Abstract
Background and purpose:
Postural hypotension is a common side-effect of L-DOPA treatment of Parkinson's disease, and may be potentiated when L-DOPA is combined with selegiline, a selective inhibitor of monoamine oxidase B (MAO-B). Rasagiline is a new, potent and selective MAO-B inhibitor, which does not possess the sympathomimetic effects of selegiline. We have studied the effects of these selective MAO inhibitors, L-DOPA and dopamine on the cardiovascular system of the rat.
Experimental approach:
Blood pressure and heart rate was measured in conscious rats following acute or chronic administration of rasagiline, selegiline and L-DOPA, by comparison with the selective MAO-A inhibitor clorgyline, or the MAO-A/B inhibitor tranylcypromine. Cardiovascular responses, catecholamine release, and modification of pressor response to dopamine were studied in pithed rats.
Key results:
In conscious rats neither rasagiline nor selegiline caused significant potentiation of the effects of L-DOPA (50, 100, 150 mg.kg−1) on blood pressure or heart rate at doses which selectively inhibited MAO-B, but L-DOPA responses were potentiated by clorgyline and tranylcypromine. In rats treated twice daily for 8 days with L-DOPA and carbidopa, selegiline (5 mg.kg−1) but not rasagiline (0.2 mg.kg−1) caused a significant hypotensive response to L-DOPA and carbidopa, although both drugs caused similar inhibition of MAO-A and MAO-B. In pithed rats, selegiline but not rasagiline increased catecholamine release and heart rate, and potentiated dopamine pressor response at MAO-B selective dose.
Conclusions and implications:
The different responses to the two MAO-B inhibitors may be explained by the amine releasing effect of amphetamine metabolites formed from selegiline.
Keywords: selegiline, rasagiline, carbidopa, clorgyline, tranylcypromine, L-DOPA, blood pressure, heart rate
Introduction
Despite advances in the development of selective dopamine receptor agonists for treatment of Parkinson's disease, L-dihydroxyphenylalanine (L-DOPA) remains the mainstay of therapy because of its high efficacy. Use of L-DOPA, however, is associated with neurological and other side effects. One of the major extra-neurological side effects of L-DOPA is postural hypotension (Calne et al., 1970); early in the clinical use of L-DOPA, however, it was observed that in depressed Parkinsonian patients who had been treated with a non-selective monoamine oxidase (MAO)-A/B inhibitor, administration of a small dose of L-DOPA elicited a pronounced hypertensive response which could be controlled with an α-adrenoceptor antagonist (Hunter et al., 1970). Following the discovery of the selective MAO-B inhibitory property of selegiline (Knoll and Magyar, 1972), it was found that this drug could be combined with L-DOPA without a hypertensive response (Birkmayer et al., 1975). Subsequently, however, selegiline in conjunction with L-DOPA was shown to cause an exaggerated orthostatic hypotension (Turkka et al., 1997; Churchyard et al., 1997, 1999; Montastruc et al., 2000).
Selegiline is metabolized in the body to L-methamphetamine and L-amphetamine (Reynolds et al., 1978), and amphetamines may therefore play a role in the cardiovascular response to selegiline/L-DOPA combination. Amphetamine-like drugs have indeed been shown to cause hypotension (Gill et al., 1967; Martin et al., 1971), as well as hypertension (Bexis and Docherty, 2006), and we have recently shown that L-methamphetamine (1 mg kg−1 intravenously (i.v.)) causes hypotension in conscious rats (Abassi et al., 2004). Rasagiline is a new selective MAO-B inhibitor which has recently been approved for treatment of Parkinson's disease, and is not metabolized to amphetamines (Finberg and Youdim, 2002; Chen and Swope, 2005). We were therefore interested to compare the effects of rasagiline and selegiline on modulation of the action of L-DOPA on the rat blood pressure. Cardiovascular responses to L-DOPA in conscious rats were studied both with and without administration of carbidopa, a peripherally acting inhibitor of aromatic amino-acid decarboxylase (AAADC). In the absence of carbidopa, any potential interactions between L-DOPA and the MAO inhibitors would be mainly at the level of peripheral blood vessels, whereas in the presence of carbidopa, effects of L-DOPA are mainly those resulting from actions within the CNS. Although patient treatment with L-DOPA is invariably carried out with the addition of a peripherally acting AAADC inhibitor, it was thought also of importance to study the effects of L-DOPA/MAO-B inhibitor combination without the AAADC inhibitor in order to evaluate the potential risk of cardiovascular side effects in the absence of effective AAADC inhibition. In order to further evaluate the effects of the two MAO-B inhibitors on the cardiovascular system, we also compared their effects on release of catecholamines, and potentiation of pressor response to dopamine, in the pithed rat preparation.
Rasagiline and selegiline are propargylamine type, irreversible MAO inhibitors, which bind covalently to the enzyme-active site. As a result, much lower doses of the inhibitors are required on chronic than on acute administration, because of the cumulative effect of successive doses on enzyme inhibition. In the current study, we used both acute and chronic treatment protocols, and in each case, low, MAO-B-selective doses of rasagiline and selegiline were used, in order to study the effects of selective inhibition of MAO-B, as well as higher doses, which inhibit the MAO-A enzyme to a progressively increasing extent. In order to achieve substantial MAO-B inhibition, higher doses were administered in the acute than in the chronic treatment protocols. Because rasagiline is 3–15 times more potent than selegiline for inhibition of MAO in rats (Youdim et al., 2001), the doses of rasagiline used in this study were one-fifth those of selegiline. The effects of the selective MAO-B inhibitors were compared with the selective MAO-A inhibitor clorgyline, and the MAO-A/B inhibitor tranylcypromine.
Methods
Animals
Sprague–Dawley-derived male rats (250–300 g body weight) were obtained from the Rappaport Medical Faculty in-house colony for experiments with conscious animals, or from Harlan (Jerusalem, Israel) in the pithed rat study. Animals were maintained at 19–21°C environmental temperature, 12/12 h light/dark cycle, and fed standard rat chow and tap water ad lib. All procedures were authorized by the Technion Animal Ethics Committee, which follows the Guidelines Concerning the Care and Use of Laboratory Animals (National Institutes of Health, USA).
