Abstract
Background and purpose:
To further assess the clinical potential of the blockade of metabotropic glutamate receptors (mGluR1) for the treatment of pain.
Experimental approach:
We characterized the effects of A-841720, a novel, potent and non-competitive mGluR1 antagonist in models of pain and of motor and cognitive function.
Key results:
At recombinant human and native rat mGluR1 receptors, A-841720 inhibited agonist-induced calcium mobilization, with IC50 values of 10.7±3.9 and 1.0±0.2 nM, respectively, while showing selectivity over other mGluR receptors, in addition to other neurotransmitter receptors, ion channels, and transporters. Intraperitoneal injection of A-841720 potently reduced complete Freund's adjuvant-induced inflammatory pain (ED50=23 μmol kg−1) and monoiodoacetate-induced joint pain (ED50=43 μmol kg−1). A-841720 also decreased mechanical allodynia observed in both the sciatic nerve chronic constriction injury and L5-L6 spinal nerve ligation (SNL) models of neuropathic pain (ED50=28 and 27 μmol kg−1, respectively). Electrophysiological studies demonstrated that systemic administration of A-841720 in SNL animals significantly reduced evoked firing in spinal wide dynamic range neurons. Significant motor side effects were observed at analgesic doses and A-841720 also impaired cognitive function in the Y-maze and the Water Maze tests.
Conclusions and implications.
The analgesic effects of a selective mGluR1 receptor antagonist are associated with motor and cognitive side effects. The lack of separation between efficacy and side effects in pre-clinical models indicates that mGluR1 antagonism may not provide an adequate therapeutic window for the development of such antagonists as novel analgesic agents in humans.
Keywords: metabotropic glutamate receptor, pain, cognitive function
Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system and mediates its actions through two distinct types of receptors, the ligand-gated cation channels termed ionotropic glutamate receptors (NMDA, AMPA/kainate receptors), and the G-protein coupled receptors termed metabotropic glutamate receptors (mGluRs). Thus far, eight mammalian mGluRs have been cloned and divided into three subgroups based on sequence similarities, coupling to G-proteins, and pharmacological properties. Group I mGluRs (mGluR1 and mGluR5 receptors) are preferentially coupled via Gαq/11 to increase intracellular inositol 1,4,5-triphosphate (InsP3) concentration, resulting in the release of intracellular Ca2+ from intracellular stores. Group II (mGluR2 and mGluR3 receptors) and Group III (mGluR4, 6, 7, 8 receptors) couple to inhibition of adenylate cyclase through Gαi/o (Conn and Pin, 1997; Hermans and Challiss, 2001).
Group I mGluRs are present in a number of key structures in the CNS including hippocampus, cerebellum, cortex, and thalamus, and are thought to play an important role in normal brain functions and neuronal development. In addition, perturbations of the glutamatergic system are thought to play a major role in many diseases such as epilepsy, anxiety, depression, brain ischemia, schizophrenia and neurodegenerative diseases (Bordi and Ugolini, 1999; Meldrum, 2000; Spooren et al., 2001). These receptors are also expressed in some of the major centers of the pain neuraxis, like the peripheral sensory neurons, spinal cord, thalamus, periaqueductal gray, amygdala and cortex (Spooren et al., 2001, 2003; Varney and Gereau, 2002). Indeed, increasing numbers of preclinical studies indicate that Group I mGluRs can modulate nociceptive processing at various levels in the nervous system and are involved in both peripheral and central sensitization associated with prolonged and chronic pain (Fundytus, 2001; Karim et al., 2001a; Neugebauer, 2002; Varney and Gereau, 2002). In addition, it has been shown that in response to injury, Group I mGluR receptors are upregulated on nociceptive specific neurons such as those of the spino-thalamic tract (Mills and Hulsebosch, 2002) and are critical for inflammation-induced hyperexcitability (Neugebauer, 2002; Han and Neugebauer, 2005). In preclinical studies, Group I mGluR receptor agonists, such as (S)-3,5-dihydroxyphenylglycine ((S)-DHPG), induce hyperalgesia and spontaneous pain (Fisher and Coderre, 1996; Young et al., 1997; Fisher et al., 1998). Furthermore, selective pharmacological agents have become available to distinguish the specific contribution to pain transmission of mGluR1 or mGluR5 receptors. With regards to mGluR5 receptors, 6-methyl-2-(phenylazo)-3-pyridinol (SIB1757) produced a full reversal of hyperalgesia in neuropathic pain in rats (Dogrul et al., 2000), while other mGluR5 receptor antagonists, including 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP), have been shown to produce antinociceptive effects in a wide range of rat nociceptive assays (Bhave et al., 2001; Spooren et al., 2001; Zhu et al., 2004; Varty et al., 2005). However, while efficacious in pain models, MPEP was shown to have a small therapeutic window with the induction of significant CNS side effects at doses only threefold higher than the antinociceptive doses (Spooren et al., 2001; Zhu et al., 2004).
In support of the role of mGluR1 receptors in pain, it has been demonstrated that antisense knockdown of mGluR1 receptors decreases spinal nociceptive neurotransmission and neuropathic hyperalgesia (Young et al., 1998; Fundytus et al., 2001; Noda et al., 2003). Similarly, antinociceptive effects have been shown with mGluR1 receptor antagonists such as 7-(hydroxylimino) cyclopropa[b]chromen-1a-carboxamide ethyl ester (CPCCOEt), AIDA, LY456236 and LY367385 in several pain models, but these agents are either relatively weak antagonists, or the evaluation of their selectivity versus other pain targets has not been fully investigated (Salt et al., 1999; Bhave et al., 2001; Kingston et al., 2002; Zhang et al., 2002; Lavreysen et al., 2003; Han and Neugebauer, 2005; Varty et al., 2005). New mGluR1 antagonists have recently been disclosed including BAY36–7620, LY-456066, 1-(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-2-phenyl-1-ethanone (R-214127) and JNJ16259685, however, these antagonists were either not assessed in animal models of pain, or were not very efficacious, especially in neuropathic pain models (De Vry et al., 2001; Kingston et al., 2003; Lavreysen et al., 2003, 2004; Han and Neugebauer, 2005; Steckler et al., 2005a).
mGluR1 receptors have also been recently suggested to play a role in the modulation of cognitive processes. Immunohistochemical studies have shown that mGluR1 receptors are highly expressed in rat brain regions such as hippocampus, olfactory bulb, cerebellum and thalamus (Shigemoto and Mizuno, 2000). In addition, in Hodgkins disease, a CNS disorder in which patients develop auto-antibodies against mGluR1 receptors, deficits are observed in cerebellar motor learning that are associated with a decreased number of Purkinje cells in the cerebellum (Coesmans et al., 2003). Furthermore, mGluR1 receptor knockout mice displayed deficits in motor coordination and were not able to learn to locate a submerged platform in a water maze test (Conquet et al., 1994). Finally, recent data using the selective and potent mGluR1 receptor antagonist JNJ16259685 have demonstrated that full occupancy of mGluR1 receptors in vivo in cerebellum and thalamus is associated with impairment of acquisition and retention in a spatial water maze task (Steckler et al., 2005b). Thus, it is critical to consider CNS-mediated side effects along with analgesic efficacy when evaluating the potential therapeutic utility of mGluR1 receptor blockade.
In this study, we report the in vitro biological and pharmacodynamic profiles of 9-Dimethylamino-3-(N-hexamethyleneiminyl)-3H-5-thia-1,3,6-triazafluoren-4-one (A-841720), a potent non-competitive mGluR1 receptor antagonist of a different and novel structural class (Zheng et al., 2005). To further investigate the potential of mGluR1 receptor blockade as a viable therapeutic approach for the treatment of chronic pain, the effects of A-841720 were investigated in rodent models of nociception. Furthermore, to assess potential target-related side effects, the effects of mGluR1 receptor blockade were also tested in models of motor and cognitive functions.
