNP, 0.5–400 nM), S-nitrosoacetylpenicillamine (SNAP, 0.5–50 nM) or 3-morpholino-sydnonimine (SIN-1, 0.8–3200 nM). Based on these control experiments, approximate EC70s of SNP (6 nM), SNAP (1.5 nM) and SIN-1 (272 nM) were re-tested for the ability to relax PE-contracted rings either alone or subsequently when mixed with NaHS at a concentration (100 μM), which by itself produced little or no direct vasorelaxant effect. Mixing of drugs in this way was carried out in plastic eppendorf vials and an aliquot of the ‘mixture' injected into the organ bath approximately 1 min later. Results are shown as % relaxation of PE-induced contraction, recorded either 1 or 5 min after administration of either drug or drug combination, (ii) PE-precontracted rings were relaxed by addition of a single dose (approx. EC70 in each case) of either SNP (6 nM), acetylcholine (ACh, 400 nM), histamine (100 μM) or isoprenaline (400 nM). At the peak of the vasorelaxant response (approx. 1 min), increasing concentrations of either NaHS (10–1600 μM), H2S gas in solution (10–400 μM) or a single concentration of cysteine (1 mM) was added and the effect on aortic tone (i.e. further relaxation or ‘contraction' – representing reversal of the vasorelaxant response of the agonist applied) was determined. Deionized water was used as a vehicle control. In some experiments, glibenclamide (10 μM), CuSO4 (200 nM) or DL-propargylglycine, (PAG, 1 mM; inhibitor of CSE-mediated H2S biosynthesis) were applied to the organ bath after the vasorelaxant agent was added and left in contact with the tissue for a further 3 min before addition of NaHS. Results are shown as % additional contraction/relaxation of aortic rings exposed to each vasorelaxant agent.
Measurement of rat blood pressure
Experiments were carried out essentially as previously described (Mok et al., 2004). Briefly, rats were anaesthetized (i.p.) with a mixture of ketamine (112.5 mg kg−1) and xylazine (15 mg kg−1). MAP was recorded from the carotid artery by means of a pressure transducer connected to a PowerLab (AD Instruments Ltd., Australia) running Chart v5. The left femoral vein was cannulated for administration of drugs. SNP (3.3–16.5 nmol kg−1) or NaHS (5–20 μmol kg−1) or a mixture thereof were injected intravenously (i.v.) as a bolus (1 ml kg−1). In some experiments, NaHS (10 or 25 μmol kg−1 min−1) or saline (NaCl, 0.9% w v−1) were additionally administered as a constant i.v. infusion (10 min, 0.5 ml kg−1 min−1) before or 3 min after i.v. injection of the NOS inhibitor, L-NG nitroarginine methyl ester (L-NAME, 25 mg kg−1). Results are shown as change in MAP and are expressed as mm Hg.
Statistical analyses
Data show mean±s.e.m with the number of experimental observations indicated in parenthesis. Statistical analysis was by one-way analysis of variance (ANOVA) followed by post hoc Tukey test. A P-value of less than 0.05 was taken to indicate statistical significance.
Drugs and chemicals
SNP and SIN-1 were purchased from the Alexis Corporation (Lausen, Switzerland). All other drugs and reagents were obtained from Sigma Ltd (St Louis, MO, USA). H2S gas was obtained from Soxal Pte. Ltd (Jurong Town, Singapore). H2S gas was bubbled for 3 min through water (1 ml) to prepare a saturated solution (98 mM). The resulting solution was diluted one in 10 with water and aliquots of H2S solution (2.5–200 μl) were thereafter rapidly transferred to the organ bath using gas-tight syringes.
Results
Effect of H2S and NO on contractility of the isolated rat aorta
The reaction between NO and H2S was first investigated using the precontracted rat aorta. In early work, the vasorelaxant effect of the H2S donor, NaHS, as well as H2S gas in solution and a range of NO donors were compared. NaHS has been very widely used by many researchers as a convenient, water-soluble H2S donor. In solution, NaHS dissociates to Na+ and HS−, which then reacts with H+ to yield H2S. Free H2S gas accounts for approximately 30% of a molar concentration of NaHS (Zhao and Wang, 2002). In order to test the assumption that NaHS acts only as an H2S donor drug, we also compared the effect of NaHS with that of authentic H2S gas in some experiments.
