Abstract
Background and purpose:
Intravenous administration of recombinant human activated protein C (rhAPC) is known to reduce lipopolysaccharide (LPS)-induced pulmonary inflammation by attenuating neutrophil chemotaxis towards the alveolar compartment. Ideally, one would administer rhAPC in pulmonary inflammation at the site of infection to minimize the risk of systemic bleeding complications. In this study, we therefore assessed the effect of inhaled rhAPC in a murine model of acute lung injury.
Experimental approach:
Mice were exposed to LPS (0.5 mg kg-1: intranasally) to induce acute lung injury. 30 minutes before and 3 hours after LPS exposure mice were subjected to vehicle or rhAPC inhalation (25 or 100 μg per mouse in each nebulization). In order to establish whether rhAPC inhalation affects neutrophil recruitment, neutrophil migration was determined in vitro using a trans-well migration assay.
Key results:
rhAPC inhalation dose-dependently decreased LPS-induced coagulation and inflammation markers in bronchoalveolar lavage fluid (BALF), reduced protein leakage into the alveolar space and improved lung function. In contrast, rhAPC did not prevent LPS-induced neutrophil recruitment into the alveolar space.
Neutrophil migration in vitro towards FCS or interleukin (IL)-8 was significantly inhibited by pretreatment with rhAPC (0.01-10 μg ml-1], whereas rhAPC (10 μg ml-1) added to the chemoattractant (modelling for topical rhAPC administration) did not affect neutrophil migration towards FCS or IL-8.
Conclusions and Implications:
rhAPC inhalation significantly diminished LPS-induced pulmonary inflammation. The benefit of inhaled rhAPC appeared not to involve attenuation of neutrophil recruitment, in contrast to its effects after intravenous administration.
Keywords: activated protein C, inhalation, pulmonary inflammation, lung function, neutrophil migration
Introduction
The natural protein C anticoagulant pathway serves as a vitally important system limiting the amplification of the coagulation response at the level of co-factors Va and VIIIa. Activation of protein C is triggered by thrombin allowing the system to serve as a direct negative feedback mechanism. In addition to its well-known anticoagulant activity, activated protein C has been described to exert potential anti-inflammatory effects (Hancock et al., 1995; Murakami et al., 1997). Furthermore, intravenous administration of recombinant human activated protein C (rhAPC) improves survival in patients with severe sepsis and/or experimental animal models of sepsis (Taylor et al., 1987; Bernard et al., 2001; Ely et al., 2003). The beneficial effect of rhAPC on the outcome of sepsis has been attributed to its capacity to inhibit different pathways involved in the pathogenesis of sepsis, like the activation of coagulation, the production of pro-inflammatory mediators and/or the adhesion of inflammatory cells to the endothelium (Hancock et al., 1995; Murakami et al., 1997; Joyce and Grinnell, 2002). However, recent analysis of the PROWESS study data does not support any systemic anti-inflammatory effect of rhAPC treatment (Dhainaut et al., 2003). In addition, rhAPC seems to have no effect on inflammatory mediators in human models of endotoxemia (Derhaschnig et al., 2003; Kalil et al., 2004). A similar lack of anti-inflammatory properties of rhAPC was observed in a human model of endotoxin-induced pulmonary inflammation (Nick et al., 2004). Intravenous administration of rhAPC however, did affect neutrophil chemotaxis, suggesting that the beneficial effects of rhAPC in severe sepsis might result from the direct inhibition of neutrophil migration (Nick et al., 2004).
Within the pulmonary compartment, acute inflammation is characterized by fibrin formation, cytokine production, protein leakage into the alveolar space due to increased capillary barrier permeability and neutrophil migration, which are all potential targets of rhAPC (Hancock et al., 1995; Murakami et al., 1997; Nick et al., 2004; Zeng et al., 2004; Finigan et al., 2005). To prevent bleeding complications due to systemic rhAPC administration, which is actually the major complication of rhAPC in sepsis trials (Bernard et al., 2001; Vincent et al., 2005), local administration of rhAPC directly in the lung would be the preferred treatment strategy for patients suffering from pulmonary inflammation. To proof or refute the hypothesis that local administration of rhAPC is beneficial in pulmonary inflammation by diminishing neutrophil influx, we established the effect of rhAPC nebulization on acute murine lung injury induced by bacterial endotoxin.
Methods
Test systems used
Animals
10-week-old, female C57Bl/6 mice (±20 g) were maintained at the animal care facility of the Academic Medical Center according to institutional guidelines. Animal procedures were carried out in compliance with Institutional Standards for Humane Care and Use of Laboratory Animals. The Animal Care and Use Committee of the Academic Medical Centre approved all experiments. In each experimental group, eight mice were used. Baseline characteristics and effects of 100 μg mouse−1 nebulization−1 (without lipopolysaccharide (LPS)) were analyzed for n=3.
