Abstract
Background and purpose:
Following transient focal stroke, rapid accumulation and activation of neutrophils in the ischaemic region is deleterious due to release of reactive oxygen species and myeloperoxidase (MPO). The purpose of this study was to examine whether AM-36, both a Na+ channel blocker and an antioxidant, afforded neuroprotection by modulating neutrophil accumulation into brain, following endothelin-1 (ET-1) induced middle cerebral artery occlusion (MCAo) in conscious rats.
Experimental approach:
AM-36 was administered at 3 and 24 h after ET-1-induced MCAo. Functional recovery was determined using grid-walking and cylinder tests. Image analysis of brain sections was used to determine infarct volume. The effect of AM-36 on neutrophil infiltration and their interaction with macrophages was examined in rats at 48 h following MCAo by both an MPO assay and double-label immunofluorescence. Blood brain barrier (BBB) breakdown was measured by the area stained by intravenous Evans Blue.
Key results:
AM-36 reduced functional deficits in both tests such that no difference existed from pre-ischaemic values at 48 h. Neutrophil infiltration, assessed by MPO activity, and infarct volume were significantly reduced following AM-36 administration by 54 and 60% respectively. Similarly, immunofluorescence revealed that AM-36 reduced neutrophil infiltration by ∼50% in selected brain regions, when compared to controls, and also modulated macrophage phagocytosis of neutrophils. Breakdown of the BBB was significantly reduced by 60% following AM-36 treatment.
Conclusions and Implications:
These findings suggest that AM-36 can directly modulate the neutrophil inflammatory response and reduce BBB breakdown following MCAo.
Keywords: AM-36, blood–brain barrier, cerebral ischaemia, endothelin, neutrophils, macrophages
Introduction
The role of polymorphonuclear leukocytes (neutrophils) in the infarcted region following cerebral ischaemia remains controversial (Hayward et al., 1996; Emerich et al., 2002), with some studies showing a neuroprotective effect after ablation of circulating neutrophils or blockade of their entry into the ischaemic region (for summary, see Beray-Berthat et al., 2003). However, other studies have failed to show that reducing neutrophil infiltration limits the progression of the ischaemic injury (for review see Emerich et al., 2002). Thus, Emerich et al. (2002) proposed that neutrophils enter the brain after the majority of ischaemic damage has occurred, mainly to remove dead cells and set the stage for tissue remodelling. Recently, Yu et al. (2004) have shown that adenosine (which accumulates in the ischaemic brain) can activate neutrophils, thereby enhancing the formation of inflammatory mediators via the respiratory burst, including superoxide anion. Additionally, neutrophil activation leads to degranulation and release of the enzyme myeloperoxidase (MPO), which leads to the production of hypochlorous acid, a potent oxidant (for review see Nathan, 2006). Findings from other studies have shown that inhibiting neutrophil infiltration into the ischaemic brain can reduce the formation of damaging ROS including ascorbyl radicals (Matsuo et al., 1995) and superoxide anions (Fabian and Kent, 1999), along with limiting the depletion of cortical glutathione (Beray-Berthat et al., 2003). Furthermore, van der Goes et al. (2001) demonstrated that superoxide anion can damage cerebral endothelial cells, disrupting the tight junctions of the blood–brain barrier (BBB), leading to increased BBB permeability. Coupled together, these findings directly implicate infiltrating neutrophils in the mechanisms of ischaemic injury (Iadecola, 2004). Furthermore, neutrophil adhesion to the endothelium can also lead to re-occlusion of the microvasculature, thus exacerbating ischaemia (see review by del Zoppo and Mabuchi, 2003).
Resolution of the neutrophil inflammatory response following cerebral ischaemia begins with the removal of neutrophils, either by necrosis or by apoptosis, followed by macrophage phagocytosis (Weston et al., 2006). Neutrophils are constitutively programmed to undergo apoptosis, with the resulting apoptotic bodies being rapidly removed through phagocytic recognition by macrophages thus preventing the lytic release of cytotoxic and immunogenic intracellular contents into the surrounding microenvironment which can subsequently damage adjacent cells (Savill and Fadok, 2000). Macrophages can also induce apoptosis in neutrophils that infiltrate an injury site, in addition to engulfing them, and this action is considered to be beneficial because recognition and engulfment of neutrophils prevents an inflammatory response (Meszaros et al., 2000).
Several drugs that target neutrophil infiltration have been developed as potential therapies for ischaemic stroke, being specifically designed to inhibit neutrophil adhesion and subsequent infiltration. Two such drugs were tested in clinical trials; a monoclonal antibody (enlimomab, R6.5) to intracellular adhesion molecule-1 (ICAM-1) and a humanized antibody (Hu23F2G or LeukArrest) to the CD18 integrin (Becker, 2002). Clinical trials with these drugs were unsuccessful due both to a lack of neuroprotective efficacy and side effects such as leukopenia, which occurs at the therapeutic doses required to achieve successful inhibition of the infiltration (Becker, 2002). There is thus a need to investigate compounds, which may inhibit the damaging effects of neutrophils through alternative mechanisms.