In these preliminary experiments, we observed that SNP, SIN-1 and SNAP (NO donors) as well as NaHS (H2S donor) and H2S solution produced concentration-related relaxation of the PE-precontracted rat aorta with EC50 values of 3.2±2.1 nM, 160±36 nM, 1.25±0.05 nM, 630±56 μM and 191.7±53.4 μM (all n⩾6). Emax values for SNP, SIN-1 and SNAP were similar (94.3±0.8, 97.4±0.8 and 97.6±1.1%, respectively, n⩾6) while both NaHS and H2S gas were less effective (62.1±5.8 and 74.3±6.6%, respectively, n=6–14).
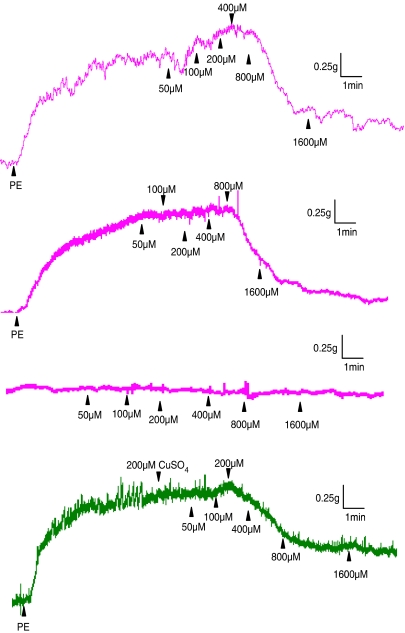
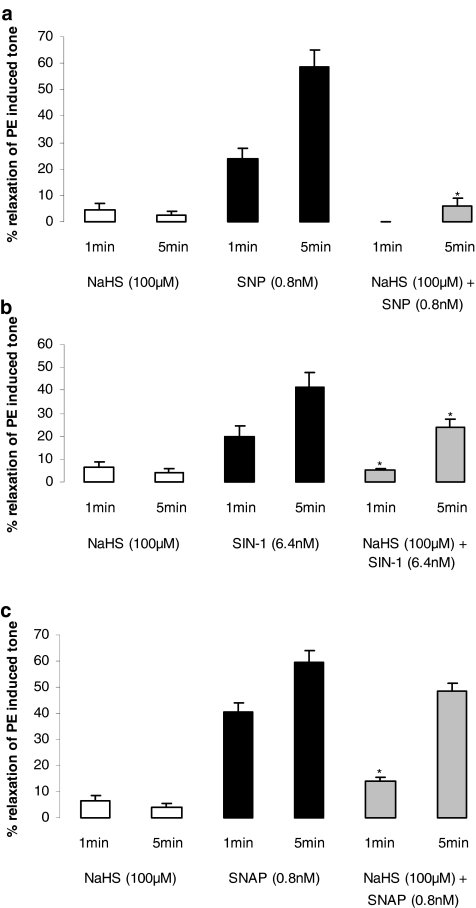
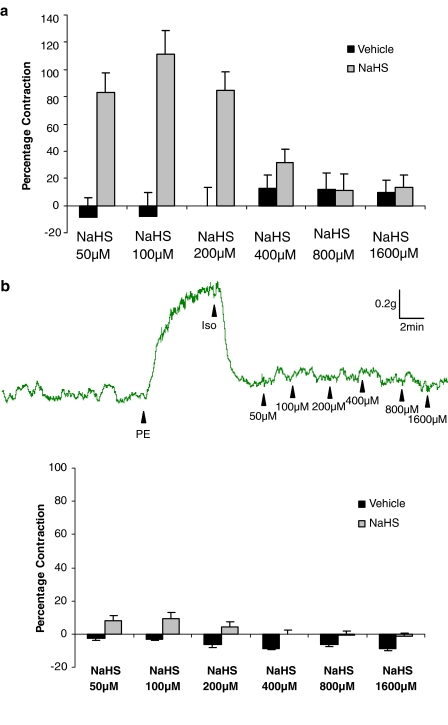
In the course of these ‘pathfinder' experiments we observed that low concentrations of NaHS (10–100 μM) produced an additional contraction, which was ‘superimposed' on that caused by PE. At such concentrations, NaHS did not relax PE-precontracted aortic rings. The additional contraction was not apparent in either endothelium-denuded rings, noncontracted vessels or precontracted rings exposed to CuSO4 (200 nM) (Table 1 and representative traces are shown in Figure 1). Thus, H2S may interact with and ‘quench' endogenous NO released from PE-precontracted aortic rings. To test this novel hypothesis, we mixed together an approximate EC50 of SNP (0.8 μM), SIN-1 (6.4 μM) or SNAP (0.8 μM) with a subthreshold concentration of NaHS (100 μM). As noted previously, this concentration of NaHS would be expected to result in a bath concentration of H2S of approximately 30 μM (i.e. at the lower end of the range of plasma concentration of this mediator). Application of the resulting ‘mixture' to PE-precontracted aortic rings resulted in markedly reduced vasorelaxant activity 1 min after drug addition. This effect also persisted (for SNP and SIN-1) until at least 5 min after addition of the ‘mixture' (Figure 2a–c).