Conscious rat blood pressure and heart rate determination
Rasagiline or selegiline was administered orally by gavage either once only (acute) or once daily for 8 or 21 days (chronic). In the acute and 8-day chronic administration protocols, indwelling polyethylene catheters were implanted in the caudal artery, and in the chronic administration protocol they were implanted in the left femoral artery (no connection between choice of catheter implantation technique and dosing protocol). For catheter implantation, rats were anaesthetized using a mixture of pentobarbital and chloral hydrate (12:60 mg kg−1 i.p.). The arterial catheters were fabricated from a short length of fine polyethylene tubing (OD 0.8 mm), for introduction into the artery, joined to a longer length of wider tubing (OD 0.96 mm), which was lead under the skin of the back to an exit point behind the neck. The catheter was filled with physiological saline (saline; 0.9% NaCl) containing heparin (100 U ml−1) and plugged with a steel pin. Penicillin (20 000 U) was administered i.m. after surgery. The animals were housed separately in polyethylene cages, and recovered in the laboratory overnight. On the morning after surgery, the arterial line was connected to a pressure transducer (Statham P23dB) via saline-filled polyethylene tubing with light physical restraint of the animal and pulsatile blood pressure trace was displayed on a physiological recorder. Mean arterial blood pressure (MAP) was measured using the formula: MAP=DBP+(PP × 0.33), where DBP=diastolic blood pressure, PP=pulse pressure. Heart rate (HR) was counted from the pulsatile pressure trace.
Drug treatments in conscious rat experiments
In acute treatment experiments, surgery for implantation of arterial catheters was completed 24 h before the experiment. The following day, rats were connected to the arterial transducer as described above and then the MAO inhibitor drug was administered by gavage. After 2 h (the time required for maximal inhibition of MAO in brain and peripheral tissues), blood pressure response was determined to oral administration of water (1 ml). After 30 min, L-DOPA administration was commenced, at doses of 50, 100 and 150 mg kg−1 with 30 min between doses.
Other rats received oral doses of selegiline and rasagiline once daily for 21 days, the last dose being just before catheter implantation on day 21. On the measurement day (day 22), arterial lines were connected to transducers as described above, and the animals were then allowed to settle down for 45 min before oral administration of water followed by L-DOPA as described above. In this protocol, responses to L-DOPA were measured 24 h after the last dose of MAO inhibitor, so that the extent of MAO inhibition would have been the same as in our previous study (Youdim et al., 2001).
In preliminary experiments, no significant changes in MAP and HR were observed in control rats following a single dose of L-DOPA (100 mg kg−1). It was therefore decided to administer the three doses of L-DOPA (50, 100 and 150 mg kg−1) in a cumulative manner, with 30 min between doses, in order to maximize the L-DOPA response. Responses to 50 and 100 mg kg−1 doses of L-DOPA are expressed as the difference between the value 30 min after administration and the baseline value, measured just before administration of the 50 mg kg−1 dose. Responses to the 150 mg kg−1 dose were measured 60 min after administration, as this was the time required for maximum development of pressor response.
In the combined L-DOPA/carbidopa (LD/CD) experiment, MAO inhibitors were administered once daily for 7 days at 0830 hours, and LD/CD (50 mg kg−1 L-DOPA and 12.5 mg kg−1 carbidopa) was administered twice daily, at 0830 and 1530 hours. Rats were anaesthetized for implantation of arterial catheters on day 7. On day 8, the MAO inhibitors were administered at the usual time, the arterial lines were then connected to transducers and the animals allowed to settle down for 1 h. Blood pressure and HR were then determined every 15 min for 45 min, followed by oral administration of the usual LD/CD combination, and MAP and HR were followed every 15 min for 2 h. In this experimental protocol, LD/CD was administered 2 h after MAO inhibitors, in order to model the human treatment situation.
Pithed rat preparation
Rats were pithed under halothane anaesthesia (3% in oxygen) and ventilated with a Harvard Apparatus (Boston, MA, USA) rodent ventilator at a stroke volume of 1 ml per 100 g body weight and a rate of 60 strokes per min. Systemic blood pressure was measured from a carotid artery, and drugs were injected via a jugular vein cannula. The animal's body temperature was maintained at 37°C by means of a thermostatically controlled warming pad.
Following completion of surgery, a period of 60 min was allowed for decay of catecholamines released during pithing. Rasagiline and selegiline were then administered at doses of 0.2 and 1.0, or 1 and 5 mg kg−1, respectively with 30 min between doses. Arterial blood samples (0.25 ml) were taken after the 60 min equilibration period and 30 min after each drug dose, and donor rat blood of the same volume was administered i.v. Blood pressure and HR values were determined after the 60 min baseline period, and 30 min after test drug administration, before blood sampling. Modification of the effects of dopamine on blood pressure and HR by rasagiline and selegiline was studied in separate experiments. Bolus doses of dopamine (5, 10, 20 and 40 μg kg−1) were administered i.v. after the 60 min equilibration period and 30 min after each dose of MAO inhibitor. Area under the diastolic blood pressure curve following each dopamine response was determined by computerized planimetry, and the response after MAO inhibitor was expressed as a ratio of the response before MAO inhibitor administration.
Determination of plasma catecholamines
Catecholamines in plasma were extracted on acid washed alumina, as described by Medvedev et al. (1990). Samples (20 μl) of the perchloric acid eluates from the alumina were injected into the mobile phase of an HPLC system equipped with a 50 × 4.6 mm; 3-μm particle Spherisorb S30DS2 reversed phase column (Regis Inc., Morton Grove, IL, USA) at 30°C. The mobile phase (1 ml min−1) consisted of 6.9 g NaH2PO4, 200 mg heptane-1-sulphonic acid (sodium salt), 80 mg disodium ethylene-diaminetetra-acetic acid (Na2EDTA) and 30 ml methanol per litre, and was adjusted to pH 2.6 using ortho-phosphoric acid. Catecholamines and metabolites in the eluate were detected using a model 5100A Coulochem detector (ESA Inc., Chelmsford, MA, USA) with model 5011 analytical cell and model 5020 guard cell (ESA, USA). Guard cell potential was set at +300 mV. The first electrode of the dual-electrode analytical cell was set at +60 mV and the second (measuring electrode) was set at −350 mV. Output of the detector was analysed using Borwin (Jasco Inc., Easton, MD, USA) software, and quantitation of peaks was carried out by comparing peak heights of samples with those of standard solutions, taking into account recovery of an internal standard (dihydroxybenzylamine).