Methods
Cell cultures and fluorometric imaging plate reader
Human mGluR1 (mGluR1α) receptors or human mGluR5 (mGluR5α) receptors were stably transfected in 1321 N1 cells (human astrocytoma), along with the rat Glutamate/Aspartate Transporter (rat GLAST). Co-expressing rat GLAST increased the agonist-induced calcium signal by lowering levels of extracellular glutamate, thereby reducing desensitization of the receptors (Desai Ma et al., 1995). Cells were routinely maintained in DMEM media (containing 10% FBS, 1% GlutaMax, 500 μg ml−1 Geneticin, 200 μg ml−1 Hygromycin B). Two days before running the experiment, cells were plated as a monolayer in 96-well Biocoat black-wall-clear bottom microplates (Becton Dickinson, Boston, MA) at the density of 30 000 cells per wells, in DMEM glutamate-free media (containing 10% FBS, 1% GlutaMax, 500 μg ml−1 Geneticin, 200 μg ml−1 Hygromycin B). On the day of the experiment, the culture medium was removed and replaced by 100 μl per well of D-PBS containing 0.04% Pluronic F127 and 4 μM Fluo-4 AM (fluorescent calcium indicator dye). After incubation for 60–90 min at room temperature, the cells were washed four times with D-PBS in a plate washer (Molecular Devices, Sunnyvale, CA, USA) to remove extracellular Fluo-4 AM. After the final wash, 150 μl of D-PBS was added to each well. When applicable, antagonists were added 3 min before addition of agonist. Changes in fluorescence were recorded over time in fluorometric imaging plate reader (FLIPR) (Molecular Devices, Sunnyvale, CA, USA) at 1-s intervals for the first minute, then at 5-s intervals throughout the course of each experiment. The peak increase in fluorescence over baseline was calculated and expressed as a percentage of maximal agonist response (in absence of antagonist). Concentration–response data were analyzed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA), and the EC50 or IC50 values were derived from a single curve fit to the mean data of multiple replicates (as indicated in legends).
Rat (Sprague Dawley) cerebellum granule neurons (prepared from 6- to 7-day old rats) in primary culture expressing mGluR1 receptors were grown for 3 days in poly-D-lysine coated-96-well Biocoat black-wall-clear-bottom microplates (Becton Dickinson, Boston, MA, USA) in defined serum-free Neurobasal A medium (containing 2% B27, 33 mM glucose, 2 mM glutamine, 50 IU ml−1 penicillin, 50 μg ml−1 streptomycin, 15 mM KCl, and 10 μM cytosine arabinoside (AraC, to prevent glial cell proliferation)) before performing the assay. We previously determined that these rat cerebellum granule neurons grown for 3 days in vitro, expressed >90% mGluR1 and <10% mGluR5 receptors (data not shown). In all experiments using these neurons, cells were preincubated with 10 μM of the selective mGluR5 receptor antagonist MPEP for 10–15 min to block mGluR5 receptors. Before the FLIPR assay, cells were washed in Hanks' Balanced Salt Solution (HBSS, Gibco, Grand Island, NY, USA), and cells were loaded in HBSS containing 0.04% Pluronic F-127 and 4 μM Fluo-4 AM for 60 min at room temperature. Subsequently, cells were washed with HBSS, and 150 μl of buffer was left in each well. Changes in fluorescence were recorded as described for the recombinant mGluR1 and mGluR5 receptors. The peak increase in fluorescence over baseline was calculated and expressed as a percentage of maximal agonist (20 μM (S)-DHPG) response (in absence of antagonist).
Human mGluR2 receptor cloned from human foetal brain was co-expressed in HEK293 cells along with rat GLAST and the alpha subunit of G16. Briefly, HEK293 grown in DMEM (low glucose without phenol red and glutamine) containing 1% GlutaMax and 10% dialyzed FCS, were transfected with Lipofectamine (Invitrogen, Karlsruhe, Germany) using linearized pcDNA3.1 (V5/His)-hmGluR2 (ScaI) and pcDNA3.1 Zeo-Gα16 IRES ratGLAST (SspI). After transfection the cells were selected in DMEM Medium, 1% GlutaMax, 10% FCS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, 800 μg ml−1 Geneticin and 250 μg ml−1 Zeozin. Functional clones were selected using intracellular Ca2+ measurement by FLIPR on cells plated on Biocoat-plates (3 × 104cells well−1) in DMEM glutamate-free medium (containing 1% GlutaMax, 10% dialyzed FCS) over night and agonist-induced response was measured the following day.
Similarly, human mGluR4 receptor (mGluR4α, cloned from human cerebellum), was co-transfected with rat GLAST and the alpha subunit of G15, in HEK293 cells using linearized pcDNA3 –hmGluR4 (SspI) and pcDNA3.1(+) Hygro-rGLAST IRES Gα15 (SspI). After transfection the cells were cultured in DMEM medium, containing 1% GlutaMax 10% dialyzed FCS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, 800 μg ml−1 Geneticin and 150 μg ml−1 hygromycin.
Human mGluR7α receptor-cDNA was cloned from fetal human brain. Briefly, HEK293 cells grown in DMEM medium containing 1% GlutaMax and 10% dialyzed FCS were transfected using Lipofectamine (Invitrogen, Karlsruhe, Germany) using linearized pcDNA3(−)-hmGluR7a (SspI). After transfection, cells were cultured in DMEM medium, containing 1% GlutaMax, 10% dialyzed FCS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, and 800 μg ml−1 Geneticin. Isolated single clones were tested for their ability to reduce 20 μM forskolin-induced production of cellular cAMP (AlphaScreen camp assay, PerkinElmer Life and Analytical Sciences, Boston, MA, USA) and subcloned by FACS sorting (FACSVantage SE, Becton Dickinson). Single cell clones were then retested for cAMP reduction, and transfected with pcDNA3.1 (+) Hygro rGLAST IRES Gα15 (SspI), as described above. Cells were selected in DMEM GlutaMax, 10% dialyzed FCS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, 800 μg ml−1 Geneticin and 150 μg ml−1 hygromycin. As clones were isolated by serial dilution and tested by FLIPR functional assay using 1 mM L-AP4, or 10 mM L-glutamate.
Binding experiments
mGluR1 receptor in vitro competition binding experiments were carried out using the selective mGluR1 receptor radioligand [3H]-R214127 (Lavreysen et al., 2003). Adult rat (Sprague Dawley) cerebella were purchased from Pel-Freez Biological (Rogers, Arkansas). Frozen tissues were thawed, homogenized using Polytron for 10 s in 50 mM Tris-HCl, pH 7.4 with Complete Protease Inhibitor (EDTA-free, Roche Applied Science Indianapolis, IN, USA) and centrifuged for 20 min at 23 500 g at 4°C. The obtained pellet was washed twice with 50 mM Tris-HCl, pH 7.4 to remove endogenous glutamate, centrifuged and homogenized again in 50 mM Tris-HCl, pH 7.4. Membrane preparations were aliquoted, frozen quickly in liquid N2, and stored at −80°C until further use. Protein concentrations were measured using BCA Protein Assay Kit (Pierce, Meriden, CT, USA). Rat cerebellum membrane preparation (40–50 μg) were incubated at 4°C for 3 min with increasing concentrations of compounds (10−11–10−4 M), in 50 mM Tris-HCl, pH 7.4, 1.2 mM MgCl2 and 2 mM CaCl2. Nonspecific binding was determined in presence of the mGluR1 receptor selective non-competitive antagonist LY-456066 (10 μM). Similarly, mGluR5-receptor binding assays (using adult Sprague Dawley rat cortex membranes) were performed using the selective mGluR5 receptor radioligand [3H]-MPEP at ambient temperature for 1 h in 30 mM Na HEPES, pH 8.0, 110 mM NaCl, 1.2 mM MgCl2, 5 mM KCl and 2.5 mM CaCl2. Nonspecific binding was determined using 10 μM of the mGluR5 receptor selective non-competitive antagonist MTEP.
In vivo studies
Male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing 200–300 g were utilized in all antinociceptive and motor experiments. Long Evans rats (Charles River, Wilmington, MA, USA) were utilized in the cognitive assays since they have pigmented eyes and good visual acuity that make them preferable for performing spatial memory testing (visual cues) as required in the cognitive assays. Animals were group-housed in Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facilities at Abbott Laboratories in a temperature-regulated environment with lights on between 0700 and 1900. Food and water were available ad libitum except during testing. All animal handling and experimental protocols were approved by an institutional animal care and use committee (IACUC). All experiments were performed during the light cycle.
Complete freund's adjuvant-induced thermal hyperalgesia
Unilateral inflammation was induced by injecting 150 μl of a 50% solution of complete Freund's adjuvant (CFA) (Sigma Chemical Co., St Louis, MO, USA) in physiological saline into the plantar surface of the right hind paw of the rat. The hyperalgesia to thermal stimulation was determined 48 h following CFA using a commercially available paw thermal stimulator (UARDG, University of California, San Diego, CA, USA). Rats were placed individually in Plexiglass cubicles mounted on a glass surface maintained at 30°C, and allowed a 15 min habituation period. A thermal stimulus, in the form of radiant heat emitted from a focused projection bulb, was then applied to the plantar surface of each hind paw. In each test session, each rat was tested in three sequential trials at approximately 5 min intervals. Paw withdrawal latencies (PWL) were calculated as the mean of the two shortest latencies. In addition to a dose–response study, a time–course of effect was evaluated, dosing 100 μmol kg−1 or vehicle and testing at 0.5, 1, 1.2, 2, 4, 6 and 24 h postdosing.