Table 1.
Effect of low concentrations of NaHS on response of endothelium-intact and endothelium-denuded, phenylephrine-precontracted aortic rings
| NaHS concentration (μM) | Endothelium intact (%) | Endothelium denuded (%) |
|---|---|---|
| 50 | 102.3±0.8 | 100.8±0.8 |
| 100 | 109.1±1.3* | 99.6±1.4 |
| 200 | 106.9±4.8* | 89.9±3.2* |
Abbreviation: NaHS, sodium hydrosulphide.
Results are expressed as % contraction over and above that induced by phenylephrine (200 nM; set at 100%). Values in excess of 100% therefore indicate contraction due to NaHS addition while values less than 100% indicate relaxation. Results show mean±s.e.m., n=5–7
P<0.05.
Figure 1.
Representative traces showing the effect of NaHS (50–1600 μM) on rat aortic rings precontracted with PE (200 nM). NaHS injection at the peak of the PE-induced contraction evoked an additional contraction in endothelium-intact (first trace) but not in endothelium-denuded (second trace) rings. NaHS did not affect nonprecontracted rings (third trace) or precontracted rings treated with CuSO4 (200 nM, fourth trace). Vertical bar indicates tension scale (g). Horizontal bar shows time (min). Drugs were injected at the arrows as indicated.
Figure 2.
Effect of NaHS (100 μM) and NO donors administered alone or after mixing (1 min) with approximately EC70s of either SNP (a; 0.8 nM), SIN-1 (b; 6.4 nM) or SNAP (c; 0.8 nM) on tone of the PE-precontracted rat aorta. Deionized water was used vehicle control. Results are expressed as % relaxation of PE-induced tone and are mean±s.e.m., n=6, *P<0.05.
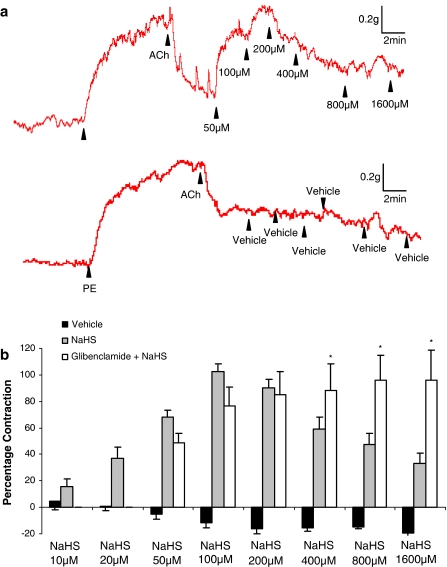
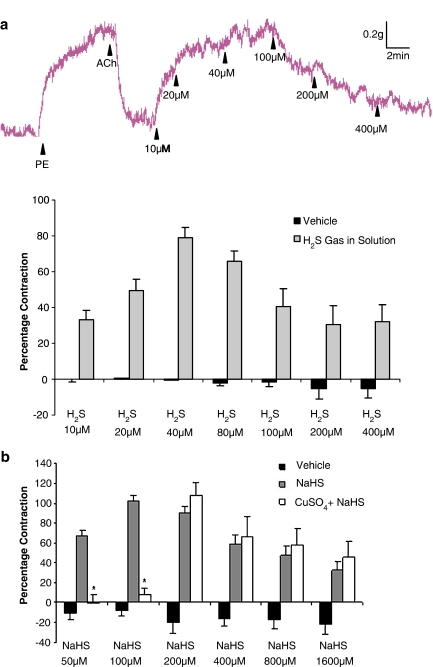
An additional series of experiments was undertaken to test the ability of NaHS to reverse the vasorelaxant effect of a range of endothelium-dependent and -independent agent. Addition of the endothelium-dependent vasorelaxant agent, ACh (400 nM) to PE-contracted aortic rings caused relaxation (69.7±3.8% of the PE response) which, in the absence of further intervention, was sustained for several minutes. Addition of increasing concentrations of either NaHS or H2S gas in solution (but not vehicle) to the ACh-relaxed aorta preparation caused a biphasic effect viz. ‘contraction' (i.e. reversal of the relaxation due to ACh) at low concentrations (10–100 μM) followed by relaxation at higher concentrations (i.e. 100–1600 μM) (Figures 3a,b and 4a).
