Abstract
Background and Purpose:
Central anti-nociceptive actions of baclofen involve activation of K+ channels. Here we assessed what types of K+ channel might participate in the peripheral anti-nociception induced by baclofen.
Experimental approach:
Nociceptive thresholds to mechanical stimulation in rat paws treated with intraplantar prostaglandin E2.(PGE2) to induce hyperalgesia were measured 3h after PGE2 injection. Other agents were also given by intraplantar injection
Key results:
Baclofen elicited a dose-dependent (15 - 240 μg per paw) anti-nociceptive effect. An intermediate dose of baclofen (60 μg) did not produce antinociception in the contralateral paw, showing its peripheral site of action. The GABAB receptor antagonist saclofen (12.5 - 100 μg per paw) antagonized, in a dose-dependent manner, peripheral antinociception induced by baclofen (60 μg), suggesting a specific effect. This antinociceptive action of baclofen was unaffected by bicuculline, GABAA receptor antagonist (80 μg per paw), or by (1,2,5,6 tetrahydropyridin-4-yl) methylphosphinic acid, GABAC receptor antagonist (20 μg per paw). The peripheral antinociception induced by baclofen (60 μg) was reversed, in a dose-dependent manner, by the voltage-dependent K+ channel blockers tetraethylammonium (7.5 - 30 μg per paw) and 4-aminopyridine (2.5 - 10 μg per paw). The blockers of other K+ channels, glibenclamide (160 μg), tolbutamide (320 μg), charybdotoxin (2 μg), dequalinium (50 μg) and caesium (500 μg) had no effect.
Conclusions and Implications:
This study provides evidence that the peripheral antinociceptive effect of the GABAB receptor agonist baclofen results from the activation of tetraethylammonium-sensitive K+ channels. Other K+ channels appear not to be involved.
Keywords: baclofen, K+ channel, peripheral antinociception, 4-aminopyridine, tetraethylammonium, sulphonylureas, saclofen, GABAB receptor
Introduction
GABA is the major inhibitory neurotransmitter in the vertebrate central nervous system. GABA receptors have been classified into three distinct subtypes GABAA, GABAB and GABAC. Both GABAA and GABAC receptors form ligand-gated chloride channels, while the GABAB receptor belongs to the G-protein-coupled receptor family whose activation causes a decrease in Ca++ and increase in K+ membrane conductance (Sigel et al., 1983; Johnston, 1997; Bowery and Enna, 2000).
Baclofen (β-[4-chlorophenyl] GABA) is a stereospecifically active agonist at the GABAB receptor (Bowery et al., 1981). It has been used therapeutically as a muscle relaxant and for the treatment of trigeminal neuralgia (Korolkovas, 1999).
In a variety of animal pain models, baclofen has been reported to produce antinociception following systemic (Shafizadeh et al., 1997; Sabetkasai et al., 1999), supraspinal (Sawynok, 1987; Shafizadeh et al., 1997; Potes et al., 2006) and spinal (Aran and Hammond, 1991; Sawynok, 1987; Malan et al., 2002) administration. In addition, studies have demonstrated the presence of GABAA and GABAB receptors in the periphery (Carlton et al., 1999; Calver et al., 2000).
Several reports have demonstrated that the opening of different potassium channels underlies the antinociceptive effect induced by activation of G-protein-coupled receptors such as the μ/δ-opioid and GABAB receptors. In the central nervous system, the opening of ATP-sensitive K+ channels seems to play a role in antinociception induced by morphine and [D-pen2,5]-enkephalin, since the sulphonylureas, glibenclamide and tolbutamide antagonize the antinociceptive effect of these drugs (Ocaña et al., 1990; Wild et al., 1991; Ocaña and Baeyens, 1993, 1994). Similarly, the peripheral antinociception induced by morphine and SNC80 is also antagonized by glibenclamide and tolbutamide (Rodrigues and Duarte 2000; Pacheco and Duarte, 2005). On the other hand, the central antinociception induced by baclofen in the tail-flick test is specifically antagonized by K+ channel blockers 4-aminopyridine (4-AP) and tetraethylammonium (TEA) (Ocaña and Baeyens, 1993).