The novel arylalkylpiperazine compound AM-36 [1-(2-(4-chloro-phenyl)-2-hydroxy)-ethyl-4-(3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl) methylpiperazine], was designed to incorporate multiple neuroprotective properties within one structure (Jarrott et al., 1999), by integrating the anti-oxidant group, butylated hydroxytoluene (BHT) into a modified form of the polyamine antagonist, eliprodil. AM-36 has been shown to have antioxidant properties (Jarrott et al., 1999; Callaway et al., 2003), Na+ channel blocking activity (Callaway et al., 2004) and is capable of inhibiting neuronal apoptosis (Callaway et al., 2001). Previous studies by Callaway et al. (1999) have demonstrated AM-36 to be neuroprotective, reducing both cortical and striatal damage when administered up to 3 h following transient forebrain ischaemia in conscious rats. An editorial comment on this paper (Clemens, 1999) emphasized the long window of therapeutic opportunity for AM-36 administration, and alluded to the possibility that mechanisms other than the sodium channel blockade and antioxidant properties may be involved in its action. Thus, we have investigated the possible effects of AM-36 administration on neutrophil infiltration into the ischaemic region following endothelin-1 (ET-1)-induced stroke in conscious rats. In addition, behavioural experiments were performed to investigate the effect of AM-36 on functional recovery and its correlation with neutrophil infiltration. The present data demonstrates that AM-36 modulates the neutrophil inflammatory response and reduces BBB breakdown following ET-1 induced stroke.
Methods
Animals
Adult male Long-Evans rats weighing 280–300 g were purchased from Monash University Animal Services and a total of 58 rats were used in this study. Rats were group-housed (four rats to a cage) under diurnal lighting with temperature maintained between 18 and 24°C and given free access to food and water, until surgery, whereupon individual rats were housed in separate cages. All animal experiments were performed in accordance with the Prevention of Cruelty to Animals Act 1986, under the guidelines of the National Health and Medical Research Council for the Care and Use of Animals for Experimental Purposes in Australia.
Surgical preparation
Rats were anaesthetized using pentobarbitone sodium (60 mg kg−1) by intraperitoneal injection (i.p.). Using the method of Sharkey et al. (1993) a 23-gauge stainless steel guide cannula was stereotaxically inserted into the piriform cortex 2 mm dorsal to the right middle cerebral artery (MCA) using the coordinates of Callaway et al. (1999), (0.2 mm anterior, −5.2 mm lateral, and −5.9 mm ventral, relative to Bregma, according to a stereotaxic atlas (Paxinos and Watson, 1986)). The cannula was secured in place with dental cement, and two small screws inserted into the skull, to assist the dental cement to adhere to the skull. The incision was closed with sutures. Rats had access to food and water ad libitum and were allowed to recover for at least 3 days before the induction of cerebral ischaemia.
Induction of focal cerebral ischaemia
Cerebral ischaemia was induced in conscious rats by occlusion of the right MCA by an ET-1 injection (120 pmol in 6 μl of saline over 6 min; American Peptide Company, Sunnyvale, CA, USA) via a 30-gauge injector needle that protruded 2 mm beyond the end of the previously implanted guide cannula. The injector was held in place by a polyethylene-tubing cuff, and the animal was placed into a clear Plexiglas box for video recording during the induction of ischaemia (Callaway et al., 1999). Characteristic indications of middle cerebral artery occlusion (MCAo) included clenching or failure to extend the contralateral forelimb and counterclockwise circling. Rats that did not exhibit these behavioural signs were deemed not to have had any occlusion of the MCA and were excluded from any further study. Sham-occlusion rats underwent cannula implantation but received a saline injection instead of an ET-1 injection and did not exhibit any behavioural reactions to the injection that would indicate a stroke.
AM-36 administration
To examine the effect of AM-36 treatment on neutrophil infiltration, rats were divided into three groups: (1) sham-MCAo (n=5), (2) ET-1+water (vehicle-treated) (n=6) and (3) ET-1+AM-36 (n=6). The effects of AM-36 have been investigated in a number of studies at different therapeutic doses, including 1 mg kg−1 i.p. (Callaway et al., 2004), 1.8 mg kg−1 i.p. (Callaway et al., 2000) and 6 mg kg−1 i.p. (Callaway et al., 1999; Callaway et al., 2003; Callaway et al., 2004), with 6 mg kg−1 i.p. found to have maximal neuroprotective effects in reducing ischaemic damage. Although higher doses of AM-36 have been examined, for instance 10 mg kg−1 i.v., these very high doses appeared to have a central action which modulates cardiovascular autonomic function, indicated by bradycardia and hypotension. These effects, however, are not seen at a dose of 6 mg kg−1 i.p. (unpublished observations). AM-36 is a lipophilic compound (Log D=4.5) and pharmacokinetic analysis of AM-36 revealed that it readily crosses the BBB with a brain to plasma ratio of 9:1 achieved within 30 min following administration, a ratio that is maintained as plasma concentrations decrease with t1/2 of 14 h (unpublished observations). Given these findings, AM-36 was administered at a dose of 6 mg kg−1 i.p. at 3 h and 24 h after the ET-1 injection, while vehicle-treated rats were injected with an equal volume of water i.p. AM-36 ditartrate was dissolved in sterile water for injection and the dose was calculated as the base.
