Abstract
Background and purpose:
Experiments were designed to determine the mechanism of the relaxation induced by tamoxifen in porcine coronary arteries at the tissue, cellular and molecular levels.
Experimental approach:
Porcine left circumflex coronary arteries were isolated and isometric tension was measured. [Ca2+]i in native endothelial cells of intact arteries was determined by a calcium fluorescence imaging technique and eNOS ser1177 phosphorylation was assayed by Western blotting.
Key results:
Tamoxifen induced an endothelium-dependent relaxation that was antagonized by ICI 182,780 and abolished by NG-nitro-L-arginine methyl ester (L-NAME) or 1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one (ODQ). L-Arginine reversed the effect of L-NAME while indomethacin was without effect. Tamoxifen-induced relaxation was attenuated by charybdotoxin (CTX) plus apamin, ouabain or by incubation in a K+-free solution. Moreover, tamoxifen triggered extracellular Ca2+-dependent increases in endothelial [Ca2+]i and this effect was abolished by ICI 182,780. Endothelium-independent relaxation to sodium nitroprusside was also inhibited by ouabain or in a K+-free solution. Furthermore, tamoxifen increased endothelial nitric oxide synthase (eNOS) phosphorylation at Ser-1177 and ICI 182,780 prevented this effect.
Conclusions and Implications:
The present results suggest that tamoxifen mainly induces endothelium-dependent relaxation and that endothelial nitric oxide (NO) is the primary mediator of this effect. NO-dependent responses may result from elevated [Ca2+]i in endothelial cells; an effect abolished by ICI 182,780. NO activates Na+/K+-ATPase in vascular smooth muscle, leading to relaxation. These results suggest that tamoxifen is able to modulate eNOS phosphorylation directly.
Keywords: tamoxifen, Na+/K+-ATPase, nitric oxide, intracellular calcium, endothelium, coronary arteries
Introduction
Tamoxifen, a synthetic nonsteroidal antioestrogen drug (the first-generation selective oestrogen receptor modulator), is used primarily to treat breast cancer (Fisher et al., 2002). Clinical investigations suggest that tamoxifen exerts oestrogen-like cardiovascular protective effects (McDonald and Stewart, 1991; McDonald et al., 1995). Tamoxifen therapy improves endothelial function in postmenopausal women (Stamatelopoulos et al., 2004) and in men with advanced atherosclerosis (Clarke et al., 2001).
Laboratory studies in animals also support the theory that tamoxifen induces oestrogen-like effects in the vascular system. Chronic tamoxifen treatment inhibits constrictor responses to endothelin-1 (Tsang et al., 2004a) and to phenylephrine (Thorin et al., 2003) in cerebral arteries of ovariectomized rats. Tamoxifen acutely inhibits voltage-gated Ca2+ currents in aortic smooth muscle cells (Song et al., 1996). Chronic tamoxifen therapy reduces mRNA expression levels of the α1c subunit of voltage-gated Ca2+ channels in rat aortae during oestrogen deficiency (Tsang et al., 2004b). Tamoxifen relaxes mammalian blood vessels in vitro but the role of the endothelium is controversial. Tamoxifen reduces rabbit coronary artery tone via oestrogen receptor-mediated endothelial nitric oxide (NO) release (Figtree et al., 2000), while endothelium-independent relaxation to tamoxifen has been reported in porcine coronary arteries (Hutchison et al., 2001). At least three mechanisms have been proposed to explain the endothelium-independent relaxation to tamoxifen: (i) activation of ATP-sensitive K+ channels in porcine coronary arteries (Hutchison et al., 2001), (ii) inhibition of protein kinase C in rabbit cerebral arteries (Wickman and Vollrath, 2000) and (iii) inhibition of mitogen-activated protein kinase in rat aortae (Park et al., 2003).
Our results reveal a novel mechanism that accounts for tamoxifen-induced acute relaxation of porcine coronary arteries. Tamoxifen caused mainly endothelium-dependent, NO-mediated relaxation. NO-dependent relaxation is associated with elevated [Ca2+]i in endothelial cells and endothelial nitric oxide synthase (eNOS) phosphorylation, which was inhibited by ICI 182 780, a classic oestrogen receptor antagonist.
The resultant NO release activates the Na+/K+-ATPase in vascular smooth muscle and causes vasorelaxation. The clinical usefulness of tamoxifen in improving endothelial function has already been established in recent studies (Clarke et al., 2001; Stamatelopoulos et al., 2004). We propose a plausible mechanism that could underlie tamoxifen's ability to stimulate organ blood flow and lower systemic vascular resistance, thereby contributing to the overall benefit of tamoxifen therapy.
Methods
All experiments were in accordance with institutional guidelines. This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85–23, revised 1996).