Figure 3.
Representative traces (a) and the concentration-related effect of NaHS (b; 50–1600 μM) on PE-precontracted aortic rings, which were partially relaxed by addition of acetylcholine (400 nM). Vertical bar indicates tension scale (g). Horizontal bar shows time (min). Drugs were injected at the arrows as indicated. Glibenclamide (b, 10 μM) was added to the organ bath 3 min before addition of NaHS. Deionized water was used as vehicle control and did not affect the ACh-mediated relaxation (P>0.05). Results show % contraction (reversal of ACh-mediated relaxation) and are mean±s.e.m., n=4–14, *P<0.05.
Figure 4.
Concentration-related effect of H2S gas in solution (a; 10–400 μM) on PE-precontracted aortic rings, which were partially relaxed by addition of acetylcholine (400 nM). Vertical bar indicates tension scale (g). Horizontal bar shows time (min). Drugs were injected at the arrows as indicated. Effect of CuSO4 on concentration-related effect of NaHS (b; 50–1600 μM) on PE-precontracted aortic rings, which were partially relaxed by addition of acetylcholine (400 nM). CuSO4 (b, 200 nM) was added to the organ bath 3 min before addition of NaHS. Deionized water was used as vehicle control and did not affect the ACh-mediated relaxation (P>0.05). Results show % contraction (reversal of ACh-mediated relaxation) and are mean±s.e.m., n=4–14, *P<0.05.
Intriguingly, inclusion into the organ bath of the KATP channel antagonist, glibenclamide (10 μM) did not significantly affect the ‘contractile' component of the response to low concentrations (i.e. 50–200 μM) of NaHS but completely abolished the subsequent relaxant effect that occurred at higher concentrations (200–1600 μM; Figure 3b). In complete contrast, inclusion into the organ bath of CuSO4 (200 nM), which converts nitrosothiols to a mixture of nitrite and nitrate, abolished the ‘contractile' component of the response to low concentrations (i.e. 50 and 100 μM) of NaHS without affecting the direct vasorelaxant effect of NaHS at higher concentrations (i.e. 200–1600 μM) (Figure 4b).
Further experiments were conducted using both histamine (endothelium-dependent) and isoprenaline (endothelium-independent) as vasorelaxant agents. Based on preliminary concentration pathfinder experiments, EC50s for histamine (45.0±14.5 μM, n=4) and isoprenaline (89.6±14.8 nM, n=4) were applied to PE-contracted rat aortic rings and NaHS added to the organ bath at increasing concentrations. Like ACh, the response to NaHS in histamine-relaxed tissues was biphasic in nature with ‘contraction' at low concentrations and relaxation at high concentrations (Figure 5a). In contrast, NaHS did not reverse the vasorelaxant effect of isoprenaline (Figure 5b).
Figure 5.
(a) Concentration-related effect of NaHS (a; 10–1600 μM) on PE-precontracted aortic rings, which were partially relaxed by addition of histamine (100 μM). Results show % contraction (reversal of histamine-mediated relaxation) and are mean±s.e.m., n=7. (b) Upper trace: representative trace showing the effect of NaHS (50–1600 μM) on PE-precontracted aortic rings, which were partially relaxed by addition of isoprenaline (400 nM). Vertical bar indicates tension scale (g). Horizontal bar shows time (min). Drugs were injected at the arrows as indicated. Lower graph: effect of NaHS (a; 50–1600 μM) on rat aortic rings, which had been precontracted with PE (200 nM) and then partially relaxed by addition of isoprenaline (400 nM). Deionized water was used a vehicle control. Results show % contraction (reversal of isoprenaline-mediated relaxation) and are mean±s.e.m., n=12.
