Abstract
Background and purpose:
Glycogen synthase kinase-3 (GSK-3) is a ubiquitous serine-threonine protein kinase that participates in a multitude of cellular processes and has recently been implicated in the pathophysiology of a number of diseases. The aim of this study was to investigate the effects of GSK-3β inhibition in a model of acute inflammation. Here, we have investigated the effects of TDZD-8, a potent and selective GSK-3β inhibitor, in a mouse model of carrageenan-induced pleurisy.
Experimental approach:
Injection of carrageenan into the pleural cavity of mice elicited an acute inflammatory response characterized by: accumulation of fluid containing a large number of neutrophils (PMNs) in the pleural cavity, infiltration of PMNs in lung tissues and subsequent lipid peroxidation, and increased production of nitrite/nitrate (NOx), prostaglandin E2 (PGE2), tumour necrosis factor-α, (TNF-α) and interleukin-1β (IL-1β). Furthermore, carrageenan induced an upregulation of the adhesion molecules ICAM-1 and P-selectin, iNOS, COX-2 as well as nitrotyrosine as determined by immunohistochemical analysis of lung tissues.
Key results:
Administration of TDZD-8 (1, 3 or 10 mg kg−1, i.p.), 30 min prior to injection of carrageenan, caused a dose-dependent reduction in all the parameters of inflammation measured.
Conclusions and Implications:
Thus, based on these findings we propose that inhibitors of the activity of GSK-3β, such as TDZD-8, may be useful in the treatment of various inflammatory diseases.
Keywords: glycogen synthase kinase-3β, acute inflammation, NF-κB, polymorphonuclear leukocytes
Introduction
Glycogen synthase kinase (GSK)-3 was first identified in 1980 as a ubiquitous serine-threonine protein kinase involved in glycogen metabolism (Frame and Cohen, 2001; Van Wauwe and Haefner, 2003). It has since been implicated in a multitude of cellular processes, ranging from cell membrane-to-nucleus signalling, gene transcription, translation and cytoskeletal organization to cell cycle progression and survival (Embi et al., 1980; Cohen and Abraham, 1999; Woodgett, 2001). Two isoforms have been isolated in mammals, GSK-3α and GSK-3β. GSK-3 is constitutively active in cells, although phosphorylation of a specific serine residue (Ser21 in GSK-3α and Ser9 in GSK-3β) located in its N-terminal domain inhibits GSK-3 activity and, hence, reduces its activity to alter cell function (Woodgett, 2001). A wide variety of extracellular stimuli, including insulin, epidermal growth factor, and fibroblast growth factor, exert their effects by inhibiting GSK-3 activity (Cross et al, 1995).
Unique to GSK-3β is its reported ability to influence the activity of the transcription factor nuclear factor (NF)-κB (Ali et al., 2001; Frame and Cohen, 2001). This concept was based on the findings that GSK-3β knockout mice showed a similar phenotype to mice in which the gene for p65 or IκB kinase 2 (and hence, NF-κB activation) had been deleted (Hoeflich et al., 2000). In both cases, disruption of the p65 gene and disruption of the murine GSK-3β gene resulted in embryonic lethality caused by severe liver degeneration (Beg et al., 1995; Li et al., 1999; Hoeflich et al., 2000). The regulatory influence of GSK-3β on the activity of NF-κB, which has since been confirmed in a range of systems (Schwabe and Brenner, 2002; Demarchi et al., 2003; Buss et al., 2004; Takada et al., 2004), is the basis for the hypothesis that GSK-3β may play a key role in the regulation of the inflammatory response.
Recent in vivo studies have demonstrated that 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8) and SB 415286, potent selective inhibitors of GSK-3β reduced the renal and liver dysfunction caused by both endotoxaemia and administration of endotoxin and peptidoglycan (Dugo et al., 2005). This study proposed that the protective effect of the GSK-3β inhibitors was due to inhibition of the phosphorylation of Ser536 on p65 (Dugo et al., 2005). In agreement with these findings, it was recently reported that GSK-3β-deficient mice challenged with LPS and given a GSK-3β inhibitor showed a reduced mortality and a decreased production of proinflammatory cytokines (Martin et al., 2005). In addition, there is also evidence that TDZD-8 reduces the development of colon injury associated with experimental colitis (Whittle et al., 2006), as well as the development of arthritis in mice (Cuzzocrea et al., 2006b, in press), and modulates secondary damage following spinal cord injury (Cuzzocrea et al., 2006b).
In the present study, to explore further the possible role of GSK-3β in the modulation of different inflammatory conditions in vivo, the effects of the selective GSK-3β inhibitor, TDZD-8 were evaluated in a well-established murine model of carrageenan-induced pleurisy. Carrageenan-induced local inflammation is a useful model to assess the contribution of mediators involved in the vascular changes associated with acute inflammation and is commonly used in the evaluation of nonsteroidal anti-inflammatory drugs. Here, we have investigated the effects of the GSK-3β inhibitor TDZD-8 on (i) polymorphonuclear (PMN) infiltration (assessing myeloperoxidase (MPO) activity), (ii) lipid peroxidation (as malondialdehyde (MDA) levels), (iii) cycloxygenase (COX)-2 expression (by immunohistochemistry), (iv) nitration of tyrosine residues as an indicator of peroxynitrite (by immunohistochemistry), (v) inducible nitric oxide synthase (iNOS) expression, (vi) NF-κB expression, (vii) apoptosis (TUNEL staining), (viii) Bax and Bcl-2 expression and (ix) lung damage (by histology).
Methods
Animals
Male CD mice (weight 20–25 g; Harlan Nossan, Milan, Italy) were used in these studies. The animals were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on the protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with EEC regulations (O.J. of E.C. L358/1 12/18/1986).
Carrageenan-induced pleurisy
Carrageenan-induced pleurisy was induced as previously described (Cuzzocrea et al., 2000). Mice were anaesthetized with isoflurane and subjected to a skin incision at the level of the sixth left intercostal space. The underlying muscle was dissected and saline (0.1 ml) or saline containing 2% λ-carrageenan (0.1 ml) was injected into the pleural cavity. The skin incision was closed with a suture and the animals were allowed to recover. At 4 h after the injection of carrageenan, the animals were killed by inhalation of CO2. The chest was carefully opened and the pleural cavity rinsed with 1 ml of saline solution containing heparin (5 U ml−1) and indomethacin (10 μg ml−1). The exudate and washing solution were removed by aspiration and the total volume measured. Any exudate, which was contaminated with blood was discarded. The amount of exudate was calculated by subtracting the volume injected (1 ml) from the total volume recovered. The leukocytes in the exudate were suspended in phosphate-buffer saline (PBS) and counted with an optical microscope in a Burker's chamber after staining with Toluidine blue.