Physiological parameters
Core body temperature was measured rectally before stroke, and at 30 min, 1, 2 and 3 h following stroke. For rats administered with AM-36, core body temperature was measured for a further 2 h after stroke, to ensure that AM-36 was not having any effect on body temperature. Each rat was weighed before surgery, before stroke induction and every day after stroke induction until the day rats were killed.
Assessment of functional outcome
The grid-walking test (Soblosky et al., 1996) and the cylinder test (Schallert et al., 2000) were used as methods for evaluation of limb deficits following ET-1-induced MCAo. Behavioural tests were conducted, pre-surgery, pre-stroke (0 h), and each subsequent day until the time of death. Each rat acted as its own control, with behavioural results being compared to pre-stroke values within the same rat. The grid-walking test was used to measure motor coordination in the forelimbs and hindlimbs. For this test, rats were placed onto a plastic-coated wire grid (3 × 3 cm) 20 cm above the ground, and video monitored over 3 min. Slow-motion video playback was used to examine rat movement across the grid. A slip of a rat's paw through the wire grid was counted as an error. The number of errors for each paw were counted and subsequently divided by the total number of steps made (for each paw) and thus expressed as the percentage of total steps. The cylinder test was used to assess limb use asymmetries between the contralateral and ipsilateral forepaws on rearing and landing following cerebral ischaemia. For this test, as previously described by Schallert et al. (2000), rats were placed into a clear plastic cylinder 20 cm in diameter and 30 cm high for a period of 2 min, and forelimb use was videotaped. The number of contacts made with the wall or floor of the cylinder (when rearing and landing, respectively) by ipsilateral, contralateral or both forepaws simultaneously were counted over a 2 min period and expressed as a percentage of total paw use.
Brain tissue processing
At 48 h after ischaemia, rats were anaesthetized with pentobarbitone sodium (60 mg kg−1 i.p.) and transcardially perfused with 250 ml of physiological saline (0.9%) at a perfusion pressure of 100 mm Hg. As previously described by Weston et al. (2006), brains were removed and using a brain matrix, cut into six 2 mm coronal slices from the olfactory bulbs, frozen and stored at −80°C until processing. Coronal cryostat sections (16 μm thick) were then cut from each of the six slices, slide mounted, and used for both image analysis and immunohistochemistry. Approximately 10 sections were taken from each of the slices, with the majority of the brain remaining in the slices used in the biochemical assay for MPO activity.
Determination of infarct volume
The infarct area was determined in unstained sections using the ballistic light method (Callaway et al., 2000), which has been shown to permit rapid and reliable measurements of infarct damage. The infarct area was measured, using a computerized image analysis system (MCID M4 image analyzer, Imaging Research Inc., St Catherines, Ontario, Canada), by tracing around the area of damage in each brain section, which appeared dark in contrast to non-lesioned areas. Infarct volume was determined by integrating the cross-sectional area of damage at each stereotaxic level and the distance between each of the levels according to the method of Osborne et al. (1987). Correction for oedema of ischaemic area was calculated as described by Leach et al. (1993).
Myeloperoxidase activity assay
The remaining slices of brain, not used for histology and immunohistochemistry were used to determine MPO activity as previously described by Weston et al. (2006). Briefly, the ischaemic and non-ischaemic hemispheres (from the six slices), for each individual rat, were pooled separately for the assay, and wet weight recorded. Pooled tissues were homogenized (1:20, wt vol−1) in 5 mM phosphate buffer (pH 6; 4°C) and centrifuged at 30 000 g for 30 min (4°C). The supernatant was discarded and the pellet washed again as described above. After decanting the supernatant, the pellet was extracted by suspension in 0.5% hexadecyltrimethylammonium bromide (Sigma Chemical Co, St Louis, MO, USA), in 50 mM potassium phosphate buffer (pH 6, 25°C) for approximately 2 min at an original wet weight to volume ratio of 1:10. Three freeze-thaw cycles were then performed with sonications (10 s, 25°C) between cycles. After the final cycle, the tissues were incubated at 4°C for 20 min and centrifuged at 12 500 g for 15 min (4°C). A 0.1 ml sample of the supernatant was mixed with 2.9 ml of 50 mM phosphate buffer (pH 6.0) containing 0.167 mg ml−1 o-dianisidine dihydrochloride (Sigma Chemical Co, St Louis, MO, USA) and 0.0005% hydrogen peroxide. The change in absorbance was measured at 460 nm using a spectrophotometer, with absorbance recorded at 15 s intervals over a 3 min period. One unit of MPO activity is defined as the amount that degrades 1 μmol of peroxide min−1 at 25°C. Tissue MPO activity was calculated using human MPO (DakoCytomation, Botany, Australia) as a standard, with units of MPO activity for each tissue sample being normalized on the basis of grams per wet weight of tissue.