Blood vessel preparation
Pig hearts were obtained fresh from a local slaughterhouse in Hong Kong. The hearts were placed on a dissecting plate filled with ice-cold Krebs solution containing (mM): 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1.2 KH2PO4 and 11 D-glucose. After clearing fatty connective tissues, the left circumflex coronary artery was cut into several rings, each about 3 mm in length. Each ring was suspended in an organ bath filled with Krebs solution oxygenated with 95% O2–5% CO2 and maintained at 37°C (pH: 7.3–7.5). The rings were stretched to an optimal tension of 15 mN, as determined by length-tension curves, and arteries were allowed to equilibrate for 90 min before the commencement of the experiment. In some rings, the endothelial layer was disrupted by gently rubbing the luminal surface with dissecting forceps. Removal of the endothelium was confirmed by a lack of relaxation to bradykinin (50 nM). Each experiment was performed on artery rings obtained from different pigs.
Isometric tension measurement
After an equilibration period of 30 min in the organ baths, each ring was initially contracted by 100 nM U46619 and subsequently relaxed by 50 nM bradykinin (vasodilatation>85%) to confirm the presence of a functional endothelium. Rings were then washed in pre-warmed Krebs solution until baseline tone was restored or readjusted if necessary.
Cumulative concentration-dependent effects of tamoxifen were studied in rings with and without endothelium, contracted by endothelin-1 or U46619. Initial experiments showed that tamoxifen-induced relaxation was reduced markedly in rings without endothelium. Therefore, the first set of experiments examined the role of endothelium-derived relaxing factors. After confirming the presence of the endothelium, coronary artery rings were contracted with endothelin-1 after tissues had been incubated for 30 min with each of the following inhibitors: 100 μM NG-nitro-L-arginine methyl ester (L-NAME) (NOS inhibitor), 3 μM 1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one (ODQ) (guanylate cyclase inhibitor), 10 μM indomethacin (cyclooxygenase inhibitor) or charybdotoxin (CTX) plus apamin, each at 50 nM. Once a steady contraction was achieved, tamoxifen was added cumulatively to the bathing solution. To further support the role for NO, the effect of L-arginine (500 μM) was tested on L-NAME-induced inhibition of tamoxifen responses.
To elucidate the possible involvement of oestrogen receptors, rings were treated with 1 μM ICI 182 780 (classic oestrogen receptor antagonist) before testing the vascular effects of tamoxifen. In other experiments, we tested the effects of a 1-h incubation of actinomycin D (RNA synthesis inhibitor) or cycloheximide (protein synthesis inhibitor) on tamoxifen-induced relaxation.
Tamoxifen-induced relaxation was inhibited by CTX plus apamin, suggesting a role for endothelium-derived hyperpolarizing factor-like factors. The effects of iberiotoxin (IBX) (large-conductance Ca2+-activated K+ (BKCa) channel blocker) or ouabain (Na+/K+ pump inhibitor) were used to examine this. Rings were incubated for 30 min with each inhibitor before the relaxant effects of tamoxifen were studied. A similar amplitude of initial tone was produced to that under control conditions (no inhibitors) by varying the concentration of endothelin-1. For comparison, the vascular effect of oestrogen was tested on rings in the absence and presence of ouabain.
To determine whether endothelium-derived NO activates vascular smooth muscle Na+-K+ pump, the inhibitors listed above were examined on endothelium-independent relaxations to sodium nitroprusside (SNP, NO donor) in rings without endothelium. The relaxant effects of tamoxifen, SNP, or nifedipine were also examined in a K+-free solution, an ionic condition that abolishes Na+/K+-ATPase activity.
Free radical scavenging assay
Possible free radical scavenging effects of tamoxifen and 17β-oestradiol were examined as previously described (Leung et al., 2006). α-Tocopherol (vitamin E) was used as a reference antioxidant. In brief, 0.5 ml of methanol containing different concentrations (3–30 μM) of tamoxifen or 17β-oestradiol were mixed in a test tube with 2.5 ml of methanol containing 75 μM 2,2-diphenyl-1-picrylhydrazyl (DPPH). DPPH is a stable free radical with a deep purple colour in an ethanol solution and has an absorbance at 517 nm, but becomes pale yellow when trapped by an antioxidant. The reaction mixture was kept in the dark at room temperature for 90 min and thereafter the absorbance was measured at 517 nm. The free radical scavenging activity was approximated using the following equation:
where Aa is the absorbance of the incubation DPPH solution without tamoxifen or 17β-oestradiol, Ab is the absorbance of the incubation mixture containing DPPH and tamoxifen or oestrogen, and Ac is the absorbance of the blank solution (methanol) with DPPH.
In situ endothelial [Ca2+]i imaging
Coronary artery rings with endothelium were fluorescently labelled by incubation of tissues for 1 h at 22°C in a Krebs solution containing 10 μM Fura-2 AM, 0.025% pluronic F-127 and 1 mM probenacid (to prevent Fura 2 secretion). After this incubation period, extracellular Fura-2 AM was removed. Native endothelial cells were then perfused for 20 min with Krebs solution at 2 ml min−1 (37°C) to permit intracellular cleavage of intracellular Fura-2 AM into active Fura-2 by esterases.