In this context, the aim of the present study was to verify the possible peripheral antinociceptive effect of baclofen using the rat paw pressure test. The specificity of baclofen in GABAB receptors was also tested through intraplantar administration of saclofen, a GABAB receptor antagonist. Furthermore, the possible involvement of K+ channels was evaluated using specific K+ channel blockers, especially those for tetraethylammonium (TEA)-sensitive K+ channels, as they were associated with the central antinociception induced by GABA (Ocaña and Baeyens, 1993).
Methods
Animals
The experiments were performed on 160–200 g male Wistar rats (N=4–15 per group) from CEBIO-UFMG (The Animal Centre of the Federal University of Minas Gerais). The rats were housed in a temperature-controlled room (23±1°C) on an automatic 12-h light/dark cycle (0600–1800 h of light phase). All testing was carried out during the light phase (0800–1500). Food and water were freely available until the beginning of the experiments. Naive rats were used throughout. All the experiments were approved by the Ethics Committee on Animal Experimentation (CETEA) of the Federal University of Minas Gerais.
Measurement of hyperalgesia
Hyperalgesia was induced by a subcutaneous (s.c.) injection of prostaglandin E2 (PGE2, 2 μg) into the plantar surface of the rat's hindpaw and measured by the paw pressure test described by Randall and Selitto (1957). The rat was carefully kept in a horizontal/normal position, by one hand of the researcher while the test paw was submitted to pressure. An analgesimeter (Ugo-Basile, Italy) with a cone-shaped paw-presser with a rounded tip was used to apply a linearly increasing force to the rat's right hindpaw. The weight in grams required to elicit nociceptive paw response was determined as the nociceptive threshold. A cutoff value of 300 g was used to prevent damage to the paws. The nociceptive threshold was measured in the right paw and determined by the average of three consecutive trials recorded before (zero time) and 3 h after PGE2 injection (peak of effect). The results were calculated by the difference between these two averages (Δ of nociceptive threshold) and expressed as grams. To reduce stress, the rats were habituated to the apparatus 1 day before the experiments.
Experimental protocol
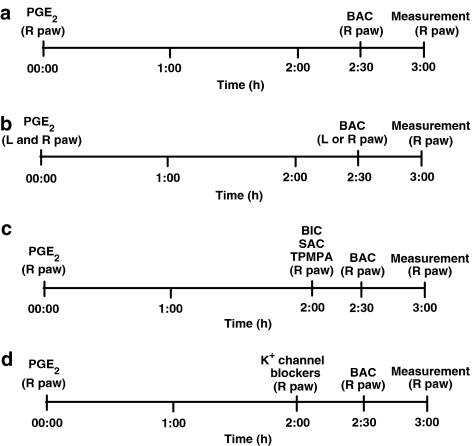
Baclofen was administered s.c. in the right hindpaw (R paw) 2.5 h after the local injection of PGE2 (Figure 1a).
Figure 1.
Schedules of drug administration: (a) baclofen; (b) exclusion of a systemic antinociceptive effect of baclofen; (c) GABA receptor antagonists; (d) K+ channels blockers.
In the protocol used to determine whether baclofen was acting outside the injected paw, PGE2 was injected into both hindpaws, while baclofen was administered into the left or right paw (L or R paw) (Figure 1b).
Saclofen, bicuculline and 1,2,5,6 tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA) were administered s.c. in the right paw 5 min before baclofen (Figure 1c).
All the K+ channel blockers were injected s.c. into the right hindpaw 30 min before baclofen (Figure 1d).
The nociceptive threshold was always measured in the right hindpaw. The protocol above was assessed in pilot experiments to determine the best moment of injection of each substance.
Statistical analysis
The data were analysed statistically by one-way analysis of variance (ANOVA) using the Bonferroni test post hoc for multiple comparisons. Probabilities less than 5% (P<0.05) were considered statistically significant.