Determination of MAO inhibition
The activities of MAO-A and -B were determined as described by Youdim et al. (2001). Tissues were homogenized in 0.3 M sucrose (one part tissue to 20 parts sucrose) using a glass-teflon motor-driven homogenizer. The crude enzyme preparation was added to 0.05 M phosphate buffer (pH 7.4) and incubated together with selegiline 0.1 μM (for determination of MAO-A) or clorgyline 0.1 μM (for determination of MAO-B) for 60 min at 37°C. Labelled substrates (14C-5-hydroxytryptamine creatinine disulphate 100 μM for determination of MAO-A, or 14C-phenylethylamine 10 μM for determination of MAO-B) were added and incubation continued for 30 or 20 min, respectively. The reaction was then stopped by addition of citric acid (2 M). Radioactive metabolites were extracted into toluene/ethyl acetate (1:1 v v−1), a solution of 2,5-diphenyloxazole was added to a final concentration of 0.4% (w v−1), and metabolite content estimated by liquid scintillation counting. Activity in the presence of drug was expressed as a percentage of that in control samples.
Statistics
Statistical analysis was carried out using one-way ANOVA (Graphpad ‘Prism') for MAO and baseline data, or two-way repeated-measures ANOVA for conscious rat experiments (Graphpad ‘Prism' or SPSS, Israel, Ltd, version 9.0). In the experiments using three doses of L-DOPA, the variables in two-way ANOVA were drug and L-DOPA dose. In the LD/CD experiment, variables were drug treatment and time after LD/CD. Paired data were compared using the Student's ‘t'-test.
Drugs
L-DOPA (Teva, Israel) was suspended in 0.25% sodium carboxymethylcellulose in water immediately before oral administration. Other drugs administered orally were dissolved in water. Drugs administered i.v. were dissolved in 0.9% saline. Carbidopa was supplied by Teva (Israel). Other drugs were obtained from Sigma (Israel). Radioactive compounds were obtained from Amersham/Searle (Arlington Heights, IL, USA) (specific activities 1.85-2.29 GBq mmol−1). Doses of rasagiline and selegiline refer to the base.
Results
Modification of blood pressure and HR responses in conscious rats
Baseline values of MAP and HR
Baseline values of MAP and HR differed between the rats used in acute and chronic drug administration experiments (Table 1), but within each experiment (i.e. acute, 8-day, 21-day treatments) the baseline values were not significantly different between the MAO-B inhibitor and control treatments. Baseline MAP values differed between the acute tranypcypromine and acute control group. For rats in the control groups given water only, values of MAP were highly significantly different (P<0.001) between the acute and either 8- or 21-day treatments but non-significantly different between 8- and 21-day groups; baseline HR values were significantly different between 21- and 1-day groups. This difference in baseline values was not related to method of arterial cannulation, as this was the same for acute and 21-day treatments, but appears to be the result of a change in the breeding colony over time (the 1-day experiments were performed several months before the 8- and 21-day experiments).
Table 1.
Baseline values of MAP and HR in conscious rat experiments
| Dose (mg kg−1 × days) | MAP (mm Hg) | HR beats (min−1) | Dose (mg kg−1 × days) | MAP (mm Hg) | HR beats (min−1) | |
|---|---|---|---|---|---|---|
| Control | Water × 1 (8) | 96±1.3 | 360±13 | Water × 8 (14) | 120±3.5a | 382±7.5 |
| Rasagiline | 0.2 × 1 (7) | 99±3 | 356±3 | 0.2 × 8 (10) | 118±3.8 | 414±9 |
| 1 × 1 (6) | 97±2.8 | 350±13 | 1 × 8 (13) | 119±2.7 | 374±5.8 | |
| Selegiline | 1 × 1 (6) | 98±2 | 360±17 | 1 × 8 (11) | 119±4.3 | 395±7.5 |
| 5 × 1 (7) | 97±2 | 364±15 | 5 × 8 (12) | 124±1.8 | 399±6.8 | |
| Clorgyline | 2 × 1 (4) | 103±4 | 360±17 | |||
| Tranylcypromine | 25 × 1 (5) | 107±3.7b | 360±21 | |||
| Water × 21 (7) | 121±4a | 459±21a | ||||
| 0.1 × 21 (8) | 113±1.9 | 414±11 | ||||
| 0.5 × 21 (8) | 116±2.7 | 398±8 | ||||
| 0.5 × 21 (6) | 116±2.4 | 440±29 | ||||
| 1 × 21 (7) | 118±3.4 | 375±18 |
Abbreviations: HR, heart rate; MAP, mean arterial blood pressure.
Values are means±s.e.mean Numbers of animals per group for each treatment shown in parentheses.
P<0.001.
P<0.01 for difference from 1-day control.
L-DOPA without decarboxylase inhibitor
Initially, the effect of acute treatment with MAO inhibitors on cardiovascular responses to L-DOPA was determined without peripheral AAADC inhibition. Modification of L-DOPA response by selective inhibition of MAO-A was determined using the MAO-A-selective inhibitor clorgyline, and the effect of combined MAO-A/B inhibition was studied using tranylcypromine. In control rats, the administration orally of water, or L-DOPA (50, 100 and 150 mg kg−1; Figure 1a and b) produced no significant change in MAP or HR. Following acute administration of clorgyline (2 mg kg−1) or tranylcypromine (25 mg kg−1), there was an increased pressor response to the highest dose level of L-DOPA (Figure 1a). Clorgyline-treated rats had a significant tachycardic response to the 100 mg kg−1 dose of L-DOPA and in tranylcypromine-treated rats, there was a significant tachycardia following the 150 mg kg−1 dose of L-DOPA (Figure 1b).
Figure 1.
(a) Pressor responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) 2 h after treatment with a single dose of clorgyline (2 mg kg−1) or tranylcypromine (25 mg kg−1). Increment above baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg−1) L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 4–5 (clorgyline, tranylcypromine) or 8 (control) rats per treatment group. **P<0.01, ***P<0.001 for difference from corresponding control value by one-way ANOVA. (b) HR responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) 2 h after treatment with a single dose of clorgyline (2 mg kg−1) or tranylcypromine (25 mg kg−1). Change from baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg−1) after L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 4–5 (clorgyline, tranylcypromine) or 8 (control) rats per treatment group. *P<0.05, **P<0.01 for difference from corresponding control value by one-way ANOVA.