Inflammatory joint pain
Unilateral knee joint inflammation was induced in the rats by a single intra-articular (i.a.) injection of sodium monoiodoacetate (MIA) (Sigma-Aldrich, St Louis, MO, USA) (3 mg in 0.05 ml sterile isotonic saline) into the joint cavity using a 26G needle under light (2–4%) halothane (Halocarbon Laboratories, River Edge, NJ, USA) anesthesia. Following injection, the animals were allowed to recover from the effects of anesthesia before returning to their cages. Four days following i.a. of MIA, hind limb weight bearing assessment was carried out as described below.
Hind limb weight bearing difference (WBD) between MIA injected and contralateral (uninjected) side was used as a behavioral measure of pain. Differences in weight bearing on the injected versus contralateral hind limb were assessed following the injection of MIA by placing the animals in an Incapacitance Tester (Linton, Norfolk, UK). The animals were restrained in a clear plexiglass chamber (6″ × 3.5″ × 3.7″), and their hind limbs were positioned over two force plates (2″ × 1.5″ each) placed side by side to measure the weight borne on each hind limb. The animals were allowed to acclimatize for a brief period of time before weight bearing readings (measured in grams) were recorded. Bilateral hind limb weight bearing, consisting of three trials (3 s per trial) was recorded for each animal and then averaged to give a mean weight bearing score for both ipsilateral and contralateral side hind limbs.
Spinal nerve (L5/L6) ligation model of neuropathic pain
A 1.5 cm incision was made dorsal to the lumbosacral plexus. The paraspinal muscles (left side) were separated from the spinous processes, the L5 and L6 spinal nerves isolated, and tightly ligated with 3–0 silk threads. Following hemostasis, the wound was sutured and coated with antibiotic ointment. The rats were allowed to recover and then placed in a cage with soft bedding for 14 days before behavioral testing for mechanical allodynia. In addition to a dose–response study, a chronic dosing study was performed, starting 14 days following spinal nerve injury. Rats received vehicle or A-841720 (100 μmol kg−1 i.p.) twice a day for 4 days and were tested on day 5 following the morning dosing.
Mechanical (tactile) allodynia was measured using calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA). Briefly, rats were placed into individual plexiglass containers and allowed to acclimate for 15–20 min before testing. Paw withdrawal threshold (PWTvonfrey) was determined by increasing and decreasing stimulus intensity and estimated using a Dixon nonparametric test. Only rats with threshold scores ⩽4.5 g were considered allodynic and utilized in further testing.
Sciatic nerve ligation model of neuropathic pain
A 1.5 cm incision was made 0.5 cm below the pelvis and the biceps femoris and the gluteus superficialis (right side) were separated. The sciatic nerve was exposed, isolated, and four loose ligatures (5–0 chromic catgut) with 1 mm spacing were placed around it. The rats were allowed to recover and then placed in a cage with soft bedding for 14 days before behavioral testing for mechanical allodynia as described above.
Electrophysiology
For electrophysiological experiments the procedures were as described previously (McGaraughty et al., 2006). Briefly, neuropathic rats (L5–L6 spinal nerve ligation model, 14 days postsurgery) were anesthetized with pentobarbital (50 mg kg−1, i.p.), and catheters were placed into the left and right external jugular veins. A laminectomy was performed to remove vertebral segments T12–L3. The animals were then secured in a stereotaxic frame supported by clamps attached to the vertebral processes on either side of the exposure site. Rat anesthesia was maintained for the duration of the experiment by a continuous infusion of propofol (8–12 mg kg−1 h−1, i.v.). Body temperature was kept at approximately 37°C by placing the animals on a circulating water blanket. Platinum-plated stainless steel microelectrodes (Frederick Haer, Brunswick, ME, USA) were used to record the activity of spinal wide dynamic range (WDR) neurons, which respond in a graded manner to noxious and innocuous stimuli. Spike waveforms were monitored throughout the experiment, digitized (32 points), and then stored for off-line analysis (Datawave Technologies, Longmont, CO, USA). Rats were stimulated three times (5 min apart) before drug administration with a von Frey hair (10 g) applied to the neuronal receptive field located on the ipsilateral hindpaw. A-841720 (3 and 10 μmol kg−1, i.v.) or vehicle was then infused over a 5–7 min period, and the von Frey hair was reapplied 5, 15 and 25 min after this infusion.
Locomotor activity and rotorod performance
Locomotor activity was recorded in an open field using photobeam activity monitors for 30 min (AccuScan Instruments, Columbus, OH, USA). Rotorod performance was measured using an accelerating rotorod apparatus (Omnitech Electronics Inc., Columbus, OH, USA). For the rotorod assay, rats were allowed a 30 min acclimation period in the testing room and then placed on a 9 cm diameter rod that increased in speed from 0 to 20 r.p.m. over a 60 s period. The time required for the rat to fall from the rod was recorded, with a maximum score of 60 s. Each rat was given three training sessions.
Y-maze
Spontaneous alternation behavior was assessed in a gray, acrylic Y maze (Piper Plastics. Libertyville, IL, USA), that consists of three arms (54 cm long × 16 cm wide × 35 cm deep) projecting from each side of a central equilateral triangle. Rats were observed, in a blind fashion, using a camera mounted on ceiling directly above the maze. On the testing day, rats were habituated to the testing room (∼60 min at 400 LUX lighting conditions). Rats were then individually placed at the end of one arm, varied across rats, and allowed to freely explore the maze for 5 min. A complete entry of the hind paws into any arm counted as an arm entry. The series of arm entries was recorded and the alternation was defined if animals entered arms three times in succession from the results of consecutive arm entering (e.g. arm A, B, C, or B, C, A). The number of overlapping entrance sequences (e.g. ABC or BCA) was defined as the number of alternations. The percentage of alternation was used a an index of working memory since working memory impairment disrupts the normal tendency of rats to explore less recently visited arms on the Y-maze. The dependent variable was percent alternation, calculated as (number of alternations divided by (total entries−2)) × 100. In the present study, the effects of A-841720 were evaluated acutely. Animals (n=8 per group) were treated with saline or A-841720 (10, 30 and 100 μmol kg−1, i.p.) 30 min before being placed into the Y-maze.
Morris water maze test
A circular water tank (180 cm diameter and 60 cm high) was filled with water made opaque using powdered milk to a depth of 47 cm. Water temperature was maintained at 26°C. A Smart II video tracking system supplier was used for data collection and analysis (San Diego Instruments Inc., San Diego, CA, USA). A 5-day protocol was used. Rats were trained to locate a submerged platform for the first four days. In addition, on day 5, rats were tested in a visible platform paradigm to control for visiomotor function. In the submerged platform task, consisting of three trials per day per rat, a white-topped platform was placed in a fixed position (NW), with the top of the platform 2 cm under the water. Rats were individually placed into the pool facing the wall of the tank at one of three random starting locations (south, west and east), and allowed to freely swim to locate the submerged platform. If the animal could not find the submerged platform within the required time period (90 s for trial 1 on day 1 and 60 s thereafter for all other trials), it was gently guided to the platform by the investigator. Each rat was allowed to stay on the platform for a fixed time (20 s for the first trial on the first day and 15 s thereafter), after which they were placed into a cage beneath a heat lamp for 5 min before commencing the next trial. One trial with a visible platform was conducted on day 5, in which the platform (located at SE) was elevated 0.8–1 cm above the water surface. Rats were placed into the water facing the wall at the farthest position from the platform (N) and allowed to freely explore the pool to find the visible platform within 60 s. In both visible and hidden platform tasks, animals were treated with saline or A-841720 (30 or 100 μmol kg−1, i.p.) 30 min prior to the first training session of each day.
Statistics
Analysis of the in vivo nociception data was carried out using analysis of variance (ANOVA) and data are presented as mean±s.e.m. For the time course of effects of A-841720 in the CFA model, and the effects of chronic dosing in SNL animals, a repeated ANOVA was performed. Where appropriate, Fisher's Protected Least Significant Difference (FPLSD) was used for post hoc analysis. ED50 values were estimated using least squares linear regression.
For the electrophysiology experiments, statistical significance from baseline firing levels was established by using a Wilcoxon's matched-pairs test. For comparison across groups, statistical significance for cell activity was established by using a Kruskal–Wallis analysis of variance (ANOVA) followed by a Mann–Whitney U-test (P<0.05).