Chemicals
The following drugs and chemicals were used: PGE2 (Sigma, USA), baclofen (Novartis AG, Switzerland), saclofen (ToCris, EUA), bicuculline (Sigma), TPMPA (Sigma), glibenclamide (Sigma, USA), tolbutamide (ICN Biomedicals, USA), charybdotoxin (Sigma, USA), dequalinium (Calbiochem, USA), TEA (Sigma, USA), 4-AP (Sigma, USA) and caesium (Mitsuwa's Pure Chemical, Japan). PGE2 (ethanol 8% in saline), baclofen, saclofen, bicuculline, TPMPA, TEA, 4-AP were dissolved in isotonic saline. The K+ channel blockers charybdotoxin, caesium and dequalinium were dissolved in demineralized water, while the sulphonylureas glibenclamide and tolbutamide were dissolved in Tween 80 vehicle (2% in saline). All drugs were dissolved immediately before use and injected in a volume of 100 μl per paw, with exception of K+ channel blockers and bicuculline, saclofen and TPMPA which were injected in a volume of 50 μl. Control naïve rats (not hyperalgesic) were injected with the vehicle for PGE2 (ethanol 8% in saline).
Results
Peripheral antinociceptive effect of baclofen
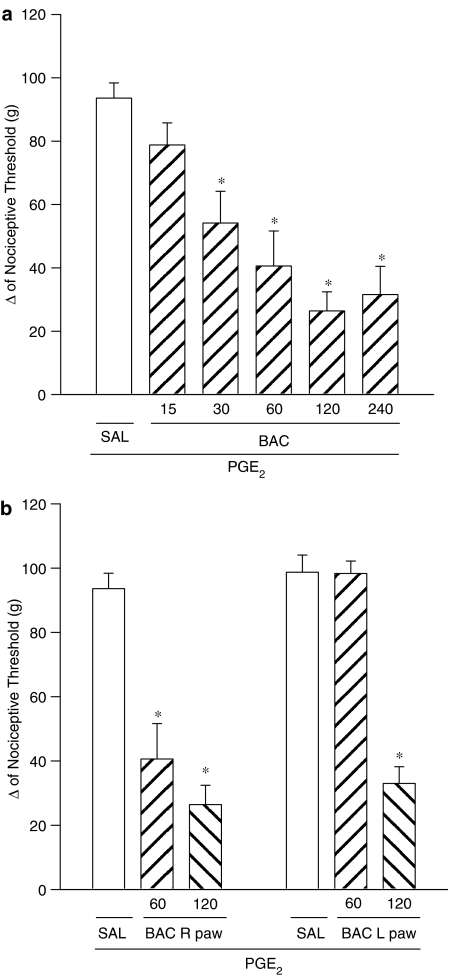
Figure 2a shows that intraplantar administration of baclofen (15, 30, 60, 120 and 240 μg) in the right paw antagonized the hyperalgesic effect of PGE2 (2 μg per paw) in a dose-dependent manner. Baclofen, at a dose of 60 μg injected into the left paw, produced no antinociceptive effect in the right paw, whereas at a dose of 120 μg injected into the left paw, baclofen did induce an antinociceptive effect in the contralateral paw (Figure 2b).
Figure 2.
Dose-dependent effect of baclofen on the nociceptive threshold in PGE2-induced hyperalgesia in rats (a) and exclusion of systemic antinociceptive effect of baclofen at dose of 60 μg (b). (a) Baclofen (15–240 μg per paw) was given 2.5 h after local administration of 100 μl of PGE2 (2 μg). (b) Baclofen (60 or 120 μg per paw) was administered into the right (R) or left (L) paw 2 h and 30 min after PGE2 (2 μg) administration into both hind paws. The antinociceptive response was measured in the paw pressure test as described in Methods. Each column represents the mean ±s.e.m. for 5–10 rats per group. *Indicates a significant difference from the PGE2+saline (SAL) injected control (P<0.05, ANOVA+Bonferroni test).
