Abstract
Background and purpose:
The chemokine receptor CCR1 is a potential target for the treatment of rheumatoid arthritis. To explore the impact of CCR1 blockade in experimental arthritis and the underlying mechanisms, we used J-113863, a non-peptide antagonist of the mouse receptor.
Experimental approach:
Compound J-113863 was tested in collagen-induced arthritis (CIA) and three models of acute inflammation; Staphylococcus enterotoxin B (SEB)-induced interleukin-2 (IL-2), delayed-type hypersensitivity (DTH) response, and lipopolysaccharide (LPS)-induced tumour necrosis factorα (TNFα) production. In the LPS model, CCR1 knockout, adrenalectomised, or IL-10-depleted mice were also used. Production of TNFα by mouse macrophages and human synovial membrane samples in vitro were also studied.
Key results:
Treatment of arthritic mice with J-113863 improved paw inflammation and joint damage, and dramatically decreased cell infiltration into joints. The compound did not inhibit IL-2 or DTH, but reduced plasma TNFα levels in LPS-treated mice. Surprisingly, CCR1 knockout mice produced more TNFα than controls in response to LPS, and J-113863 decreased TNFα also in CCR1 null mice, indicating that its effect was unrelated to CCR1. Adrenalectomy or neutralisation of IL-10 did not prevent inhibition of TNFα production by J-113863. The compound did not inhibit mouse TNFα in vitro, but did induce a trend towards increased TNFα release in cells from synovial membranes of rheumatoid arthritis patients.
Conclusions and implications:
CCR1 blockade improves the development of CIA, probably via inhibition of inflammatory cell recruitment. However, results from both CCR1-deficient mice and human synovial membranes suggest that, in some experimental settings, blocking CCR1 could enhance TNF production.
Keywords: CCR1, J-113863, chemokines, TNFα, IL-10, collagen-induced arthritis, inflammation, rheumatoid arthritis
Introduction
The chemokine receptor, CCR1, is a member of a receptor superfamily that binds multiple CC-chemokines, the most representative being CCL3/macrophage inflammatory protein-1α (MIP-1α) and CCL5/RANTES (Murphy et al., 2000). The receptor is expressed on neutrophils and monocytes (Lee et al., 2000), T cells (Su et al., 1996), immature dendritic cells (Sozzani et al., 1997), platelets (Clemetson et al., 2000) and resident tissue cells (macrophages, fibroblasts, mast cells and osteoclasts) (Menten et al., 2002). The wide cellular distribution of CCR1, together with its role in both cell migration and activation suggest that it may have a pleiotropic role in immune and inflammatory disorders. To study the role of CCR1 in a particular experimental disease, a number of strategies have been used, including modified chemokines, DNA vaccines (Youssef et al., 2000), anti-CCR1 antibodies (Tokuda et al., 2000), or CCR1 knockout mice (Gao et al., 1997; Topham et al., 1999; Blease et al., 2000; Gao et al., 2000). Those studies suggested that CCR1 is a potential therapeutic target in multiple sclerosis (Rottman et al., 2000), transplant rejection (Gao et al., 2000), or allergic airway diseases (Blease et al., 2000).
The role of CCR1 in arthritis has been addressed by using MetRANTES, a dual CCR1/CCR5 receptor antagonist (Proudfoot et al., 1999), which ameliorates the progression of rat adjuvant- (Shahrara et al., 2005) and mouse collagen-induced arthritis (CIA) (Plater-Zyberk et al., 1997) models. Recently, it has been reported that the lack of CCL3/MIP-1α, a ligand of both CCR1 and CCR5, plays an essential role in the development of CIA (Chintalacharuvu et al., 2005). These studies indicated that dual CCR1/CCR5 neutralization might be effective to control arthritis. Whether CCR1 blockade alone is effective is unknown, since no results with CCR1 knockout mice with arthritis are available.
Small molecule antagonists of chemokine receptors are also excellent tools to assess the contribution of a specific receptor to an experimental model of disease. The compounds to be tested in animals have to bind the receptor of the species of choice, usually a rodent. However, chemokine receptor antagonists, like other G-protein coupled receptor (GPCR) antagonists (Horuk, 2003), often exhibit species selectivity, and most of them only display a high affinity for the human receptor (Liang et al., 2000a). This finding may explain why, despite intense experimental activity in drug discovery on chemokine receptor antagonists, in vivo results from only a few compounds are available. A CCR1 antagonist, BX-471 from Berlex (Liang et al., 2000b), has been evaluated in models of transplant rejection (Horuk et al., 2001), renal fibrosis (Anders et al., 2002; Vielhauer et al., 2004), lupus nephritis (Anders et al., 2004) and, more recently, fungal asthma (Carpenter et al., 2005). However, no data using this compound in an experimental model of arthritis have been published. While this compound is a potent antagonist of the human receptor, it exhibits only a moderate antagonistic effect on rat and mouse CCR1 receptors (Horuk et al., 2001).
The aim of this present study was to evaluate the impact of the pharmacological blockade of CCR1 on experimental models of inflammation, including murine arthritis, using a small molecule antagonist of CCR1 that exhibited good affinity for both the human and mouse CCR1 receptor. J-113863 (1-(1-cycloocten-1-ylmethyl)-4-(2,7-dichloroxanthen-9-ylcarboxamido)-1-ethylpiperidinium iodide) from Banyu Pharmaceuticals (Naya and Saeki, 2001) has IC50 values of 0.9 and 5.8 nM for human and mouse CCR1 receptors, respectively (Naya et al., 2001). In a previous work, we demonstrated that this compound is able to inhibit cell infiltration in the air pouch of mice challenged with carrageenan (Garcia-Ramallo et al., 2002). Herein, we show that pharmacological antagonism of CCR1 by J-113863 prevents the progression of established CIA, probably via the inhibition of the migration of inflammatory cells into joints and not by a direct effect on T cells or macrophages. As we show that the lack of CCR1 can, in some circumstances, potentiate TNFα synthesis and/or release, this effect should be explored before a CCR1 antagonist progresses into clinical trials.
Methods
Animals
All mice strains used throughout our studies (Swiss, C57Bl/6, Balbc, DBA-1), as well as adrenalectomized and sham-operated mice were purchased from Harlan Ibérica (St Feliu de Codines, Spain). The origin of the CCR1 knockout mice on a Balbc background have been described elsewhere (Blease et al., 2000). CCR1 knockout mice on a C57Bl/6 background were supplied by Taconics (Germantown, NY, USA). All the experimental procedures contained in this paper followed the Spanish legislation on ‘Protection of animals used in experimental and other scientific purposes' in agreement with the European regulations.