Double-labelled immunofluorescence for neutrophils and macrophages
As previously described by Weston et al. (2006), slide-mounted sections were fixed in 4% paraformaldehyde for 30 min. All sections were subject to a pre-block in 10% normal goat serum (NGS), 0.5% bovine serum albumin and 0.3% Triton X-100 in phosphate buffered saline (PBS; 0.1 M, pH 7.4) for 1 h at room temperature, and then washed in PBS. All washes mentioned herein were 3 × 10 min. Sections were then incubated overnight at 4°C in rabbit polyclonal polymorphonuclear neutrophil (PMN) anti-sera at 1:100 000 (Accurate Chemicals, Westbury, NY, USA) for neutrophils and mouse ED1 monoclonal antibody at 1:300 (Accurate Chemicals, Westbury, NY, USA) for macrophages in PBS containing 2% NGS and 0.3% Triton X-100. Tissue sections were washed and subsequently incubated with Alexa Fluorophore 488 nm donkey anti-mouse IgG at 1:500 (Invitrogen, Mount Waverley, Australia) and Texas Red Fluorophore 595 nm goat anti-rabbit IgG at 1:500 (Vector Laboratories, Burlingame, CA, USA) in PBS containing 2% NGS and 0.3% Triton X-100 for 90 min at room temperature. Sections were again washed in PBS, prior to being coverslipped with anti-fade mountant (Vector Laboratories, Burlingame, CA, USA). In control experiments, primary antibodies were omitted to verify the absence of uncontrolled secondary antibody binding.
Quantification of immunohistochemistry
Immunoreactive (IR) cells, in dual-stained sections, were visualized in a 20 × field sections under an Olympus BX-51 fluorescence photomicroscope, and counted using UTHSCSA Image Tool (version 3) (The University of Texas, San Antonio, TX, USA). The effects of AM-36 were examined by counting the total number of IR neutrophils in the ischaemic and non-ischaemic hemispheres of six predetermined cerebral regions (striatum, motor cortex, forelimb, hindlimb, parietal and barrel cortical regions), across six stereotaxic levels (4.2 to −5.2 mm relative to Bregma; (Paxinos and Watson, 1986). A mean was calculated for each cerebral region, of the total number PMN IR neutrophils and the total number of PMN IR neutrophils colocalized with ED1.
Confocal imaging
Slide-mounted sections stained with both PMN anti-sera and ED1 monoclonal antibody (see above) were imaged with a Leica TCS-NT (Argon laser, Leica Microsystems, Wetzlar, Germany) inverted confocal microscope using 100 × /1.0 oil immersion lens. In dual-channel imaging, photomultiplier sensitivities were set to a level at which bleed-through effects from one channel to the other were negligible. For simultaneous imaging of PMN and ED1, immunostaining controls were used to adjust the photomultiplier levels separately for PMN and ED1, and the same settings were used during imaging of double immunostained sections.
Evaluation of BBB integrity
A separate group of rats was used to investigate the integrity of the BBB using Evans Blue extravasation, according to Belayev et al. (1996). Evans Blue binds to albumin, which normally is too large a molecule to cross the BBB, but which enters the brain when the BBB permeability increases following cerebral ischaemia (Uyama et al., 1988). A time-course of Evans Blue extravasation into the brain was examined at 3 (n=6), 24 (n=6), 48 (n=6) and 72 h (n=6) after ET-1 induced MCAo in conscious rats. To investigate whether AM-36 affected Evans Blue extravasation, two additional groups (sham MCAo (n=6) and AM-36 treated (n=6)) were examined at 48 h. Evans Blue (2% in saline, 4 ml kg−1) was injected intravenously at 48 h after the onset of MCAo. The chest was subsequently opened under pentobarbitone sodium (120 mg kg−1 i.p.) anaesthesia 2 h later. Rats were transcardially perfused with physiological saline (0.9%) at 100 mm Hg pressure. After decapitation, brains were removed and frozen over liquid nitrogen and stored at −80°C until processing. Coronal cryostat sections (16 μm thick) were cut and slide mounted, from 11 pre-determined levels ranging from +4.2 mm to −4.8 mm relative to Bregma (Paxinos and Watson, 1986). Evans Blue extravasation was visualized using an Olympus BX-51 fluorescence photomicroscope. The area of Evans Blue extravasation was measured using imaging analysis system for each of the 11 levels (SCION software system, SCION Corporation, Frederick, MD, USA).