Isolated human neutrophils
Neutrophils were isolated from blood of healthy volunteers using Polymorph-prep (Axis-Shield, Oslo, Norway) according to the manufacturers' recommendations.
Experimental design
Experimental procedure
LPS was administered intranasally according to previously described methods (Maris et al., 2004). LPS was diluted in 0.9% sterile saline (50 μl) to a final concentration of 200 μg ml−1 (i.e. 10 μg per mouse). rhAPC (25 or 100 μg per mouse for each nebulization; (dose based on previous publications (Yasui et al., 2001; Yuda et al., 2004) or vehicle (sterile saline) was inhaled by nebulization 30 min before and 3 h after LPS exposure. In a second series of experiments, the additional activity of repeated dosing of 100 μg rhAPC (30 min before and 1, 3 and 5 h after LPS) was addressed in an attempt to saturate any potential effects of natural inhibitors of this drug.
rhAPC or vehicle was aerosolized (airflow 5 l min−1) for 3 min using a plastic chamber attached to an Aeroneb pro-nebulizer (Medicare, Uitgeest, The Netherlands), after which mice were maintained in the chamber for an additional 7 min.
Lung function measurement
Lung function characteristics in response to LPS were determined using whole body plethysmography as described previously (Shore et al., 2001). Briefly, mice were individually (awake and unrestrained) placed in a plethysmograph (Buxco Electronics Inc., Wilmington, NC, USA). Pressure fluctuations in the plethysmograph caused by breathing of the mice were continuously monitored for 10 min and subsequently these pressure fluctuations were quantified using the algorithm for enhanced pause (Penh), which can be interpreted as an index of flow limitation as it has been shown to correlate with pulmonary resistance (Johnston et al., 2005). Averaged Penh values were therefore, determined for each mouse before (baseline) and 2 and 4 h after LPS administration and were used to compare results across treatment groups.
Bronchoalveolar lavage
Animals were killed 6 h after LPS challenge by intraperitoneal injection of 0.3 ml Hypnorm Midazolam and bronchoalveolar lavage (BAL) was performed by instilling four times 0.4 ml aliquots of saline by a 22-gauge Abbocath-T catheter into the trachea via a midline incision (Leemans et al., 2002).
Total cell numbers were counted with a Burker-Turk hemocytometer (Emergo, Landsmeer, The Netherlands). BAL fluid differential counts were done on cytospin preparations stained with Giemsa.
Enzyme-linked immunosorbent assay
Tumor necrosis factor (TNFα), Interleukin-6 (IL-6) and cytokine-induced neutrophil chemoattractant (KC) were measured by enzyme-linked immunosorbent assay (ELISAs) according to manufacturers' instructions (R&D Systems, Minneapolis, MN, USA). Detection limits were 39 pg ml−1 (IL-6), 36 pg ml−1 (TNFα) and 17 pg ml−1 (KC).
Thrombin-antithrombin complexes (TATc) were determined as a measurement of activation of coagulation (Weijer et al., 2004). TATc were measured with a mouse-specific ELISA. In short, rabbits were immunized with mouse thrombin or rat antithrombin. Anti-thrombin antibodies were used as capture antibody, digoxigenin (DIG)-conjugated anti-antithrombin antibodies were used as detection antibodies, Horseradish peroxidase (HRP) labelled sheep anti-DIG F(ab)-fragments (Boehringer Mannheim GmbH, Germany) were used as staining enzyme, and TMB (Sigma) was used as substrate. Dilutions of mouse serum (Sigma) were used for the standard curve, yielding a detection limit of 1 ng ml−1 (Schoenmakers et al., 2004).
Total protein concentration
BAL fluid total protein levels were determined using a bicinchoninic acid (BCA) protein assay (Pierce, Omnilabo International, The Netherlands) according to manufacturers' instructions with BSA as standard.
Myeloperoxidase activity assay
Myeloperoxidase (MPO) activity was measured as a marker for granulocyte activity within lung tissue (after BAL procedure) as described previously (Knapp et al., 2003). Briefly, lungs were homogenized in potassium phosphate buffer, pH 7.4. After centrifugation, cells were lysed in potassium phosphate buffer pH 6.0 containing 0.5% hexadecyltrimethyl ammoniumbromide (HETAB) and 10 mM EDTA. MPO activity was determined by measuring the H2O2 dependent oxidation of 3,3′5,5 tetramethylbenzidine (TMB). The reaction was stopped with glacial acetic acid followed by reading the absorbance at 655 nm. MPO activity was expressed as units of MPO activity per gram lung tissue per min ((OD655 × dilution factor minute−1) tissue weight−1). All reagents were purchased from Sigma. MPO activity was expressed as units per gram lung tissue per min.