The endothelial [Ca2+]i imaging method used was as described elsewhere (Leung et al., 2006). Briefly, after Fura-2 loading, the ring was longitudinally cut open and pinned (luminal side up) to a block of silicone elastomer, which was fixed onto the base plate of a custom-made flow chamber. The base plate was then covered with a gasket and cover glass (24 × 32 mm), held in place by screws. There was a 1-mm gap between vessel lumen and cover glass allowing for free flow of buffer. After tissue mounting, the chamber was placed on an inverted microscope and perfused with Krebs solution (37°C) at 2 ml min−1. The specimen was illuminated (Polychrome IV light source) on the stage of a Ix70 Olympus microscope, fitted with a 20X Olympus water immersion objective. The Fura-2 loaded tissue was alternately excited at 340 and 380 nm, and images of the respective 510 nm emissions were collected at 1-s intervals using MetaFluor v4.6 software (Universal Imaging Corp., West Chester, PA, USA). The emitted light was transmitted to a collecting device and then to a cooled charge coupled device (CCD) camera. Illumination through the Polychrome IV light source and acquisition by the CCD camera were controlled by MetaFluor software v4.6. Video frames containing images of cell fluorescence were digitized at a resolution of 512 horizontal × 480 vertical pixels. Imaging analysis was performed using a MetaFluor imaging system. After background subtraction, the fluorescence ratio (F340/F380) was obtained by dividing, pixel by pixel, the images at 340 nm and 380 nm. Changes in this ratio reflected changes in [Ca2+]i and the ratiometric method eliminated potential artefacts caused by variations in cell thickness, intracellular dye distribution or photobleaching.
Western blot analysis
At the end of the tension measurement experiments, porcine coronary artery rings were snap frozen in liquid nitrogen and subsequently homogenized with ice-cold radioimmunoprecipitation assay lysis buffer containing 1 μM leupetin, 5 μM aprotonin, 100 μM phenylmethylsulphonyl fluoride, 1 mM sodium orthovanadate, 1 mM ethylene glycol-bis-(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mM ethylenediamine-N,N,N′,N′-tetraacetic acid, 1 mM sodium fluoride and 2 mg ml−1 β-glycerolphosphate. The lysates were sonicated on ice for 30 min and then centrifuged at 20 000 g for 20 min. The supernatant was collected and analysed for protein concentration using the Lowry method (Bio-Rad, Hercules, CA, USA). Sample buffer containing 5% β-mercaptoethanol was added and the sample was then denatured by boiling for 10 min. For each sample, 80 μg protein was separated with 7.5% sodium dodecyl sulphate–polyacryamide gel, together with the prestained and biotinylated size markers. The resolved proteins were electrophorectically transferred to a nitrocellulose immobilon-P polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA) using a mini-trans-blot cell (Bio-Rad) at 100 V for 60 min at 4°C. The membranes were blocked with 1% bovine serum albumin dissolved in phosphate buffer saline 0.5% tween-20 for 1 h at room temperature. Primary antibodies against total eNOS (1:500) and eNOS phosphorylated at Ser-1177 (1:1000) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and Upstate (Charlottesville, VA, USA), respectively, and a secondary anti-rabbit antibody conjugated to horseradish peroxidase was from (DakoCytomation, Glostrup, Denmark) at a dilution of 1:3000. The membranes were developed with an enhanced chemiluminescence detection system (ECL reagents; Amersham Pharmacia, Pittsburgh, PA, USA) and exposed on X-ray films (Fuji). Densitometry was performed using a documentation program (Flurochem, Alpha Innotech Corp., San Leandro, CA, USA). eNOS corresponding to a 145-kDa band was visualized with reference to molecular weight markers.
Chemicals
Bradykinin, 9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α (U46619), L-NAME, ODQ, L-arginine, indomethacin, tamoxifen, 17β-oestradiol, endothelin-1, CTX, IBX, apamin, ouabain and SNP were from Sigma (St Louis, MO, USA); ICI 182 780 from Tocris (Bristol, UK). Stock solution was prepared in dimethylsulphoxide (DMSO) for indomethacin, tamoxifen, oestrogen and ICI 182 780, and kept at −20°C. Desired dilution was made before the experiment. DMSO (0.1% v/v) did not affect endothelin-1-contracted rings.
Data analysis
Data are mean±s.e.m. and n refers to the number of artery rings studied from different pigs. Several rings prepared from the same artery were investigated in parallel. The relaxation was calculated as percentage reduction in constrictor-induced tone in each ring. Concentration–response curves were analysed by nonlinear curve fitting using Graphpad software (Version 3.0) and statistical differences between curves were analysed by two-way ANOVA followed by Bonferroni post-tests. The negative logarithm of the relaxant concentration that produced 50% maximal relaxation (pD2) was approximated. P-values less than 0.05 was regarded as statistically significant.
Results
Relaxant responses
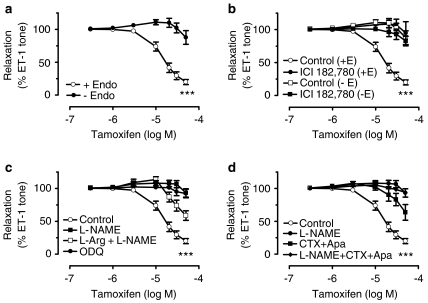
Tamoxifen relaxed endothelium intact coronary arterial rings that were contracted by either endothelin-1 or U46619 with similar sensitivity and potency (pD2: 4.84±0.06 and 4.78±0.04, P<0.05). Removal of the endothelium markedly reduced tamoxifen-induced relaxation (Emax: 83.2±3.7% with endothelium and 14.7±8.8% without endothelium, P<0.001, Figure 1a). The residual small relaxation in artery rings without endothelium was unaffected by IBX (n=5). ICI 182 780 antagonized tamoxifen-induced relaxation in rings with endothelium and was without effect in endothelium-free arteries (Figure 1b).