Statistical analyses
Data are expressed as mean±s.e.m. Infarct area was examined by ANOVA followed by Student–Newman–Keuls method, and infarct volumes were compared by Student's t-test. ANOVA followed by Student–Newman–Keuls method was used for analysis of the MPO activity, the examination of BBB permeability and physiological parameters (temperature and body weight). Behavioural tests were analysed using ANOVA followed by Bonferroni post-test being used for analysis of individual differences. Cell counts of PMN and ED1 IR cells were assessed by Kruskal–Wallis nonparametric ANOVA followed by Dunn's post-test. Percentage differences between vehicle and AM-36-treated rats were compared by Kruskal–Wallis nonparametric ANOVA followed by Dunn's post-test. A value of P<0.05 was considered to be significant.
Results
Effect of AM-36 on functional outcomes
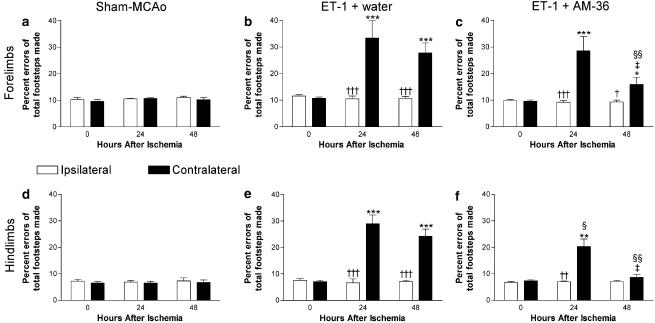
The grid-walking test and cylinder test were used to assess functional recovery following AM-36 administration. Grid-walking assessments revealed increased errors when stepping with the forelimb and hindlimb contralateral to the ET-1 MCAo (Figure 1b, c, e and f) following both vehicle and AM-36 treatment. AM-36 administration, however, significantly reduced the number of hindlimb errors at 24 h after ET-1 administration, while at 48 h, AM-36 reduced the number of errors in both the contralateral forelimb and hindlimb (Figure 1c and f) when compared to vehicle-treated rats (Figure 1b and e) (with the result at 48 h in the hindlimbs showing no significant difference from sham-occluded rats). Furthermore, AM-36 administration significantly reduced the number of errors made when stepping with contralateral forelimbs and hindlimbs at 48 h when compared with 24 h (Figure 1c and f). Sham-MCAo rats showed negligible differences with time or between paws (Figure 1a and d).
Figure 1.
Effects of AM-36 on grid-walking. Data are expressed as errors made as a percentage of total steps taken with contralateral and ipsilateral forelimbs (a–c) and hindlimbs (d–f) after cerebral ischaemia induced by ET-1. Each rat acted as its own control, and results after ischaemia were compared with pre-ischaemia scores (0 h after ischaemia). *P<0.05, **P<0.01, ***P<0.001 relative to the 0 h score in the same limb; †P<0.05, ††P<0.01, †††P<0.001 compared with opposite limb at the same time; ‡P<0.01 relative to the result at 24 h in the same limb; §P<0.01, §§P<0.001 compared with ET-1+water group in the same limb (ANOVA followed by Bonferroni post-test). Data are mean±s.e.m. n=5–6 in each group.
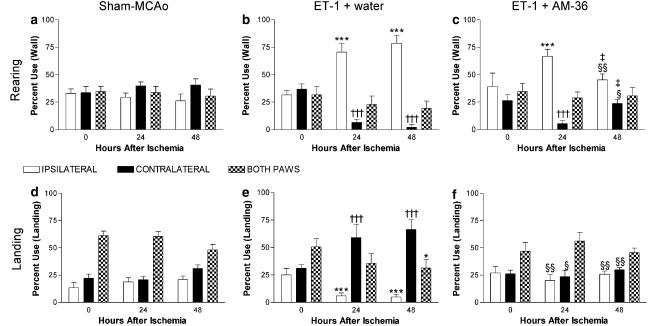
Sham-MCAo rats showed no limb use asymmetries when either rearing or landing in the cylinder test (Figure 2a and d). Following MCAo, however, rats showed significant limb use asymmetries when rearing and landing, with increased use of the ipsilateral forelimb on rearing and an increased use of the contralateral forelimb on landing. Vehicle-treated rats showed significant limb use asymmetries when rearing at both 24 and 48 h when compared to pre-ischaemia forelimb use (Figure 2b), while AM-36-treated rats only showed significant limb use asymmetries at 24 h, with forelimb use at 48 h showing no difference from pre-ischaemia values (Figure 2c). The landing preference of AM-36-treated rats (Figure 2f) showed no significant change from pre-ischaemia preferences or sham-occlusion rats. Conversely, vehicle-treated rats showed significant limb use asymmetries on landing at both 24 and 48 h, as indicated by the increased contralateral forelimb preference (Figure 2e).