In rats treated acutely with rasagiline (0.2, 1 mg kg−1) or selegiline (1, 5 mg kg−1), there was no significant change in MAP or HR following any of the L-DOPA doses used (Figure 2a and b). In animals treated chronically (21 days) with selegiline (0.5, 1.0 mg kg−1) or rasagiline (0.1, 0.5 mg kg−1), there was also no significant change in MAP or HR following the three challenge doses of L-DOPA when all treatments were compared by repeated-measures two-way ANOVA. When all responses in each drug treatment group were compared with the control group by ANOVA, a marginally significant increase in MAP was seen in the group given 0.5 mg kg−1 rasagiline (P=0.049, F1,13=4.71), but no significant difference between rasagiline and control was seen for each L-DOPA dose separately by post hoc test (Figure 3a and b).
Figure 2.
(a) Pressor responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) 2 h after treatment with a single oral dose of rasagiline (rasa) 0.2 or 1 mg kg−1, or selegiline (sele) 1 or 5 mg kg−1. Increment of MAP above baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg-1) after L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 5–7 (rasagiline, selegiline) or 8 (control) rats per treatment group. (b) HR responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) 2 h after treatment with a single oral dose of rasagiline (rasa) 0.2 or 1 mg kg−1, or selegiline (sele) 1 or 5 mg kg−1. Change in HR from baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg−1) after L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 5–7 (rasagiline, selegiline) or 8 (control) rats per treatment group.
Figure 3.
(a) Pressor responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) following treatment with a daily dose of rasagiline (rasa) 0.2 or 1 mg kg−1, or selegiline (sele) 1 or 5 mg kg−1 for 3 weeks. Increment of MAP above baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg−1) after L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 5–7 (rasagiline, selegiline) or 8 (control) rats per treatment group. *P<0.05. (b) HR responses of conscious rats to oral administration of L-DOPA (50, 100 and 150 mg kg−1) following treatment with a daily dose of rasagiline (rasa) 0.2 or 1 mg kg−1, or selegiline (sele) 1 or 5 mg kg−1 for 3 weeks. Increment of MAP above baseline values shown at 30 min (50, 100 mg kg−1 L-DOPA) or 60 min (150 mg kg−1) after L-DOPA, which was the time of maximal blood pressure response. Mean±s.e.mean shown for 5–7 (rasagiline, selegiline) or 8 (control) rats per treatment group.
Rats given L-DOPA and clorgyline or tranylcypromine showed mild behavioural stimulation (increased alertness and locomotor activity), but no overt behavioural or other effects were seen following L-DOPA and the MAO-B inhibitors. The extent of MAO inhibition by rasagiline and selegiline following once only, and once daily for 21 days administration has been previously reported from our laboratory, under exactly the same conditions of rat strain and holding as used in this study (Youdim et al., 2001). Following a single dose, rasagiline would have produced more than 90% inhibition of MAO-B, and 0 or 20% inhibition of MAO-A at 0.2, 1.0 mg kg−1 respectively. On chronic treatment, the degree of inhibition would have been more than 90% for MAO-B, and 30 or 40% for MAO-A at 0.1 and 0.5 mg kg−1, respectively. The single dose of selegiline (1 and 5 mg kg−1) would have produced 65 and 90% inhibition of MAO-B, respectively, and 0% inhibition of MAO-A, whereas the chronic administration (0.5 and 1 mg kg−1) would have produced 80% inhibition of MAO-B and 0–20% inhibition of MAO-A.
L-DOPA and carbidopa
In an additional experiment in conscious rats, rasagiline or selegiline were administered for 8 days, together with LD/CD combination, and cardiovascular response to LD/CD was measured on day 8. There was no significant difference between baseline levels of MAP and HR measured on day 8 in the 45 min before LD/CD administration between the control group and those in rats treated with rasagiline or selegiline (Table 1). In this experiment, there was a trend for MAP to decrease following LD/CD, and this effect was not significantly altered in animals treated with 0.2 or 1 mg kg−1 rasagiline (see Figure 4a). HR was slightly increased by LD/CD, and this effect was not altered by the lower dose of rasagiline (0.2 mg kg−1) but was significantly enhanced by the higher dose (1 mg kg−1; Figure 4b). In rats treated with the lower dose of selegiline (1 mg kg−1), there was no significant change in the MAP or HR responses to LD/CD, but in the rats treated with the higher dose (5 mg kg−1) the depressor response to LD/CD, but not the tachycardic response, was significantly enhanced (Figure 5a and b). As the conditions in the LD/CD experiment were different (8 days administration, addition of LD/CD), the extent of inhibition of brain MAO-A and MAO-B was determined in these animals. Mean data for brain MAO inhibition are shown in Figure 6a. In all MAO inhibitor-treated rats, MAO-B was inhibited by more than 90% but the extent of MAO-A inhibition was greater with rasagiline than selegiline.
Figure 4.
(a) Change in MAP following a single oral dose of LD/CD combination (50/12.5 mg kg−1) administered at time 0 in rats treated daily for 7 days with water (control; n=14), or rasagiline (rasa) 0.2 (n=10) or 1 (n=13) mg kg−1 once daily, and LD/CD twice daily. Water or rasagiline was administered 1 h before start of blood pressure recording on day 8. (b) Change in HR following a single oral dose of LD/CD combination (50/12.5 mg kg−1) administered at time 0 in rats treated daily for 7 days with water (control; n=14), or rasagiline (rasa) 0.2 (n=10) or 1 (n=13) mg kg−1 once daily, and LD/CD twice daily. Water or rasagiline was administered 1 h before start of blood pressure recording on day 8. ***P<0.001 (two-way ANOVA with repeated measures) for difference from control between 75 and 120 min after LD/CD.
Figure 5.
