Abstract
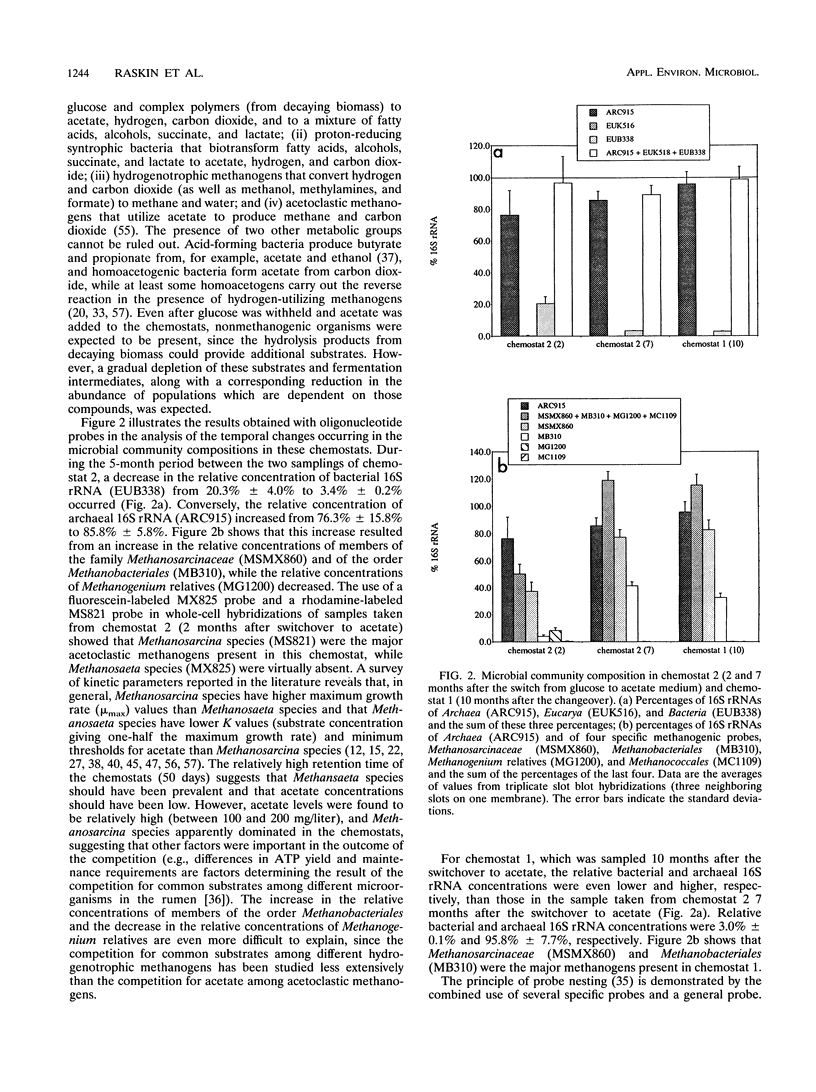
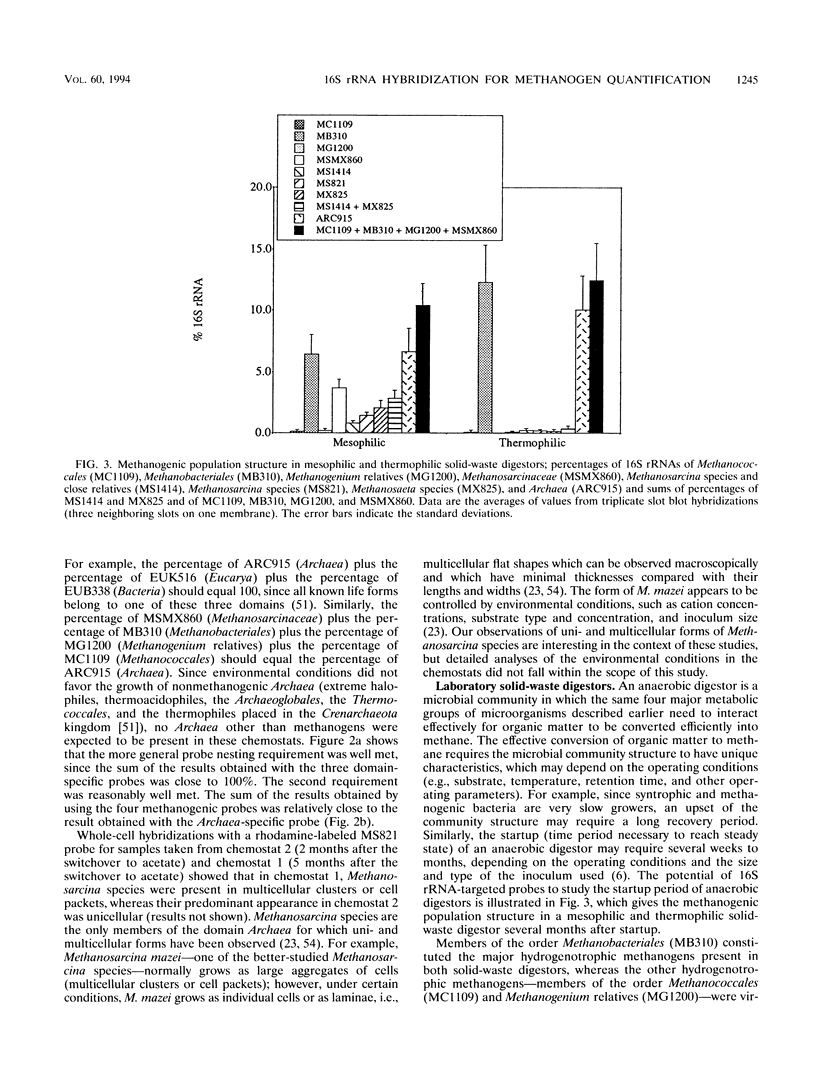
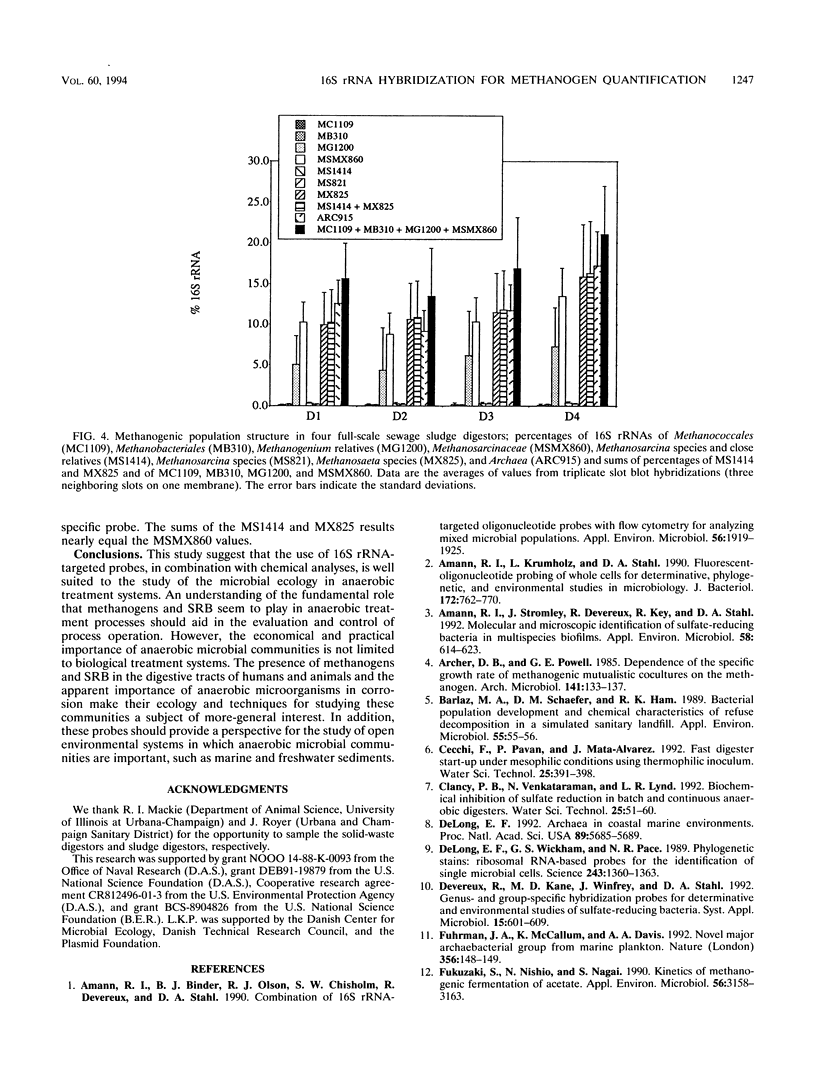
The microbial community structure of anaerobic biological reactors was evaluated by using oligonucleotide probes complementary to conserved tracts of the 16S rRNAs of phylogenetically defined groups of methanogens. Phylogenetically defined groups of methanogens were quantified and visualized, respectively, by hybridization of 32P- and fluorescent-dye-labeled probes to the 16S rRNAs from samples taken from laboratory acetate-fed chemostats, laboratory municipal solid waste digestors, and full-scale sewage sludge digestors. Methanosarcina species, members of the order Methanobacteriales, and Methanosaeta species were the most abundant methanogens present in the chemostats, the solid-waste digestors, and the sewage sludge digestors, respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. I., Krumholz L., Stahl D. A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990 Feb;172(2):762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. I., Stromley J., Devereux R., Key R., Stahl D. A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992 Feb;58(2):614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlaz M. A., Schaefer D. M., Ham R. K. Bacterial population development and chemical characteristics of refuse decomposition in a simulated sanitary landfill. Appl Environ Microbiol. 1989 Jan;55(1):55–65. doi: 10.1128/aem.55.1.55-65.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F. Archaea in coastal marine environments. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., McCallum K., Davis A. A. Novel major archaebacterial group from marine plankton. Nature. 1992 Mar 12;356(6365):148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- Fukuzaki S., Nishio N., Nagai S. Kinetics of the methanogenic fermentation of acetate. Appl Environ Microbiol. 