Figure 2.
Effects of AM-36 on limb use asymmetries when rearing (a–c) and landing (d–f) in the cylinder test after ET-1 induced MCAo. Data are mean±s.e.m. expressed as a percentage of total forelimb use. Each rat acted as its own control, and results after stroke were compared with pre-ischaemia scores (0 h after ischaemia). ***P<0.001 ipsilateral paw relative to 0 h score in same paw; †††P<0.001 contralateral paw relative to 0 h in the same paw; *P<0.05 both paws compared to 0 h; §P<0.01, §§P<0.001 AM-36-treated rats compared to vehicle rats at the same time; ‡P<0.01 relative to the result at 24 h in the same limb (ANOVA followed by Bonferroni post-test). n=5–6 in each group.
Effect of AM-36 on histopathological outcome
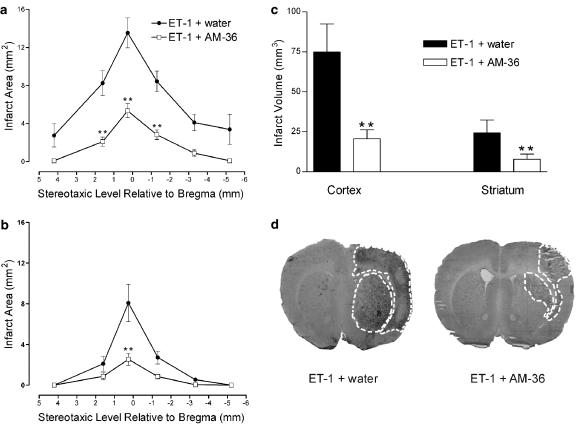
The infarct area (Figure 3a and b) and the infarct volume (Figure 3c) of both the cortex and striatum were significantly reduced by approximately 60% following AM-36 administration, when compared with vehicle-treated rats. In particular, AM-36 administration significantly reduced the size of the infarct within the forelimb, hindlimb and parietal regions of the cortex, ipsilateral to occluded artery, while the barrel region of the somatosensory cortex demonstrated consistent damage in both vehicle and AM-36-treated rats (data not shown). Sham-MCAo rats only showed damage associated with the injection tract. Representative pictures taken from image analysis, shown in Figure 3d (0.20 mm relative to Bregma; Paxinos and Watson, 1986), indicate the reduction in infarct area following AM-36 administration, especially in the sensorimotor cortex.
Figure 3.
Effect of AM-36 on infarct area (a, b) and total infarct volume (c) in both the cortex (a) and striatum (b) induced by ET-1, with data presented as mean±s.e.m. of infarct area measured at six predetermined coronal planes through the brain. **P<0.01 (a, b) compared with vehicle-treated control rats (Two way repeated measures ANOVA followed by Student–Newman–Keuls method) for infarct area. **P<0.01 (c) compared with vehicle-treated control rats (Student's t-test). Infarct area and volume is significantly reduced following AM-36 treatment. Representative images generated from unstained sections, using the MCID, showing reduced infarct size from vehicle-treated (ET-1+water) rats following treatment with AM-36 (d). Infarct area in right hemisphere induced by ET-1 can be visualized as the dark area and is marked by the white dotted line on each of the sections. n=5–6 rats in each treatment group.
Following stroke induction there was no significant change in core rectal temperature between vehicle (38.6±0.1°C), AM-36 (38.3±0.2°C) or sham-MCAo rats (38.4±0.2°C). AM-36 had no effect on body temperature 37.5±0.2°C (pre-AM-36 administration) versus 37.5±0.1°C (post-AM-36). Vehicle and AM-36-treated rats showed a significantly decreased body weight at both 24 (−18.7±7.5 versus −11.5±4.9 g) and 48 h (−23.2±8.5 versus −14.2±5.1 g) respectively, after stroke induction. Vehicle-treated rats showed a significantly greater decrease in body weight than AM-36-treated rats, compared with pre-stroke body weights. No change in body weight was seen in sham-occlusion rats after saline injection.