Experimental design
A dose–response curve was performed investigating the effect of TDZD-8 (1, 3 or 10 mg kg−1) on the development of carrageenan-induced pleurisy. Mice were randomized into eight groups. Sham animals were subjected to the surgical procedure alone, receiving a bolus injection of saline (1 mg kg−1 i.p.) instead of carrageenan, and pretreated 30 min prior with either vehicle (10% dimethylsulphoxide (DMSO) 1 mg kg−1 i.p.) or TDZD-8 (1, 3 or 10 mg kg−1 i.p.). The remaining mice were subjected to carrageenan-induced pleurisy (as described above) and pretreated with an intraperitoneal (i.p.) bolus of vehicle (10% DMSO 1 ml kg−1) or 1, 3 or 10 mg kg−1 TDZD-8. N=10 per group. The doses of TDZD-8 1, 3 and 10 mg kg−1 used here were based on previous in vivo studies (Dugo et al., 2005, Cuzzocrea et al., 2006a, 2006b)
Histological examination
Lung tissues samples were taken 4 h after injection of carrageenan. Lung tissues samples were fixed for 1 week in 10% (w/v) PBS-buffered formaldehyde solution at room temperature, dehydrated using graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, USA). Sections were then deparaffinized with xylene, stained with hematoxylin and eosin. All sections were studied using Axiovision Ziess (Milan, Italy) microscope.
Measurement of cytokines
Tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) levels were evaluated in the exudates, 4 h after the induction of pleurisy by carrageenan injection, as previously described (Cuzzocrea et al., 1999a). The assay was carried out using a colorimetric commercial ELISA kit (Calbiochem-Novabiochem Corporation, Milan, Italy).
Measurement of nitrite–nitrate concentration
Total nitrite in exudates, an indicator of nitric oxide (NO) synthesis, was measured as previously described (Cuzzocrea et al., 2001). Briefly, the nitrate in the sample was first reduced to nitrite by incubation with nitrate reductase (670 mU ml−1) and NADPH (160 μM) at room temperature for 3 h. The total nitrite concentration in the samples was then measured using the Griess reaction, by adding 100 μl of Griess reagent (0.1% (w/v) naphthylethylendiamide dihydrochloride in H2O and 1% (w/v) sulphanilamide in 5% (v/v) concentrated H3PO4; vol. 1:1) to the 100 μl sample. The optical density at 550 nm (OD550) was measured using ELISA microplate reader (SLT-Lab Instruments, Salzburg, Austria). Nitrite concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrite prepared in H2O.
Measurement of PGE2 in the pleural exudate
The amount of prostaglandin E2 (PGE2) present in the pleural fluid of mice, collected 4 h after carrageenan administration, was assayed with a colorimetric, commercial kit (Calbiochem-Novabiochem Corporation, La Jolla, CA, USA).
Immunohistochemical localization of ICAM-1, P-selectin, iNOS, nitrotyrosine, COX-2, Bax and Bcl-2
At the end of the experiment, the tissues were fixed in 10% (w/v) PBS-buffered formaldehyde and 8 μm sections were prepared from paraffin embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min. The sections were permeablized with 0.1% (w/v) Triton X-100 in PBS for 20 min. Nonspecific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin- or avidin-binding sites were blocked by sequential incubation for 15 min with biotin and avidin (DBA, Milan, Italy), respectively. Sections were incubated overnight with (1) purified goat polyclonal antibody directed towards P-selectin which reacts with mice; or (2) with purified hamster anti-mouse ICAM-1 (CD54) (1:500 in PBS, w/v) (DBA, Milan, Italy) or (3) with anti-nitrotyrosine rabbit polyclonal antibody (1:500 in PBS, v/v) or with anti-COX-2 antibody (1:500 in PBS, v/v) or (4) with anti-iNOS antibody (1:500 in PBS, v/v) or (5) with anti-Bax antibody (1:500 in PBS, v/v) or (6) with anti-Bcl-2 antibody (1:500 in PBS, v/v). Sections were washed with PBS, and incubated with secondary antibody. Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin–biotin peroxidase complex (DBA, Milan, Italy). In order to confirm that the immunoreaction for the nitrotyrosine was specific some sections were also incubated with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM) to verify the binding specificity. To verify the binding specificity for ICAM-1, P-selectin, COX-2, Bax or Bcl2, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations, no positive staining was found in the sections indicating that the immunoreaction was positive in all the experiments carried out.
MPO activity
Myeloperoxidase (MPO) activity, an indicator of PMN accumulation, was determined as previously described (Mullane et al., 1985). At the specified time following injection of carrageenan, lung tissues were obtained and weighed, each piece homogenized in a solution containing 0.5% (w/v) hexadecyltrimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20 000 g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM hydrogen peroxide. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide min−1 at 37°C and was expressed in milliunits per gram weight of wet tissue.
MDA measurement
Malondialdehyde (MDA) levels in the lung tissue were determined as an indicator of lipid peroxidation as previously described (Ohkawa et al., 1979). Lung tissue collected at the specified time, was homogenized in 1.15% (w/v) KCl solution. A 100 μl aliquot of the homogenate was added to a reaction mixture containing 200 μl of 8.1% (w/v) SDS, 1.5 ml of 20% (v/v) acetic acid (pH 3.5), 1.5 ml of 0.8% (w/v) thiobarbituric acid and 700 μl distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3000 g for 10 min. The absorbance of the supernatant was measured using spectrophotometry at 650 nm.