Neutrophil migration assay
Isolated human neutrophils were washed and labelled for 1 h with 10 μM Cell Tracker Green in serum-free RPMI medium. The dye was fixed by 1 h incubation in medium with 10% serum, and subsequently cells were washed in PBS. To assess the direct cellular effect of rhAPC on neutrophil migration, cells (1 × 105) were preincubated with rhAPC (0–10 μg ml−1) for 20 min, washed and transferred to 8 μM pore size HTS FluoroBlok Cell Culture Inserts (BD Bioscience, San Jose, CA, USA) which were inserted in fitting 24-wells plates containing RPMI supplemented with fetal calf serum (FCS) (20%) or interleukin-8 (IL-8) (100 ng ml−1) as chemoattractant. To determine the chemoattractive capacity of rhAPC, in a different set of experiments, migration of cells (not pretreated with rhAPC) towards FCS (20%) or IL-8 (100 ng ml−1) supplemented with or without 10 μg ml−1 rhAPC was assessed. After inserting the Cell Culture Inserts into the 24-well plate with chemoattractants, fluorescence values representing the number of cells on the bottom side of the insert were read during 25 cycles (each cycle comprising four readings spanning 2 min) at 37°C on a Series 4000 CytoFluor Multi-Well Plate Reader (Perseptive Biosystems, Framingham, MA, USA). The raw fluorescence data were corrected for background fluorescence and fading of the fluorophore. No-attractant controls were subtracted for each condition to correct for any effects not due to active migration to the chosen attractant. Data are represented as percentage migration relative to the control (no rhAPC pretreatment (right) or no rhAPC supplemented the chemoattractant (left)) and are shown as mean±s.e.
Data analysis and statistical procedures
Statistical analyses were conducted using GraphPad Prism version 3.00, GraphPad software (San Diego, CA, USA). Data are expressed as means±s.e. Comparison between two groups was analyzed using (one-tailed) Mann–Whitney U-tests.
Drugs, chemicals, reagents and other material
LPS (Escherichia coli O55:B5) was obtained from Sigma (St Louis, MO, USA). rhAPC (Xigris) was obtained from Eli Lilly (Houten, The Netherlands). Hypnorm was purchased from Janssen Pharmaceutical (Geel, Belgium) and Midazolam was obtained from Roche (Almere, The Netherlands). ELISA kits for TNF, Il-6 and KC was purchased from R&D Systems (Minneapolis, MN, USA). Bicinchoninic acid (BCA) protein assay was supplied by Pierce, Omnilabo International (Breda, The Netherlands). Polymorph-prep was purchased from Axis-Shield (Oslo, Norway). Cell Tracker Green was obtained from Molecular Probes (Eugene, OR, USA).
Results
rhAPC inhalation reduces cytokine and TATc levels in BALF
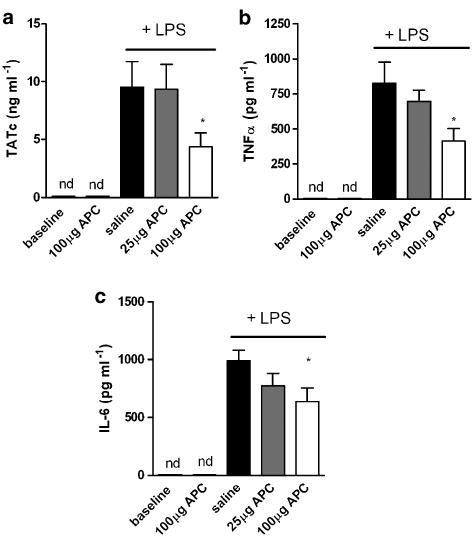
Intra-alveolar fibrin deposition and extensive inflammation are two specific characteristics of endotoxin-induced pulmonary inflammation and therefore we determined the effect of inhaled rhAPC on TATc, IL-6 and TNFα levels in bronchoalveolar lavage fluid (BALF). Intranasal LPS administration induced TATc, IL-6 and TNFα levels, which were below detection limit in untreated mice. Treatment with 100 μg rhAPC significantly decreased LPS-induced TATc levels in BALF (Figure 1a). In addition, rhAPC dose-dependently decreased LPS induced levels of TNFα (Figure 1b) and IL-6 (Figure 1c). rhAPC treatment of control mice not subjected to LPS had no effect on TATc and/or cytokine levels (below the detection level).
Figure 1.
Effect of rhAPC inhalation on coagulant and inflammatory mediators during acute pulmonary inflammation. (a) TATc levels in BALF as a marker for the activation of coagulation. TNFα (b) and IL-6 (c) levels in BALF represent the production of pro-inflammatory mediators. Mediators were measured 6 h after LPS administration and for baseline levels (no LPS administration) of mice treated with vehicle (saline) or 100 μg rhAPC. Data shown are mean±s.e.; *P<0.05 vs vehicle.