For the Y-maze study, the alteration rates were compared using one-way ANOVA followed by Dunnett's multiple comparison test for post hoc analysis. For the water maze study, dependent variables were latency to find the hidden or visible platform and distance traveled to find the platform. Repeated measures ANOVA was used in hidden platform training sessions (latency and path length data) from day 1 through day 4, followed by Dunnett's multiple comparison test as a post hoc test (Prism, GraphPad software Inc., San Diego CA, USA).
Compounds
Synthesis of A-841720 (9-dimethylamino-3-(N-hexamethyleneiminyl)-3H-5-thia-1,3,6-triazafluoren-4-one) was described by Zheng et al. (2005). R-214127 (Lavreysen et al., 2003), MTEP (Spooren et al., 2003) and LY-456066 (Kingston et al., 2003) were used as reference compounds and were synthesized at Abbott Laboratories (Abbott Park, USA). L-Glutamate, L-quisqualate, (S)-DHPG, L-AP4, CPCCOEt, SIB1757 were obtained from Tocris (Bristol, UK). A-841720 was dissolved in 10% dimethyl sulphoxide (DMSO) and 90% polyethylene glycol (PEG400) for intraperitoneal (i.p.) administration using a volume of 2 ml kg−1 for antinociceptive and motor assays and in 10% PEG400 in water for the Y and water maze studies. Except where noted, A-841720 was injected 30 min (i.p.) before nociceptive or side effect testing, in agreement with its pharmacokinetic profile.
Materials
[3H]-MPEP (51.0 Ci mmol−1) was purchased from Tocris (Bristol, UK). [3H]-R-214127 (9 Ci mmol−1, radiochemical purity >96.4%) a selective non-competitive mGluR1 receptor radioligand described by Lavreysen et al. (2003) was synthesized at Abbott Laboratories (Abbott Park, USA). Fluo-4 AM and Pluronic F-127 were purchased from Molecular Probes (Eugene, OR, USA). Neurobasal media, DMEM, D-PBS, Geneticin, hygromycin B, GlutaMax and tissue culture reagents were from Invitrogen/Life Technology (Carlsbad, CA, USA). All other chemicals were purchased from Sigma (St Louis, MO, USA), unless otherwise noted (Figure 1).
Figure 1.
Chemical structures of A-841720, CPCCOEt, LY-456066, and R-214127.
Results
Characterization of fluorescence responses from transfected human mGluR1 and human mGluR5 receptors
Human mGluR1 and human mGluR5 receptors were co-expressed along with rat Glutamate/Aspartate Transporter (GLAST) in 1321 N1 cells, as described in Methods. The nonselective agonist L-glutamate induced a rapid, transient, calcium release that peaked within 20 s of agonist addition, and decreased toward baseline within 2 min of agonist addition (Figure 2). A similar profile was obtained with the recombinant human mGluR5 receptor (data not shown). The nonselective agonist L-quisqualate and the Group I mGluRs selective agonist (S)-DHPG were tested in both cell lines. At human mGluR1 and human mGluR5 receptors, the rank of order potencies in FLIPR functional assays were L-quisqualic acid >L-glutamate >(S)-DHPG (Table 1). There was no effect of the three agonists on calcium signaling in 1321 N1 nontransfected cells, or transfected with rat GLAST alone (data not shown).
Figure 2.
Activation of human mGluR1 receptor-mediated calcium release by L-glutamate. Representative L-glutamate-induced FLIPR tracing from recombinant human mGluR1 receptor expressed in 1321 N1 cells. Arrow indicates the addition time of the agonist. Basal fluorescence (∼1000 relative fluorescence (counts) units) was subtracted from total fluorescence as indicated under Methods.
Table 1.
Characterization of mGluR receptor-agonists in human mGluR1 and mGluR5 receptor-expressing cell lines
|
Human mGluR1 |
Human mGluR5 |
|||
|---|---|---|---|---|
| EC50 (nM) | Efficacy (%) | EC50 (nM) | Efficacy (%) | |
| L-glutamate | 3835±384 | 100 | 3568±742 | 100 |
| L-quisqualate | 70.3±6.4 | 108 | 19.9±2.9 | 107 |
| (S)-DHPG | 12 066±1263 | 108 | 6657±584 | 99 |
Agonist activities were assayed by concentration–response curves of various compounds and normalized to 10 μM L-glutamate (100%). Potencies are shown as mean EC50 values±s.e.m. for 8–12 determinations.
In vitro pharmacological characterization of A-841720 at human mGluR1 and human mGluR5 receptors
A-841720 produced concentration-dependent inhibition of 10 μM L-glutamate-induced calcium release at human mGluR1 receptors, with IC50 of 10.7 nM, and behaved as a full antagonist, completely blocking the calcium response to 10 μM L-glutamate (Table 2). A-841720 was more than 1400-fold more potent than the classical selective mGluR1 receptor antagonist CPCCOEt (Table 2). A-841720 was also about fivefold more potent in this assay than two other prototypic selective mGluR1 receptor antagonists tested, LY-456066 (IC50=52.0 nM) and R-214127 (IC50=49.7 nM) (Table 2). The selective mGluR5 receptor antagonists (MPEP, MTEP and SIB1757) did not inhibit agonist-induced calcium signals in cells expressing human mGluR1 receptors.
Table 2.
Comparison of A-841720 and prototypic mGluR1 and mGluR5 receptor antagonists, in mGluR1 and mGluR5 receptor assays
|
Mean±s.e.m. (n) |
|||||||
|---|---|---|---|---|---|---|---|
| A-841720 | LY456066 | R-214127 | CPCCOEt | MPEP | MTEP | SIB1517 | |
| Human mGluR1 FLIPRa | 10.7±3.9 | 52±6.0 | 49.7±7.2 | 14288±3017 | >10000 | >10000 | >10000 |
| (100%; n=13) | (100%; n=20) | (97%; n=10) | (82%; n=12) | (n=20) | (n=14) | (n=12) | |
| Human mGluR5 FLIPRa | 343±59.5 | >10000 | >10000 | >10000 | 29.1±5.2 | 46±8.9 | 2117±261 |
| (89%; n=13) | (n=13) | (n=10) | (n=12) | (98%; n=12) | (98%; n=16) | (99%; n=12) | |
| Rat mGluR1 Bindingb | 1.05±0.6 | 28.0±1.1 | n.d. | 11910±1644 | >10000 | >10000 | >10000 |
| (103%; n=10) | (101%; n=12) | (68%; n=3) | (n=12) | (n=12) | (n=6) | ||
| Rat mGluR1 FLIPRa | 1.0±0.2 | 10.0±2.2 | 1.8±0.3 | 5321±869 | >10000 | >10000 | >10000 |
| (96%; n=6) | (95%; n=6) | (94%; n=5) | (76%; n=3) | (n=5) | (n=5) | (n=3) | |
| Rat mGluR5 Bindingb | 200.0±17 | >10000 | >10000 | >10000 | 5.8±0.6 | 11.5±2.5 | 791.0±216 |
| (94%; n=14) | (n=4) | (n=4) | (n=7) | (110%; n=11) | (107%; n=6) | (111%; n=4) | |
Concentration–response data were generated using 10 μM L-glutamate for human mGluR1 and mGluR5 FLIPR assays, and 20 μM (S)-DHPG for rat mGluR1 FLIPR assays, respectively. Data are expressed as mean.
IC50 values (nM).
Ki values (nM)±s.e.m., and ‘n' represents the number of independent determinations.
In the human mGluR5 receptor FLIPR functional assay, A-841720 concentration dependently inhibited agonist-induced calcium release, but with lower potency (IC50=343 nM, Table 2). A-841720 was about six to eightfold less potent than the two selective mGluR5 receptor antagonists MPEP (IC50=29.1 nM) and MTEP (IC50=46.0 nM), respectively. As expected, the selective mGluR1 receptor antagonists did not block agonist-induced response in cells expressing recombinant mGluR5 receptors (Table 2).
A-841720 is a non-competitive antagonist at human mGluR1 and human mGluR5 receptors
To analyze the mode of action of A-841720 on group I mGluR receptors, concentration–response curves for stimulation of calcium release in presence of the full agonist L-quisqualate were compared in absence or presence of increasing concentration of A-841720. In the human mGluR1 receptor FLIPR assay, 10 nM A-841720 shifted the log concentration-response curve to the right (Figure 3a). Increasing concentrations of the antagonist induced a profound reduction in the amplitude of calcium release evoked by L-quisqualate alone. 30 nM of A-841720 reduced the agonist-induced maximal response by 38%, and 100 nM of the antagonist completely abolished L-quisqualate-induced response. These results indicated that A-841720 is a non-competitive antagonist, with respect to the agonist-binding site.