Figure 1.
(a) Concentration–response curves for tamoxifen-induced relaxation in endothelin-1-contracted porcine coronary artery rings with (+Endo) and without (−Endo) endothelium. (b) Inhibitory effect of ICI 182 780 on the tamoxifen dilatation. (c) Inhibition of tamoxifen-induced relaxation by L-NAME and ODQ in rings with endothelium and partial reversal by L-arginine. (d) Inhibitory effects of CTX plus apamin or in combination with L-NAME in rings with endothelium. Results are mean±s.e.m. of 6–8 experiments. Statistical difference is indicated by *** (P<0.001) compared with control curve.
Effects of inhibitors of NO-dependent relaxation
The markedly reduced relaxation to tamoxifen in endothelium-free arteries suggested a primary contribution of endothelium-derived relaxing factors to vasodilatation. Pretreatment with L-NAME or ODQ abolished endothelium-dependent relaxation to tamoxifen (Figure 1c), with L-NAME and ODQ being equally effective. Exposure to L-arginine, the precursor of NO biosynthesis, partially reversed the L-NAME-induced inhibition (Figure 1c). In contrast, indomethacin did not modify relaxation to tamoxifen (pD2: 4.84±0.06 in control and 4.89±0.08 in indomethacin, n=6–8, P>0.05), thus ruling out a role for vasorelaxing prostanoids (data not shown).
Effects of K+ channel blockers and ouabain
Tamoxifen-induced relaxation was inhibited by CTX plus apamin, but this inhibition was not as great as that produced by L-NAME (Figure 1d). Cotreatment with L-NAME, CTX plus apamin augmented the inhibitory effects caused by the K+ channel blockers (Figure 1d).
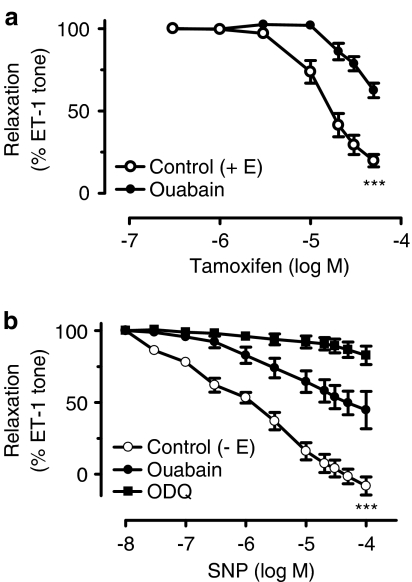
The role of the Na+-K+-ATPase pump was assessed by treating the rings with ouabain or by incubation in a K+-free solution. Ouabain reduced the relaxant effect of tamoxifen (Figure 2a). Removal of extracellular K+ also inhibited tamoxifen-induced relaxation in rings with endothelium (Figure 3a).
Figure 2.
(a) Effects of ouabain on tamoxifen-induced relaxation in rings with endothelium. (b) Effects of ouabain and ODQ on SNP-induced relaxation in rings without endothelium. Results are mean±s.e.m. of 6–7 experiments. Statistical difference is indicated by *** (P<0.001) as compared with control curve.
Figure 3.
(a) Tamoxifen-induced relaxation in a K+-free bathing solution in rings with endothelium and (b) SNP relaxation in rings without endothelium. For comparison, the effects of ouabain are included in both graphs. (c) The effects of CTX and apamin on SNP-induced relaxation in rings without endothelium. Results are mean±s.e.m. of 5–6 experiments. Statistical difference is indicated by *** (P<0.001) as compared with control curve.
SNP-induced relaxation
In endothelium-free coronary artery rings, SNP produced relaxations (pD2 of 5.47±0.69). This relaxation was attenuated by ouabain and abolished by ODQ (Figure 2b). SNP-induced relaxation was also impaired in a K+-free solution (Figure 3b). The effects of ouabain and a K+-free solution were identical (Figure 3b). In contrast, neither ouabain nor removal of extarcellular K+ affected 0.1 μM nifedipine-induced relaxation in rings without endothelium (relaxation: 45.4±9.5% in control; 52.7±7.9% in ouabain; and 47.0±4.6% in zero K+, n=6–7, P>0.05 compared with control). L-NAME did not affect SNP-induced relaxation (n=4). The vasodilatation to SNP was not affected by CTX plus apamin either in endothelium-denuded porcine coronary arteries with control (Figure 3c).