1990 Oct;56(10):3158–3163. doi: 10.1128/aem.56.10.3158-3163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Macfarlane G. T., Cummings J. H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988 Aug;65(2):103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Isa Z., Grusenmeyer S., Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol. 1986 Mar;51(3):572–579. doi: 10.1128/aem.51.3.572-579.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Guyot J. P., Wolfe R. S. Methanogenesis from sucrose by defined immobilized consortia. Appl Environ Microbiol. 1984 Jan;47(1):1–6. doi: 10.1128/aem.47.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. D., Poulsen L. K., Stahl D. A. Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol. 1993 Mar;59(3):682–686. doi: 10.1128/aem.59.3.682-686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Monica J., Zinder Stephen H. Isolation and Characterization of a Thermophilic Bacterium Which Oxidizes Acetate in Syntrophic Association with a Methanogen and Which Grows Acetogenically on H(2)-CO(2). Appl Environ Microbiol. 1988 Jan;54(1):124–129. doi: 10.1128/aem.54.1.124-129.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer L. E., Macario A. J., Conway de Macario E. Lamina, a novel multicellular form of Methanosarcina mazei S-6. J Bacteriol. 1992 Jan;174(1):309–314. doi: 10.1128/jb.174.1.309-314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Zinder S. H. Kinetics of Acetate Utilization by Two Thermophilic Acetotrophic Methanogens: Methanosarcina sp. Strain CALS-1 and Methanothrix sp. Strain CALS-1. Appl Environ Microbiol. 