In a final series of experiments, we tested whether endogenous H2S (synthesized by aortic rings from added cysteine) was also able to react with and quench endogenous NO. To this end, we added cysteine (1 mM) to PE-precontracted rat aortic preparations which, as above, had been relaxed with ACh. Like NaHS, cysteine also reversed the ACh-mediated relaxation (i.e. contracted; (77.7±6.5%, n=11) the aorta under these conditions. This contraction was partially inhibited (42.2±8.8%, n=7) by inclusion into the organ bath of PAG, the inhibitor of CSE (1 mM). Cysteine (1mM) did not affect the tone of noncontracted aortic rings (data not shown).
Effect of H2S and NO on MAP of the anaesthetized rat
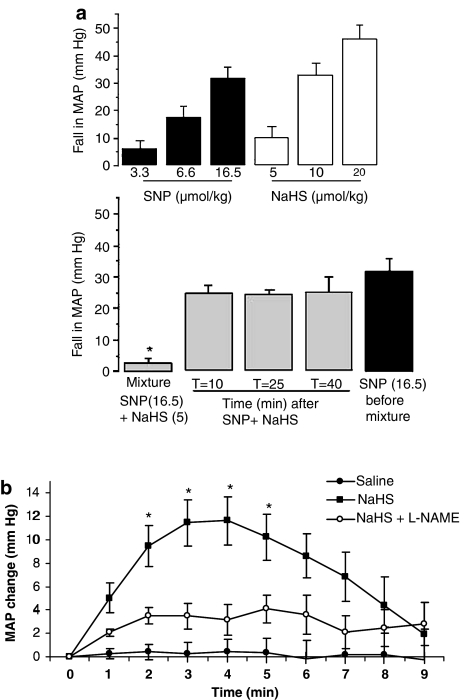
Whether NO and H2S might also react together in the intact animal was studied by monitoring the blood pressure lowering effect of NaHS both alone, and in combination with SNP, in the anaesthetized rat. Baseline MAP of animals used in these experiments was 105.5±4.1 mm Hg (n=26). Bolus (i.v.) injection of either SNP or NaHS caused dose-related falls in blood pressure (Figure 6a). Brief mixing followed by coadministration of a mixture of SNP (by itself a dose which produced approximate 35 mm Hg fall in blood pressure) and NaHS (by itself a dose which produced less than 10 mm Hg fall) resulted in an almost complete loss of vasodepressor activity (Figure 6a) again suggesting quenching of released NO by H2S derived from NaHS. Administration of the same dose of SNP alone (i.e. without NaHS) at timed intervals (10–40 min) after injection of the ‘mixture' revealed complete restoration of the vasodepressor effect of injected SNP even at the earliest time point.
Figure 6.
(a) Upper graph: effect of SNP (3.3–16.5 nmol kg−1, i.v.) and NaHS (5–20 μmol kg−1, i.v.) on mean arterial blood pressure (MAP) of the anaesthetized rat. Lower graph: effect of a mixture of SNP (16.5 nmol kg−1) and NaHS (5 μmol kg−1) on blood pressure and subsequent time-related recovery of the response to SNP administered alone. For comparison the response to SNP (16.5 nmol kg−1) injected before administration of the mixture is also shown. Results show reduction in MAP (mm Hg) % and are mean±s.e.m, n=4–6, *P<0.05 (cf. SNP alone). (b) Time-dependent effect of an i.v. infusion (10 min) of either saline, NaHS (10 μmol kg−1 min−1) or NaHS (10 μmol kg−1 min−1 administered 3 min after i.v. injection of L-NAME, 25 mg kg−1) on mean arterial pressure (MAP) of the anaesthetized rat. The effect of the NaHS infusion was inhibited by pretreatment with L-NAME (P<0.05). Results show reduction in MAP (mm Hg) % and are mean±s.e.m., n=4–6.