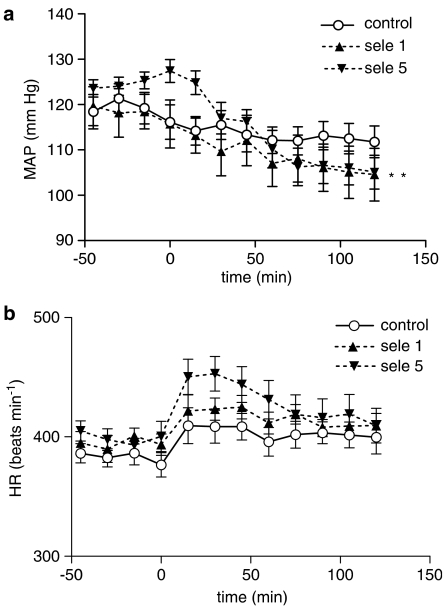
(a) Change in MAP following a single oral dose of LD/CD combination (50/12.5 mg kg−1) administered at time 0 in rats treated daily for 7 days with water (control; n=14), or selegiline (sele) 1 (n=11) or 5 (n=12) mg kg−1 once daily, and LD/CD twice daily. Water or selegiline was administered 1 h before start of blood pressure recording on day 8. **P<0.01 (two-way ANOVA with repeated measures) for difference from control between 75 and 120 min after L-DOPA (selegiline 5 mg kg−1). (b) Change in mean HR following a single oral dose of LD/CD combination (50/12.5 mg kg−1) administered at time 0 in rats treated daily for 7 days with water (control; n=14), or selegiline (sele) 1 (n=11) or 5 (n=12) mg kg−1 once daily, and LD/CD twice daily. Water or selegiline was administered 1 h before start of blood pressure recording on day 8.
Figure 6.
Inhibition of MAO-A and -B by rasagiline and selegiline. (a) Cerebral cortical MAO in rats treated for 8 days with selegiline or rasagiline (once) and LD/CD (twice) daily. N=5–8 (rasagiline) and 5–10 (selegiline). (b) Hepatic MAO in pithed rats. **P<0.01 for difference between rasagiline 0.2 mg kg−1 and selegiline 1.0 mg kg−1 by ANOVA. N=9 (saline) and 11 (selegiline). % Inhibition of MAO activity=100−((MAO activity in treated/MAO activity in control tissues) × 100).
Modification of blood pressure responses and catecholamine release in the pithed rat
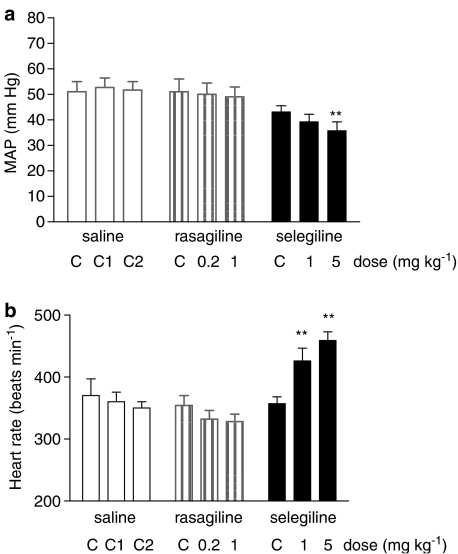
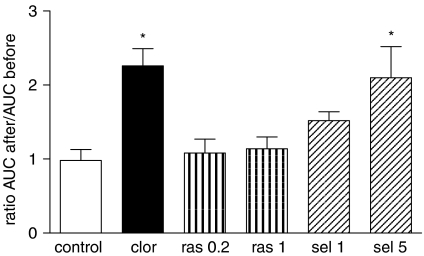
Following i.v. injection of rasagiline and selegiline in pithed rats hepatic MAO was inhibited to a similar extent (Figure 6b), although the extent of MAO-B inhibition by the lower dose of rasagiline (0.2 mg kg−1) was significantly less than that produced by the lower dose of selegiline (1.0 mg kg−1). A dose of 1 mg kg−1 of each compound caused >90% inhibition of MAO-B and about 20% inhibition of MAO-A. Selegiline caused a significant increase in HR at the two doses used (1 and 5 mg kg−1; Figure 7) and a significant fall in blood pressure at the higher dose. In six out of 11 experiments, selegiline caused a slowly developing increase in pulse pressure, although no significant effect on mean pulse pressure was seen for the whole group of animals. Rasagiline had no significant effect on blood pressure or HR at either dose used (Figure 7), and did not cause a similar effect on pulse pressure to that of selegiline in any of the nine experiments in which it was administered. Selegiline caused a significant increase in plasma noradrenaline at 1 and 5 mg kg−1, and a significant increase in plasma adrenaline concentrations at 5 mg kg−1 (Figure 8), whereas rasagiline was without significant effect on plasma catecholamines. I.v. injection of dopamine (5–40 μg kg−1) produced a brief pressor response whose extent and duration was potentiated by clorgyline at a dose which caused >90% inhibition of MAO-A with minimal inhibition of MAO-B (Figure 9). Because both extent and duration of response were increased by MAO-A inhibition, responses were expressed as area under the curve. Pressor responses to dopamine were not potentiated by rasagiline, but were significantly enhanced by selegiline at the dose of 5 mg kg−1 (Figure 9). As the extent of potentiation of the different doses of dopamine was the same, only the result at 20 μg kg−1 is shown in Figure 9 for sake of clarity.
Figure 7.
Effects of rasagiline and selegiline on blood pressure (a) and HR (b) of pithed rats. Baseline values (C) were measured 60 min after pithing. Post-drug values were measured 30 min after second and third injections of saline (C2, C3), or after rasagiline (0.2, 1.0 mg kg−1) or selegiline (1, 5 mg kg−1). **P<0.01 for difference from baseline values by ANOVA with repeated measures. N=8 (saline) or 11 (rasagiline, selegiline).
Figure 8.
Plasma concentrations of noradrenaline (a) and adrenaline (b) in pithed rats. Baseline values (C) were measured 60 min after pithing. Second and third values measured 30 min after saline injections (C2, C3) or after rasagiline (0.2, 1.0 mg kg−1) or selegiline (1, 5 mg kg−1) injections i.v. *P<0.05, **P<0.01 for difference from baseline values by ANOVA with repeated measures and t-test post hoc.
Figure 9.
Modification of pressor responses to dopamine by clorgyline (2 mg kg−1), rasagiline (0.2, 1 mg kg−1) and selegiline (1, 5 mg kg−1) in pithed rats. *P<0.05 by ANOVA (Dunnett's test).