LPS-induced lung injury is improved by rhAPC inhalation
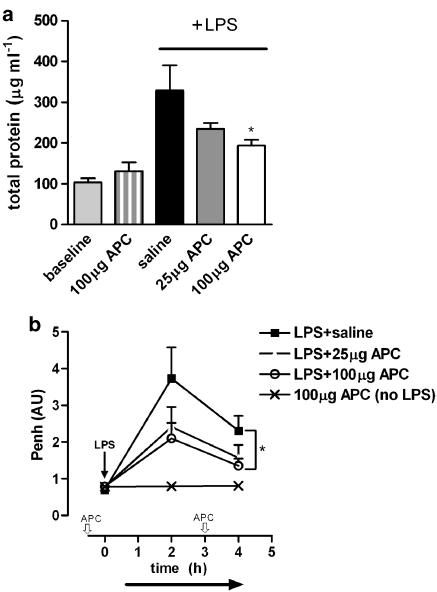
In order to assess whether the inhibition of activation of coagulation and extensive inflammation by rhAPC prevents LPS-induced lung injury, total protein levels in BALF were determined as marker for endothelial–epithelial leakage. LPS inhalation increased BALF protein levels approximately threefold. As shown in Figure 2a, rhAPC inhalation dose dependently inhibited the LPS-induced increase in protein concentration in BALF. rhAPC inhalation without LPS administration did not affect total protein concentration. To determine the functional consequence of rhAPC-dependent prevention of endothelial–epithelial leakage, lung function was analyzed using whole body plethysmography. LPS inhalation increased the Penh (index of airflow limitation indicative of pulmonary resistance) 2 h after LPS administration, which was dose dependently diminished by rhAPC (Figure 2b). At 4 h after LPS, Penh levels were lower than at 2 h but this reduced effect was further decreased by 100 μg rhAPC (Figure 2b). The administration of rhAPC alone (without LPS treatment) did not affect the level of Penh.
Figure 2.
Effect of rhAPC inhalation on lung function during acute pulmonary inflammation. (a) LPS-induced protein leakage represented as the concentration of total protein within BALF measured 6 h after LPS administration. Indicated on the left are baseline levels (no LPS administration) of mice treated with vehicle or 100 μg APC. (b) LPS-induced pulmonary resistance measured by plethysmography and represented as Penh, shown in arbitrary units (AU). rhAPC was given 30 min before and 3 h after LPS (indicated in the x axis,  ), whereas Penh was determined before and 2 and 4 h after LPS administration. Effect of APC treatment on LPS-induced protein leakage and pulmonary resistance was determined by statistical comparison to saline-treated animals. Data shown are mean±s.e.; *P<0.05 vs vehicle.
), whereas Penh was determined before and 2 and 4 h after LPS administration. Effect of APC treatment on LPS-induced protein leakage and pulmonary resistance was determined by statistical comparison to saline-treated animals. Data shown are mean±s.e.; *P<0.05 vs vehicle.
Effect of increased rhAPC administration on TATc, TNFα and IL-6
In plasma, activated protein C is inhibited by specific protease inhibitors (α1-antitrypsin, protein C inhibitor). As these inhibitors are also present in the lung compartment (Olsen et al., 1975; Fujimoto et al, 2003), we wondered whether APC inactivation might limit the protective effect and whether additional rhAPC administration might increase rhAPC effectiveness. To this end, rhAPC was administered, not only 30 min before and 3 h after LPS exposure but also 1 and 5 h after LPS exposure. As shown in Table 1, the increased treatment strategy again significantly reduced TATc, TNFα and IL-6 levels compared to saline administration. However, the coagulant and inflammatory markers were decreased to levels not different from those achieved with the initial rhAPC treatment schedule (data from Figure 1 shown in Table 1 (A) for comparison), suggesting that additional rhAPC treatment did not further improve lung inflammation.
Table 1.
Effect of increased treatment with rhAPC on LPS-induced TNFα, IL-6 and TATc levels in BALF
| (A) rhAPC inhalation | (B) Increased rhAPC inhalation | |||
|---|---|---|---|---|
| Saline | 100 μg APC | Saline | 100 μg APC | |
| TNFα (pg/ml) | 733.8±84.44 | 440.0±71.91* | 797.9±67.14 | 448.8±33.79*** |
| IL-6 (pg/ml) | 954.2±92.31 | 604.1±125.0* | 1088±167.2 | 587.2±152.3* |
| TATc (ng/ml) | 9.55±2.20 | 4.39±1.19* | 8.40±0.76 | 5.15±0.75* |
(A) 100 μg rhAPC administered 30 min before and 3 h after LPS exposure compared to saline treatment also represented in Figure 1. (B) 100 μg rhAPC administered 30 min before and 1, 3 and 5 h after LPS exposure compared to saline treatment. Data shown are mean±s.e.