Antagonism of baclofen-induced antinociception by saclofen
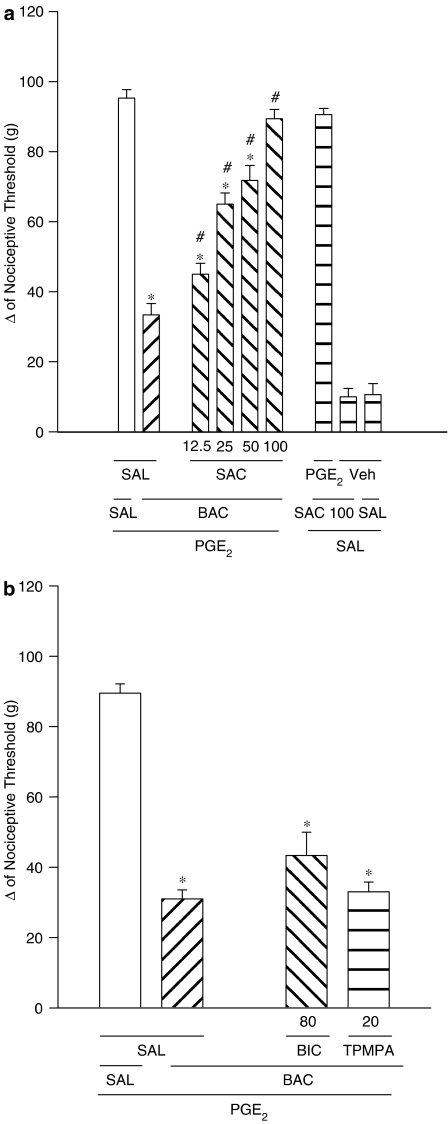
The intraplantar injection of saclofen (12.5, 25, 50 and 100 μg) reduced the peripheral antinociception induced by baclofen (60 μg per paw; Figure 3a) in a dose-dependent manner. Saclofen did not modify the nociceptive threshold in control animals (vehicle only) nor induce any overt behavioural effects at the doses used.
Figure 3.
Effect of intraplantar administration of (a) saclofen (SAC) and in (b), bicuculline (BIC) and 1,2,5,6 tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA) on the peripheral antinociception induced by baclofen in hyperalgesic paws (PGE2, 2 μg). Saclofen (12.5–100 μg), BIC (80 ng) and TPMPA (20 μg), were administered 5 min before baclofen (60 μg per paw). Each column represents the mean±s.e.m. for 5–8 rats per group. *,#Indicate significant differences compared to PGE2+SAL+SAL- and PGE2+BAC+SAL-injected groups, respectively (P<0.05, ANOVA+Bonferroni test). Veh=vehicle (ethanol 8% in saline).
Effect of bicuculline and TPMPA on baclofen-induced antinociception
As shown in Figure 3b neither bicuculline (80 ng per paw) nor TPMPA (20 μg per paw) reduced the antinociceptive effects of baclofen (60 μg per paw). These drugs caused no effects when used alone (data not shown).
Antagonism of baclofen-induced antinociception by TEA and 4-AP
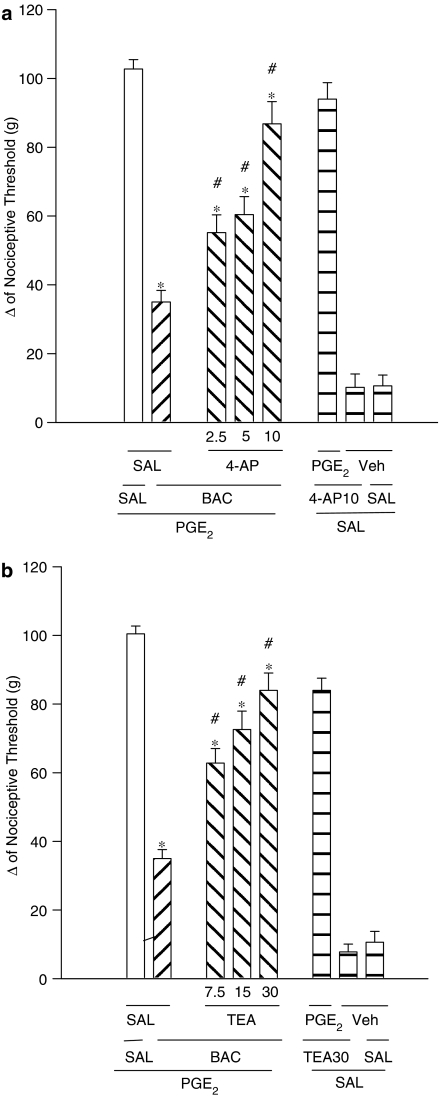
TEA (7.5, 15 and 30 μg per paw) and 4-AP (2.5, 5 and 10 μg per paw) significantly reduced the baclofen-induced peripheral antinociception (60 μg per paw) in a dose-dependent manner (Figures 4a and b). Neither of the K+ channel blockers tested significantly modified the nociceptive threshold in control animals nor did they induce any overt behavioural effects.
Figure 4.