Western blot analysis
Lung tissues were disrupted by homogenization on ice in a buffer containing: HEPES 20 mM, MgCl2 1.5 mM, NaCl 420 mM, EDTA 1 mM, EGTA 1 mM, ditiothreitol 1 mM, phenylmethyl sulphonylfluoride 0.5 mM, trypsin inhibitor 15 μg ml−1, pepstatin 3 μg ml−1, leupeptin 2 μg ml−1, benzidamin 40 μM, nonidet P-40 1% and glycerol 20%. Protein concentration was estimated by the Bio-Rad protein assay using bovine serum albumin as standard. Equal amounts of protein (70 μg) were dissolved in Laemmli's sample buffer, boiled and subjected to sodium dodecylsulphate-polyacrylamide gel electrophoresis minigel (8% polyacrylamide). The blot was performed by transferring proteins from a slab gel to nitrocellulose membranes at 240 mA for 40 min at room temperature. The filter was then blocked with 1 × PBS, 5% (w/v) nonfat dried milk for 40 min at room temperature and subsequently probed with specific mAbs against iNOS (BD Laboratories, 1:2000), or COX-2 (Cayman Chemical, 1:500), or Bax (Santa Cruz Biotechnology, 1:100), or Bcl-2 (Santa Cruz Biotechnology Inc., CA, USA, 1:100), or IκB-α (Santa Cruz Biotechnology, 1:1000), or Phospho-NF-κB p65 (serine 536) (Cell Signaling, 1:1000) in 1 × PBS, 5% w/v nonfat dried milk, 0.1% Tween-20 at 4°C, overnight. The secondary antibody (anti-mouse IgG or anti-rabbit IgG or anti-goat IgG peroxidase conjugated, Jackson Immuno Research, Laboratories Inc. 1:5000) was incubated for 1 h at room temperature. Subsequently, the blot was extensively washed with PBS, developed using enhanced chemiluminescence detection reagents (Amersham), according to the manufacturer's instructions and exposed to Kodak X-Omat film. The protein bands of iNOS (∼130 kDa), COX-2 (∼70 kDa), on X-ray film were scanned and densitometrically analysed with a model GS-700 imaging densitometer (Bio-Rad Laboratories, Segrate, Milan, Italy).
TUNEL assay
Terminal deoxynucleotidyltransferase-mediated UTP end labelling (TUNEL) assay was conducted by using a TUNEL detection kit according to the manufacturer's instructions (Apotag, HRP kit DBA, Milan, Italy). Briefly, sections were incubated with 15 μg ml−1 proteinase K for 15 min at room temperature and then washed with PBS. Endogenous peroxidase was inactivated by 3% H2O2 for 5 min at room temperature and then washed with PBS. Sections were immersed in terminal deoxynucleotidyltransferase (TdT) buffer containing deoxynucleotidyl transferase and biotinylated dUTP in TdT buffer, incubated in a humid atmosphere at 37°C for 90 min, and then washed with PBS. The sections were incubated at room temperature for 30 min with anti-horseradish peroxidase-conjugated antibody, and the signals were visualized with diaminobenzidine.
Materials
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company Ltd (Poole, Dorset, UK). TDZD-8 was obtained from Calbiochem (Merck Biosciences Ltd, Beeston, Nottingham, UK). All other chemicals were of the highest commercial grade available. All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter, Italy).
Statistical evaluation
All values in the figures and text are expressed as mean±standard error of the mean (s.e.m.) of n observations. For the in vivo studies n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments (histological or immunohistochemistry coloration) performed on different experimental days on the tissue sections collected from all the animals in each group. The results were analysed by one-way ANOVA followed by a Bonferroni posthoc test for multiple comparisons. A P-value <0.05 were considered significant. and individual group means were then compared with Student's unpaired t test. A P-value <0.05 was considered significant.
Results
Effects of TDZD-8 on carrageenan-induced pleurisy
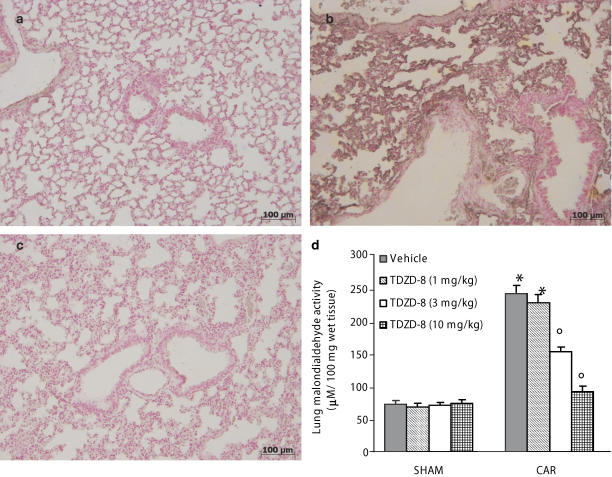
When compared with lung sections taken from saline-treated animals (sham group Figure 1a), histological examination of lung sections taken from mice treated with carrageenan revealed significant tissue damage and oedema (Figure 1b), as well as infiltration of PMNs within the tissues (see arrows Figure 1b1). TDZD-8 (3, 10 mg kg−1) reduced the degree of lung injury (Figure 1d–e respectively). However, the lowest dose of TDZD-8 (1 mg kg−1) did not reduce the degree of lung injury (Figure 1c). Furthermore, injection of carrageenan elicited an acute inflammatory response characterized by the accumulation of fluid (oedema) in the pleural cavity (Table 1) containing large amounts of PMNs (Table 1). Pretreatment with TDZD-8 attenuated carrageenan-induced oedema formation and PMN infiltration in a dose-dependent manner (Table 1). The reductions in oedema formation and PMN infiltration were significant at the higher doses of TDZD-8 (3 and 10 mg kg−1; Table 1).
Figure 1.
Effect of TDZD-8 on histological alterations of lung tissue 4 h after carrageenan-induced injury. No histological alterations were observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pre-treated with vehicle demonstrated oedema, tissue injury (b) as well as infiltration of the tissue with neutrophils (see arrows b1). Carrageenan-treated animals pretreated with TDZD-8 (5 mg kg−1 i.p.) (d) or at (10 mg kg−1 i.p.) (e) demonstrated reduced lung injury and neutrophil infiltration. On the contrary, TDZD-8 (1 mg kg−1) did not reduce the degree of lung injury (c). Original magnification: × 125. The figure is representative of at least three experiments performed on different experimental days.
Table 1.
Effect of TDZD-8 on carrageenan-induced inflammation, TNF-α and IL-1β production in the pleural exudate
| Volume exudate (ml) | PMNs infiltration (million cells/mouse) | TNF-α (pg/ml) | IL-1β (pg/ml) | |
|---|---|---|---|---|
| Sham+vehicle | 0.07±0.06 | 0.6±0.2 | 5.0±0.6 | 7.0±2.3 |
| Sham+TDZD-8 (1 mg kg−1) | 0.09±0.07 | 0.8±0.12 | 8.0±0.4 | 9.0±3.2 |
| Sham+TDZD-8 (3 mg kg−1) | 0.1±0.05 | 0.5±0.15 | 6.0±0.5 | 11.0±2.9 |
| Sham+TDZD-8 (10 mg kg−1) | 0.11±0.04 | 0.7±0.13 | 7.0±0.4 | 8.0±4.2 |
| CAR+Vehicle | 1.0±0.1* | 9.0±0.5* | 47±5* | 131±9* |
| CAR+ TDZD-8 (1 mg kg−1) | 0.9±0.11* | 8.0±0.35* | 42±3* | 112±11** |
| CAR+TDZD-8 (3 mg kg−1) | 0.6±0.05** | 5.0±0.3** | 21±4.5** | 82±5.0** |
| CAR+TDZD-8 (10 mg kg−1) | 0.24±0.09** | 3.5±0.28** | 17±2.5** | 45±6.5** |
Abbreviations: IL-1β, interleukin-1β; PMN, polymorphonuclear; TDZD-8, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione; TNF-α, tumour necrosis factor-α.