Figure 3.
A-841720 is a non-competitive antagonist at human Group I mGluR receptors. Concentration–responses curves for L-quisqualate recorded (a) under control conditions, and in presence of 10, 30, 100 nM of A-841720 in human mGluR1 receptor FLIPR assay. (b) Concentration–responses curves for L-quisqualate in human mGluR5 receptor FLIPR assay recorded under control conditions, and in presence of 30, 300 and 1000 nM of A-841720. Values are expressed as percent of 10 μM L-quisqualate-induced calcium release. Data points represent the mean±s.e.m. from at least three to four independent experiments performed in duplicate.
In the human mGluR5 receptor FLIPR assay, higher concentrations of A-841720 were needed to reduce the maximal response induced by L-quisqualate, when compared to human mGluR1 receptor (Figure 3b). Antagonist (30 nM) did not affect the agonist-induced calcium release. In all, 300 and 1000 nM A-841720 induced about 18 and 46% reduction maximal response evoked by L-quisqualate, respectively. A-841720 also behaved as non-competitive antagonist at human mGluR5 receptor.
A-841720 is a potent antagonist at native rat mGluR1 receptors
To further characterize A-841720, the antagonist was tested in rat mGluR1 and rat mGluR5 receptors by binding and FLIPR functional assays. Rat mGluR1 receptor competition binding assays were performed using the selective and non-competitive mGluR1 receptor radioligand [3H]-R214127, and membranes prepared from adult rat cerebellum. A-841720 bound with high affinity to rat mGluR1 receptor, with Ki=1 nM (Table 2). LY-456066 was about 28-fold less potent than A-841720, and CPCCOEt displaced [3H]-R214127 with micromolar potency. As expected, the selective mGluR5 receptor antagonists MPEP, MTEP and SIB1757 did not displace [3H]-R214127. Compared to the reference antagonists tested, the ranking order potency was: A-841720>LY-456066>>>CPCCOEt>MPEP=MTEP=SIB1757.
Rat mGluR5 receptor competition binding studies were done using adult rat cortex membrane preparation, and the selective non-competitive mGluR5 receptor radioligand [3H]-MPEP. In this assay, A-841720 displaced [3H]-MPEP with relatively low potency (Ki=200 nM, Table 2), showing about 200-fold separation between rat mGluR5 and rat mGluR1 receptors. A-841720 was also about 17-fold and 34-fold less potent than the two selective mGluR5 receptor antagonists MTEP and MPEP, respectively.
To assess the functional interaction of A-841720 with rat mGluR1 receptor, inhibition of agonist-evoked calcium release in rat cerebellum granule primary cultures was carried out. Importantly, this FLIPR assay allowed the functional characterization of agonists and antagonists in a native neuronal environment. In these neurons, (S)-DHPG activated mGluR1 receptors with EC50 of 2.9±0.1 μM (n=10). A-841720 showed concentration-dependent inhibition of (S)-DHPG-induced calcium release with IC50=1 nM (Table 2), and during the continuous recording of FLIPR signal, A-841720 did not show any intrinsic activity up to 10 μM (Figure 4). A-841720 showed similar potency as R-214127, and is about 10-fold more potent than LY-456066, and about 5300-fold more potent than CPCCOEt (Table 2).
Figure 4.
Effect of A-841720 on Group I selective agonist (S)-DHPG-induced calcium release in rat cerebellum primary culture, measured by FLIPR. Representative tracing of (S)-DHPG-induced calcium release in control condition, and following inhibition by A-841720 in rat cerebellum primary culture. A-841720 was added in a concentration-dependent fashion 3 min before the addition of the agonist (S)-DHPG, as described in experimental procedures. Arrows indicate times of addition of the compounds.
A-841720 is a highly selective mGluR1 receptor antagonist
A-841720 (tested at 10 μM), when evaluated in a radioligand-binding screen that contained representative of most GPCRs, as well as ligand- and voltage-gated ion channel binding sites (CEREP Discovery, Paris, France), did not show significant displacement (<30% specific radioligand displacement). Additionally, A-841720 was weak or inactive in functional FLIPR based assays against other pain targets including human TRPV1 (IC50>10 μM), human P2X7 (IC50=9 μM, 100% maximal inhibition), as well as in in vitro binding assays for human CB2 (Ki>0.9 μM, 75% maximal inhibition), and human nicotinic receptors α4β2 (Ki>10 μM) and α7 (Ki>10 μM).
To further characterize the selectivity of action of A-841720, its effects on other metabotropic glutamate receptor subtypes were examined. The same measures of receptor activation as for mGluR1 and mGluR5 receptors were used (i.e., FLIPR and calcium release). For these experiments, the Gi/o-coupled mGluR2, mGluR4 and mGluR7 receptors were co-expressed with the ubiquitous Gα15 or Gα16 proteins (see Methods). A-841720 did not inhibit agonist-induced calcium release in HEK293 expressing mGluR2, mGluR4 or mGluR7 receptors (data not shown). Taken together, these results indicated that A-841720 is a potent selective mGluR1 receptor antagonist.
Effect of A-841720 on CFA-induced chronic inflammatory thermal hyperalgesia
CFA injection into the hind paw induced a significant decrease in paw withdrawal latency (PWL) to thermal stimulation (PWL control: 9.61±0.14 s vs PWL inflamed: 5.57±0.43 s, P<0.01), demonstrating the development of thermal hyperalgesia (Figure 5). A-841720 dose-dependently and fully relieved CFA-induced thermal hyperalgesia following i.p. administration, with ED50 of 23 μmol kg−1. There was a significant drug effect (F(3,40)=33.97; P<0.0001) and side of testing effect (F(1,40)=120.10; P<0.0001) with side-drug interaction (F(3,40)=3.02; P<0.05). Under the same conditions, 10 μmol kg−1, i.p. (a dose that did not induce locomotor side effects) or 30 μmol kg−1 i.p. (a dose associated with 50% decrease in locomotor activity) A-841720 did not produce antinociceptive effects on the uninjured paws tested for thermal nociception. However, at the highest dose (100 μmol kg−1, i.p.) that is associated with significant locomotor side effects in both motor side effect assays, A-841720 showed an increase in PWL. These data suggest that A-841720 is devoid of antinociceptive effects against acute thermal nociception. In this model, the significant antihyperalgesic effects of A-841720 at 30 or 100 μmol kg−1, i.p. peaked at 30 min postinjection and were still ∼50% at 6 h (data not shown).
Figure 5.
Antinociceptive effects of A-841720 following i.p. administration in the CFA model of chronic inflammatory pain testing for thermal hyperalgesia. Two days following CFA injection, A-841720 was injected 30 min (i.p.) before testing. Filled circles represent paw withdrawal latencies ipsilateral to the injury; filled squares represent paw withdrawal latencies contralateral to the injury. Data represent mean±s.e.m. **P<0.01 as compared to vehicle-treated animals. ++P<0.01 as compared to CFA-treated paw (n=6 per group).
MIA-induced inflammatory joint pain
MIA injection into the knee joint induced inflammation of the joint associated with weight bearing differences (WBD) between the injured and noninjured hind limbs. Four days after MIA injection, a substantial WBD was observed between the injured and noninjured hind limbs (56.6±6.6 g). When tested at 10 μmol kg−1, A-841720 did not significantly decrease MIA-induced increase in WBD (P>0.05). However, A-841720 significantly decreased MIA-induced increase in WBD at higher doses (F(3,23)=5.09, P<0.01), producing 44±7% effect at 30 μmol/kg and 62±4% effect at 100 μmol kg−1, with an ED50 of 43 μmol kg−1 i.p.
Spinal nerve injury-induced mechanical allodynia
Spinal nerve injury ((L5–L6) spinal nerve ligation (SNL model)) induced a decrease in paw withdrawal threshold (PWTvonfrey) to mechanical stimulation with von Frey monofilaments 2 weeks following injury (control 14.23±0.31 g; injured 2.93±0.18 g, P<0.01) demonstrating the development of mechanical allodynia. A-841720 reduced spinal nerve injury-induced mechanical allodynia (F(5,54)=53.9, P<0.01) with an ED50 of 27 μmol kg−1 (P<0.01, Figure 6a), producing a complete reduction at 100 μmol kg−1 i.p.
Figure 6.
Antinociceptive effects of A-841720 following i.p. administration in (a) L5–L6 spinal nerve ligation model (SNL); or (b) sciatic nerve chronic constriction injury model of neuropathic pain. In these experiments, A-841720 was administered 30 min before testing. Filled circles represent paw withdrawal thresholds ipsilateral to the nerve injury. Data represent mean±s.e.m. **P<0.01 as compared to vehicle-treated animals (n=8 per group).