Discussion and conclusions
The effects of L-DOPA on cardiovascular function are complex, and involve several receptor systems, some of which exert opposing effects. The drug exerts its effects mainly via conversion to dopamine, but the possibility of an action on its own receptors, must also be considered (Misu and Goshima, 1993). The initial biotransformation of the L-DOPA molecule is its decarboxylation by AAADC with the formation of dopamine. This reaction can occur in catecholaminergic neurons and elsewhere, owing to the ubiquitous occurrence of AAADC (Melamed et al., 1980). When L-DOPA is administered without a peripherally acting AAADC inhibitor dopamine will therefore be formed in a variety of peripheral tissues.
Cardiovascular effects of dopamine in the whole animal and man are predominantly the result of activation of β-adrenoceptors and dopamine receptors at lower dose levels, with an increasing activation of α-adrenoceptors at higher doses (Goldberg and Rajfer, 1985). Dopamine activates both D1- and D2-type receptors in the periphery. Agonist effects at D1-type receptors cause vasodilatation by direct action at vascular smooth muscle cells; agonist effects at presynaptic D2-type receptors cause vasodilatation indirectly, by reduction in release of noradrenaline, and agonist effects at post-synaptic D2 receptors may reduce D1-mediated vasodilatation by inhibition of adenylate cyclase (Goldberg and Kohli, 1983; Goldberg and Rajfer, 1985; Kobayashi et al., 1994; Zanzottera et al., 1998). In addition, dopamine displaces noradrenaline from neuronal storage sites (Henning and Rubenson, 1970; Dayan and Finberg, 2003). The pressor responses to dopamine were shown by Nichols and Ruffolo (1987) to be mediated by activation of α1 and α2 adrenoceptors. Although the pressor responses are not affected by reserpinization, the chronotropic responses are reduced. Cardiovascular effects of L-DOPA in the whole organism will be the resultant of actions of dopamine formed in the CNS as well as peripheral effects on the heart and vascular system.
In the present study in conscious rats, L-DOPA administered orally caused nonsignificant changes in MAP and HR, at doses of 50–150 mg kg−1. Following parenteral administration, L-DOPA causes pressor responses in rats. Henning and Rubenson (1970) observed a pressor response from L-DOPA administered i.p. (50–200 mg kg−1) in conscious Sprague–Dawley rats. In conscious Wistar rats given L-DOPA i.v., doses up to 50 μmol kg−1 h−1 (i.e. 1 mg kg−1 h−1) caused no change in blood pressure, but evoked a vasodilatatory response in mesenteric, renal and skeletal muscle vascular beds, which is presumably compensated by a positive inotropic cardiac response, as HR did not change (Drieman et al., 1994). At the dose of 200 μmol kg−1 h−1, increased MAP and tachycardia was seen. Corresponding responses were seen with dopamine. The effects of L-DOPA administered i.v. will be considerably greater than those resulting from the same dose administered orally, owing to the extensive first-pass metabolism of L-DOPA in the gastrointestinal tract and liver. Following administration of clorgyline or tranylcypromine, L-DOPA caused pronounced pressor and tachycardic responses (Figure 1), as is also seen in human subjects treated with non-selective inhibitors of MAO-A and -B (Hunter et al., 1970). These responses probably represent increased activation of vasoconstrictor α-adrenoceptor-mediated effects, which overcome the D1/D2-mediated vasodilatatory peripheral effects of dopamine released from the L-DOPA.
When combined with a peripherally acting inhibitor of AAADC (MK486), administration of L-DOPA to conscious rats caused a reduction in blood pressure (Henning and Rubenson, 1970). In spontaneously hypertensive rats (Yamori et al., 1972), L-DOPA caused a depressor response which was more prolonged in the presence of an MAO inhibitor, and was also magnified by pretreatment with a peripherally acting inhibitor of AAADC (Yamori et al., 1972). The depressor response to L-DOPA with MK486 was blocked by transection of the CNS below, but not above, the brain stem (Henning et al., 1972), which, together with the increased tissue noradrenaline level in brains of rats treated with L-DOPA, pointed to a role of brain stem noradrenaline levels in causing the fall in blood pressure (Yamori et al., 1972).
In the current study, MAP decreased following administration of LD/CD in all groups of rats, but it is not possible to conclude that this hypotensive effect is due to LD/CD, because all animals in the experiment received LD/CD. In rats treated with selegiline (5 mg kg−1), the hypotensive effect following LD/CD was significantly greater than that seen in control rats. Mean MAP in rasagiline-treated rats given LD/CD did not change significantly from those given saline and LD/CD, but HR increased in the rats treated with rasagiline 1 mg kg−1. Both doses of selegiline and rasagiline caused nearly complete inhibition of brain MAO-B, but the inhibition of brain MAO-A by rasagiline at 0.2 mg kg−1 was equivalent to that produced by selegiline at the dose of 5 mg kg−1 (26%). The higher dose of rasagiline caused a tachycardic response following LD/CD, but this dose caused a greater degree of inhibition of MAO-A (65%). On the other hand, rasagiline-treated rats did not show a hypotensive response following LD/CD as opposed to that seen in rats pretreated with selegiline, so this response is possibly related to the amphetamine-like properties of selegiline. These results indicate that rasagiline may possess a smaller hypotensive potential than selegiline when the drugs are given together with LD/CD.
When rasagiline and selegiline were administered i.v. to pithed rats, it was found that a dose of 1 mg kg−1 of both inhibitors caused a similar pattern of inhibition of hepatic MAO-A and MAO-B, in spite of the fact that rasagiline is 5–10 times more potent than selegiline by the oral route. This is probably because of the high degree of first-pass hepatic metabolism of selegiline (Laine et al., 2000). A single dose of selegiline (1 mg kg−1), however, sufficient to selectively inhibit MAO-B but not MAO-A, caused sympathomimetic effects in the pithed rat, as evidenced by increased HR and elevated plasma noradrenaline concentration. The higher dose of selegiline (5 mg kg−1) also increased adrenaline release, and potentiated the pressor response to dopamine. By contrast, rasagiline produced none of these sympathomimetic effects, although at 1 mg kg−1 it produced a similar degree of MAO-A and MAO-B inhibition to that produced by selegiline 5 mg kg−1. The sympathomimetic actions of selegiline may be explained by the amine-releasing effect of its amphetamine metabolites, as described previously (Simpson, 1978; Finberg et al., 1980a, 1980b).