In separate experiments, we also examined the possibility that a similar reaction may take place between NaHS and endogenous, endothelium-derived NO. A slow i.v. infusion of NaHS (25 μmol kg−1 min−1) resulted in a marked fall in MAP, which was sustained throughout the 10 min infusion period. In contrast, a slow i.v. infusion of a lower dose of NaHS (i.e. 10 μmol kg−1 min−1) resulted in a small (approx. 10–15 mm Hg) but significant rise in blood pressure which peaked 4 min into the infusion period and began to decline thereafter (Figure 6b). Intriguingly, this unexpected vasopressor effect of NaHS was abolished in animals given L-NAME to inhibit endogenous NO biosynthesis (Figure 6b). These data suggest that low concentrations of H2S may also quench endogenous, endothelium-derived NO in vivo as well as in vitro.
Discussion
We have previously shown that NO and H2S react together in aqueous solution to form an, as yet unidentified, novel nitrosothiol molecule. This conclusion centred on the use of donors of NO, such as SNP or SNAP, and of a donor of H2S, NaHS, and was based on a combination of data using electron paramagnetic resonance, NO amperometry and measurement of nitrite concentration (Whiteman et al., 2006). In the present study, we have evaluated the pharmacological consequences of such an interaction between NO and H2S in terms of the regulation of blood vessel responsiveness both in vitro and in vivo.
Effect of H2S and NO on contractility of the isolated rat aorta
SNP, SIN-1, SNAP, NaHS and H2S gas in solution all produced concentration-related relaxation of the PE-precontracted rat aorta. It is noteworthy that both NaHS and H2S solution produced similar effects in this tissue. However, NaHS proved to be some 3.2 times less potent in molar terms than H2S gas since, as indicated earlier, only approximately 30% of NaHS solution is present as free H2S gas.
Preliminary experiments using the PE-precontracted rat aorta revealed that low concentrations (approx. 10–100 μM) of NaHS produced a further contraction on top of that caused by PE whereas higher concentrations (>100 μM) caused relaxation. NaHS did not contract ‘low tone' (i.e. nonprecontracted tissues) in our hands. By far the predominant effect of NaHS on mammalian blood vessels both in vitro and in vivo reported to date has been vasorelaxation (Zhao et al., 2001) due to opening of vascular KATP channels (Zhao and Wang, 2002). However, high (possibly nonphysiological) concentrations of NaHS (i.e. >1 mM) have been shown to produce modest contraction of the ‘low tone' rat aortic rings as well as a complex tri-phasic (contraction/relaxation/contraction) pattern of responses in the PE-pretreated rat pulmonary artery (Dombkowski et al., 2004). These effects may be due to a change in pH (alkalinization) of the medium at such high concentrations of NaHS. However, the ability of H2S to further contract PE-pretreated rat aorta at concentrations (i.e. 10–50 μM), which are known to occur naturally in mammalian blood and tissue (Richardson et al., 2000; Zhong et al., 2003; Mok et al., 2004) has not previously been reported and may be of physiological and or pathophysiological significance.
As noted previously we have recently shown that H2S reacts chemically with NO to form a novel nitrosothiol (Whiteman et al., 2006). Based on a number of lines of pharmacological evidence we now propose that a similar reaction underpins the vascular contractile effect of low concentrations of NaHS observed in the present study. Firstly, NaHS did not contract aortic rings, which had been rubbed to remove the endothelial cells. The contractile effect of H2S is therefore not a direct action on vascular smooth muscle cells but an indirect effect involving endothelial cells. Contraction of the rat aorta (and other blood vessels) in the organ bath is associated with stretch-induced activation of endothelial cell NOS and release of vasorelaxant NO (Dainty et al., 1990; Ohno et al., 1990). Furthermore, drugs which deplete NO under these circumstances, such as NOS inhibitors (Moore et al., 2000), are well known to cause an additional contraction of blood vessels. Accordingly, in situ quenching of released NO by H2S may underlie the contractile response to NaHS in precontracted (but not noncontracted) and in endothelium-intact but not endothelium-depleted vessels.
Secondly, mixing NaHS with either SNP, SIN-1 or SNAP resulted in a markedly diminished vasorelaxant effect of each NO donor thus providing direct evidence that H2S is able to quench and thereby inactivate NO in vitro. Also, carrying out the experiment in this way rules out the possibility that ‘cross talk' between the two gases occurs by a tissue-based mechanism, for instance, effects on intracellular transduction pathways. Furthermore, the finding that H2S reduced the relaxant effect of three chemically very distinct NO donor molecules points yet again to a direct chemical interaction between H2S (derived from NaHS) and NO (derived from NO donors) rather than an indirect effect on, for example, NO liberation from the donor drug.