The anti-allodynic effects of A-841720 (100 μmol kg−1, i.p.) were sustained after subchronic dosing (twice daily for 5 days). A-841720 produced a 78±10% effect (P<0.01) in reducing mechanical allodynia when tested on day 1, and still produced a 65±6% effect (P<0.01 vs vehicle group) when tested on the fifth day of chronic dosing in SNL animals (data not shown). No significant differences were observed between effects observed at day 1 and day 5 (repeated-measures (F(4,40)=0.51, NS)).
Sciatic nerve injury-induced mechanical allodynia
Chronic constriction injury (CCI) of the sciatic nerve produced a decrease in PWTvonfrey to mechanical stimulation with von Frey monofilaments 2 weeks following surgery (control 13.49±0.41 g; injured 3.04±0.21 g, P<0.01) demonstrating the development of mechanical allodynia. A-841720 dose-dependently reduced sciatic nerve injury-induced mechanical allodynia (F(5,53)=43.70, P<0.01). A-841720 produced a 55±10% reduction at 30 μmol kg−1 (i.p.), and a 96±4.5% reduction at 100 μmol kg−1 (i.p.), with an ED50 of 28 μmol kg−1 (P<0.01, Figure 6b).
Effects of A-841720 on evoked firing of spinal WDR neurons in SNL rats
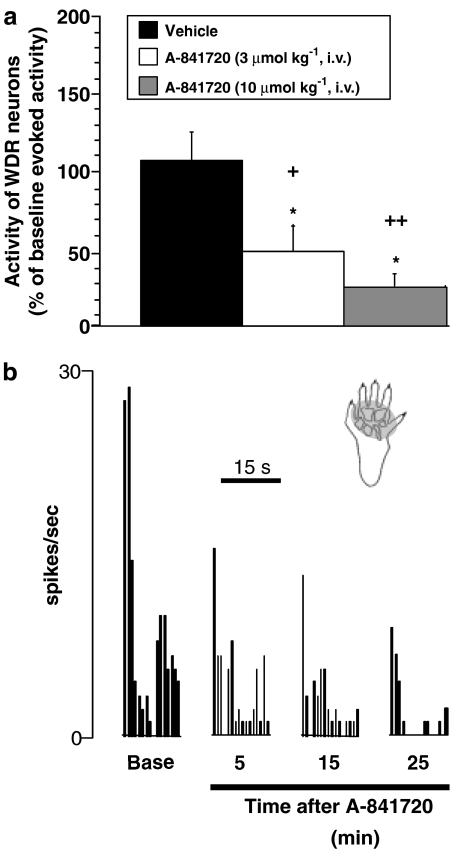
To further understand the mode of action for systemic A-841720, we examined its effects on the evoked firing of wide dynamic range (WDR) neurons in anesthetized neuropathic rats (SNL model). Discharge activity was recorded from 18 WDR neurons located 789.8±56.3 μm from the surface of the spinal cord. During baseline recording, application of a von Frey hair (10 g) to the neuronal receptive field evoked 178.5±18.2 spikes in 15 s. As shown in Figure 7, intravenous administration of 3 and 10 μmol kg−1 of A-841720 dose-dependently reduced evoked WDR firing compared to baseline levels (χ2(2)=7.73 (P<0.05)). The greatest reduction (74.9±7.6%) in von Frey-evoked WDR firing occurred 25 min after the injection of 10 μmol kg−1 of A-841720. A-841720 at 10 μmol kg−1 had a quick onset of action reducing evoked activity by 63.6±10.6% from baseline levels only 5 min after injection (P<0.05; Figure 7).
Figure 7.
Administration of A-841720 (3 and 10 μmol kg−1, i.v.) significantly reduced von Frey hair-evoked WDR activity in SNL rats. Data were collected 25 min after injection. (a) Representative ratemeter showing the responses of a single WDR neuron to von Frey hair stimulation (10 g for 15 s) both before (base) and after the administration of A-841720 (10 μmol kg−1, i.v.). (b) Shaded region on the paw represents the neuronal receptive field. *P<0.05 vs baseline evoked activity; +P<0.05, ++P<0.01 vs vehicle-treated group (n=5–7 per group).
Effects of A-841720 on motor activity
A-841720 produced a significant decrease in spontaneous exploratory activity at 30 μmol kg−1 (53±3%) and at 100 μmol kg−1, i.p. (68±5%) (F(3,31)=13.5, P<0.01), as measured by the rat total horizontal activity (number of beams crossed) in a novel open field (Figure 8a). A-841720 had no significant effect on motor coordination as measured by the rotarod assay, at doses up to 30 μmol kg−1, i.p. but a mild decrease (11±3%) in latency to fall from an accelerating rod was present at 100 μmol kg−1, i.p. (F(3,31)=7.2, P<0.01) (Figure 8b). Rats were fully awake, responsive to stimuli, and retained the righting reflex at all doses tested.
Figure 8.
Effect of A-841720 in locomotor (a and b) and cognitive (c and d) function assays. (a) Effect of A-841720 in the spontaneous locomotion assay. Data represent mean horizontal activity±s.e.m. *P<0.05 relative to vehicle controls (n=8 per group). (b) Effect of A-841720 in the rotarod assay of motor coordination. Data represent mean latency to fall ±s.e.m. *P<0.05 relative to vehicle controls (n=8 per group). (c) Effect of A-841720 in the Y-maze cognitive test following. Data represent mean % alternation score±s.e.m. *P<0.05 relative to vehicle controls (n=8 per group). (d) Effect of A-841720 in the Morris Water maze cognitive test. A-841720 was given 30 min before the first trial each day. Graph shows the mean±s.e.m. path length to find the hidden platform within each day (from day 1–4). Animals receiving A-841720 traveled a longer distance to find the hidden platform. *P<0.05; **P<0.01; ***P<0.001 versus vehicle-treated rats (n=10 per group).
Effect of A-841720 on cognitive tests
In the Y-maze test that measures spatial working memory, vehicle-treated rats exhibited an 80% alternation rate. The administration of A-841720 impaired spontaneous alternation (F(3,28)=3.6; P<0.05) reaching significance at the 100 μmol kg−1 dose, i.p. (Figure 8c).
In the water maze test, all animals gradually learned to locate the submerged platform. A-841720-treated animals were significantly slower to find the platform than vehicle control animals when considering both path length (repeated-ANOVA day 1: (F(3,87)=5.237; P<0.05); day 2: (F(3,87)=13.70; P<0.0001); day 3: (F(3,87)=15.63; P<0.0001); day 4: (F(3,87)=22.70; P<0.05)) and latency (repeated-ANOVA day 1: (F(3,87)=1.169; P>0.05); day 2: (F(3,87)=5.0070; P<0.001); day 3: (F(3,87)=7.093; P<0.001); day 4: (F(3,87)=14.19; P<0.0001)) measurements. Post hoc analysis showed that animals treated with A-841720 (30 and 100 μmol kg−1 doses) not only traveled significantly longer distance to find hidden platform relative to controls (P<0.01 on day 1 (100 dose) and P<0.01 on day 2, 3, and 4 for both doses); but also spent significantly longer time to reach the platform (P<0.01 on day 2 (100 μmol kg−1 dose) and P<0.01 on day 3 and 4 for both doses; Figure 8d). However, in a visible platform test conducted on day 5, A-841720 did not affect animals' path length or escape latency compared to vehicle controls (path length: (F(3,27)=0.3053; P>0.05); latency (F(3,87)=0.4349; P>0.05); data not shown).
Pharmacokinetic profile of A-841720
The pharmacokinetic profile of A-841720 in rats was characterized by high plasma clearance (CLp=3.6 l h−1 kg), a large volume of distribution (Vd=4.5 l kg−1), and a long plasma elimination half-life (T1/2=3 h, i.v.). Plasma and brain samples were also harvested 45 min following A-841720 i.p. administration. Mean plasma levels of A-841720 were 70, 313, and 1015 ng ml−1 at the 10, 30 and 100 μmol kg−1 doses, respectively. Mean brain levels were 217, 851 and 2642 ng g−1 over the same dose range, demonstrating linear plasma and brain exposure and good CNS penetration (brain/plasma ratio of about 3:1 for all doses).
Discussion
The major aim of these studies was to compare directly the consequences of blocking mGluR1 receptors on pain transmission, on locomotor function, and on cognitive function. Specifically, the mGluR1 receptor antagonist A-841720 was administered systemically, and tested in chronic pain models. In addition, the drug was tested in two models of learning and memory (Y-maze and Morris water maze tests), and in two models of locomotor function, that is spontaneous exploratory behavior and rotorod assay.