Dopamine is a substrate for both type A and type B MAO in the rat, and the two enzyme forms show a similar affinity for this substrate (Strolin-Benedetti et al., 1989). The fact that the pressor effect of dopamine was potentiated by clorgyline but not by rasagiline is further evidence for a noradrenaline-releasing action of dopamine, as the degradative metabolism of dopamine would be affected to the same degree by clorgyline and rasagiline, but clorgyline would enhance the non-exocytotic amine-releasing effect of dopamine by increasing the cytosolic neuronal noradrenaline pool (the form of MAO associated with sympathetic neurons is MAO-A; Jarrott and Iversen, 1971). The fact that significant vasodepressor responses were not seen following any dose of L-DOPA in the MAO-inhibited conscious rats may be an additional indication of the major role of noradrenaline released by dopamine in mediating the cardiovascular responses to both L-DOPA and dopamine.
The mechanism of potentiation of dopamine's pressor effect by selegiline would require further study, but may be the result of inhibition of amine uptake with resultant increased post-synaptic effect of dopamine on α-adrenoceptors. Selegiline has also been claimed to facilitate neuronal noradrenaline and dopamine release (Knoll et al., 1996; Knoll, 1998). In human Parkinsonian patients treated with selegiline, increased levels of plasma noradrenaline were seen following L-DOPA administration (Stryjer et al., 2005).
In conclusion, the present study shows that only minimal cardiovascular effects occur in the conscious rat following selective MAO-B inhibition and high doses of L-DOPA without AAADC inhibition. This study also accentuates our previous findings of lack of sympathomimetic effect of rasagiline by contrast with the sympathomimetic effects of selegiline. Hypotensive effects of selegiline/L-DOPA combination are probably mediated by CNS mechanisms, as they were not seen in the absence of peripheral AAADC inhibition. Extrapolation of these data to human Parkinsonian patients, however, is limited because of the sympathetic denervation which accompanies this disease (Goldstein et al., 2000).
Acknowledgments
This study was supported by a research grant from Teva Inc.
Abbreviations
- AAADC
aromatic amino-acid decarboxylase
- HR
heart rate
- L-DOPA
(L)-3,4-dihydroxyphenylalanine
- LD/CD
L-DOPA/carbidopa combination
- MAO
monoamine oxidase
- MAP
mean arterial blood pressure
Conflict of interest
JPMF and MBHY will profit from the sale of rasagiline, which is currently marketed by Teva Pharmaceutical Industries Ltd, Israel.
References
- Abassi ZA, Binah O, Youdim MBH. Cardiovascular activity of rasagiline, a selective and potent inhibitor of mitochondrial monoamine oxidase B: comparison with selegiline. Br J Pharmacol. 2004;143:371–378. doi: 10.1038/sj.bjp.0705962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer W, Riederer P, Youdim MBH, Linauer W. The potentiation of the anti-akinetic effect after L-DOPA treatment by an inhibitor of MAO-B, deprenyl. J Neural Transm. 1975;35:303–326. doi: 10.1007/BF01253131. [DOI] [PubMed] [Google Scholar]
- Calne DB, Brennan J, Spiers ASD, Stern GM. Hypotension caused by L-Dopa. BMJ. 1970;1:474–475. doi: 10.1136/bmj.1.5694.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Swope DM. Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease. J Clin Pharmacol. 2005;45:878–894. doi: 10.1177/0091270005277935. [DOI] [PubMed] [Google Scholar]
- Churchyard A, Mathias CJ, Lees AJ. Autonomic effects of selegiline: possible cardiovascular toxicity in Parkinson's disease. J Neurol Neurosurg Psychiat. 1997;63:228–234. doi: 10.1136/jnnp.63.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard A, Mathias CJ, Lees AJ. Selegiline-induced postural hypotension in Parkinson's disease: a longitudinal study on the effects of drug withdrawal. Movement Disord. 1999;14:246–251. doi: 10.1002/1531-8257(199903)14:2<246::aid-mds1008>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dayan L, Finberg JPM. L-DOPA increases noradrenaline turnover in central and peripheral nervous systems. Neuropharmacology. 2003;45:524–533. doi: 10.1016/s0028-3908(03)00190-4. [DOI] [PubMed] [Google Scholar]
- Drieman JC, Van Kan FJPM, Thijssen HHW, Van Essen H, Smits JFM, Struijker Doudier HAJ. Regional haemodynamic effects of dopamine and its prodrugs L-dopa and gludopa in the rat and in the glycerol-treated rat as a model for acute renal failure. Br J Pharmacol. 1994;111:1117–1122. doi: 10.1111/j.1476-5381.1994.tb14860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg JPM, Sabbagh A, Youdim MBH.Pharmacology of selective propargyl ‘suicide' inhibitors of monoamine oxidase Enzymes and Neurotransmitters in Mental Disease 1980aWiley: London; 205–219.In: Usdin TL, Sourkes E, Youdim MBH (eds) [Google Scholar]
- Finberg JPM, Tenne M, Youdim MBH.Selective irreversible propargyl derivative inhibitors of monoamine oxidase (MAO) without the cheese effect Monoamine Oxidase Inhibitors - The State of the Art 1980bWiley: London; 31–43.In: Youdim MBH, Paykel ES (eds) [Google Scholar]
- Finberg JPM, Youdim MBH. Pharmacological properties of the anti-Parkinson drug rasagiline; modification of endogenous brain amines, reserpine reversal, serotonergic and dopaminergic behaviours. Neuropharmacology. 2002;43:1110–1118. doi: 10.1016/s0028-3908(02)00216-2. [DOI] [PubMed] [Google Scholar]
- Gill JR, Jr, Masson DT, Bartter FC. Effects of hydroxyamphetamine on the function of the sympathetic nervous system in normotensive subjects. J Pharmacol Exp Ther. 1967;155:288–295. [PubMed] [Google Scholar]
- Goldberg LI, Kohli JD. Peripheral dopamine receptors: a classification based on potency series and peripheral antagonism. Trends Pharmacol Sci. 1983;2:64. [Google Scholar]