Data are means±s.e. m. of 10 mice for each group.
P<0.01 versus sham.
P<0.01 versus carrageenan.
Effects of TDZD-8 on the expression of adhesion molecules (ICAM-1, P-selectin)
Staining of lung tissue sections obtained from saline-treated mice with anti-ICAM-1 antibody showed a specific staining along bronchial epithelium demonstrating that ICAM-1 is constitutively expressed (Figure 2a). No positive staining for P-selectin was found in lung tissue sections from saline-treated mice (Figure 2a). At 4 h after carrageenan injection, the staining intensity for ICAM-1 substantially increased along the vessels (Figure 2b) mainly localized in the vascular endothelium (Figure 2b1). Lung tissue sections obtained from carrageenan-treated mice showed positive staining for P-selectin localized in the vessels (Figure 3b). No positive staining for ICAM-1 or P-selectin was observed in the lungs of carrageenan-treated mice pretreated with TDZD-8 (10 mg kg−1) (Figures 2c and 3c, respectively). As this expression of adhesion molecules appeared to correlate with an influx of leukocytes into the lung tissue, we investigated the effect of TDZD-8 on neutrophil infiltration by measurement of MPO activity. This activity was significantly elevated at 4 h after carrageenan administration in vehicle-treated mice (Figure 3d). Pretreatment with TDZD-8 attenuated neutrophil infiltration into the lung tissue in a dose-dependent fashion, and was significant at the 3 and 10 mg kg−1 doses (Figure 3d).
Figure 2.
Effect of TDZD-8 on the immunohistochemical localization of ICAM-1 expression in the lung after carrageenan injection. No positive staining for ICAM-1 was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pretreated with vehicle showed intense positive staining for ICAM-1 along the vessels (b) as well as the bronchial epithelium (b1). The degree of positive staining for ICAM-1 was markedly reduced in lung sections obtained from mice pretreated with 10 mg kg−1 TDZD-8 mice (c). Original magnification: × 125. The figure is representative of at least three experiments performed on different experimental days.
Figure 3.
Effect of TDZD-8 on carrageenan-induced P-selectin expression and PMN infiltration in the lung. No positive staining for P-selectin was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pre-treated with vehicle showed intense positive staining for P-selectin along the vessels (b). The degree of positive staining for P-selectin was markedly reduced in tissue sections obtained from mice pre-treated with 10 mg kg−1 TDZD-8 (c). MPO activity, index of PMN infiltration, was significantly elevated at 4 h after carrageenan (CAR) administration in vehicle-treated mice (d). TDZD-8 (1, 3 and 10 mg kg−1 i.p.) significantly reduced MPO activity in the lung in a dose-dependent fashion (d). The figure is representative of at least three experiments performed on different experimental days. Data are expressed as mean±s.e.m. from n=10 mice for each group. *P<0.01 versus sham group. °P<0.01 versus carrageenan.
Effects of TDZD-8 on carrageenan-induced NO production
No positive staining for iNOS was observed in the lung tissues obtained from the sham group (Figure 4a). Immunohistochemical analysis of lung sections obtained from carrageenan-treated mice revealed positive staining for iNOS (Figure 4b). In contrast, no staining for iNOS was found in the lungs of carrageenan-treated mice that had been treated with TDZD-8 (10 mg kg−1) (Figure 4c). A significant increase in iNOS expression 4 h after carrageenan injection, as assayed by Western blot analysis, was also detected in lungs obtained from mice subjected to carrageenan-induced pleurisy (Figure 5a, see densitometry analysis Figure 5a1). TDZD-8 (10 mg kg−1) treatment significantly attenuated this iNOS expression (Figure 5a, see densitometry analysis Figure 5a1). NO levels were also significantly increased in the exudate obtained from mice administered carrageenan (Figure 4d). Pretreatment of mice with TDZD-8 significantly reduced (in a dose-dependent fashion) NO exudates levels (Figure 4d). No significant reduction of NO exudates levels was found in the animal treated with TDZD-8 at the lower dose (1 mg kg−1; Figure 4d).
Figure 4.
Effect of TDZD-8 on carrageenan-induced iNOS expression and NO formation in the lung. No positive staining for iNOS was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pretreated with vehicle showed positive staining for iNOS, localized mainly in inflammatory cells (b). The degree of positive staining for iNOS was markedly reduced in tissue sections obtained from mice pretreated with 10 mg kg−1 TDZD-8 (c). Nitrite and nitrate levels, stable NO metabolites, were significantly increased in the pleural exudates at 4 h after carrageenan (CAR) administration (d). TDZD-8 (1, 3 and 10 mg kg−1i.p.) significantly reduced the carrageenan-induced elevation of nitrite and nitrate exudates levels in a dose dependent manner. The figure is representative of at least three experiments performed on different experimental days. Data are expressed as meanc±s.e.m. from n=10 mice for each group. *P<0.01 versus sham group. °P<0.01 versus carrageenan.
Figure 5.
A representative blot of iNOS (a) and COX-2 (b) expression in the lung after carrageenan (CAR) injection. A significant increase in iNOS (a, a1) and COX-2 (b, b1) expression, assayed by Western blot analysis, was detected in lungs obtained from mice subjected to carrageenan-induced pleurisy. Pretreatment with TDZD-8 10 mg kg−1 significantly attenuated iNOS (a, a1) and COX-2 (b, b1) expression in the lung tissues. A representative blot of tissue lysates (a and b) obtained from five animals per group is shown and densitometry analysis of all animals is reported. The results in panel a1 and b1 are expressed as mean±s.e.m. from n=5/6 lungs for each group. *P<0.01 versus carrageenan.