Antagonism induced by intraplantar administration of (a) 4-AP and (b) TEA of the peripheral antinociception produced by baclofen in hyperalgesic paws (PGE2, 2 μg). 4-AP (2.5–10 μg) or TEA (7.5–30 μg) were administered 30 min before baclofen (60 μg per paw). Each column represents the mean±s.e.m. for 5–15 rats per group. *,#Indicate significant differences compared to PGE2+SAL+SAL- and PGE2+BAC+SAL-injected groups, respectively (P<0.05, ANOVA+Bonferroni test). Veh=vehicle (ethanol 8% in saline).
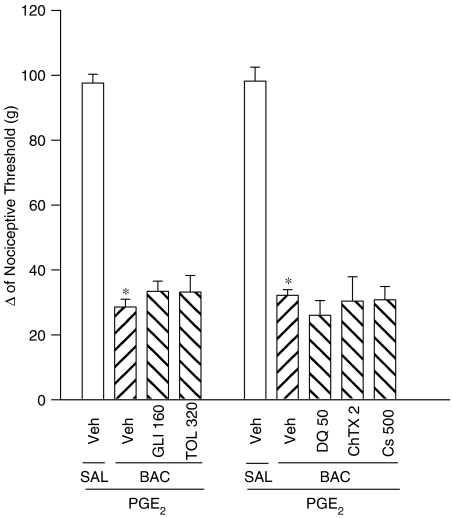
Effect of glibenclamide, tolbutamide, charybdotoxin, dequalinium and caesium on baclofen-induced antinociception
Glibenclamide (160 μg) and tolbutamide (320 μg) injected into the paw did not reduce baclofen-induced antinociception (Figure 5). Charybdotoxin (2 μg), dequalinium (50 μg) and caesium (500 μg) also failed to counteract the antinociception induced by baclofen (Figure 5). These drugs did not induce hyperalgesia or antinociception by themselves (data not shown).
Figure 5.
Effect of intraplantar administration of glibenclamide (GLI), tolbutamide (TOL) charybdotoxin (ChTX), dequalinium DQ0 and caesium (Cs) on the peripheral antinociception produced by baclofen in hyperalgesic paws (PGE2, 2 μg). Drugs (the doses shown are μg per paw) were administered 30 min before baclofen (60 μg per paw). Each column represents the mean±s.e.m. for 4–7 rats per group. * and # Indicate significant differences compared to PGE2+SAL+SAL- and PGE2+BAC+SAL-injected groups, respectively (P<0.05, ANOVA+Bonferroni test). Veh=vehicle (ethanol 8% in saline).
Discussion and conclusions
The participation of the GABAergic system in pain modulation has been extensively studied at both systemic and central levels. However, there are only a few studies associating GABA and peripheral antinociception. In the present study, baclofen, a GABAB receptor agonist, induced a dose-dependent and peripheral antinociceptive effect on PGE2-induced hyperalgesia.
The possibility that baclofen at a dose of 60 μg per paw produced antinociception by acting at sites outside the paw was excluded, since its administration into the left paw did not alter hyperalgesia in the contralateral paw. In these experiments, PGE2 was administered in the left paw, so that this administration site would present similar conditions to that in the right paw, with an equal possibility that these agents would reach receptors outside the injected paw. Baclofen is known to induce muscle-relaxant effects when administered systemically or centrally (Malcangio and Bowery, 1996) but, in the present study this was shown not to be the case, since baclofen induced a local effect. Furthermore, data from the literature demonstrated that the doses of baclofen which produced antinociception did not induce motor incoordination (Bowery, 1997; Patel et al., 2001; Balerio and Rubio, 2002).
The existence of GABAA and GABAB receptors in the periphery has been previously described (Carlton et al., 1999; Calver et al., 2000). A source of endogenous GABA for these peripheral receptors might be glutamate-containing primary afferent fibres. This amino acid is present in more than 90% of afferent primary fibres (Battaglia and Rustioni, 1988) and is converted by glutamic acid decarboxylase (GAD) into GABA (Malcangio and Bowery, 1996). In 2004, Stoyanova (2004) demonstrated the presence of GABA in primary afferent neurons of feline sensory ganglia: trigeminal and dorsal root ganglia.