- Goldberg LI, Rajfer SI. Dopamine receptors: applications in clinical cardiology. Circulation. 1985;722:245–248. doi: 10.1161/01.cir.72.2.245. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Li ST, Bruce S, Metmen LV, Cannon RO., III Cardiac sympathetic denervation in Parkinson's disease. Ann Intern Med. 2000;13:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- Henning M, Rubenson A. Central hypotensive effect of L-3, 4-dihydroxyphenylalanine in the rat. J Pharm Pharmacol. 1970;22:553–560. doi: 10.1111/j.2042-7158.1970.tb10570.x. [DOI] [PubMed] [Google Scholar]
- Henning M, Rubenson A, Trolin G. On the localization of the hypotensive effect of l-dopa. J Pharm Pharmacol. 1972;24:447–451. doi: 10.1111/j.2042-7158.1972.tb09030.x. [DOI] [PubMed] [Google Scholar]
- Hunter KR, Boakes AJ, Laurence DR, Stern GM. Monoamine oxidase inhibitors and L-dopa. BMJ. 1970;719:388. doi: 10.1136/bmj.3.5719.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrott B, Iversen LL. Noradrenaline metabolizing enzymes in normal and sympathetically denervated vas deferens. J Neurochem. 1971;18:1–6. doi: 10.1111/j.1471-4159.1971.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Knoll J. (−)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain. Pharmacol Toxicol. 1998;82:57–66. doi: 10.1111/j.1600-0773.1998.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Knoll J, Magyar K.Some puzzling effects of monoamine oxidase inhibitors Monoamine Oxidase: New Vistas, Adv Biochem Psychopharmacol 1972Raven Press: New York; 393–408.In: Costa E, Sandler M (eds)Vol. 5, [PubMed] [Google Scholar]
- Knoll J, Miklya I, Knoll B, Marko R, Kelemen K. (−)Deprenyl and (−)1-phenyl-2-propylaminopentane, [(−)PPAP] act primarily as potent stimulants of action potential-transmitter release coupling in the catecholaminergic neurons. Life Sci. 1996;58:817–827. doi: 10.1016/0024-3205(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Cavallotti D, Ricci A, Amenta F. Localisation of dopamine D2-like receptors in pulmonary artery of the human and rabbit but not of the rat. Eur J Pharmacol. 1994;261:229–236. doi: 10.1016/0014-2999(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Laine K, Anttila M, Huuponen R, Maki-Ikola O, Heinonen E. Multiple-dose pharmacokinetics of selegiline and desmethylselegiline suggest saturable tissue binding. Clin Neuropharmacol. 2000;23:22–27. doi: 10.1097/00002826-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioural effects of amphetamine, metamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–260. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Medvedev OS, Esler M, Angus JA, Cox HS, Eisenhofer G. Simultaneous determination of plasma noradrenaline and adrenaline kinetics: responses to nitroprusside-induced hypotension and 2-deoxyglucose-induced glucopenia in the rabbit. Naunyn-Schmiedeberg's Arch Pharmacol. 1990;341:192–199. doi: 10.1007/BF00169730. [DOI] [PubMed] [Google Scholar]
- Melamed E, Hefti F, Wurtman RJ. Nonaminergic striatal neurons convert exogenous L-DOPA to dopamine in Parkinsonism. Ann Neurol. 1980;8:558–563. doi: 10.1002/ana.410080603. [DOI] [PubMed] [Google Scholar]
- Misu Y, Goshima Y. Is L-DOPA an endogenous neurotransmitter. Trends Pharmacol Sci. 1993;14:119–123. doi: 10.1016/0165-6147(93)90082-u. [DOI] [PubMed] [Google Scholar]
- Montastruc JL, Chaumerliac C, Desboeuf K, Manika M, Bagheri H, Rascol O, et al. Adverse drug reactions to selegiline: a review of the French pharmacovigilance database. Clin Neuropharmacol. 2000;23:271–275. doi: 10.1097/00002826-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Nichols AJ, Ruffolo RR. Evaluation of the alpha and beta adrenoceptor-mediated activities of the novel, orally active inotropic agent, ibopamine, in the cardiovascular system of the pithed rat: comparion with epinine and dopamine. J Pharmacol Exp Ther. 1987;242:455–463. [PubMed] [Google Scholar]
- Reynolds GP, Elsworth JD, Blau K, Sandler M, Lees AJ, Stern GM. Deprenyl is metabolized to methamphetamine and amphetamine in man. Br J Clin Pharmacol. 1978;6:542–545. doi: 10.1111/j.1365-2125.1978.tb00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LL. Evidence that deprenyl, a type B monoamine oxidase inhibitor, is an indirectly acting sympathomimetic amine. Biochem Pharmacol. 1978;27:1591–1595. doi: 10.1016/0006-2952(78)90490-2. [DOI] [PubMed] [Google Scholar]
- Strolin-Benedetti M, Sanson G, Bona L, Gallina M, Persiani S, Tipton KF. The oxidation of dopamine and epinine by the two forms of monoamine oxidase from rat liver. J Neural Transm. 1989;52 Suppl:233–238. doi: 10.1007/978-3-7091-6499-0_22. [DOI] [PubMed] [Google Scholar]
- Stryjer R, Klein C, Treves TA, Rabey JM. The effects of acute loading with levodopa and levodopa with selegiline on blood pressure and plasma norepinephrine levels in chronic Parkinson's disease patients. Acta Neurol Scand. 2005;111:89–94. doi: 10.1111/j.1600-0404.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Turkka J, Suominen K, Tolonen U, Sotaniemi K, Myllyla VV. Selegiline diminishes cardiovascular autonomic responses in Parkinson's disease. Neurology. 1997;48:662–667. doi: 10.1212/wnl.48.3.662. [DOI] [PubMed] [Google Scholar]
- Yamori Y, De Jong W, Yamabe H, Lovenberg W, Sjoerdsma A. Effects of l-dopa and inhibitors of decarboxylase and monoamine oxidase on brain noradrenaline levels and blood pressure in spontaneously hypertensive rats. J Pharm Pharmacol. 1972;24:690–695. doi: 10.1111/j.2042-7158.1972.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Gross A, Finberg JPM. Rasagiline[N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br J Pharmacol. 2001;132:500–506. doi: 10.1038/sj.bjp.0703826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanzottera D, Ferlenga P, Marchini F, Semeraro C. Pharmacological evidence for the presence of a peripheral postjunctional D2-like dopamine receptor in rabbit splenic artery. Brit J Pharmacol. 1998;123:730–736. doi: 10.1038/sj.bjp.0701651. [DOI] [PMC free article] [PubMed] [Google Scholar]