Effects of TDZD-8 on carrageenan-induced nitrotyrosine formation and lipid peroxidation
Immunohistochemical analysis of lung sections obtained from mice treated with carrageenan revealed positive staining for nitrotyrosine (Figure 6b). In contrast, no positive staining for nitrotyrosine was found in the lungs of carrageenan-treated mice, which had been treated with TDZD-8 (10 mg kg−1) (Figure 6c). There was no staining for nitrotyrosine (Figure 6a) in lungs obtained from the sham group of mice. In addition, at 4 h after carrageenan-induced pleurisy, MDA levels were also measured in the lungs as an indicator of lipid peroxidation. As shown in Figure 6d, MDA levels were significantly increased in the lungs of carrageenan-treated mice. Lipid peroxidation was significantly attenuated in a dose-dependent fashion by the i.p. injection of TDZD-8 (Figure 6d). No significant reduction of MDA levels was found in the animal treated with TDZD-8 at the lowest dose (1 mg kg−1; Figure 6d).
Figure 6.
Effect of TDZD-8 on carrageenan-induced nitrotyrosine formation and lipid peroxidation in the lung. No positive staining for nitrotyrosine was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pre-treated with vehicle showed positive staining for nitrotyrosine, localized mainly in inflammatory cells (b). There was a marked reduction in the immunostaining for nitrotyrosine in the lungs of carrageenan-treated mice pretreated with 10 mg kg−1 TDZD-8 (c). MDA levels, an index of lipid peroxidation, were significantly increased in lung tissues 4 h after carrageenan (CAR) administration (d). TDZD-8 (1, 3 and 10 mg kg−1i.p.) significantly reduced the carrageenan-induced elevation of MDA tissues levels in a dose-dependent manner. The figure is representative of at least three experiments performed on different experimental days. Data are expressed as mean±s.e.m. from n=10 mice for each group. *P<0.01 versus sham group. °P<0.01 versus carrageenan.
Effects of TDZD-8 on carrageenan-induced prostaglandin formation
Although staining was absent in tissue obtained from the sham group of animals (Figure 7a), immunohistochemical analysis of lung sections obtained from carrageenan-treated mice revealed positive staining for COX-2, which was primarily localized in alveolar macrophages (Figure 7b). In contrast, no positive COX-2 staining was found in the lungs from carrageenan-treated mice that had been treated with TDZD-8 (10 mg kg−1) (Figure 7c). Western blot analysis of lung homogenates obtained from carrageenan-treated mice also revealed an increase of COX-2 expression, which was significantly attenuated in the lungs of mice treated with TDZD-8 (10 mg kg−1) (Figure 5b, see densitometry analysis Figure 5b1). In addition, COX-2 activity was also assessed by measurement of the increased formation of PGE2 in the pleural exudate. The level of PGE2 found in the pleural exudate of carrageenan-treated mice pretreated with vehicle was significantly greater than in those treated with the higher doses of TDZD-8 (Figure 7d). COX-1 was also detected by immunohistochemical analysis in the lung sections obtained from mice treated with carrageenan, however, the degree of staining was similar to that observed in the lungs of sham animals (data not shown). The degree of staining for COX-1 in lungs of carrageenan-treated mice treated with TDZD-8 (10 mg kg−1) was similar to those observed in lungs obtained from carrageenan-treated mice and sham mice (data not shown).
Figure 7.
Effect of TDZD-8 on carrageenan-induced COX-2 expression and PGE2 production in the lung. No positive staining for COX-2 was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pretreated with vehicle showed positive staining for COX-2, localized mainly in inflammatory cells (b). There was a marked reduction in the immunostaining for COX-2 in the lungs of carrageenan-treated mice pretreated with 10 mg kg−1 TDZD-8 (c). PGE2 levels were significantly increased in the pleural exudates at 4 h after carrageenan (CAR) administration (d). TDZD-8 (1, 3 and 10 mg kg−1i.p.) significantly reduced the carrageenan-induced elevation of PGE2 exudate levels in a dose-dependent manner. The figure is representative of at least three experiments performed on different experimental days. Data are expressed as mean±s.e.m. from n=10 mice for each group. *P<0.01 versus sham group. °P<0.01 versus carrageenan.
Effects of TDZD-8 on the release of proinflammatory cytokine induced by carrageenan
When compared to sham animals, injection of carrageenan resulted in an increase in the levels of TNF-α and IL-1β in the pleural exudates (Table 1). The release of TNF-α and IL-1β was attenuated, in a dose-dependent manner, by treatment with TDZD-8 (Table 1). This reduction in TNF-α and IL-1β exudate levels was significant at the 3 and 10 mg kg−1 doses (Table 1).
Effect of TDZD-8 on IκB-α degradation and phosphorylation of Ser536 on p65
To investigate the cellular mechanisms by which treatment with TDZD-8 may attenuate the development of carrageenan-induced pleurisy, we evaluated both IκB-α degradation and phosphorylation of Ser536 on the NF-κB subunit p65. The presence of IκB-α in homogenates of lung tissues was investigated by immunoblot analysis at 4 h after carrageenan administration. A basal level of IκB-α was detected in the lung tissues of sham-animals (Figure 8a, see densitometry analysis, Figure 8a1), whereas in carrageenan-treated mice IκB-α levels were substantially reduced (Figure 8a, see densitometry analysis, Figure 8a1). TDZD-8 (10 mg kg−1) prevented carrageenan-induced IκB-α degradation, the IκB-α levels observed in these animals were similar to those of the sham group (Figure 8a, see densitometry analysis, Figure 8a1). We also evaluated the phosphorylation of Ser536 on the NF-κB subunit p65 by Western blot analysis in lung samples collected 4 hours after carrageenan administration. A significant increase in the phosphorylation of Ser536 was observed in lung tissues collected from carrageenan-treated mice (Figure 8b, see densitometry analysis, Figure 8b1). Treatment with the GSK-3β inhibitor significantly reduced the phosphorylation of p65 on Ser536 (Figure 8b, see densitometry analysis, Figure 8b1).
Figure 8.
Representative Western blots showing the effects of TDZD-8 on IκB-α degradation (a, a1) and phosphorylation of Ser536 on NF-κB subunit p65 (b, b1) after carrageenan (CAR) injection. A representative blot of lysates (a, b) obtained from five animals per group is shown and densitometry analysis of all animals is reported. The results in panel a1, b1 are expressed as mean±s.e.m. from n=5/6 lung tissues for each group. *P<0.01 versus carrageenan.
Effects of TDZD-8 on apoptosis in lung tissues after carrageenan-induced pleurisy
To investigate whether acute lung inflammation is associated with apoptotic cell death we measured TUNEL-like staining in lung tissues. At 4 h after carrageenan administration, lung tissues demonstrated a marked appearance of dark brown apoptotic cells and intercellular apoptotic fragments (Figure 9a, a1,a2,a3). In contrast, no apoptotic cells or fragments were observed in the tissues obtained from carrageenan-mice treated with TDZD-8 10 mg kg−1 (Figure 9b). Similarly, no apoptotic cells were observed in lungs of sham-treated mice (Figure 9c). A positive control is also included (Figure 9d).