BBB opening
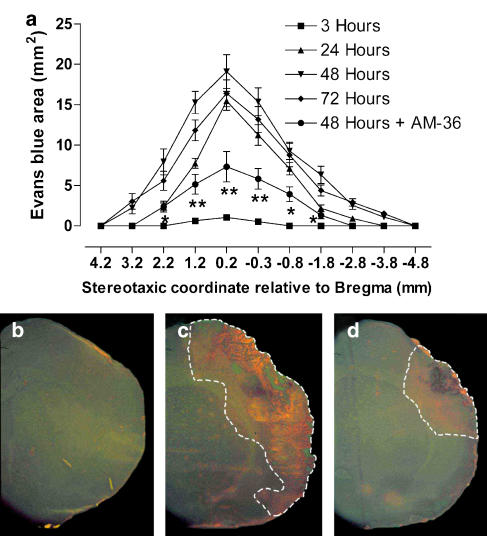
Measurement of the area stained by Evans Blue, given i.v., was used to determine the time course of BBB opening, following ET-1-induced transient focal ischaemia. The area stained by Evans Blue significantly increased from 3 to 48 h, where tissue Evans Blue extravasation was maximal in the ischaemic hemisphere, after the onset of MCAo (Figure 4a). At 72 h, Evans Blue extravasation was less than that at 48 h (Figure 4a). AM-36 administration significantly reduced the extravasation of Evans blue by ∼60% when compared to vehicle-treated rats (Figure 4a). Representative images (Figure 4b–d) show the increase in Evans Blue extravasation at 48 h in vehicle rats (Figure 4c) when compared to sham-occluded rats (Figure 4b). AM-36 administration reduced the extravasation of Evans Blue (Figure 4d), seen as a reduced area of fluorescence, when compared to vehicle-treated rats.
Figure 4.
Time course of Evans Blue extravasation in rat brain from 3 to 72 h after ET-1 induced MCAo (a) including the effect of AM-36 when administered up to 48 h, with data presented as mean±s.e.m. of area stained by Evans Blue. The area stained by Evans Blue significantly increased from 3 to 48 h, and then decreased at 72 h (P<0.001, 3 versus 24 h; P<0.05, 24 versus 48 h; P<0.05, 48 versus 72 h). *P<0.05, **P<0.01 AM-36 compared to ET-1 group at 48 h (Two way repeated measures ANOVA followed by Student–Newman–Keuls method). n=6 rats in each group. Representative sections through the ipsilateral cortex ( × 2) viewed following Evans Blue injection at 48 h (b–d), showing reduced Evans Blue extravasation with AM-36 administration (d) when compared with vehicle-treated rats (c). There was no extravasation of Evans Blue in the sham-MCAo rats (b). Area of Evans Blue extravasation is indicated by white dotted line.
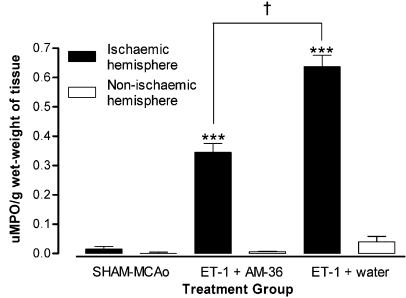
Effect of AM-36 on myeloperoxidase activity
AM-36 administration significantly reduced MPO activity in the ischaemic hemisphere by 54% when compared to vehicle-treated rats (Figure 5). Both ischaemic hemispheres of AM-36-treated and vehicle-treated rats displayed significantly greater MPO activity when compared to the non-ischaemic hemisphere. Vehicle-treated rats displayed a significantly greater MPO activity in the non-ischaemic hemisphere when compared with sham-MCAo rats. MPO activity was negligible in both hemispheres of sham-MCAo rats, and in the non-ischaemic hemisphere of AM-36-treated rats.
Figure 5.
MPO activity was measured in the ischaemic and non-ischaemic hemispheres of ET-1+ water, ET-1+AM-36 and sham-MCAo groups. Values are mean±s.e.m., expressed as units of MPO activity per gram wet-weight of brain tissue, for n=5–6 rats in each group. AM-36 treatment significantly reduced the MPO activity in the ischaemic hemisphere when compared to the ET-1+water group. ***P<0.0001 ischaemic hemisphere versus non-ischaemic hemisphere for ET-1+AM-36 and ET-1+water groups; †P<0.05 AM-36 treatment group compared with the vehicle-treated group (ANOVA followed by Student–Newman–Keuls method).