Thirdly, while exogenous H2S (derived from NaHS) quenches the vasorelaxant effect of exogenous NO (derived from SNP, SIN-1 or SNAP), it was clearly important to determine whether NaHS exhibits similar activity against endogenous NO (derived from the vascular endothelium). To this end, an alternative experimental approach was used in which low concentrations (10–1600 μM) of NaHS were added to PE-pretreated aortic rings, which had been partially relaxed using a submaximal concentration of either SNP (for comparison) or ACh or histamine (both endothelium/NO-dependent vasodilators in this tissue, Van de Voorde and Leusen, 1983; Moore et al., 1990). In all three cases, a concentration-related reversal of vasorelaxation (i.e. a ‘physiological' contraction) was noted. The effect of NaHS was rapid in onset and a full restoration of the PE-induced tone (i.e. complete reversal) was achieved. Prior exposure of aortic rings to CuSO4 (which is known to convert nitrosothiols to nitrite and nitrate; Stubauer et al., 1991) prevented the contractile without affecting the relaxant effect of NaHS thus providing further evidence for the production of a nitrosothiol within the organ bath. CuSO4 was used in these experiments rather then HgCl2 (another agent of choice for converting nitrosothiol to nitrite in tissue homogenates, etc) due to the endothelial damage and stripping effect of the latter compound (Golpon et al., 2003). In stark contrast, NaHS did not reverse the relaxant effect of isoprenaline that produces a cAMP-mediated, endothelium-independent and thus NO-independent relaxation response in this tissue. The relaxation response at higher concentrations of NaHS was, in turn, reduced by glibenclamide suggesting that this effect followed opening of vascular smooth muscle KATP channels.
The potential physiological significance of an NO/H2S interaction would be enhanced if it could be shown to occur between endogenous (i.e. vessel-derived) H2S and NO. The precise role of endogenous H2S in the control of vascular reactivity remains to be elucidated. Homogenates of rat aorta and tail artery do convert exogenous cysteine to H2S in vitro as a result of CSE enzyme activity (Zhao et al., 2001, 2003). The vascular effects of cysteine have also been the subject of scrutiny over many years. Although cysteine has little or no direct vascular activity it does reduce the relaxant effect of ACh in PE-contracted rat aorta rings (Ellis et al., 2000) and the effect of SNP in the rabbit aorta (Jia and Furchgott, 1993). In the present experiments we demonstrated that cysteine (like NaHS) also reversed ACh-mediated vasorelaxation of PE-contracted rat aortic rings in a manner, which was partially reversed by prior application of PAG, an inhibitor of CSE. Thus, we propose that cysteine added to the organ bath is converted by CSE enzyme activity in aortic rings to endogenous H2S, which then reacts with and partly quenches the ACh-evoked, endothelium-derived NO to bring about a contraction of the tissue.
Effect of H2S and NO on MAP of the anaesthetized rat
Mixing together effective vasodepressor doses of both NaHS and SNP resulted in complete loss of vasodepressor activity of the mixture in the anaesthetized rat. No change in sensitivity towards injected SNP was apparent at 10 min after injection of the mixture suggesting that the effect of NaHS was not secondary to inhibition of the effect of NO on vascular smooth muscle. Thus, we conclude that, as in aortic rings in vitro, the nitrosothiol product of this reaction exhibits negligible vasodilator activity in vivo. We also show that an i.v. infusion of NaHS (25 μmol kg−1 min−1) lowered blood pressure in the rat while infusion of a low dose of NaHS (10 μmol kg−1 min−1) increased blood pressure. The effect was relatively small (10–15 mm Hg) but was statistically significant and was maintained for several minutes. That such vasopressor activity of NaHS was abolished in animals pretreated with L-NAME to inhibit endogenous NO biosynthesis suggests that H2S (derived from NaHS) reacts with and quenches endogenous endothelium-derived NO thereby resulting in loss of NO-derived vasodilator tone and a rise in blood pressure.