As stated in the introduction, Group I mGluR receptors are expressed in the major centers of the pain neuraxis (peripheral sensory neurons, spinal cord, thalamus, periaqueductal gray), as well as brain regions involved in the processing of nociceptive information (amygdala, cortex) (Spooren et al., 2001, 2003; Varney and Gereau, 2002). Indeed, an increasing number of preclinical studies indicate that Group I mGluR receptors can modulate nociceptive processing at various levels in the nervous system and are involved in both peripheral and central sensitization (Fundytus, 2001; Karim et al., 2001a; Neugebauer, 2002; Varney and Gereau, 2002). For example, the expression of mGluR1 receptor is increased in rats following spinal cord injury, and this may mediate the chronic central pain induced by the injury (Mills and Hulsebosch, 2002). While no data are reported in the literature using mGluR1 receptor knockout mice in animal models of pain, antisense knock down of mGluR1 receptors reduced the development of neuropathic pain, acute thermal pain and formalin-induced nociception (Young et al., 1998; Noda et al., 2003). Similarly, anti-mGluR1 receptor antibodies induced antinociceptive effects in models of chronic pain (Fundytus et al., 1998). In addition to expression and knock down studies, an increasing number of preclinical studies suggested that mGluR1 receptor antagonists might hold great promise for the treatment of pain (Neugebauer, 2002). Similar to mGluR1 receptors, mGluR5 receptor blockade using selective antagonists like MPEP, produced antinociceptive effects in animal model of inflammatory and neuropathic pain (Karim et al., 2001b; Walker et al., 2001a, 2001b; Fisher et al., 2002; Hudson et al., 2002; Zhu et al., 2004, 2005; Varty et al., 2005). Spinal administration of anti-mGluR5 receptor antibodies reduced cold hyperalgesia in neuropathic pain (Fundytus et al., 1998), and electrophysiological recording indicated that mGluR5 receptors may be involved in spinal nociception (Bordi and Ugolini, 1999).
A-841720 is a novel non-competitive mGluR1 receptor antagonist, structurally unrelated to glutamate-derived mGluR receptor ligands. In vitro, A-841720 inhibited receptor activity without affecting the binding of agonist: the antagonist concentration-dependently displaced the noncompetitive mGluR1 receptor radioligand [3H]-R214127 and furthermore, increasing the concentration of A-841720 decreased the efficacy of L-quisqualate to induce calcium release in the human mGluR1 receptor FLIPR assay. The same effects were observed using the non-competitive mGluR5 receptor radioligand [3H]-MPEP in a binding assay, and in human mGluR5 receptor FLIPR assay, however, with lower efficacies compared to human mGluR1 receptor.
At recombinant human and native rat mGluR1 receptors, A-841720 non-competitively inhibited L-glutamate-induced calcium mobilization, with IC50 values of 10.7 and 1.0 nM, respectively, while showing >30- and 200-fold selectivity over human and rat mGluR5 receptors, respectively (Table 2). In addition, A-841720 inhibited [3H]-R214127 binding to membranes prepared from adult rat cerebellum with Ki=1.05 nM. A-841720 did not antagonize human mGluR2, mGluR4 and mGluR7 receptors and was shown to be inactive at a large number of receptors/uptake/channels, some of which known to be involved in pain transmission. In addition, A-841720 was shown to have good pharmacokinetic properties following systemic administration with linear blood and brain exposure with a ratio of about 3–1 brain to plasma for all doses tested. Taken together, these data show that A-841720 is a potent and selective mGluR1 receptor antagonist with in vitro and pharmacokinetic properties adequate for further evaluating the role of mGluR1 receptors in in vivo models of pain, locomotion and cognitive functions.
In the present study, A-841720 fully reversed chronic inflammation-induced thermal hyperalgesia and pain associated with joint inflammation. It is worth noting that glutamate has been shown to increase in the hind paw of rats during nociceptive sciatic nerve stimulation or the formalin test, as well as in the synovial fluid of arthritic patients (Omote et al., 1998; deGroot et al., 2000; McNearney et al., 2000). In addition to its effects on inflammatory pain, A-841720 also reversed mechanical allodynia observed in two models of nerve injury. This finding is in agreement with studies showing that anti-mGluR1 receptor antibodies decreased neuropathic pain in rats (Fundytus et al., 1998), and that knock down of mGluR1 protein expression by intrathecal injection of mGluR1 receptor antisense is accompanied by a reduction in cold and heat hyperalgesia as well as mechanical allodynia in the CCI model of neuropathic pain (Fundytus, 2001). Interestingly, in the present study, we showed that systemic administration of A-841720 reduced spinal neuronal responses to stimulation in anesthetized neuropathic animals, indicating that an mGluR1 receptor antagonist can modulate spinal cord nociceptive transmission. Furthermore, it has been previously shown that delivery of mGluR1 receptor antagonists into localized brain areas produces significant analgesic effects. Delivery of the weak mGluR1 receptor antagonist CPCCOEt through microdialysis probes into the central nucleus of the amygdala of arthritic rats resulting in decreased vocalizations (Han and Neugebauer, 2005), illustrated the role of mGluR1 in amygdala-mediated pain behavior. Taken together, these data show that A-841720 produces a broad-spectrum analgesic profile, at least in part due to a central site of action.
Given the wide distribution of mGluR1 receptors in CNS areas such as hippocampus, cortex, striatum, amygdala and cerebellum, in addition to analgesic effects, mGluR1 receptor antagonists have the potential to produce CNS side effects such as impairment of motor and cognitive functions. In the present study, A-841720 induced locomotor deficits in both the spontaneous locomotor assay and to a lesser extent in the rotorod assay of motor coordination. These results are consistent with the results obtained with the mGluR1 receptor knockout mice showing them to have severe deficits in motor coordination (Riedel et al., 2003). Although we cannot rule out that the effects of A-841720 in animal models of nociception we observed could at least be partially mediated by a decrease in motor function, the fact that A-841720 decreased evoked firing of spinal nociceptive neurons in neuropathic rats (Figure 7) supports a clear role for mGluR1 receptors in modulating nociception independently of secondary effects on locomotor function.
mGluR1 receptor knockout mice also exhibit a deficit in learning the delay eye-blink conditioning task (Riedel et al., 2003) and an impaired learning capacity in a Morris water maze test (Conquet et al., 1994). In addition, long-term potentiation (LTP) in hippocampal mossy fibres was greatly reduced in these animals, in agreement with the demonstration that hippocampal mGluR1 receptors are necessary for corticostriatal LTP or depression (LTD) (Gubellini et al., 2003). In keeping with the mGluR1 receptor knockout data, systemic A-841720 induced a significantly lower spontaneous alternation rate in the Y-maze assay and a significantly increased latency and path length to locate the submerged platform in the Morris Water maze test. Although the vehicle used in the Y-maze study was not saline, our present results indicated that the vehicle did not influence the animal performance, showing both mean arm entries (16.63±1.57) and alteration rate (80.96±3.26) very comparable to those obtained with saline (Jia Bao Pan, personal observation). These two studies suggest a deficit in both short-term spatial working memory and spatial reference learning at doses that are fully effective in pain models. Importantly, in the Morris water maze test, A-841720-injected animals spent the same amount of time and traveled similar distance to reach a visible platform as the control animals, demonstrating that the deficit in the submerged platform task was not due to a deficit in sensorimotor processes. The effects on cognitive function observed with A-841720 confirmed and extended observation made with weaker and less selective mGluR1 receptor antagonists. For example, LY 367385 was shown to disrupt memory formation in spatial learning tasks, such as radial arm maze (Naie and Manahan-Vaughan, 2005). More recently, JNJ16259685, a potent and selective mGluR1 receptor antagonist, was shown to produce impairment of acquisition and retention in a spatial water maze task at doses shown to produce full occupancy of brain mGluR1 receptors in vivo (Lavreysen et al., 2004; Steckler et al., 2005b). Indeed, at 0.63–2.5 mg kg−1 s.c., doses for which full occupancy of the mGluR1 receptor in both the cerebellum and the thalamus was detected (Lavreysen et al., 2004), JNJ16259685 significantly impaired the performance of mice in several different versions of the water maze test (Steckler et al., 2005b). We cannot completely rule out that the effects of A-841720 on cognitive function could be attributed to the blockade of mGluR5 receptors in vivo. However, MPEP has been shown to produce CNS sides effects at threefold higher doses than fully efficacious analgesic doses (Zhu et al., 2004) and to have relatively small effects in the rat water maze and in an operant delayed matching to position task (Ballard et al., 2005). In addition, blockade of mGluR5 receptors was also shown to produce less disruption of spatial learning than mGluR1 receptor blockade (Steckler et al., 2005b). Overall, these data support the overall finding that blockade of mGluR5 receptor produces a different in vivo profile from that produced by blockade of mGluR1 receptor, suggesting that the effects observed with A-841720 are primarily mGluR1 receptor mediated.