1989 Feb;55(2):488–491. doi: 10.1128/aem.55.2.488-491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L. K., Ballard G., Stahl D. A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993 May;59(5):1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin L., Stromley J. M., Rittmann B. E., Stahl D. A. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol. 1994 Apr;60(4):1232–1240. doi: 10.1128/aem.60.4.1232-1240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior E., Watson-Craik I. A., Kasali G. B. Control/promotion of the refuse methanogenic fermentation. Crit Rev Biotechnol. 1990;10(2):93–118. doi: 10.3109/07388559009068262. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Acetate as sole carbon and energy source for growth of methanosarcina strain 227. Appl Environ Microbiol. 1980 May;39(5):993–999. doi: 10.1128/aem.39.5.993-999.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Flesher B., Mansfield H. R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988 May;54(5):1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Fardeau M. L., Hatchikian C. E., Le Gall J., Belaich J. P. Energetics of Growth of a Defined Mixed Culture of Desulfovibrio vulgaris and Methanosarcina barkeri: Interspecies Hydrogen Transfer in Batch and Continuous Cultures. Appl Environ Microbiol. 1983 Nov;46(5):1152–1156. doi: 10.1128/aem.46.5.1152-1156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. M., Hickey R. F., Zeikus J. G. Characterization of metabolic performance of methanogenic granules treating brewery wastewater: role of sulfate-reducing bacteria. Appl Environ Microbiol. 1991 Dec;57(12):3438–3449. doi: 10.1128/aem.57.12.3438-3449.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R., Macario A. J., Conway de Macario E. Immunochemical differences among Methanosarcina mazei S-6 morphologic forms. J Bacteriol. 1992 Jul;174(14):4683–4688. doi: 10.1128/jb.174.14.4683-4688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