Pharmacokinetics of J-113893 in mice
J-113863 was given by i.p. injection to 18 Swiss mice at 3 or 10 mg kg−1, in a volume of 10 ml kg−1. At several time points, blood was extracted from retro-orbital plexus into heparinized tubes, centrifuged and plasma samples frozen until analysis. Three different mice were used for each time point. Aliquots of 30 μl of plasma were diluted with 200 μl of a solution containing 0.2% TFA. After centrifugation, 70 μl of the supernatant were mixed with the same volume of water, and 10 μl of this mixture was analyzed by means of HPLC/MS.
Collagen-induced arthritis
DBA-1 male mice of 10–12 weeks of age were immunized with 0.1 ml of collagen II (chicken collagen, Sigma, TresCantos, Madrid, Spain) emulsified in complete Freund's adjuvant, injected at the base of the tail (collagen concentration, 2 mg ml−1). After 21 days, mice received an i.p. injection of 0.1 mg of collagen II in saline. The arthritis was monitored by scoring inflamed joints in each paw as described previously (Ross et al., 1997) with slight modifications: 0=no inflammation; 1=mild erythema and swelling of individual digits; 2=moderate erythema and swelling of the joint; 3=severe erythema and swelling of the entire paw; 4=severe erythema and ankylosis of the paw. The arthritic index is the sum of the scores of the four mouse paws; the maximum possible score being 16. Animals were observed every day and, when the first symptom of arthritis was evident, each mouse received an i.p. injection of vehicle or the CCR1 antagonist at 3 or 10 mg kg−1, once daily for 11 days. Mice were monitored daily during the period of treatment by an observer unaware of the treatment. At 1 day after the last administration of the compound (day 12), blood from the retro-orbital plexus was collected in heparinized tubes and animals killed. Plasma samples were frozen and levels of cytokines (TNFα, IL-1, sTNFRII, IL-10) and chemokines (CCL-3/MIP-1α and CCL2/MCP-1) determined by ELISA (R&D Systems, Abingdon, UK), according to the manufacturer's instructions. Plasma levels of anti-collagen IgG1 and IgG2A antibodies were determined by direct ELISA, as described previously (Williams et al., 1992).
The effect of treatments on joint inflammation and cartilage and bone damage were evaluated histologically, following a modification of the protocol described previously (Lawlor et al., 2001). From each mouse, both hindpaws were removed, fomalin-fixed, decalcified and wax-embedded before sectioning and staining with hematoxilin–eosin. The joint damage score was recorded by assessing the degree of pannus formation, cartilage erosion, and bone destruction. For each parameter a scale ranging from 0 (normal) to 4 (severe) was used.
Delayed-type hypersensitivity
Male Swiss mice were sensitized with 2,4-dinitrofluorobenzenic acid (DNFB, Sigma) by applying 0.1 ml of a solution in olive oil:acetone (4:1, v:v) to the shaved abdomen for 2 consecutive days. After 5 days later, mice received vehicle or test compound at several doses by i.p. injection, followed 30 min later by a solution of 10 μl of DNFB 0.2% in acetone in their left ears. Animals were killed 24 h later, ear biopsies of 8 mm in diameter were obtained and wet weight determined.
SEB-induced IL-2
Balbc male mice fasted overnight and with water ad libitum received vehicle or several doses of the CCR1 antagonist (i.p.), followed 30 min later by an i.p. injection of Staphylococcus aureus enterotoxin B (SEB) (S4881, Sigma) disolved in saline at a dose of 50 μg per mouse. After 3 h, mice were anesthesized and blood from the retro-orbital plexus collected in heparinized tubes. Samples were centrifuged and supernatants frozen until analysis. Mouse IL-2 levels in the samples were determined by ELISA (R&D Systems, Abingdon, UK) according to the manufacturer's instructions.
In vivo LPS assay
Swiss mice fasted overnight with water ad libitum received vehicle or the CCR1 antagonist at several doses (i.p.) followed 30 min later by an i.p. injection of 5 mg kg−1 of LPS from Escherichia coli 0111:B4 (Sigma). After 1½ h later, blood from the retro-orbital plexus was collected into heparinized tubes, centrifuged and samples frozen until analysis. Plasma levels of TNFα and IL-10 were determined by ELISA (R&D Systems) according to the manufacturer's instructions. In experiments with CCR1 knockout mice, animals with both Balbc and C57 backgrounds were studied. Corresponding age- and sex-matched mice of Balbc or C57 backgrounds were used as controls.
LPS assay in IL-10-depleted mice
Swiss male mice received an i.p. injection containing 250 μg of an anti-IL-10 antibody (JESS 2A5, BD Pharmingen, San Diego, CA, USA) or the corresponding isotype-matched control (R3-34, rat anti-mouse IgG1). After 2 h later, vehicle or 10 mg kg−1 of J-113863 were administered and the LPS assay was performed as described.
LPS assay in adrenalectomized mice
Adrenalectomized mice and sham-operated controls were treated with vehicle or 10 mg kg−1 of J-113863 and, 30 min later, the LPS assay was performed as described. A group of animals treated with rolipram, a PDE4 inhibitor, (10 mg kg−1, i.p., 30 min before LPS) was included as a positive control.
TNFα synthesis in mouse macrophages in vitro
Macrophages were isolated by washing the peritoneal cavity of 10 anesthesized mice with 2 ml of PBS. Cells were pooled, centrifuged and seeded in 48-well plates at a density of 5 × 105 cells per well in RPMI medium plus 1% FBS. Samples were incubated in the presence of vehicle (DMSO) or the CCR1 antagonist at 10−5 to 10−9 M, together with 1 μg ml−1 LPS for 24 h. The final concentration of DMSO in the samples was 0.5%. Supernatants were obtained by centrifugation and TNFα and IL-10 contents analyzed by ELISA (R&D Systems) following the manufacturer's instructions.