Further support for a role of GABA in peripheral antinociception came from behavioural studies. For example, gabapentin, a GABA-mimetic drug, induced peripheral antinociceptive effect in the formalin test (Carlton and Zhou, 1998) and in formalin-induced secondary hyperalgesia (Motta et al., 2004). Carlton et al. (1999) also demonstrated that muscimol, a GABAA receptor agonist, induced peripheral antinociception in the formalin test.
Saclofen was used in the present study, in order to confirm the involvement of the GABAB receptor in peripheral antinociception induced by baclofen. The results demonstrated that this antagonist was able to prevent the peripheral antinociceptive effect induced by baclofen in a dose-dependent manner. Other studies also demonstrated the involvement of the GABAB receptors in central antinociception induced by baclofen. In a variety of nociceptive tests, GABAB receptor antagonists such as phaclofen, 2-hydroxysaclofen (Aran and Hammond, 1991; Shafizadeh et al., 1997) and CGP 35348 (Malcangio et al., 1991; Dirig and Yaksh, 1995; Sabetkasai et al., 1999) reversed the baclofen-induced antinociception. In contrast, the present results show that neither the GABAA receptor antagonist bicuculline nor the GABAC receptor antagonist TPMPA reversed the antinociceptive effect induced by baclofen, results which further support the argument that the antinociceptive response induced by baclofen in the paw pressure test is mediated by a GABAB mechanism.
The present results demonstrated that the peripheral antinociception induced by baclofen was reversed, in a dose-dependent manner, by two K+ channel blockers, 4-AP and TEA. According to Alexander et al. (2001), 4-AP and TEA, aside from blocking the voltage-dependent K+ channels (Kv), also block G-protein-regulated inward rectifier K+ channels (GIRK). Studies have shown that the antinociception induced by baclofen was reduced in GIRK2 subunit knockout mice (Blednov et al., 2003) and in mice pretreated for several days with an antisense that lowered Kv1.1 gene expression (Galeotti et al., 1997). In a pharmacological study, Ocaña and Baeyens (1993) demonstrated that 4-AP and TEA antagonized the central antinociceptive effect of baclofen. The current results also agree with those who described 4-AP as more potent in blocking the voltage-dependent K+ channels than TEA (Cook and Quast, 1990).
In contrast, the sulphonylureas glibenclamide and tolbutamide, specific blockers of ATP-sensitive K+ channels, exhibited no effect in the peripheral antinociception induced by baclofen. These data are supported by those of Ocaña and Baeyens (1993) who reported that the central antinociception induced by baclofen was not modified by intracerebroventricular administration of the sulphonylurea, gliquidone. It is important to emphasize that experiments from our group have already demonstrated that the sulphonylureas (at the same dose as that used for baclofen) reversed the peripheral antinociceptive effect of morphine (Rodrigues and Duarte, 2000), dibutyryl cyclic GMP (Soares and Duarte, 2001) and SNC80 (Pacheco and Duarte, 2005). Consequently, the present results suggest that ATP-sensitive K+ channels are not involved in the peripheral antinociception induced by baclofen.
Dequalinium, a selective blocker of small conductance Ca2+-activated K+ channels (Castle et al., 1993; Dunn, 1994) and charybdotoxin, a blocker of large conductance Ca2+-activated K+ channels (Mackinnon and Miller, 1988) also failed to antagonize the peripheral antinociceptive effect induced by baclofen. Alves et al. (2004) demonstrated that NS1619, a specific opener of large conductance Ca2+-activated K+ channels, did not produce antinociceptive action in hyperalgesia induced by PGE2, suggesting that these channels may not exist in peripheral tissues or, more probably, that they were not activated under the current experimental conditions. In contrast, Ortiz et al. (2002) studying the involvement of K+ channels in the antinociceptive action of diclofenac in the formalin test demonstrated that charybdotoxin was effective.
In summary, the data of the present study suggested that activation of peripheral GABAB receptors by baclofen induces antinociception via the opening of the voltage-dependent K+ channel or the G-protein-coupled inwardly rectifying K+ channel. Other K+ channel types such as large and small conductance Ca2+-activated and ATP-sensitive K+ channels appear not to be involved. This is the first demonstration of the peripheral antinociceptive effect of baclofen and of one of its possible mechanisms of action.
Acknowledgments
We were supported by a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Pesquisa (CNPq).