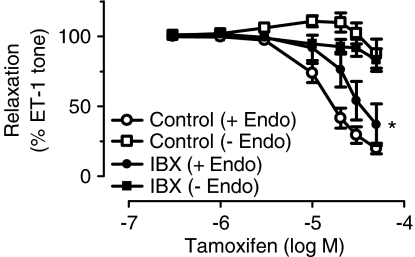
Effects of IBX, a BKCa channel blocker
Tamoxifen-induced relaxation was significantly inhibited by IBX (20 or 30 μM) in rings with endothelium (Figure 4), but this inhibition was not as great as that produced by L-NAME (Figure 1d). IBX did not affect the endothelium-independent relaxation of tamoxifen (Figure 4).
Figure 4.
The effect of IBX on tamoxifen-induced relaxation in rings with and without endothelium. Results are mean±s.e.m. of 6–7 experiments. Statistical difference is indicated by * (P<0.05) as compared with control curve.
Effects of actinomycin D and cycloheximide
Actinomycin D (10 μM) and cycloheximide (10 μM) did not influence tamoxifen-induced relaxation in rings with endothelium (pD2: 4.70±0.13 in control; 4.69±0.29 in actinomycin D; 4.45±0.51 in cycloheximide, n=5–7, P>0.05 compared with controls) (data not shown).
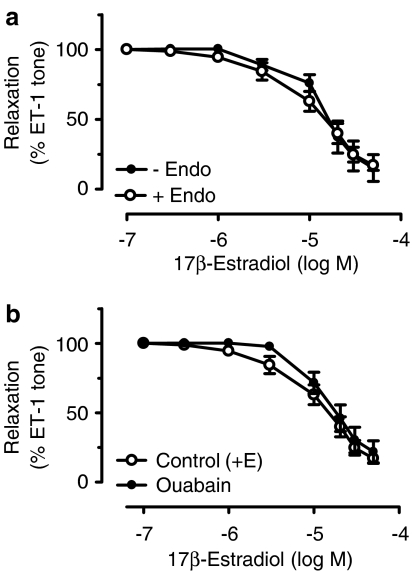
Relaxant effect of 17β-oestradiol
17β-Oestradiol caused relaxations in rings contracted by endothelin-1; the responses were similar in rings with (pD2: 4.70±0.13) and without endothelium (pD2: 4.48±0.21, n=6–7, P>0.05, Figure 5a). Ouabain (100 μM) did not modify relaxation to 17β-oestradiol (pD2: 4.70±0.13 in control and 4.53±0.19 in ouabain, n=6–7, P>0.05, Figure 5b).
Figure 5.
(a) Relaxation induced by 17β-oestradiol in endothelin-1-contracted rings with and without endothelium. (b) The effect of 100 μM ouabain on 17β-oestradiol-induced relaxation in rings with endothelium. Results are mean±s.e.m. of five experiments.
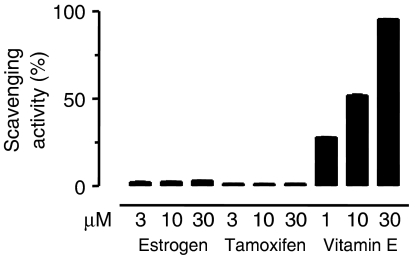
Free radical scavenging effect
Concentrations of tamoxifen or oestrogen which caused coronary artery vasodilatation did not have any free radical scavenging activity (Figure 6). Under the conditions of the assay, vitamin E produced concentration-dependent increases in free radical scavenging activity (Figure 6).
Figure 6.
The free radical-scavenging activity of various concentrations (1, 3 and 10 μM) of tamoxifen, 17β-oestradiol, and vitamin E. Results are mean±s.e.m. of six experiments in each group.
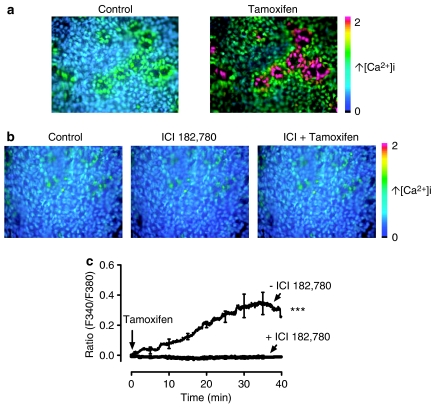
Effects on [Ca2+]i in coronary artery endothelial cells
En face fluorescence images from viable individual endothelial cells were clearly visible (Figure 7a). These signals were absent after removal of the endothelium. Increases in [Ca2+]i due to the addition of tamoxifen occurred only in arterial tissues with an intact endothelium (Figure 7a). Treatment with ICI 182 780 abolished the [Ca2+]i increases produced by 30 μM tamoxifen in endothelial cells (Figure 7b). The bradykinin-stimulated rise in endothelial [Ca2+]i was unaffected by ICI 182 780 (data not shown). Figure 7c shows that perfusion of tamoxifen triggered a gradual rise in [Ca2+]i and this effect was abolished by 1 μM ICI 182 780.
Figure 7.