In conclusion, A-841720, a new mGluR1 receptor antagonist can induce full efficacy in both chronic neuropathic and inflammatory pain models. These effects are in part due to the ability of A-841720 to decrease nociceptive transmission at the spinal cord level. However, the effects of the fully analgesic dose of A-841720 were also associated with significant deficits in both motor and cognitive functions. The lack of separation between the doses inducing analgesia and those inducing significant CNS side effects in preclinical models indicates that antagonism of mGluR1 receptors may not provide a sufficient separation between efficacy and side effects for the development of novel analgesic agents in humans.
Acknowledgments
The authors thank Dr Bruce Surber for preparation of [3H]-R214127, Dr Reinhold Mueller for providing the human mGluR7 cell lines, and Dr Kennan Marsh and Dr Michael Jarvis for helpful discussion. Jill Wetter is also gratefully acknowledged for her technical assistance with the pharmacokinetic studies.
Abbreviations
- A-841720
9-Dimethylamino-3-(N-hexamethyleneiminyl)-3H-5-thia-1,3,6-triazafluoren-4-one
- CFA
complete Freund's adjuvant
- CPCCOEt
7-(hydroxylimino) cyclopropa[b]chromen-1a-carboxamide ethyl ester
- FLIPR
fluorometric imaging plate reader
- HEK
human embryonic kidney
- InsP3
inositol 1,4,5-triphosphate
- mGluR
metobotropic glutamate receptor
- MIA
monoiodoacetate
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine
- OA
osteoarthritis
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- R-214127
1-(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-2-phenyl-1-ethanone
- (S)-DHPG
(S)-3,5-dihydroxyphenylglycine
- SIB1757
6-methyl-2-(phenylazo)-3-pyridinol
- SL-327
α-[Amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl) benzeneacetonitrile
Conflict of interest
The authors state no conflict of intrest.
References
- Ballard TM, Wooley ML, Prinssen E, Huwvler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology. 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53:325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- De Vry J, Horvath E, Schreiber R. Neuroprotective and behavioral effects of the selective metabotropic glutamate mGlu(1) receptor antagonist BAY 36–7620. Eur J Pharmacol. 2001;428:203–214. doi: 10.1016/s0014-2999(01)01296-1. [DOI] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Desai Ma BJ, Mayne NG, Schoepp DD. Cloning and expression of a human metabotropic glutamate receptor 1 alpha: enhanced coupling on co-transfection with a glutamate transporter. Mol Pharmacol. 1995;48:648–657. [PubMed] [Google Scholar]
- Dogrul AOM, Lai J, Malan TP, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- Fisher K, Cahill CM, Coderre TJ. Intrathecal administration of the mGluR compound, (S)-4CPG, attenuates hyperalgesia and allodynia associated with sciatic nerve constriction injury in rats. Pain. 1998;77:59–66. doi: 10.1016/S0304-3959(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain. 1996;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Chabot JG, Osbourne MG, Lefebvre CD, Dray A, Henry JL, et al. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Dray A, Henry JL, Coderre TJ. In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. Neuroreport. 1998;9:731–735. doi: 10.1097/00001756-199803090-00031. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Costa C, Tropepi D, Bernardi G, et al. Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology. 2003;44:8–16. doi: 10.1016/s0028-3908(02)00214-9. [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, et al. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22:2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Bhave G, Gereau RW. Metabotropic glutamate receptors on peripheral sensory neuron terminals as targets for the development of novel analgesics. Mol Psychiat. 2001a;6:615–617. doi: 10.1038/sj.mp.4000961. [DOI] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RW. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001b;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Griffey K, Johnson MP, Chamberlain MJ, Kelly G, Tomlinson R, et al. Inhibition of group I metabotropic glutamate receptor responses in vivo in rats by a new generation of carboxyphenylglycine-like amino acid antagonists. Neurosci Lett. 2002;330:127–130. doi: 10.1016/s0304-3940(02)00751-6. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Linglin L, Tsui HT, Baez M, Tomlinson R, Hong EJ, et al. A novel series of potent and selective non-competitive antagonists of metabotropic glutamate receptor 1 Society for Neuroscience Abstract # 2003575.2
- Lavreysen H, Janssen C, Bischoff F, Langlois X, Leysen JE, Lesage AS. [3H]R214127: a novel high-affinity radioligand for the mGlu1 receptor reveals a common binding site shared by multiple allosteric antagonists. Mol Pharmacol. 2003;63:1082–1093. doi: 10.1124/mol.63.5.1082. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nobrega-Pereira SLanglois X, Blokland S, Somers M, et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Faltynek CR, Jarvis MF. Systemic and site-specific effects of A-425619, a selective TRPV1 receptor antagonist, on wide dynamic range neurons in CFA-treated and uninjured rats. J Neurophysiol. 2006;95:18–25. doi: 10.1152/jn.00560.2005. [DOI] [PubMed] [Google Scholar]
- McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;7:739–745. [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutri. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-D-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci. 2005;21:411–421. doi: 10.1111/j.1460-9568.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Metabotropic glutamate receptors – important modulators of nociception and pain behavior. Pain. 2002;98:1–8. doi: 10.1016/s0304-3959(02)00140-9. [DOI] [PubMed] [Google Scholar]
- Noda K, Anzai T, Ogata M, Akita H, Ogura T, Saji M. Antisense knockdown of spinal-mGluR1 reduces the sustained phase of formalin-induced nociceptive responses. Brain Res. 2003;987:194–200. doi: 10.1016/s0006-8993(03)03330-4. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Salt TE, Turner JP, Kingston AE. Evaluation of agonists and antagonists acting at Group I metabotropic glutamate receptors in the thalamus in vivo. Neuropharmacology. 1999;38:1505–1510. doi: 10.1016/s0028-3908(99)00081-7. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Mizuno N.Metabotropic receptors-immunocytochemical and in situ hybridization analysis Handbook of Chemical Neuroanatomy; Metabotropic Glutamate Receptors: Immunocytochemical and in situ Hybridization Analysis 2000Elsevier; 63–98.In: Ottersen OP and Storm-Mathisen J (eds)Vol. 18, [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- Steckler T, Lavreysen H, Oliveira AM, Aerts N, Van Craenendonck H, Prickaerts J, et al. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology. 2005a;179:198–206. doi: 10.1007/s00213-004-2056-7. [DOI] [PubMed] [Google Scholar]
- Steckler T, Oliveira AF, Van Dyck C, Van Craenendonck H, Mateus AM, Langlois X, et al. Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task. Behav Brain Res. 2005b;164:52–60. doi: 10.1016/j.bbr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Varney MA, Gereau RW. Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Target. 2002;1:283–296. doi: 10.2174/1568007023339300. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology. 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, et al. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001a;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001b;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Young MR, Blackburn-Munro G, Dickinson T, Johnson MJ, Anderson H, Nakalembe I, et al. Antisense ablation of type I metabotropic glutamate receptor mGluR1 inhibits spinal nociceptive transmission. J Neurosci. 1998;18:10180–10188. doi: 10.1523/JNEUROSCI.18-23-10180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MR, Fleetwood-Walker SM, Dickinson T, Blackburn-Munro G, Sparrow H, Birch PJ, et al. Behavioural and electrophysiological evidence supporting a role for group I metabotropic glutamate receptors in the mediation of nociceptive inputs to the rat spinal cord. Brain Res. 1997;777:161–169. [PubMed] [Google Scholar]
- Zhang L, Lu Y, Chen Y, Westlund KN. Group I metabotropic glutamate receptor antagonists block secondary thermal hyperalgesia in rats with knee joint inflammation. J Pharmacol Exp Ther. 2002;300:149–156. doi: 10.1124/jpet.300.1.149. [DOI] [PubMed] [Google Scholar]
- Zheng GZ, Bhatia P, Daanen J, Kolasa T, Patel M, Latshaw S, et al. Structure-activity relationship of triazafluorenone derivatives as potent and selective mGluR1 antagonists. J Med Chem. 2005;48:7374–7388. doi: 10.1021/jm0504407. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Hsieh G, El-Kouhen O, Wilson SG, Mikusa JP, Hollingsworth PR, et al. Role of central and peripheral mGluR5 receptors in post-operative pain in rats. Pain. 2005;114:195–202. doi: 10.1016/j.pain.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, III, et al. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506:107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]