Abbreviations
- PGE2
prostaglandin E2
- TEA
tetraethylammonium
- TPMPA
(1,2,5,6 tetrahydropyridin-4-yl) methylphosphinic acid
Conflict of interest
The authors state no conflict of interest.
References
- Alexander S, Peters J, Mathie A, Mackenzie G, Smith A.Nomenclature Supplement Trends Pharmacol Sci (Suppl) 2001118–125.12th edn
- Alves DP, Tatsuo MAF, Leite R, Duarte IDG. Diclofenac-induced peripheral antinociception is associated with ATP-sensitive K+ channels activation. Life Sci. 2004;74:2577–2591. doi: 10.1016/j.lfs.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Aran S, Hammond DL. Antagonism of baclofen-induced antinociception by intratecal administration of phaclofen or 2-hydroxy-saclofen, but not δ-aminovaleric acid in the rat. J. Pharmacol Exp Ther. 1991;257:360–368. [PubMed] [Google Scholar]
- Balerio G, Rubio MC. Baclofen analgesia: involvement of the GABAergic system. Pharmacol Res. 2002;46:281–286. doi: 10.1016/s1043-6618(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277:302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channel. Proc Natl Acad Sci. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG.Pharmacology of GABAB receptors The GABA Receptors 1997Humana Press: Totowa, NJ; 221–236.In: Bowery NG, Enna SJ (eds)2nd edn [Google Scholar]
- Bowery NG, Doble A, Hill DR, Hudson AL, Shaw JS, Turnbull MJ, et al. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol. 1981;71:53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Enna SJ. Gamma-aminobutyric (B): first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. [PubMed] [Google Scholar]
- Calver AR, Medhurst AD, Robbins MJ, Pangalos MN. The expression of GABA (B1) and GABA (B2) receptor subunits in the CNS differs from that in peripheral tissues. Neuroscience. 2000;100:155–157. doi: 10.1016/s0306-4522(00)00262-1. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviours following local peripheral injection of gabapentin. Pain. 1998;76:201–207. doi: 10.1016/s0304-3959(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA (A) receptors: evidence for peripheral afferent despolarization. Neuroscience. 1999;93:713–722. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Castle NA, Hayllet DG, Morgan J, Mjenkinson DH. Dequalinium: a potent inhibitor of apamin-sensitive K+ channels in hepatocytes and of nicotine responses in skeletal muscle. Eur J Pharmacol. 1993;236:210–217. doi: 10.1016/0014-2999(93)90590-e. [DOI] [PubMed] [Google Scholar]
- Cook NS, Quast U.Potassium channel pharmacology Potassium channels: structure, classification, function, and therapeutic potential 1990Chichester: Ellis Horwood Limited; 181–225.In: Cook NS (eds) [Google Scholar]
- Dirig DV, Yaksh TL. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J Pharmacol Exp Ther. 1995;275:219–227. [PubMed] [Google Scholar]
- Dunn PM. Dequalinium, a selective blocker of the slow after hyperpolarization in rat sympathetic neurones in culture. Eur J Pharmacol. 1994;252:189–194. doi: 10.1016/0014-2999(94)90596-7. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Papucci L, Capaccioli S, Quattrone A, Bartolini A. An antisense oligonucleotide on the mouse sharker-like potassium channel Kv 1.1 gene prevents antinociception induced by morphine and baclofen. J Pharmacol Exp Ther. 1997;281:941–949. [PubMed] [Google Scholar]
- Johnston GAR.Molecular Biology, pharmacology, and physiology of GABAC receptors The GABA receptors 1997Humana Press: Tutowa, NJ; 297–323.In: Enna SJ, Bowery NG (eds) [Google Scholar]
- Korolkovas A.Miorrelaxantes Dicionário Terapêutico Guanabara. Edição 1999/2000 1999Editora Guanabara Koogan: Rio de Janeiro; In: Korolkovas A (eds)pp 5.1–5.5 [Google Scholar]