Isolation of cells from synovial membrane tissue
Mononuclear cells were obtained from synovial tissue specimens taken during joint replacement surgery, provided by the Orthopedic/Plastic Surgery Department, Charing Cross Hospital, London, UK. Tissue was teased into small pieces and digested in medium containing 0.15 mg ml−1 DNAase type I (Sigma, UK) and 5 mg ml−1 collagenase (Roche, UK) for 1–2 h at 37°C. Cells were passed through a nylon mesh to exclude cell debris, washed and resuspended in RPMI plus 10% heat inactivated FCS at a density of 2 × 106 cells ml−1.
Effect of J-113863 on cytokine production by synovial membrane cells
Cells cultured in 96-well plates at a density of 2 × 105 cells per well were incubated with increasing concentrations of the test compound or vehicle (0.5% DMSO). After 48 h, supernatants were harvested. Concentrations of IL-10 and TNFα were determined by ELISA following the manufacturer's instructions.
Statistical analysis
An unpaired Student's t-test was used to determine the differences between vehicle- and J-113863-treated samples both in vivo and in vitro. P<0.05 was considered statistically significant. Nonlinear regression analysis of the data and calculation of ED50 were performed using Prism 4.01 (GraphPad Software, San Diego, CA, USA).
Materials
The CCR1 antagonist, J-113863 (1-(1-cycloocten-1-ylmethyl)-4-(2,7-dichloroxanthen-9-ylcarboxamido)-1-ethylpiperidinium iodide), and the PDE4 inhibitor rolipram (4-[3-(cyclopentyloxy)-4-methoxy-phenyl]-2-pyrrolidinone) have been synthesized at the Medicinal Chemistry Department of Almirall. Dexamethasone was supplied by Sigma (St Louis, MO, USA). Rat anti-mouse IL-10 monoclonal antibody and rat anti-mouse IgG1 isotype were supplied by BD Pharmingen (San Diego, CA, USA).
Results
Pharmacokinetics of J-113863 in mice
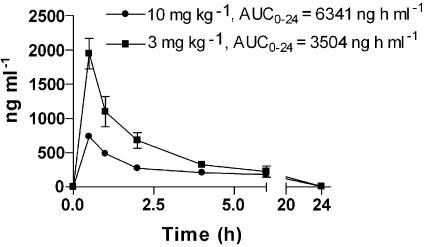
The CCR1 antagonist J-113863 is a quaternary ammonium compound and is poorly absorbed when given orally (Naya et al., 2001). Thus, the pharmacokinetic profile of J-113863 was evaluated by giving the compound i.p. at 3 or 10 mg kg−1. Figure 1 depicts the plasma concentrations of J-113863 throughout a period of 24 h, with the area under the curve (AUC) values indicated for each dose. According to our studies of CCL3/MIP1α binding to mouse bone marrow cells (data not shown), these pharmacokinetics support the use of the two doses of the compound, given i.p., in a once a day regime for in vivo testing, ensuring CCR1 blockade without accumulation of the compound following repeated administrations.
Figure 1.
Pharmacokinetic profile of J-113863 after a single i.p. injection of 3 or 10 mg kg−1 to mice. The values of the area under the curve for each dose during the period studied (AUC0-24) are also shown. Three animals were used for each time point.
Effect of the CCR1 antagonist J-113863 on murine arthritis
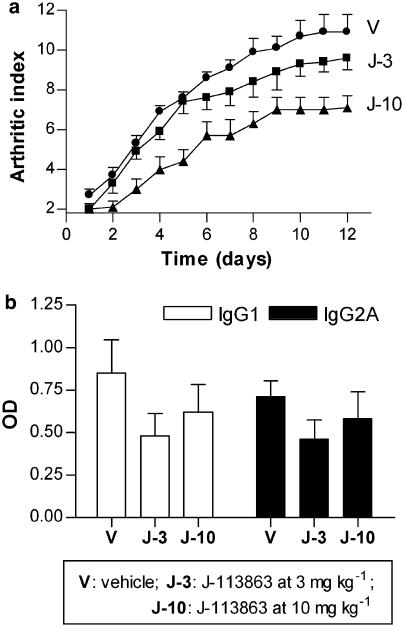
To assess the therapeutic impact of CCR1 blockade in CIA, DBA-1 mice received, from the first day of clinical manifestation of arthritis, a daily administration of vehicle or the CCR1 antagonist, at 3 or 10 mg kg−1, by i.p. injection for 11 days. We measured the effect of the compound on both paw inflammation and anti-collagen II antibodies (Figure 2). As shown in Figure 2a, the compound dose-dependently inhibited the clinical manifestation of the disease (arthritic index) from the beginning of the treatment. Figure 2b shows the impact of the treatment in the levels of IgG1 and IgG2A anti-collagen II antibodies. Although the compound moderately reduced antibody titers when compared to vehicle-treated mice, no statistical differences were attained, in part due to the high intra-assay variability.
Figure 2.
Effect of J-113863 on collagen-induced arthritis. DBA-1 mice with established disease received either vehicle (V) or the test drug for 11 days by i.p. route. (a) Effect of J-113863 at 3 mg kg−1 (J-3) and 10 mg kg−1 (J-10) on the clinical manifestations of the disease (arthritic index). (b) Effect of the compound on anti-collagen II antibodies of the IgG1 and IgG2A subtypes, measured at the end of the experiment. Results shown are representative of three independent experiments using seven mice per treatment group. *P<0.05; **P<0.01 vs vehicle, Student's t-test.
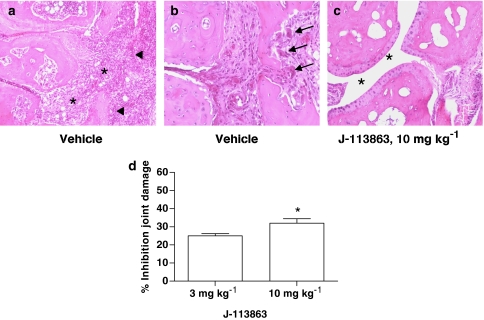
The impact of CCR1 blockade on arthritis was also assessed histologically. As shown in the top panel of Figure 3, mice treated with 10 mg kg−1 of the CCR1 antagonist exhibited a significant inhibition of cell infiltration in the joint space (Figure 3c, asterisks) compared to vehicle-treated mice (Figure 3a, asterisks). Whereas mice treated with vehicle showed a prominent pannus (Figure 3a, arrowheads) and the presence of numerous osteoclasts (Figure 3b, arrows) indicative of bone destruction, treatment with J-113863 remarkably improved both parameters. The inhibition of the joint damage score (Figure 3d) reached statistical significance vs vehicle, with the 10 mg kg−1 dose.