In conclusion, we show here that combination of H2S and NO leads to the formation of a novel molecule (perhaps a nitrosothiol as suggested previously), which does not relax blood vessels either in vitro or in vivo. One consequence of this reaction is that, by reducing endogenous NO, H2S (a presumed vasorelaxant/vasodilator) triggers a ‘paradoxical' contraction of rat aortic rings in vitro and an increase in rat blood pressure in vivo. Consequently, the formation of this novel molecule most likely represents a means for biological inactivation or perhaps sequestration of released NO. Although H2S does exhibit vasorelaxant activity in its own right (by opening vascular smooth muscle KATP channels) it is markedly less potent than NO with effects usually apparent only at concentrations above 200 μM while plasma concentrations of this gas in both man and animals are generally in the range 30–60 μM (Richardson et al., 2000). As such, we believe that a principal physiological role of H2S, released from the vasculature, may be to regulate local concentrations of NO rather than to dilate blood vessels directly. Reports in the literature providing evidence for putative physiological and/or pathophysiological role(s) of H2S may need to be re-evaluated in light of the present findings and their interpretation.
Acknowledgments
We thank the Office of Life Science (OLS) of the National University of Singapore and the Biomedical Research Council (BMRC) of Singapore for financial support. We also thank the Agency for Science, Technology and Research (A*STAR) (Singapore) for the award of National Graduate Scholarships to MY and YYPM.
Abbreviations
- CBS
cystathionine β synthetase
- cGMP
cyclic 3′5′ guanosine monophosphate
- CSE
cystathionine γ lyase
- DMSO
dimethylsulphoxide
- MAP
mean arterial blood pressure
- NOS
nitric oxide synthase
- PAG
DL-propargylglycine
Conflict of interest
The authors state no conflict of interest.
References
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty IA, Mcgrath JC, Spedding M, Templeton AG. The influence of the initial stretch and the agonist-induced tone on the effect of basal and stimulated release of EDRF. Br J Pharmacol. 1990;100:767–773. doi: 10.1111/j.1476-5381.1990.tb14090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol. 2004;286:R678–R685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- Ellis A, Li CG, Rand MJ. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br J Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpon HA, Puchner A, Barth P, Welte T, Wichert P, Feddersen CO. Nitric oxide-dependent vasorelaxation and endothelial cell damage caused by mercury chloride. Toxicology. 2003;192:179–188. doi: 10.1016/s0300-483x(03)00303-2. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Jia L, Furchgott RF. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1993;267:371–378. [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Mok YYP, Shirhan M, Cheong YP, Wang ZZ, Bhatia M, Moochhala SM, et al. Role of hydrogen sulfide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulfide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147 Suppl 1:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, al-Swayeh OA, Chong NWS, Evans RA, Gibson A. L-NG-nitroarginine (L-NOARG) – a novel, L-arginine reversible inhibitor of endothelium-dependent vasodilatation. Br J Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, al-Swayeh OA, Clifford RH, del Soldato P. A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br J Pharmacol. 2000;129:343–350. doi: 10.1038/sj.bjp.0703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, Bhatia M, Moochhala S. Hydrogen sulphide: from the smell of the past to the gas of the future. Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Ohno M, Ochiai M, Taguchi J, Hara K, Akatsuka N, Kurokawa K. Stretch may enhance the release of endothelium-derived relaxing factor in rabbit aorta. Biochem Biophys Res Commun. 1990;173:1038–1042. doi: 10.1016/s0006-291x(05)80890-3. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Richardson CJ, Magee EA, Cummings JH. A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin Chim Acta. 2000;293:115–125. doi: 10.1016/s0009-8981(99)00245-4. [DOI] [PubMed] [Google Scholar]
- Stubauer G, Giuffre A, Sarti P. Mechanism of S-nitrosothiol formation and degradation mediated by copper ions. J Biol Chem. 1991;274:28128–28133. doi: 10.1074/jbc.274.40.28128. [DOI] [PubMed] [Google Scholar]
- Van De Voorde J, Leusen I. Role of the endothelium in the vasodilator response of rat thoracic aorta to histamine. Eur J Pharmacol. 1983;87:113–120. doi: 10.1016/0014-2999(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Wang R. The gasotransmitter role of hydrogen sulfide. Antiox Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Siau JL, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide; an endogenous peroxynitrite ‘scavenger'. J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Ling L, Kostetski I, Chu SH, Siau JL, Bhatia M, et al. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric and hydrogen sulfide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypert. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]