Calcium levels in en face preparations of endothelial cells of porcine intact coronary arteries measured by ratiometric fluorescence imaging. Images show increases in [Ca2+]i in situ induced by tamoxifen (a) and the effect 1 μM ICI 182 780 (b). (c) Time course for the effect of tamoxifen on endothelial [Ca2+]i in the absence and presence of ICI 182 780. Results are mean±s.e.m. of 5–6 experiments from different pigs. Statistical difference is indicated by *** (P<0.001) between groups.
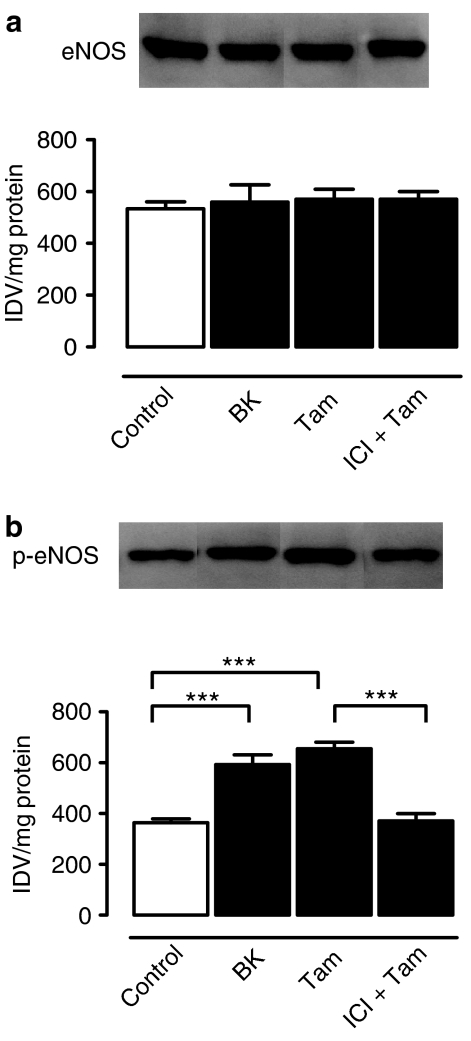
Effects on Phosphorylation of eNOS at Ser-1177
eNOS was post-translationally activated by tamoxifen (30 μM), as reflected by significantly increased phosphorylation of eNOS at Ser-1177 in arteries with endothelium, as compared with unstimulated arteries. The increased eNOS phosphorylation levels were reduced to unstimulated levels by treatment with 1 μM ICI 182 780 (Figure 8b). Bradykinin (50 nM) also increased the eNOS phosphorylation (Figure 8b). The total amount of eNOS protein was unaffected by either tamoxifen or bradykinin (Figure 8a).
Figure 8.
Effects of tamoxifen (30 μM) and bradykinin (50 nM) on total eNOS protein expression levels (a) and eNOS phosphorylation at Ser-1177 (b). The effects of ICI 182 780 were also determined on eNOS protein expression and eNOS phosphorylation. Results are mean±s.e.m. of five experiments on arteries from different pigs. Statistical difference is indicated by *** (P<0.001) between groups.
Discussion
Our study demonstrates that (i) tamoxifen but not 17β-oestradiol produces endothelium-dependent relaxations in porcine isolated coronary arteries; (ii) upon stimulation by tamoxifen, endothelium-derived NO activates smooth muscle Na+/K+ ATPase activity to cause a vasodilatation that was sensitive to ouabain and removal of extracellular K+ and was abolished by inhibition of the cyclic GMP signaling pathway; (iii) tamoxifen also activates endothelial BKCa channels to cause a vasodilatation that was sensitive to IBX; (iv) tamoxifen-induced NO-dependent vasodilatation is related to increased endothelial [Ca2+]i in intact coronary arteries in situ; (v) tamoxifen stimulates eNOS phosphorylation at Ser-1177 and (vi) tamoxifen does not possess free radical scavenging activity. Treatment with ICI 182 780 prevented tamoxifen-induced relaxation and abolished the stimulatory effects of tamoxifen on endothelial [Ca2+]i and eNOS phosphorylation, suggesting a significant contribution of ICI 182 780-sensitive oestrogen receptors to the acute vascular action of tamoxifen.
NO, one of the most important endothelium-dependent regulators of vascular tone and arterial blood pressure, relaxes vascular smooth muscle cells via cyclic GMP-mediated mechanisms. Our results clearly support an important role for endothelial NO in tamoxifen-induced vasodilatation of porcine coronary arteries. Removal of a functional endothelium markedly attenuated tamoxifen-induced relaxation with the maximal relaxation being reduced from 83.2±3.7 to 14.7±8.8%.
Furthermore, tamoxifen-induced relaxation was sensitive to inhibition of the NO/cyclic GMP-dependent pathway. L-NAME or ODQ reduced tamoxifen-induced relaxation to similar extents. The reduced relaxation after blockade of the NO/cyclic GMP signaling pathway was similar to the relaxation in endothelium denuded rings. L-Arginine, the precursor of NO synthesis, attenuated L-NAME-induced inhibition of relaxation. Release of relaxing prostanoids was unlikely to be involved in the response as the vasodilatation to tamoxifen was not affected by indomethacin.