Figure 3.
Effect of the CCR1 antagonist on paw histology. Microscopic images (× 10 for (a, c); 20 × for (b)) representative of the paw of arthritic mice treated with vehicle or 10 mg kg−1 of J-113863 illustrating the joint space (asterisks), the invasive pannus (arrowheads), and the presence of osteoclasts (arrows). (d) Inhibition of the joint damage score by J-113863 at 3 and 10 mg kg−1 vs vehicle. Results shown are representative of three independent experiments using seven mice per treatment group. *P<0.05 vs vehicle-treated mice, Student's t-test.
We were interested in studying if the compound had any impact on cytokine and chemokine synthesis. We determined the levels of TNFα, the soluble TNF receptor II (sTNFRII), IL-10 and the chemokines, CCL2/MPC-1 and CCL3/MIP-1α, in plasma, as well as levels of TNFα in paw homogenates, at the end of the experiment. Among the different mediators assayed, only plasma sTNFRII was detected. The levels of this protein were higher in vehicle-treated arthritic mice (9.1±0.89 ng ml−1) than in healthy mice (4.9±0.96 ng ml−1), but they were unaffected by treatment with J-113863 (9.63±0.5 and 10.3±1.3 ng ml−1 at 3 and 10 mg kg−1, respectively).
Effect of J-113863 on acute models of inflammation
Macrophages and T cells are the main drivers of the pathogenesis of CIA and both are known to express CCR1. We were interested in studying if J-113863 had a direct effect on these cell types by using representative animal models. The information obtained from these models could help to understand the possible mechanisms underlying the antiarthritic effect of the compound. To this end, we tested the compound in two T-cell dependent models; the delayed-type hypersensitivity (DTH) reaction induced by DNFB, and the synthesis of IL-2 following an i.p. injection of SEB. The results (Table 1) showed that J-113863 was inactive in the two models studied, whereas dexamethasone, our reference control, was active.
Table 1.
Effect of J-113863 on the DTH response induced by DNFB in the ears of mice and on the plasma levels of IL-2 induced by SEB
| Treatment | Dose (mg kg−1) | DTH Biopsy weight (mg) mean±s.e.m. | SEB IL-2 (pg ml−1) mean±s.e.m. |
|---|---|---|---|
| Vehicle | — | 15.1±1.3 | 3563±53 |
| J-113863 | 3 | 14.9±1.0 | 3650±89 |
| 10 | 15.5±2.0 | 3554±196 | |
| 30 | 14.9±1.3 | 3380±180 | |
| Dexamethasone | 3 | 6.9±0.7* | 1400±120* |
Dexamethasone was used as a reference standard. Results shown are representative of two independent experiments using six mice per group.
P<0.05 vs vehicle-treated mice, Student's t-test.
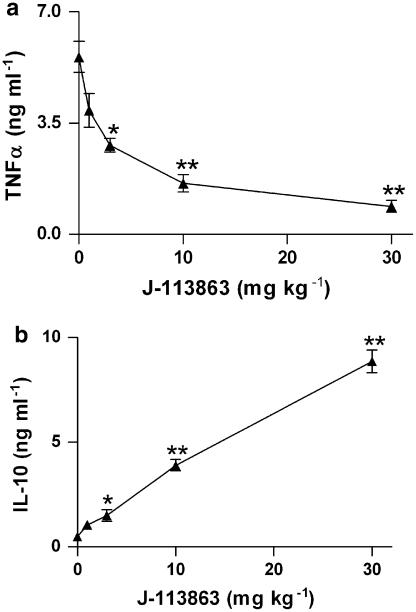
We used a third acute in vivo model, LPS-induced TNFα production, to determine if the antagonism of CCR1 inhibited output of this relevant cytokine. As shown in Figure 4a, the compound inhibited the levels of plasma TNFα in a dose-dependent manner when administered before LPS injection, with an ED50 of 3 mg kg−1. We also determined IL-10 levels from the same samples and found that this cytokine increased in parallel with the dose, as shown in Figure 4b. In animals not challenged with LPS, plasma levels of TNFα and IL-10 were undetectable both in vehicle- and J-113863-treated mice. This indicated that compound J-113863 induced the release of IL-10 in vivo only in the presence of LPS.
Figure 4.
Effect of J-113863 on the plasma levels of TNFα (a) and IL-10 (b) at 1.5 h after LPS challenge to Swiss mice. Results are the mean and s.e.m. of three independent experiments, each using five animals per treatment group. *P<0.05, **P<0.01 vs vehicle-treated mice, Student's t-test.