P<0.05
P<0.0001 vs saline treatment.
Effect of rhAPC on LPS-induced neutrophil recruitment
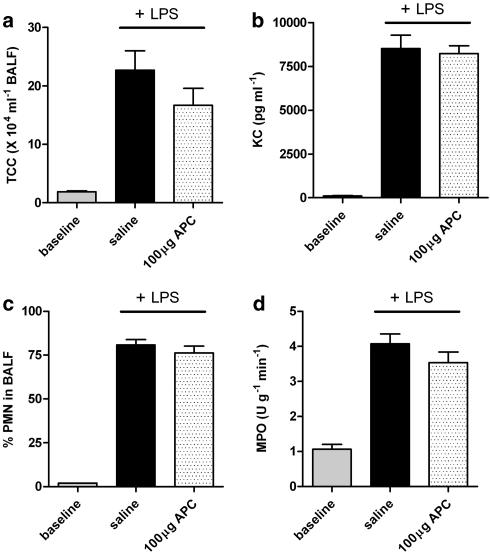
As the intravenous administration of rhAPC has proven beneficial in experimentally induced-pulmonary inflammation through inhibition of neutrophil chemotaxis (Nick et al., 2004), we subsequently determined KC levels (a murine chemo-attractant for neutrophils) in BALF, recruitment of inflammatory cells towards the alveolar space and lung MPO activity. Intranasal administration of LPS increased the total number of cells recovered from BALF by almost 10-fold. As shown in Figure 3a, rhAPC inhalation had no effect on total white blood cell influx into the alveolar space and did not affect KC levels in BALF (Figure 3b). In addition, the percentage of polymorphonuclear (PMN) cells in BALF was not affected by rhAPC treatment compared to saline treatment (Figure 3c). Hence, rhAPC has no apparent effect on neutrophil migration towards the alveolar space. To determine whether rhAPC inhalation affects neutrophil recruitment to the interstitium or the activity of neutrophils in the pulmonary microvasculature in response to LPS, we measured lung MPO activity after undergoing the BAL procedure. Figure 3d demonstrates that rhAPC had no apparent effect on neutrophil activity.
Figure 3.
Effect of rhAPC on inflammatory cell recruitment during acute pulmonary inflammation. (a) TCC; total cell count in BALF. (b) Levels of neutrophil chemoattractant KC. (c) % of polymorphonuclear (PMN) cells in BALF. (d) MPO activity as a marker for granulocyte activity within lung tissue after BAL. 100 μg rhAPC mouse−1 or saline administered 30 min before and 1, 3 and 5 h after LPS exposure. Baseline represent levels in LPS untreated mice. Data shown are mean±s.e.
rhAPC in the chemotactic compartment does not affect neutrophil migration
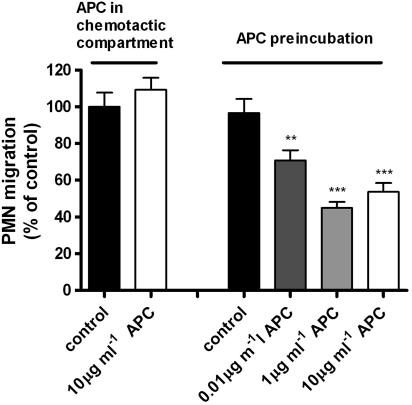
To obtain in vitro evidence for the fact that rhAPC administered to the alveolar space does not reduce migration of neutrophils from the vascular compartment into the alveolar space, we performed trans-well migration assays. Freshly isolated peripheral blood neutrophils migrate towards FCS and IL-8. The addition of rhAPC (10 μg ml−1) to either FCS (Figure 4) or IL-8 (data not shown) or rhAPC (in medium) alone (not shown) in the bottom well had no effect on neutrophil migration. As positive control for our experimental set-up, we pretreated neutrophils with rhAPC before applying them to the trans-well migration assay. As indicated in Figure 4, preincubation with 0–10 μg ml−1 rhAPC dose-dependently inhibited neutrophil migration (>50 % for 1 μg ml−1 rhAPC).
Figure 4.
Effect of rhAPC on neutrophil migration. The pretreatment of peripheral blood neutrophils with rhAPC dose-dependently inhibited migration towards FCS (20%) (right side of the figure). The addition of rhAPC to the chemoattractant (FCS) did not influence neutrophil migration (left side of the figure). Results represent the number of cells migrated as percentage of control (migration towards FCS without rhAPC, corrected for baseline fluorescence) and are shown as mean±s.e. **P<0.01, ***P<0.0001 vs control.