Figure 9.
Effect of TDZD-8 on carrageenan-induced apoptosis as measured by TUNEL-like staining. Positive staining was observed in lung sections taken from carrageenan-treated mice pretreated with vehicle (a, a1, a2, a3). In contrast, tissues obtained from carrageenan-treated mice pretreated with 10 mg kg−1 TDZD-8 demonstrated no apoptotic cells or fragments (b). Almost no apoptotic cells were observed in lungs of sham mice treated with TDZD-8 (10 mg kg−1 c). A positive control is also included (d). The figure is representative of at least three experiments performed on different experimental days.
Western blot analysis and immunohistochemistry for Bax and Bcl-2
The presence of Bax in lung homogenates was investigated by Western blot 4 h after carrageenan administration. A basal level of Bax was detected in lung tissues obtained from sham-treated animals (Figure 10a, see densitometry analysis, Figure 10a1). Bax levels were substantially increased in the lung tissues from carrageenan-treated mice (Figure 10a, see densitometry analysis, Figure 10a1). On the contrary, TDZD-8 (10 mg kg−1) treatment prevented the carrageenan-induced Bax expression (Figure 10a, see densitometry analysis, Figure 10a1).
Figure 10.
Representative Western blots showing the effects of TDZD-8 on Bax (a, a1) and Bcl-2 (b, b1) expression in lung tissue after carrageenan (CAR) injection. A representative blot of lysates (a, b) obtained from five animals per group is shown and densitometry analysis of all animals is reported. The results in panel a1, b1 are expressed as mean±s.e.m. from n=5/6 lung tissues for each group. *P<0.01 versus sham group. °P<0.01 versus carrageenan.
To detect Bcl-2 expression, whole extracts from lung tissues of mice were also analysed by Western blot analysis. A basal level of Bcl-2 expression was detected in lung tissues from sham-treated mice (Figure 10b, see densitometry analysis, Figure 10b1). At 4 h after carrageenan administration, Bcl-2 expression was significantly reduced (Figure 10b, see densitometry analysis, Figure 10b1). Treatment of mice with TDZD-8 (10 mg kg−1) significantly attenuated carrageenan-induced inhibition of Bcl-2 expression (Figure 10b, see densitometry analysis, Figure 10b1).
Lung samples were also collected 4 h after carrageenan administration in order to determine the immunohistological staining for Bax and Bcl-2. Lung tissues taken from sham-treated mice did not stain for Bax (Figure 11a) whereas lung sections obtained from carrageenan-treated mice exhibited positive staining for Bax (Figure 11b and b1). TDZD-8 (10 mg kg−1) treatment reduced the degree of positive staining for Bax in the lung of mice subjected to carrageenan-induced pleurisy (Figure 11c).
Figure 11.
Effect of TDZD-8 on carrageenan-induced Bax expression in the lung. No positive staining for Bax was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a). Lung sections taken from carrageenan-treated mice pretreated with vehicle showed positive staining for Bax (b) localized mainly in the inflammatory cells (b1). The degree of positive staining for Bax was markedly reduced in lung sections obtained from mice pretreated with 10 mg kg−1 TDZD-8 mice (c). The figure is representative of at least three experiments performed on different experimental days.
In addition, lung sections from sham-treated mice demonstrated positive staining for Bcl-2 (Figure 12a and a1) whereas in carrageenan-treated mice Bcl-2 staining was significantly reduced (Figure 12c). TDZD-8 (10 mg kg−1) treatment significantly attenuated the loss of positive staining for Bcl-2 in mice subjected to carrageenan-induced pleurisy (Figure 12b and b1).
Figure 12.
Effect of carrageenan and TDZD-8 on Bcl-2 expression in the lung. Positive staining for Bcl-2 was observed in lung sections taken from sham mice treated with TDZD-8 (10 mg kg−1 a, a1). The degree of positive staining for Bcl-2 was markedly reduced in lung sections obtained from carrageenan-mice treated with vehicle (c). Pretreatment with TDZD-8 10 mg kg−1 significantly attenuated the reduction in Bcl-2 expression caused by carrageenan (b, b1).
Discussion and conclusions
This study provides evidence that TDZD-8 attenuates: (i) the development of carrageenan-induced pleurisy, (ii) the infiltration of the lung with PMNs, (iii) the degree of lipid peroxidation in the lung, (iv) the expression of ICAM-1 and P-selectin, (v) COX-2 expression (by immunohistochemistry and western blot analysis), (vi) the nitration of tyrosine residues, (vii) iNOS expression (vii) NF-κB expression (ix) apoptosis (x) Bax and Bcl-2 expression and (xi) the degree of lung injury caused by injection of carrageenan. All of these findings support the view that TDZD-8 attenuates the degree of acute inflammation in the mouse. What, then, is the mechanism by which TDZD-8 reduces acute inflammation?
The proof of a role of GSK-3β in the regulation of acute lung injury is of special interest because several transcription factors important to the regulation of acute inflammation serve as substrates for GSK-3β. Among these is the transcription factor NF-κB, whose function is strikingly altered by GSK-3β (Hoeflich et al., 2000; Buss et al., 2004). NF-κB plays a central role in the regulation of many genes responsible for the generation of mediators or proteins in inflammation. These include the genes for TNF-α, IL-1β, iNOS and COX-2 (Verma, 2004). The discovery in 1997 that inhibition of the activation of NF-κB may be useful in conditions associated with local or systemic inflammation (Ruetten and Thiemermann, 1997) stimulated the search for agents that prevent the activation of NF-κB. The extent to which GSK-3β activates or blocks NF-κB signalling remains unclear. Hoeflich et al. (2000) first demonstrated that deletion of GSK-3β had no effect on the TNF-α-induced IκB-α degradation or on the nuclear translocation of the subunit p65, but prevented the activation of NF-κB by an unknown mechanism. On the other hand, other studies provided evidence for an inverse association between the activity of GSK-3β and NF-κB signalling. A recent study has shown that GSK-3β-dependent phosphorylation of a specific serine residue (Ser468) on p65 blocks the activation of NF-κB and that inhibition of GSK-3β was associated with increased p65 activity (Buss et al., 2004). We report here that carrageenan caused a significant increase in the phosphorylation of Ser536 on p65 in the lung tissues at 4 h, whereas treatment with the GSK-3β inhibitor TDZD-8 significantly reduced this phosphorylation. Moreover, we also demonstrate that the selective and potent GSK-3β inhibitor TDZD-8 inhibited IκB-α degradation. Taken together, the balance between pro-inflammatory and prosurvival roles of NF-κB may depend on the phosphorylation status of p65 and GSK-3β may play a central role in this process. However, the reasons for the apparent discrepancies in the modulatory effects of GSK-3β on NF-κB activity remain to be fully elucidated.