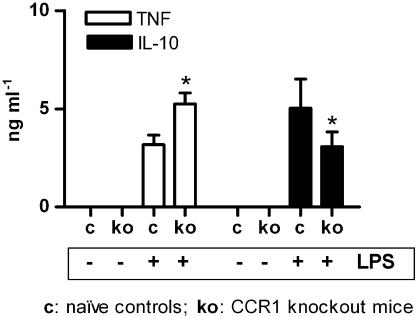
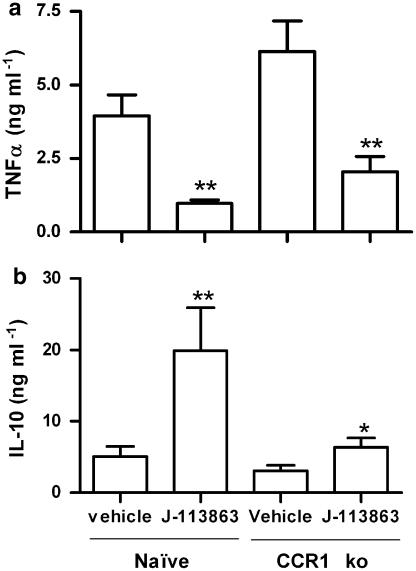
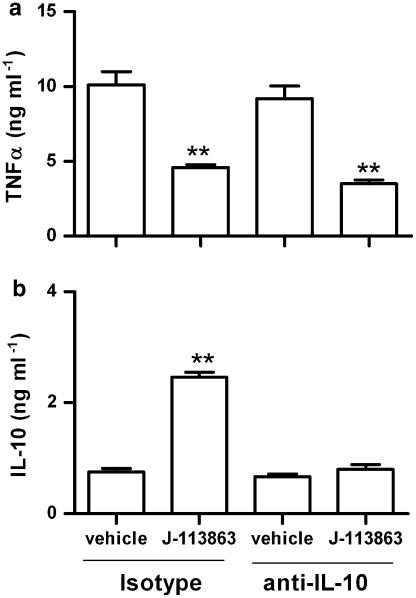
To determine if the effect of J-113863 on the LPS model was due to CCR1 antagonism, we next performed the LPS assay in CCR1 knockout mice and their corresponding background controls (WT). As shown in Figure 5, no detectable plasma levels of TNFα or IL-10 were found in untreated CCR1 null mice or WT controls in the absence of LPS, indicating that the lack of CCR1 did not cause per se an alteration on these cytokines. However, after LPS challenge, the levels of TNFα were higher and the levels of IL-10 lower in CCR1 knockout mice than in naive controls (Figure 5). Although the data presented here was obtained using knockout mice on a Balbc background, identical results were obtained when CCR1−/− mice on a C57Bl/6 background were used (data not shown). The results obtained with CCR1 knockout mice were the opposite from those obtained with J-113863 and indicated both that the lack of this chemokine receptor was detrimental for the control of the LPS-induced inflammation and that the ability of J-113863 to inhibit TNFα was unrelated to CCR1. To confirm the latter point, we administered the compound to CCR1 knockout mice before the LPS challenge and determined the levels of TNFα and IL-10. As shown in Figure 6, the compound retained its ability to decrease TNFα and increase IL-10 in animals lacking CCR1. However, whereas the decrease of TNFα caused by J-113863 was the same in naive and CCR1 null mice, the magnitude of IL-10 release was lower in the knockout mice. These results suggested that inhibition of TNFα production was probably not a consequence of IL-10 induction. To confirm this point, we performed the LPS assay in mice treated with an anti-IL-10 antibody before the drug administration. As shown in Figure 7a, the decrease of TNFα obtained with J-113863 was identical in animals treated with the control isotype and in those treated with the antibody, despite the difference in the levels of IL-10 in the two groups. At the dose used, the anti-IL-10 antibody depleted about 70% of the IL-10 in the LPS-treated mice (Figure 7b).
Figure 5.
Effect of CCR1 deficiency on the plasma levels of TNFα and IL-10. Control (c) and CCR1 knockout mice (ko) were challenged with saline or LPS; plasma levels of TNFα and IL-10 were analyzed. Results are the means and s.e.m. of three independent experiments, each using five animals/treatment group. *P<0.05 ko vs control mice, Student's t-test.
Figure 6.
Effect of vehicle or J-113863 at 10 mg kg−1 on the plasma levels of TNFα (a) and IL-10 (b) in CCR1 ko mice challenged with LPS. Results are the means and s.e.m. of three independent experiments, each using five animals per treatment group. *P<0.05, **P<0.01 vs corresponding vehicle-treated mice, Student's t-test.
Figure 7.
Effect of anti-IL-10 treatment on the TNF inhibition by J-113863 in LPS-challenged mice. Mice were treated with an anti-IL-10 antibody or corresponding isotype before the administration or vehicle or compound J-113863 at 10 mg kg1. LPS was injected and plasma contents of TNFα (a) and IL-10 (b) measured. Results shown are representative of two independent experiments using five animals per group. **P<0.01 vs corresponding vehicle-treated mice, Student's t-test.
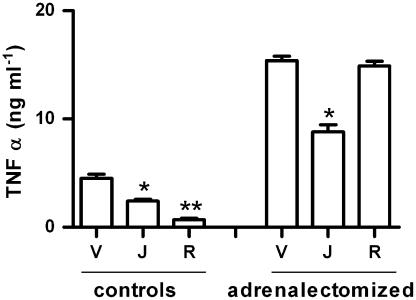
We next evaluated if the compound's effect was due to the activation of the hypothalamus—pituitary—adrenal (HPA) axis. To address this possibility, we used adrenalectomized animals. As Figure 8 shows, adrenalectomy per se causes an increase in the TNFα levels, as it has been previously reported (Cheng et al., 1997), but it has no impact on the inhibition of TNFα by J-113863. However, our positive control, rolipram (10 mg kg−1), dramatically inhibited TNFα production in controls (by 80%), and had no effect in the adrenalectomized mice.
Figure 8.
Involvement of the HPA axis in the effect of J-113863 on TNFα and IL-10. Adrenalectomized or sham-operated mice received vehicle (V) or 10 mg kg−1 of J-113863 (J) or rolipram (also 10 mg kg−1; R) followed, half an hour later, by an LPS challenge. Results shown are representative of two independent experiments using five animals per group. *P<0.05, **P<0.01 vs corresponding vehicle-treated mice, Student's t-test.
Effect of J-113863 on TNFα production in mouse cells in vitro
To study the effect of J-113863 on the synthesis of TNFα by mouse cells in vitro, we used cultured peritoneal macrophages isolated from naïve mice and treated them with LPS plus vehicle or the compound for 24 h. As shown in Table 2, the compound had no effect on the synthesis of TNFα in vitro, suggesting that the inhibition observed in vivo is not due to a direct effect on macrophages.
Table 2.
Effect of J-113863 on TNFα synthesis induced by LPS in mouse peritoneal macrophages in culture
| Treatment | (μM) | TNFα (pg ml−1) mean±s.d. |
|---|---|---|
| LPS+vehicle | — | 2507±274 |
| LPS+J-113863 | 0.01 | 2620±260 |
| 0.1 | 2445±175 | |
| 1 | 2497±220 |
Results shown are representative of three independent experiments performed in triplicate.
Effect of J-113863 on cytokine synthesis by synovial membranes
To estimate the potential of the CCR1 antagonist to alter cytokine synthesis in human arthritis, we used cells isolated from synovial membranes obtained from arthritic patients. One important feature of this system is that these cells release cytokines spontaneously into the medium, without the need for an external stimulus such as LPS. As Table 3 shows, J-113863 had no significant effect on TNFα production, but a trend towards an increase vs vehicle was observed. The compound moderately increased IL-10 levels in a non dose-dependent fashion. The maximum concentration of compound tested in this set of experiments was 10−7 M because at this concentration a 100% inhibition of migration of CCR1+ cells in chemotaxis assays was obtained (data not shown).
Table 3.