A combination of the K+ channel blockers, CTX and apamin inhibited tamoxifen-induced relaxation, albeit to a lesser degree compared with the effect of L-NAME. This observation indicates that the CTX/apamin-sensitive K+ channels are involved in the stimulation of NO production in response to tamoxifen, since the inhibition of the vasodilatation was similar in rings treated with L-NAME alone or in combination with CTX plus apamin. Moreover, our results show that the effect of CTX plus apamin is similar on the SNP-induced relaxation in intact and endothelium-denuded porcine coronary arteries. We thus concluded that the KCa channels activated by tamoxifen-stimulated release NO are located in endothelial cells and not smooth muscle cells.
The relaxant effect of tamoxifen was abolished by ICI 182 780, suggesting that this effect is probably mediated by ICI 182 780-sensitive oestrogen receptors. In contrast, neither actinomycin D nor cycloheximide affected tamoxifen-induced relaxation, indicating that the acute effect of tamoxifen was mediated by a nongenomic action on endothelial cells of porcine coronary arteries. These results are consistent with those from a previous study using pig proximal coronary arteries (Hutchison et al., 2001).
The biosynthesis of NO by eNOS from its precursor L-arginine is a Ca2+-dependent process. Endothelial NO-dependent vasodilators stimulate NO release by increasing endothelial [Ca2+]i. Our study is the first to present evidence that tamoxifen increases [Ca2+]i in native endothelial cells of intact coronary arteries. This stimulation effect was abolished by ICI 182 780. It is probable that increased Ca2+ influx may be an important early step in the tamoxifen-induced NO release from endothelial cells; an effect that appears to involve eNOS phosphorylation at Ser-1177 since ICI 182 780 also prevented tamoxifen-induced eNOS phosphorylation.
Another novel finding of our study is the demonstration that the mechanism of the NO-dependent relaxation to tamoxifen is ouabain-sensitive. We compared the tamoxifen-induced vasodilatation with that produced by SNP, an NO donor and observed that ouabain (an inhibitor of Na+/K+-ATPase) attenuated tamoxifen-induced relaxation to the same degree as that produced by L-NAME. Combined treatment with both inhibitors did not have an additive effect. The relaxation to SNP in rings without endothelium was also inhibited by ouabain; while the removal of extracellular K+ from the bathing solution reduced tamoxifen-induced relaxation to the same degree as did ouabain. The magnitude of the vasodilatation to SNP in a K+-free solution was comparable with the response after the addition of ouabain to the bathing solution. An important role for the Na+/K+-ATPase in mediation of SNP dilatation has previously been demonstrated in pig coronary arteries (Cogolludo et al., 2001). Our results, in conjunction with previous findings, suggest that NO could regulate Na+-K+-ATPase activity in arteries (Gupta et al., 1994, 1996). Our results also indicate that NO stimulation of ouabain-sensitive Na+/K+-ATPase is mediated via the cyclic GMP signaling pathway. In support of this is the finding that ODQ, a selective inhibitor of soluble guanylate cyclase, nearly abolished vasodilatation to both tamoxifen (in rings with endothelium) and to SNP (in rings without endothelium). These findings suggest that the ouabain-sensitive site is probably located on vascular smooth muscle cells. Thus, we provide indirect evidence for the role of Na+/K+-ATPase in tamoxifen-induced, NO-mediated relaxation of porcine coronary arteries.
Our study also demonstrates important differences in the vasodilatation produced by tamoxifen and 17β-oestradiol. Thus, acute application of tamoxifen caused vasodilatation of porcine coronary arteries by stimulating endothelial NO release while 17β-oestradiol acted mainly on vascular smooth muscle cells. Tamoxifen-induced vasodilatation occurs via a NO-dependent, ouabain-sensitive pathway while ouabain had no effect on 17β-oestradiol-induced relaxation.
Recent studies suggest that endothelium-derived superoxide inhibits the stimulatory effect of NO on vascular Na+/K+-ATPase activity (Gupta et al., 2002). Our in vitro study in intact coronary arteries suggests that tamoxifen does not exhibit a significant free radical scavenging effect.
Taken together, the results from this study provide novel evidence for the involvement of ouabain-sensitive mechanisms in tamoxifen-induced vasodilatation of porcine isolated left circumflex coronary arteries. Acute administration of tamoxifen has previously been shown to relax a variety of systemic blood vessels (Figtree et al., 2000; Wickman and Vollrath, 2000; Hutchison et al., 2001; Park et al., 2003) while chronic treatment with tamoxifen reduced cerebrovascular tone (Tsang et al., 2004b) and modulated mRNA expression of voltage-sensitive Ca2+ channels and K+ channels in ovariectomized female rats (Tsang et al., 2004a). These findings, combined with those of this study, support the beneficial effects of tamoxifen in arterial tone and coronary artery disease (McDonald and Stewart, 1991; McDonald et al., 1995; Clarke et al., 2001).
Acknowledgments
This study was supported by grants from Research Grants Council of Hong Kong SAR (CUHK 4366/03M and CUHK 4362/04M), Li Ka Shing Institute of Health Sciences, CUHK Focused Investment Scheme, and the Canadian Heart and Stroke Foundation (IL). HSL and LMY (postgraduate students) and FPL (a postdoctoral fellow) were partially supported by the Hong Kong RGC grant.