Discussion and conclusions
Several studies have indicated that intravenous administration of APC is protective against experimental pulmonary inflammation (Murakami et al., 1996, 1997; Nick et al., 2004; van der Poll et al., 2005). Systemic administration of rhAPC is, at least in patients with systemic disease, associated with an increased risk of bleeding complications (Bernard et al., 2001). In addition, rhAPC may affect endothelial responsiveness (Hooper et al., 2001; Uchiba et al., 2004; Zeng et al., 2004; Finigan et al., 2005) which is not necessarily the primary objective in the treatment of pulmonary inflammation. Moreover, non-invasive local administration of a (potential) protector against lung injury will be more attractive in clinical practice. Therefore, we studied the effect of rhAPC inhalation on endotoxin-induced pulmonary inflammation in mice.
The local administration of endotoxin to mice results in an acute pulmonary reaction, which is characterized by a persistent pro-coagulant state, extensive inflammation and increased endothelial and epithelial permeability. Together these effects result in the influx of protein-rich oedema fluid into the airspace, impaired gas exchange and increased pulmonary resistance (Ware and Matthay, 2000). In this study, we show that the endotoxin-induced increase in pulmonary resistance is dose-dependently attenuated by rhAPC inhalation. This is evident from a significant decrease in Penh (index of airflow limitation). Although the exact relationship between Penh and pulmonary resistance has recently been questioned (Bates and Irvin, 2003; Adler et al., 2004), Penh provides information regarding the integrated ventilatory and mechanical responses of the entire respiratory tract (Johnston et al., 2005). Hence, in accordance with the observation that rhAPC inhalation decreases total protein concentration in BALF, we conclude that rhAPC improves endotoxin-induced decreased lung function. In line with these results, it should be noted that local APC administration has previously been demonstrated to attenuate experimental lung fibrosis (Yasui et al., 2001) (Shimizu et al., 2003) and asthmatic inflammation (Yuda et al., 2004). These studies underline the beneficial effect of local APC administration in (models of) lung injury, supporting our hypothesis that local administration of rhAPC is beneficial in acute (endotoxin-induced) pulmonary inflammation.
Although rhAPC treatment diminished coagulation and cytokine levels in BALF, which resulted in improvement of lung function, rhAPC treatment does not prevent LPS-induced neutrophilic inflammatory response. Specific protease inhibitors such as α1-antitrypsin and protein C inhibitor inhibit activated protein C in plasma (Espana et al., 1991). As these inhibitors are also present in the lung (Olsen et al., 1975; Fujimoto et al., 2003), we hypothesized that immediate APC inactivation might limit its protective effect. However, increased rhAPC treatment has no additional protective effect in this model of pulmonary inflammation, which implied that rhAPC levels after 2 nebulizations (100 μg rhAPC) are sufficient to confer maximal rhAPC-induced protection. Overall this suggests that rhAPC inhalation might be beneficial in inflammatory lung disease but only offers partial protection.
Only a small fraction (1–2%) of cells within the alveolar interstitial space normally consists of neutrophils. During bacterial-endotoxin induced pulmonary inflammation however, the cellular composition of the alveolar space dramatically changes with 90% of cells being neutrophils (Puneet et al., 2005). The activated neutrophils play a critical role in host defense but also participate in the inflammatory reaction by secreting inflammatory mediators and cytotoxic enzymes contributing to the induction of tissue injury. The beneficial effects of rhAPC in severe sepsis are anticipated to result from the direct inhibition of neutrophil migration from the vasculature to the site of infection (Sturn et al., 2003; Nick et al., 2004), thereby attenuating the induction of tissue injury. Interestingly, in this study, rhAPC inhalation diminished lung injury without evidently affecting neutrophil migration.
To explore the unexpected results concerning the effect of rhAPC on neutrophil migration in vivo, we hypothesized that rhAPC in the pulmonary compartment does not affect neutrophil chemotaxis and that direct contact between rhAPC and neutrophils (as after intravascular administration) are essential for inhibiting neutrophil migration.
Indeed, we could confirm that pre-incubation of neutrophils with rhAPC inhibits migration towards either FCS or IL-8 (Sturn et al., 2003). In addition we could show that rhAPC does not affect neutrophil migration when present in the chemotactic compartment (either alone or when added to the chemoattractants FCS or IL-8). Thus, rhAPC administration into the alveolar space does not influence neutrophil influx into the alveolar compartment. In accordance with our in vivo data, this suggests that the protective effect of locally (intrapulmonary) administered rhAPC is independent of neutrophil migration and merely results from decreased thrombin formation (Yasui et al., 2001) and/or attenuated cytokine production (Grey et al., 1994) (Yuda et al., 2004). Interestingly, intravenous administration of rhAPC reduced neutrophil accumulation in the broncho-alveolar space without influencing cytokine/chemokines release (Nick et al., 2004). Intravenous rhAPC administration, in the same study, however did inhibit thrombin formation (i.e. reduced TAT levels) in the broncho-alveolar space (van der Poll et al., 2005).