Effect of J-113863 on the spontaneous release of TNFα and IL-10 by cultured synovial membrane cells from arthritic patients
| Treatment | (M) | TNFα(ng ml−1) mean±s.d. | IL-10 (ng ml−1) mean±s.d. |
|---|---|---|---|
| Vehicle | — | 1.32±0.74 | 1.58±0.17 |
| J-113863 | 10−9 | 1.55±0.89 | 2.21±0.62* |
| 10−8 | 1.66±0.83 | 2.21±0.82* | |
| 10−7 | 1.94±0.72 | 1.90±0.46 |
Results shown are the mean and s.d. of samples from two independent experiments performed in triplicate.
P<0.05 vs vehicle (Student's t-test).
Discussion and conclusions
Accumulating evidence supports the notion that chemokines are important mechanistic participants in the pathobiology of a number of human disorders (Godessart and Kunkel, 2001; Schwarz and Wells, 2002). The use of chemokine receptor antagonists in experimental models of acute or chronic inflammation has become an increasingly high profile endeavor both from the standpoint of novel drug development and as valuable tools for target validation and proof of principle. However, there have been a number of pitfalls in the development of antagonists for this family of GPCRs, including low hit ratios in high throughput screens, lack of high affinity for non-human receptors, and cross-reactivity with other non-chemokine GPCRs (Horuk, 2003; Terricabras et al., 2004). Nonetheless, investigations in this area of chemokine biology are still very active, and several compounds are being developed to target a number of chemokine receptors, in particular CCR1, CCR5, CXCR2, CXCR3 and CXCR4 (Godessart, 2005).
A number of previous investigations have demonstrated that CCR1 and its ligands are expressed in association with the progression of experimental and human arthritis (Thornton et al., 1999; Katschke et al., 2001; Koch, 2005). Thus, we wanted to assess the impact of CCR1 blockade in an experimental model of arthritis; to this end we used a low molecular weight compound which antagonizes both human and mouse receptors with a low nanomolar affinity. The compound was able to reduce the course of established arthritis in a dose-dependent manner, measured using clinical and histological criteria. A moderate reduction in anti-collagen antibodies was found which did not reach statistical significance. The most prominent effect of J-113863 was the reduction of the cellularity in the joints observed in histological samples. These results are in agreement with the inhibition of cell migration to an inflamed mouse air pouch that we have previously reported with this compound (Garcia-Ramallo et al., 2002). Our results expand a preliminary study showing that J-113863 inhibited paw inflammation when administered at 3 mg kg−1 to arthritic mice (Naya and Saeki, 2001).
J-113863 has been reported to be inactive against a panel of 19 human targets, including some chemokine receptors, such as CCR2 and CCR5, as well as the LTB4 or TNFα receptors (Naya and Saeki, 2001). The compound is also a potent antagonist of the human CCR3 (Sabroe et al., 2000), but a weak antagonist of the mouse CCR3, with an IC50 100 times higher than that of CCR1 (460 vs 5.8 nM) (Naya et al., 2001). To confirm that CCR3 antagonism was not involved in the antiarthritic effect of J-113863, we have tested compound BX-471, a selective CCR1 antagonist extensively used in the literature (Liang et al., 2000a; Carpenter et al., 2005), in the CIA model. Our studies indicate that this compound inhibited the progression of arthritis when administered to DBA-1 mice at 10 mg kg−1 (24% inhibition) and 30 mg kg−1 (35% inhibition) by i.p. injection, once daily (Godessart, unpublished results). Moreover, during the preparation of this manuscript, it has been reported that two CCR1 antagonists from Novartis, compounds A4B7 and A1B1, inhibit paw inflammation in CIA when administered orally (Revesz et al., 2005). Altogether, these results support the proposition that antagonism of CCR1 could be a valid therapeutic approach for human arthritis.
Our studies evaluating the effect of J-113863 in two models of acute inflammation dependent on T cells suggest that the antiarthritic effect of the compound is not explained by a direct effect on these cells. The critical role that TNFα plays in the pathology of rheumatoid arthritis (Feldmann et al., 2004), prompted us to investigate the connection between CCR1 blockade and TNFα production in vivo. The dose-dependent decrease of TNFα production observed with J-113863 in the LPS model is in contradiction with the increase in TNFα observed in CCR1-deficient mice. To our knowledge, our studies are the first to show that the lack of this chemokine receptor gene is detrimental in the LPS model. A recent publication using CCL3/MIP-1α null mice has demonstrated that the absence of this chemokine had no effect on TNFα levels following endotoxin challenge. These results do not contradict our findings with CCR1 knockout mice and TNFα, since the lack of CCL3 may have an impact on other chemokine receptors, such as CCR1, CCR3 or CCR5 (Menten et al., 2002).
Taking into account that CCR1 is expressed on neutrophils, it is not surprising that the lack of CCR1 has a negative impact in certain experimental conditions depending on this cell type. This is the case of certain fungal infections (Gao et al., 1997) or nephrotoxic nephritis (Topham et al., 1999). In our hands, when the LPS assay is performed in neutropenic mice or in mice treated with an anti-CXCR2 antiserum, a three- to seven-fold increase in TNFα levels vs control mice is observed (Godessart, unpublished observations). These results are in agreement with those obtained in the CCR1 null mice and suggested that neutrophils are needed for the resolution of inflammation in the LPS model. We hypothesized that the inhibition of TNFα observed with J-113863 was unrelated to CCR1 antagonism, and this was clearly demonstrated by studies combining CCR1 null mice and J-113863. Given the relevance of TNFα as a therapeutic target, it was worth trying to elucidate the mechanism responsible for the effect of J-113863.
The lack of effect of the compound on TNFα synthesis by cultured mouse macrophages indicated the target of the in vivo effect was not this cell type. Some small molecules inhibit output of TNFα in vivo by inducing IL-10 release (Rongione et al., 1997) and/or by activating the HPA axis (Pettipher et al., 1997). Our studies conclusively demonstrated that J-113863 had no effect on the HPA axis. However, a partial involvement of IL-10 cannot be excluded, as a complete depletion of the cytokine was not achieved, even at higher doses of the anti-IL-10-antibody (data not shown).