Abbreviations
- BKCa channel
large-conductance Ca2+-activated K+ channel
- CTX
charybdotoxin
- eNOS
endothelial nitric oxide synthase
- IBX
iberiotoxin
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Clarke SC, Schofield PM, Grace AA, Metcalfe JC, Kirschenlohr HL. Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation. 2001;103:1497–1502. doi: 10.1161/01.cir.103.11.1497. [DOI] [PubMed] [Google Scholar]
- Cogolludo AL, Perez-Vizcaino F, Zaragoza-Arnaez F, Ibarra M, Lopez-Lopez G, Lopez-Miranda V, et al. Mechanisms involved in SNP-induced relaxation and [Ca2+]i reduction in piglet pulmonary and systemic arteries. Br J Pharmacol. 2001;132:959–967. doi: 10.1038/sj.bjp.0703894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figtree GA, Webb CM, Collins P. Tamoxifen acutely relaxes coronary arteries by an endothelium-, nitric oxide-, and estrogen receptor-dependent mechanism. J Pharmacol Exp Ther. 2000;295:519–523. [PubMed] [Google Scholar]
- Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chough E, Daley J, Oates P, Tornheim K, Ruderman NB, et al. Hyperglycemia increases endothelial superoxide that impairs smooth muscle cell Na+-K+-ATPase activity. Am J Physiol Cell Physiol. 2002;282:C560–C566. doi: 10.1152/ajpcell.00343.2001. [DOI] [PubMed] [Google Scholar]
- Gupta S, McArthur C, Grady C, Ruderman NB. Role of endothelium-derived nitric oxide in stimulation of Na+-K+-ATPase activity by endothelin in rabbit aorta. Am J Physiol. 1994;266:H577–H582. doi: 10.1152/ajpheart.1994.266.2.H577. [DOI] [PubMed] [Google Scholar]
- Gupta S, Phipps K, Ruderman NB. Differential stimulation of Na+ pump activity by insulin and nitric oxide in rabbit aorta. Am J Physiol. 1996;270:H1287–H1293. doi: 10.1152/ajpheart.1996.270.4.H1287. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. J Cardiovasc Pharmacol. 2001;38:657–665. doi: 10.1097/00005344-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Leung HS, Yao X, Leung FP, Ko WH, Chen ZY, Gollasch M, et al. Cilnidipine, a slow-acting Ca2+ channel blocker, induces relaxation in porcine coronary artery: role of endothelial nitric oxide and [Ca2+]i. Br J Pharmacol. 2006;147:55–63. doi: 10.1038/sj.bjp.0706450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CC, Alexander FE, Whyte BW, Forrest AP, Stewart HJ. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomised trial. The Scottish Cancer Trials Breast Group. BMJ. 1995;311:977–980. doi: 10.1136/bmj.311.7011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CC, Stewart HJ. Fatal myocardial infarction in the Scottish adjuvant tamoxifen trial. The Scottish Breast Cancer Committee. BMJ. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim B, Kim J, Won KJ, Lee S, Kwon S, et al. Tamoxifen induces vasorelaxation via inhibition of mitogen-activated protein kinase in rat aortic smooth muscle. J Vet Med Sci. 2003;65:1155–1160. doi: 10.1292/jvms.65.1155. [DOI] [PubMed] [Google Scholar]
- Song J, Stanley PR, Zhang F, Joshi D, Gappy S, Sowers JR, et al. Tamoxifen (estrogen antagonist) inhibits voltage-gated calcium current and contractility in vascular smooth muscle from rats. J Pharmacol Exp Ther. 1996;277:1444–1453. [PubMed] [Google Scholar]
- Stamatelopoulos KS, Lekakis JP, Poulakaki NA, Papamichael CM, Venetsanou K, Aznaouridis K, et al. Tamoxifen improves endothelial function and reduces carotid intima-media thickness in postmenopausal women. Am Heart J. 2004;147:1093–1099. doi: 10.1016/j.ahj.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Thorin E, Pham-Dang M, Clement R, Mercier I, Calderone A. Hyperreactivity of cerebral arteries from ovariectomized rats: therapeutic benefit of tamoxifen. Br J Pharmacol. 2003;140:1187–1192. doi: 10.1038/sj.bjp.0705547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Chan FL, Wong CM, Chen ZY, Laher I, et al. Estrogen and tamoxifen modulate cerebrovascular tone in ovariectomized female rats. Hypertension. 2004b;44:78–82. doi: 10.1161/01.HYP.0000131659.27081.19. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Wong CM, Chan FL, Chen ZY, Huang Y. Differential regulation of K+ and Ca2+ channel gene expression by chronic treatment with estrogen and tamoxifen in rat aorta. Eur J Pharmacol. 2004a;483:155–162. doi: 10.1016/j.ejphar.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wickman G, Vollrath B. Effects of tamoxifen on oxyhemoglobin-induced cerebral vasoconstriction. Eur J Pharmacol. 2000;390:181–184. doi: 10.1016/s0014-2999(99)00907-3. [DOI] [PubMed] [Google Scholar]