There is good evidence that TNF-α and IL-1β help to propagate the extension of a local or systemic inflammatory process (Saklatvala, 1986; Henderson and Pettipher, 1989; Piguet et al., 1992; Wooley et al., 1993). Various studies have clearly reported that inhibition of TNF-α formation significantly prevent the development of the inflammatory process (Mageed et al., 1998; Mukherjee et al., 2005). This study demonstrates that TDZD-8 attenuates the production of TNF-α and IL-1β in pleural exudates of carrageenan-treated mice. Therefore, the inhibition of the production of TNF-α and IL-1β by TDZD-8 described in the present study most likely reflects its inhibitory effects on the activation of NF-κB. Indeed, the expression of MCP-1 and IL-6 were reduced in TNF-α stimulated mouse embryonic fibroblasts lacking GSK-3β, however, restoration of GSK-3β activity in these cells was able to in turn restore responsiveness to TNF-α treatment and elevate levels of these NF-κB regulated genes (Steinbrecher et al., 2005).
The promoter regions of murine and human COX-2 genes contain binding sites for NF-κB (Sirois et al., 1993; Appleby et al., 1994). Expression of the COX-2 gene is activated by oxidant stress (Feng et al., 1995) and reactive oxygen intermediates cause activation of NF-κB (Schreck et al., 1991), suggesting NF-κB is one of the transcription factors involved. The increase in prostaglandin formation (COX activity) by murine osteoblasts (cell line MC3T3-E1) involves activation of NF-κB (Wadleigh and Herschman, 1999). There is good evidence that an enhanced formation of prostanoids following the induction of COX-2 contributes to the pathophysiology of local inflammation (Salvemini et al., 1995; Sautebin et al., 1998) and also that selective inhibitors of COX-2 exert potent anti-inflammatory effects (Cuzzocrea et al., 2002). We demonstrate here that the carrageenan-induced increase in PGE2 levels is attenuated by pretreatment with TDZD-8. The enhanced formation of PGE2 is secondary to the expression of COX-2 protein, as (i) there was no increase in the expression of COX-1 protein (detected by immunohistochemistry) after carrageenan injection and (ii) selective inhibitors of COX-2 activity including NS-398 and SC-58125 (celecoxib) abolished the increase in PGE2 caused by carrageenan (Cuzzocrea et al., 2002). Thus, we propose that TDZD-8 reduced the expression of COX-2 protein in the lung caused by carrageenan.
Furthermore, we observed that acute lung inflammation (4 h after carrageenan administration) induced the appearance of P-selectin on the endothelial vascular wall and upregulated the surface expression of ICAM-1 on endothelial cells. Treatment with TDZD-8 abolished the expression of P-selectin and the upregulation of ICAM-1 without effecting constitutive levels of ICAM-1 on endothelial cells. These results demonstrate that inhibition of the GSK-3β pathway may interrupt the interactions between neutrophils and endothelial cells both at the early rolling phase mediated by P-selectin and at the late firm adhesion phase mediated by ICAM. The lack of increased expression of the adhesion molecule in the lung tissue of carrageenan-treated mice given TDZD-8 correlated with the reduction of leucocyte infiltration, as assessed by the specific granulocyte enzyme MPO, and with the attenuation of the lung tissue damage as evaluated by histological examination. Activation and accumulation of leukocytes is one of the initial events of tissue injury due to release of oxygen free radicals, arachidonic acid metabolites and lysosomal proteases (Salvemini et al., 2002).
Enhanced formation of NO by iNOS may contribute to the inflammatory process (Tracey et al., 1995; Wei et al., 1995; Salvemini et al., 1996; Cuzzocrea et al., 1999b). This study demonstrates that TDZD-8 attenuates the expression of iNOS in the lung in carrageenan-treated mice. This reduction in the expression of iNOS by TDZD-8 may contribute to the attenuation of nitrotyrosine formation and lipid peroxidation in the lung in carrageenan-treated animals. Nitrotyrosine formation, along with its detection by immunostaining, was initially proposed as a relatively specific marker (‘footprint') for the detection of the endogenous formation of peroxynitrite (Beckman, 1996). There is, however, recent evidence that certain other reactions can also induce tyrosine nitration for example, reaction of nitrite with hypochlorous acid and the reaction of MPO with hydrogen peroxide can both lead to the formation of nitrotyrosine (Eiserich et al., 1998). Increased nitrotyrosine staining is therefore considered as an indicator of ‘increased nitrosative stress' rather than a specific marker of the generation of peroxynitrite.
Generation of free radicals and nitric oxide by activated macrophages has also been implicated in causing oligodendrocyte apoptosis (Merrill et al., 1993). We have demonstrated that treatment with TDZD-8 attenuates the degree of apoptosis, measured by TUNEL detection kit, in the lung at 4 h after carrageenan administration. There is evidence that direct overexpression of GSK-3β is known to induce apoptosis in different cell lines, and specific inhibitors of GSK-3β are able to ameliorate this apoptotic response (Pap and Cooper, 1998).
It is known that pathways which inhibit GSK-3β activity, such as PI-3K or Wnt signalling, often lead to the induction of the NF-κB cell survival pathway (Bournat et al., 2000). Indeed, GSK-3β is a major target of Akt/PKB (van Weeren et al., 1998), which is activated by the PI-3K-mediated signalling pathway (Ozes et al., 1999). Cellular systems that have been implicated in the regulation of astrocyte apoptosis include the PI-3K pathway (Kim et al., 2001). Inhibition of this signalling cascade has been shown to lead to cell death in several models (Carbott et al., 2002), and this has been attributed, at least in part, to the reduction in activity of PI-3K's major physiological target, Akt. Loss of Akt activity in turn results in the transduction of several proapoptotic signals including sequestration of Bcl-2 and enhanced activation of an Akt substrate, GSK-3β (Pap and Cooper, 1998). We identified proapoptotic transcriptional changes, including upregulation of proapoptotic Bax and downregulation of antiapoptotic Bcl-2, using Western blot assay and by immunohistochemical staining. We report in the present study for the first time that the treatment with TDZD-8 in acute lung injury decreased features of apoptotic cell death after carrageenan administration, suggesting that protection from apoptosis may be a prerequisite for anti-inflammatory approaches. In particular, we demonstrated that the treatment with TDZD-8 lowered the signal for Bax in treated group when compared with lung sections obtained from carrageenan-treated mice, while on the contrary, the signal for Bcl-2 was more highly expressed in TDZD-8-treated mice than in carrageenan-treated mice. This means that TDZD-8 by inhibiting NF-κB prevents the loss of the antiapoptotic pathway. It also reduced the activation of the proapoptotic pathway through a mechanism that is still to be discovered. Taken together, the results of the present study enhance our understanding of the role of GSK-3β in the pathophysiology of acute inflammation. Our results imply that inhibitors of the activity of GSK-3β may be useful in the therapy of inflammation.