- Mackinnon R, Miller C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J Gen Physiol. 1988;91:335–349. doi: 10.1085/jgp.91.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TF, Mata BS, Porreca F. Spinal GABAA and GABAB receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ghelardini C, Giotti A, Malmberg-Aiello P, Bartolini A. CGP 35348, a new GABAB antagonist, prevents antinociception and muscle-relaxant effect induced by baclofen. Br J Pharmacol. 1991;103:1303–1308. doi: 10.1111/j.1476-5381.1991.tb09784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PG, Veiga APC, Francischi JN, Tatsuo MAKF. Evidence for participation of GABAA receptors in a rat model of secondary hyperalgesia. Eur J Pharmacol. 2004;483:233–239. doi: 10.1016/j.ejphar.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Ocaña M, Baeyens M. Differential effects of K+ channel blockers on antinociception induced by α2-adrenoceptor, GABAB and k-opioid receptor agonists. Br J Pharmacol. 1993;110:1049–1054. doi: 10.1111/j.1476-5381.1993.tb13919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña M, Baeyens M. Role of ATP-sensitive K+ channels in antinociception induced by R-PIA, an adenosine A1 receptor agonist. Naunyn-Schmiedeberg's Arch Pharmacol. 1994;350:57–62. doi: 10.1007/BF00180011. [DOI] [PubMed] [Google Scholar]
- Ocaña M, Del Pozo E, Barrios M, Baeyens M. An ATP-dependent potassium channel blocker antagonizes morphine analgesia. Eur J Pharmacol. 1990;186:377–378. doi: 10.1016/0014-2999(90)90466-j. [DOI] [PubMed] [Google Scholar]
- Ortiz MI, Torres-López JE, Castañeda-Hernández G, Rosas R, Vidal-Cantú GC, Granados-Soto V. Pharmacological evidence for the activation of K+ channels by diclofenac. Eur J Pharmacol. 2002;438:85–91. doi: 10.1016/s0014-2999(02)01288-8. [DOI] [PubMed] [Google Scholar]
- Pacheco DF, Duarte IDG. δ-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channels. Eur J Pharmacol. 2005;512:23–28. doi: 10.1016/j.ejphar.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Patel S, Naeem S, Kesinglnad A, Froestl W, Capogna M, Urban L, et al. The effects of GABAB agonists and gabapentin on mechanical hiperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90:217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- Potes CS, Neto FL, Castro-Lopes JM. Administration of baclofen, a gamma-aminobutyric acid type B agonist in the thalamic ventrobasal complex, attenuates allodynia in monoarthritic rats subjected to the ankle-bend test. J Neurosci Res. 2006;83:515–523. doi: 10.1002/jnr.20737. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissues. Arch Int Pharmacodyn. 1957;111:409–419. [PubMed] [Google Scholar]
- Rodrigues ARA, Duarte IDG. The peripheral antinociceptive effect induced by morphine is associated with ATP sensitive K+ channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabetkasai M, Ahang S, Shafaghi B, Zarrindast MR. Baclofen-induced antinociception and nicotinic receptor mechanism. Pharmacol Toxicol. 1999;85:247–251. doi: 10.1111/j.1600-0773.1999.tb02017.x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. GABAergic mechanisms of analgesia: An update. Pharmacol Biochem Behav. 1987;26:463–474. doi: 10.1016/0091-3057(87)90148-1. [DOI] [PubMed] [Google Scholar]
- Shafizadeh M, Semmanian S, Zarrinsdast MR, Hashemi B. Involvement of GABAB receptors in the antinociception induced by baclofen in the formalin test. Gen Pharmacol. 1997;28:611–615. doi: 10.1016/s0306-3623(96)00241-8. [DOI] [PubMed] [Google Scholar]
- Sigel E, Stephenson FA, Mamalaki C, Barnard EA. A γ-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex: purification and partial characterization. J Biol Chem. 1983;258:6965–6971. [PubMed] [Google Scholar]
- Soares AC, Duarte IDG. Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K+ channels in the PGE2-induced hyperalgesic paw. Br J Pharmacol. 2001;134:127–131. doi: 10.1038/sj.bjp.0704224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova I. γ-Aminobutiric acid immunostaining in trigeminal, nodose and spinal ganglia of the cat. Acta Histochem. 2004;106:309–314. doi: 10.1016/j.acthis.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wild KD, Vanderah T, Mosberg HI, Porreca F. Opioid δ receptor subtypes are associated with different potassium channels. Eur J Pharmacol. 1991;193:135–136. doi: 10.1016/0014-2999(91)90215-c. [DOI] [PubMed] [Google Scholar]