An alternative explanation for the discrepancy between the in vitro and in vivo effects of J-113863 on TNFα could be that the source of the cytokine in both experiments is different. The LPS model has been traditionally used to measure the potential of a drug to block TNFα synthesis in vivo. In this model, the peak of the cytokine is achieved at 1.5 h, with almost undetectable levels observed at 4 h. The same time course is observed in human volunteers following an i.v. LPS injection (Branger et al., 2002). This time course is clearly different when monocytes or macrophages are treated in vitro with LPS, where a plateau of TNFα is reached after 6–24 h (our unpublished observations). These discrepancies would suggest that TNFα in the in vivo model comes mainly from a cellular source where this cytokine may be stored pre-synthesized (i.e., Kupffer cells), allowing a rapid release, whereas in monocytes/macrophages it requires de novo protein synthesis. Further studies are needed to determine if TNFα inhibition by this CCR1 antagonist has an effect on Kupffer cells.
To explore if CCR3 was involved in the anti-TNF effect of J-113863, we tested compound BX-471 in the same model. Intriguingly, the compound also induced IL-10 and inhibited TNFα output, the latter with an ED50 of 28 mg kg−1, after i.p. injection (Godessart, unpublished results). We are currently exploring what other properties these two, structurally different, compounds have in common, apart from CCR1 antagonism.
The results obtained with J-113863 on the spontaneous production of TNFα by synovial membrane cells are intriguing. Although they were not statistically significant, they suggest that the blockade of CCR1 may potentially increase TNFα production in arthritis. These results are compatible with those obtained in vivo with CCR1 null mice. Whether this effect is exclusive for this compound or is common to all CCR1 antagonists is unknown. To our knowledge, this is the first time a chemokine receptor antagonist has been tested in this relevant system.
Results obtained in the CIA model are assumed to predict clinical efficacy in humans. However, this connection is still missing in the CCR1 field. To our knowledge, the only CCR1 antagonist tested in rheumatoid arthritis patients, CP-481,715 from Pfizer (Gladue et al., 2003; Haringman et al., 2003), did not demonstrate significant clinical efficacy in a 6-week phase II clinical trial (Gladue et al., 2004). No data on animals is available for this compound, probably because it does not bind the mouse receptor. Although there are several CCR1 antagonists under development for arthritis, there is no information concerning their efficacies in any animal model. The most advanced compound in development appears to be BX-471, but its target disease is multiple sclerosis.
This work illustrates the complexity of target validation using small molecule antagonists of chemokine receptors. It is clear that these compounds are worthy of continued investigation and may prove efficacious in altering the progression of chronic disease even when administered after onset. However, the mechanism(s) whereby these compounds alter pathology in different diseases may be via a circuitous route, which may continue to make the development of specific, efficacious, high affinity small molecular weight antagonists for chemokine receptors a challenging endeavor.
Acknowledgments
We acknowledge Cristina Balagué and Rosa López (Biology) for the determination of the anti-collagen II antibodies, Raquel Fernández (ADME) for the pharmacokinetic studies, and Virginia Pes and Encarna Jiménez (Biology) for their excellent technical assistance. These studies were funded in part by NIH grants HL031237 and HL074024.
Abbreviations
- CIA
collagen-induced arthritis
- DTH
delayed-type hypersensitivity
- DNFB
4-dinitro-fluorobenzene
- HPA
hypothalamus-pituitary-adrenal
- J-113863
1-(1-cycloocten-1-ylmethyl)-4-(2,7-dichloroxanthen-9-ylcarboxamido)-1-ethylpiperidinium iodide
- MIP-1α
macrophage inflammatory protein-1α
- SEB
Staphylococcus enterotoxin B
Conflict of interest
Mercè Amat, Neus Prats, Emma Terricabras, Jorge Beleta and Núria Godessart are employees of Almirall.
References
- Anders H, Belemezova E, Eis S, Segerer S, Vielhauer V, Perez de Lerma G, et al. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephro. 2004;15:1504–1513. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- Anders H, Vielhauer V, Frink M, Linde Y, Cohen C, Blattner SM, et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Inves. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease K, Mehrad B, Standiford T, Lukacs N, Kunkel S, Chensue S, et al. Airway remodeling is absent in CCR1−/− mice during chronic fungal allergic airway disease. J Immunol. 2000;165:1564–1572. doi: 10.4049/jimmunol.165.3.1564. [DOI] [PubMed] [Google Scholar]
- Branger J, Van Den Blink B, Weijer S, Madwed J, Bos C, Gupta A, et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- Carpenter K, Ewing J, Schuh J, Ness T, Kunkel S, Aparici M, et al. Therapeutic targeting of CCR1 attenuates established chronic fungal asthma in mice. Br J Pharmacol. 2005;145:1160–1172. doi: 10.1038/sj.bjp.0706243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Watson J, Pazoles C, Eskra J, Griffiths R, Cohan V, et al. The phosphodiesterase type 4 (PDE4) inhibitor CP-80,633 elevates plasma cyclic AMP levels and decreases tumor necrosis factor-alpha (TNFalpha) production in mice: effect of adrenalectomy. J Pharmacol Exp Ther. 1997;280:621–626. [PubMed] [Google Scholar]
- Chintalacharuvu S, Wang J, Giaconia J, Venkataraman C. An essential role for CCL3 in the development of collagen antibody-induced arthritis. Immunol Lett. 2005;100:202–204. doi: 10.1016/j.imlet.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Clemetson K, Clemetson J, Proudfoot A, Power C, Baggiolini M, Wells T. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- Feldmann M, Brennan F, Williams R, Woody J, Maini R. The transfer of a laboratory based hypothesis to a clinically useful therapy: the development of anti-TNF therapy of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2004;18:59–80. doi: 10.1016/j.berh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Gao W, Topham P, King J, Smiley S, Csizmadia V, Lu B, et al. Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Invest. 2000;105:35–44. doi: 10.1172/JCI8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Wynn T, Chang Y, Lee E, Broxmeyer H, Cooper S, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramallo E, Marques T, Prats N, Beleta J, Kunkel S, Godessart N. Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J Immunol. 2002;169:6467–6473. doi: 10.4049/jimmunol.169.11.6467. [DOI] [PubMed] [Google Scholar]