Overall these data illustrate that the mechanism by which rhAPC protects against inflammatory lung disease depends on the targeted cells. Endothelial cells as well as lymphocytes (Feistritzer et al., 2006) (Sturn et al., 2003) express endothelial protein C receptor (EPCR). This receptor is believed to be responsible for the cellular effects of APC. Systemic administration of rhAPC targets the vascular system, thus targeting EPCR on neutrophils (Sturn et al., 2003) and endothelial cells (Joyce and Grinnell, 2002), resulting in reduced neutrophil migration. Inhalation of rhAPC on the contrary, targets the alveolar barrier resulting in a neutrophil-independent protection.
Although this suggests that different modes of administration of rhAPC during pulmonary inflammation may account for independent protective mechanisms, it remains to be established whether inhaled rhAPC is actually unable to pass the alveolar barrier and secondarily target the vascular system. However, since inhaled APC was in fact unable to inhibit neutrophil migration we do not expect an impact of possible barrier passage.
Several issues should be kept in mind when interpreting our data. First, in the present study we induced lung inflammation by the intranasal administration of LPS, which is obviously different from the clinical situation of acute pulmonary inflammation. As a consequence, we can only speculate about rhAPC inhalation as treatment strategy for acute lung inflammation or pneumonia. Second, in the present study, we have administered rhAPC before the induction of the pulmonary inflammatory response. It has been shown that blocking the extrinsic coagulation cascade in mice even 6 h after LPS exposure, attenuates lung injury and inflammatory mediator release (Miller et al., 2002), suggesting that inhibition of the pro-coagulant state by rhAPC could also be protective after an inflammatory insult.
In conclusion, local rhAPC treatment by inhalation inhibits murine pulmonary inflammation and improves lung function. This implicates that rhAPC inhalation may be an attractive treatment strategy in pulmonary inflammatory diseases. As neutrophil migration is not affected by inhaled rhAPC, the mode of action of inhaled rhAPC is different from intravenous rhAPC, which adds to the complexity of the molecular working mechanisms of rhAPC.
Acknowledgments
We thank Joost Daalhuisen for expert technical assistance. Hugo Ten Cate is a Clinical Established Investigator of the Dutch Heart Foundation.
Abbreviations
- (rh)APC
(recombinant human) activated protein C
- BALF
bronchoalveolar lavage fluid
- FCS
fetal calf serum
- IL-6
interleukin-6
- IL-8
interleukin-8
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- Penh
enhanced pause
- TATc
thrombin-antithrombin complex
- TNFα
tumor necrosis factorα
Conflict of interest:
The authors states no conflict of interest.
References
- Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol. 2004;97:286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94:1297–1306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Derhaschnig U, Reiter R, Knobl P, Baumgartner M, Keen P, Jilma B. Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia. Blood. 2003;102:2093–2098. doi: 10.1182/blood-2003-02-0416. [DOI] [PubMed] [Google Scholar]
- Dhainaut JF, Yan SB, Margolis BD, Lorente JA, Russell JA, Freebairn RC, et al. Drotrecogin alfa (activated) (recombinant human activated protein C) reduces host coagulopathy response in patients with severe sepsis. Thromb Haemost. 2003;90:642–653. doi: 10.1160/TH02-11-0270. [DOI] [PubMed] [Google Scholar]
- Ely EW, Laterre PF, Angus DC, Helterbrand JD, Levy H, Dhainaut JF, et al. Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis. Crit Care Med. 2003;31:12–19. doi: 10.1097/00003246-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Espana F, Gruber A, Heeb MJ, Hanson SR, Harker LA, Griffin JH. In vivo and in vitro complexes of activated protein C with two inhibitors in baboons. Blood. 1991;77:1754–1760. [PubMed] [Google Scholar]
- Feistritzer C, Mosheimer BA, Sturn DH, Riewald M, Patsch JR, Wiedermann CJ. Endothelial protein C receptor-dependent inhibition of migration of human lymphocytes by protein C involves epidermal growth factor receptor. J Immunol. 2006;176:1019–1025. doi: 10.4049/jimmunol.176.2.1019. [DOI] [PubMed] [Google Scholar]
- Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Gabazza EC, Hataji O, Yuda H, D'Alessandro-Gabazza CN, Nakano M, et al. Thrombin-activatable fibrinolysis inhibitor and protein C inhibitor in interstitial lung disease. Am J Respir Crit Care Med. 2003;167:1687–1694. doi: 10.1164/rccm.200208-905OC. [DOI] [PubMed] [Google Scholar]
- Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol. 1994;153:3664–3672. [PubMed] [Google Scholar]
- Hancock WW, Grey ST, Hau L, Akalin E, Orthner C, Sayegh MH, et al. Binding of activated protein C to a specific receptor on human mononuclear phagocytes inhibits intracellular calcium signaling and monocyte-dependent proliferative responses. Transplantation. 1995;60:1525–1532. doi: 10.1097/00007890-199560120-00026. [DOI] [PubMed] [Google Scholar]
- Hooper WC, Phillips DJ, Renshaw MA. Activated protein C induction of MCP-1 in human endothelial cells: a possible role for endothelial cell nitric oxide synthase. Thromb Res. 2001;103:209–219. doi: 10.1016/s0049-3848(01)00319-x. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol. 2005;288:L390–L397. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288–S293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- Kalil AC, Coyle SM, Um JY, LaRosa SP, Turlo MA, Calvano SE, et al. Effects of drotrecogin alfa (activated) in human endotoxemia. Shock. 2004;21:222–229. doi: 10.1097/01.shk.0000116778.27924.79. [DOI] [PubMed] [Google Scholar]
- Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165:1445–1450. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- Maris NA, van der Sluijs KF, Florquin S, de Vos AF, Pater JM, Jansen HM, et al. Salmeterol, a beta2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1122–L1128. doi: 10.1152/ajplung.00125.2003. [DOI] [PubMed] [Google Scholar]
- Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, et al. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 2002;26:650–658. doi: 10.1165/ajrcmb.26.6.4688. [DOI] [PubMed] [Google Scholar]
- Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, et al. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87:642–647. [PubMed] [Google Scholar]
- Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, et al. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am J Physiol. 1997;272:L197–L202. doi: 10.1152/ajplung.1997.272.2.L197. [DOI] [PubMed] [Google Scholar]
- Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- Olsen GN, Harris JO, Castle JR, Waldman RH, Karmgard HJ. Alpha-1-antitrypsin content in the serum, alveolar macrophages, and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest. 1975;55:427–430. doi: 10.1172/JCI107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–L15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers SH, Groot AP, Florquin S, Reitsma PH, Spek CA. Blood cell-derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol Dis. 2004;32:325–333. doi: 10.1016/j.bcmd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Gabazza EC, Taguchi O, Yasui H, Taguchi Y, Hayashi T, et al. Activated protein C inhibits the expression of platelet-derived growth factor in the lung. Am J Respir Crit Care Med. 2003;167:1416–1426. doi: 10.1164/rccm.200206-515OC. [DOI] [PubMed] [Google Scholar]
- Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2001;164:602–607. doi: 10.1164/ajrccm.164.4.2001016. [DOI] [PubMed] [Google Scholar]
- Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102:1499–1505. doi: 10.1182/blood-2002-12-3880. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Jr, Chang A, Esmon CT, D'Angelo A, Vigano-D'Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, et al. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Levi M, Nick JA, Abraham E. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med. 2005;171:1125–1128. doi: 10.1164/rccm.200411-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005;33:2266–2277. doi: 10.1097/01.ccm.0000181729.46010.83. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Weijer S, Schoenmakers SH, Florquin S, Levi M, Vlasuk GP, Rote WE, et al. Inhibition of the tissue factor/factor VIIa pathway does not influence the inflammatory or antibacterial response to abdominal sepsis induced by Escherichia coli in mice. J Infect Dis. 2004;189:2308–2317. doi: 10.1086/421031. [DOI] [PubMed] [Google Scholar]
- Yasui H, Gabazza EC, Tamaki S, Kobayashi T, Hataji O, Yuda H, et al. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am J Respir Crit Care Med. 2001;163:1660–1668. doi: 10.1164/ajrccm.163.7.9911068. [DOI] [PubMed] [Google Scholar]
- Yuda H, Adachi Y, Taguchi O, Gabazza EC, Hataji O, Fujimoto H, et al. Activated protein C inhibits bronchial hyperresponsiveness and Th2 cytokine expression in mice. Blood. 2004;103:2196–2204. doi: 10.1182/blood-2003-06-1980. [DOI] [PubMed] [Google Scholar]
- Zeng W, Matter WF, Yan SB, Um SL, Vlahos CJ, Liu L. Effect of drotrecogin alfa (activated) on human endothelial cell permeability and Rho kinase signaling. Crit Care Med. 2004;32:S302–S308. doi: 10.1097/01.ccm.0000128038.49201.8c. [DOI] [PubMed] [Google Scholar]