Acknowledgments
This study was supported by grant from a University Minister grant. The authors thank Giovanni Pergolizzi and Carmelo La Spada for their excellent technical assistance during this study, Mrs Caterina Cutrona for secretarial assistance and Miss Valentina Malvagni for editorial assistance with the manuscript. CT is a Senior Fellow of the British Heart Foundation (FS 96/018).
Abbreviations
- DMSO
dimethylsulphoxide
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- MDA
malondialdehyde
- MPO
myeloperoxidase
- NF-κB
transcription factor nuclear factor
- NO
nitric oxide
- NOx
nitrite/nitrate
- PBS
phosphate-buffer saline
- PGE2
prostaglandin E2
- PMNs
neutrophils
- TDZD-8
4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione
- GSK-3
glycogen synthase kinase-3
- TUNEL
Terminal deoxynucleotidyltransferase-mediated UTP end labelling
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-κB in PC12 cells. J Neurosci Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- Carbott DE, Duan L, Davis MA. Phosphoinositol 3 kinase inhibitor, LY294002 increases bcl-2 protein and inhibits okadaic acid-induced apoptosis in Bcl-2 expressing renal epithelial cells. Apoptosis. 2002;7:69–76. doi: 10.1023/a:1013517013069. [DOI] [PubMed] [Google Scholar]
- Cohen J, Abraham E. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:116–121. doi: 10.1086/314839. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Costantino G, Mazzon E, Caputi AP. Beneficial effects of raxofelast (IRFI 016), a new hydrophilic vitamin e-like antioxidant, in carrageenan-induced pleurisy. Br J Pharmacol. 1999b;126:407–414. doi: 10.1038/sj.bjp.0702275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, et al. Glycogen Synthase Kinase-3{beta} inhibition reduces secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2006a;318:79–89. doi: 10.1124/jpet.106.102863. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, Van De Loo FA, et al. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162:1859–1866. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Di Paola R, Muià C, Crisafulli C, Dugo L, et al. Glycogen Synthase Kinase-3β inhibition attenuate the degree of arthritis caused by type II collagen in the mouse Clin Immunol 2006b12057–67.in press [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Sautebin L, Dugo L, Serraino I, De Sarro A, et al. Protective effects of Celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem Pharmacol. 2002;63:785–795. doi: 10.1016/s0006-2952(01)00908-x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- Cuzzocrea S, Sautebin L, De Sarro G, Costantino G, Rombola L, Mazzon E, et al. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J Immunol. 1999a;163:5094–5104. [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa b1/p105 stability. J Biol Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- Dugo L, Collin M, Allen DA, Patel NS, Bauer I, Mervaala EM, et al. GSK-3 beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J Clin Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Pettipher ER. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor a in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol. 1989;75:306–310. [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Kim MS, Cheong YP, So HS, Lee KM, Kim TY, Oh J, et al. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem Pharmacol. 2001;61:779–786. doi: 10.1016/s0006-2952(01)00541-x. [DOI] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–332. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Mageed RA, Adams G, Woodrow D, Podhajcer OL, Chernajovsky Y. Prevention of collagen-induced arthritis by gene delivery of soluble p75 tumour necrosis factor receptor. Gene Therapy. 1998;5:1584–1592. doi: 10.1038/sj.gt.3300785. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mukherjee P, Yang SY, Wu B, Song Z, Myers LK, Robbins PD, et al. Tumour necrosis factor receptor gene therapy affects cellular immune responses in collagen induced arthritis in mice. Ann Rheum Dis. 2005;64:1550–1556. doi: 10.1136/ard.2004.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;92:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Grau GE, Vesin C, Loetscher H, Genz R, Lesslauer W. Evolution of collagen arthritis is arrested by treatment with anti-tumour necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. J Immunol. 1992;77:510–514. [PMC free article] [PubMed] [Google Scholar]
- Ruetten H, Thiemermann C. Effect of calpain inhibitor I, an inhibitor of the proteolysis of IkB, on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Br J Pharmacol. 1997;121:695–704. doi: 10.1038/sj.bjp.0701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor a stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Manning PZ, Zweifel BS, Seibert K, Connor J, Currie MG, et al. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulm Pharmacol Ther. 2002;15:439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Wyatt P, Bourdon DM, Marino MH, Manning PT, et al. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautebin L, Ialenti A, Di Rosa M. Relationship between nitric oxide and prostaglandins in carrageenin pleurisy. Biochem Pharmacol. 1998;55:1113–1117. doi: 10.1016/s0006-2952(97)00530-3. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- Sirois J, Levy LO, Simmons DL, Richards JS. Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem. 1993;268:12199–12206. [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, III, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;19:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Nakane M, Kuk J, Budzik G, Klinghofer V, Harris R, et al. The nitric oxide synthase inhibitor, L-NG-monomethylarginine, reduces carrageenan-induced pleurisy in the rat. J Pharmacol Exp Ther. 1995;273:1295–1299. [PubMed] [Google Scholar]
- Van Wauwe J, Haefner B. Glycogen synthase kinase-3 as drug target: From wallflower to center of attention. Drug News Perspect. 2003;16:557–565. doi: 10.1358/dnp.2003.16.9.829337. [DOI] [PubMed] [Google Scholar]
- Van Weeren PC, De Bruyn KM, De Vries-smits AM, Van Lint J, Burgering BM. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;2:57–61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadleigh DJ, Herschman HR. Transcriptional regulation of the cyclooxygenase-2 gene by diverse ligands in murine osteoblasts. Biochem Biophys Res Commun. 1999;264:865–870. doi: 10.1006/bbrc.1999.1606. [DOI] [PubMed] [Google Scholar]
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Whittle BJR, Varga C, Pòsa A, Molnàr A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3β. Br J Pharmacol. 2006;147:575–582. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;100:RE12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Whalen JL, Chapman DL, Berger AE, Richard KA, Aspar DG, et al. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993;36:1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]