- Gladue R, Tylaska L, Brissette W, Lira P, Kath J, Poss C, et al. CP-481,715, a potent and selective CCR1 antagonist with potential therapeutic implications for inflammatory diseases. J Biol Chem. 2003;278:40473–40480. doi: 10.1074/jbc.M306875200. [DOI] [PubMed] [Google Scholar]
- Gladue R, Zwillich S, Clucas A, Brown M. CCR1 antagonists for the treatment of autoimmune diseases. Current Opinion Invest Drugs. 2004;5:499–504. [PubMed] [Google Scholar]
- Godessart N. Chemokine receptors: attractive targets for drug discovery. Ann NY Acad Sci. 2005;1051:647–657. doi: 10.1196/annals.1361.109. [DOI] [PubMed] [Google Scholar]
- Godessart N, Kunkel S. Chemokines in autoimmune disease. Curr Opin Immunol. 2001;13:670–675. doi: 10.1016/s0952-7915(01)00277-1. [DOI] [PubMed] [Google Scholar]
- Haringman J, Kraan M, Smeets T, Zwinderman K, Tak P. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:715–721. doi: 10.1136/ard.62.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods. 2003;29:369–375. doi: 10.1016/s1046-2023(02)00361-4. [DOI] [PubMed] [Google Scholar]
- Horuk R, Clayberger C, Krensky A, Wang Z, Grone H, Weber C, et al. A non-peptide functional antagonist of the CCR1 chemokine receptor is effective in rat heart transplant rejection. J Biol Chem. 2001;276:4199–4204. doi: 10.1074/jbc.M007457200. [DOI] [PubMed] [Google Scholar]
- Katschke K, Jr, Rottman J, Ruth J, Qin S, Wu L, Larosa G, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Koch A. Chemokines and their receptors: future targets. Arthritis Rheum. 2005;52:710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- Lawlor K, Campbell I, O'Donnell K, Wu L, Wicks I. Molecular and cellular mediators of interleukin-1-dependent acute inflammatory arthritis. Arthritis Rheum. 2001;44:442–450. doi: 10.1002/1529-0131(200102)44:2<442::AID-ANR63>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lee S, Brummet M, Shahabuddin S, Woodworth T, Georas S, Leifermann K, et al. Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes. J Immunol. 2000;164:3392–3401. doi: 10.4049/jimmunol.164.6.3392. [DOI] [PubMed] [Google Scholar]
- Liang M, Mallari C, Rosser M, Ng H, May K, Monahan S, et al. Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. J Biol Chem. 2000a;275:19000–19008. doi: 10.1074/jbc.M001222200. [DOI] [PubMed] [Google Scholar]
- Liang M, Rosser M, Ng H, May K, Bauman J, Islam I, et al. Species selectivity of a small molecule antagonist for the CCR1 chemokine receptor. Eur J Pharmacol. 2000b;389:41–49. doi: 10.1016/s0014-2999(99)00863-8. [DOI] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Murphy P, Baggiolini M, Charo I, Hebert C, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Naya A, Saeki T. J-113863. Chemokine CCR1 receptor antagonist. Drugs future. 2001;26:121–127. [Google Scholar]
- Naya A, Snaya A, Sagara Y, Ohwaki K, Saeki T, Ichikawa D, et al. Design, synthesis, and discovery of a novel CCR1 antagonist. J Med Chem. 2001;44:1429–1435. doi: 10.1021/jm0004244. [DOI] [PubMed] [Google Scholar]
- Pettipher E, Eskra J, Labasi J. The inhibitory effect of rolipram on TNF-alpha production in mouse blood ex vivo is dependent upon the release of corticosterone and adrenaline. Cytokine. 1997;9:582–586. doi: 10.1006/cyto.1997.0205. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C, Hoogewerf A, Proudfoot A, Power C, Wells T. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–120. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot A, Buser R, Borlat F, Alouani S, Soler D, Offord R, et al. Amino-terminally modified RANTES analogues demonstrate differential effects on RANTES receptors. J Biol Chem. 1999;274:32478–32485. doi: 10.1074/jbc.274.45.32478. [DOI] [PubMed] [Google Scholar]
- Revesz L, Bollbuck B, Buhl T, Eder J, Esser R, Feifel R, et al. Novel CCR1 antagonists with oral activity in the mouse collagen induced arthritis. Bioorg Med Chem Lett. 2005;15:5160–5164. doi: 10.1016/j.bmcl.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Rongione A, Kusske A, Ashley S, Reber H, McFadden D. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70:107–112. doi: 10.1006/jsre.1997.5071. [DOI] [PubMed] [Google Scholar]
- Ross S, Williams R, Mason L, Mauri C, Marinova-Mutafchieva L, Malfait A, et al. Suppression of TNF-alpha expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- Rottman J, Slavin A, Silva R, Weiner H, Gerard C, Hancock W. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur J Immunol. 2000;30:2372–2377. doi: 10.1002/1521-4141(2000)30:8<2372::AID-IMMU2372>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Peck M, Jan Van Keulen B, Jorritsma A, Simmons G, Clapham P, et al. A small molecule antagonist of chemokine receptors CCR1 and CCR3. J Biol Chem. 2000;275:25985–25992. doi: 10.1074/jbc.M908864199. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Wells T. New therapeutics that modulate chemokine networks. Nature Rev Drug Discovery. 2002;1:347–358. doi: 10.1038/nrd795. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Proudfoot A, Woods J, Ruth J, Amin M, Park C, et al. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–1919. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- Su S, Mukaida N, Wang J, Nomura H, Matsushima K. Preparation of specific polyclonal antibodies to a C-C chemokine receptor, CCR1, and determination of CCR1 expression on various types of leukocytes. J Leukoc Biol. 1996;60:658–666. doi: 10.1002/jlb.60.5.658. [DOI] [PubMed] [Google Scholar]
- Terricabras E, Benjamin C, Godessart N. Drug discovery and chemokine receptor antagonists: eppur si muove. Autoimmun Rev. 2004;3:550–556. doi: 10.1016/j.autrev.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Thornton S, Duwel L, Boivin G, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum. 1999;42:1109–1118. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tokuda A, Itakura M, Onai N, Kimura H, Kuriyama T, Matsushima K. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol. 2000;164:2745–2751. doi: 10.4049/jimmunol.164.5.2745. [DOI] [PubMed] [Google Scholar]
- Topham P, Csizmadia V, Soler D, Hines D, Gerard C, Salant D, et al. Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest. 1999;104:1549–1557. doi: 10.1172/JCI7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, et al. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- Williams L, Feldman M, Maini R. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Maor G, Wildbaum G, Grabie N, Gour-Lavie A, Karin N. C-C chemine-encoding DNA vaccines enhance breakdown of tolerance to their gene products and treat ongoing adjuvant arthritis. J Clin Invest. 2000;106:361–371. doi: 10.1172/JCI9109. [DOI] [PMC free article] [PubMed] [Google Scholar]