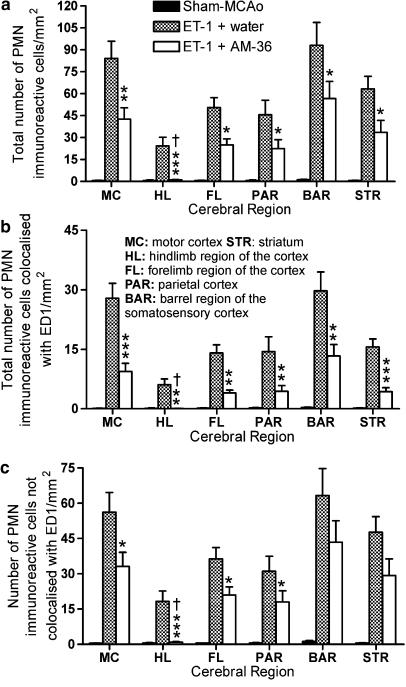
Effect of AM-36 on neutrophil inflammatory response
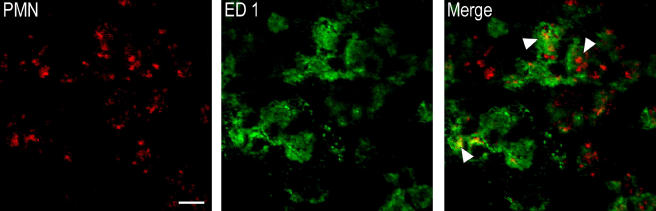
AM-36-treated rats showed a significantly reduced number of PMN IR cells in all forebrain regions examined, including the barrel cortical region that showed consistent damage between AM-36- and vehicle-treated rats, when compared to vehicle-treated rats (Figure 6a). AM-36 treatment significantly affected the interaction of neutrophils and macrophages, seen as differences in the colocalization of PMN and ED1 IR between vehicle- and AM-36-treated rats (Figure 6b and c). AM-36 treatment significantly reduced the total number of PMN IR cells that were colocalized with ED1 (Figure 6b), which corresponded to a 10% decrease in the percentage of PMN cells colocalized with ED1 when compared with vehicle-treated control rats (Table 1). AM-36 treatment, when compared to vehicle-treated rats, also significantly reduced the total number of PMN IR cells that were not colocalized with ED1 in all cerebral regions except the barrel region, where no significant difference existed (Figure 6c). However, the percentage of the total PMN IR cells not colocalized with ED1 was significantly increased by approximately 10% following AM-36 treatment, when compared to vehicle-treated rats (Table 1), except in the motor cortex, where no difference existed. Representative images of the dual labelling between PMN and ED1 demonstrate the engulfment of neutrophils by phagocytic macrophages in the cortex of ET-1 controls at 48 h, as seen in the merged images in Figure 7.
Figure 6.
Cell counts of polymorphonuclear neutrophil (PMN) anti-sera and ED1 immunoreactive (IR) cells following double staining at 48 h after ET-1 induced middle cerebral artery occlusion (MCAo) in selected cerebral regions. PMN IR cells were increased at 48 h after MCAo. (a) Total number of PMN IR cells at 48 h following MCAo in different cerebral cortex regions. AM-36 treatment significantly reduced the total number of PMN IR cells in all of the regions investigated when compared to vehicle-treated rats. AM-36-treated rats were significantly different from sham-occluded rats in all regions except the hindlimb region of the cortex. (b) The total number of PMN IR cells that were colocalized with ED1. AM-36 administration reduced the total number of PMN IR cells that were colocalized with ED1 in all cerebral regions examined, except the hindlimb region of the cortex, which again showed no difference from sham-occluded rats. (c) The total number of PMN IR cells not colocalized with ED1 was also reduced by AM-36, however, there was no significant difference between ET-1 and ET-1+AM-36 treatment in the barrel region of the somatosensory cortex and the striatum. Bars are presented as mean±s.e.m. of the number of IR cells in each cerebral region. *P<0.05, **P<0.01, *** P<0.001 AM-36 compared with vehicle-treated rats; †P>0.05 AM-36 compared with sham-occluded control rats (Kruskal–Wallis ANOVA with Dunn's post-test). n=6 for both ET-1+water and ET-1+AM-36-treated rats and n=5 for sham-occluded control rats.
Table 1.
Neutrophil – macrophage interactions
| Brain region |
% of total PMN IR cells colocalized with ED1 |
% of total PMN IR cells not colocalized with ED1 |
||
|---|---|---|---|---|
| ET-1+water | ET-1+AM-36 | ET-1+water | ET-1+AM-36 | |
| Hindlimb cortex | 24±1.7 | 3±2.5*** | 76±1.7 | 97±2.5*** |
| Forelimb cortex | 27±0.8 | 16±0.6*** | 73±0.8 | 85±0.9** |
| Striatum | 25±0.7 | 11±1.2*** | 75±3.2 | 89±1.3* |
| Motor cortex | 31±2.0 | 19±2.2*** | 69±2.0 | 75±4.3 |
| Parietal cortex | 29±1.9 | 16±3.3** | 71±2.0 | 84±3.3** |
| Barrel cortex | 32±2.0 | 25±2.8* | 68±2.0 | 75±2.8 |
Changes in percentage following AM-36 administration in the number of PMN IR cells colocalized or not colocalized with ED1 in different cerebral regions. AM-36 treatment significantly reduced the total number PMN IR cells that were colocalized with ED1, by approximately 10–15% when compared with vehicle-treated rats. Conversely, the percentage of the total number of PMN IR cells not colocalized with ED1 was increased by approximately 10–15% following AM-36 treatment in all cerebral regions except the motor cortex, where no significant difference existed. Data is presented as mean±s.e.m.
P<0.05
P<0.001
P<0.0001 AM-36 compared with vehicle-treated rats (Kruskal–Wallis ANOVA with Dunn's post-test). n=5–6 in each group.
Figure 7.
Confocal imaging of double immunostained sections shows colocalization of PMN (red) IR bodies within ED1 (green) IR cells in the cortex of an ET-1+water control rat at 48 h following cerebral ischaemia. This image represents macrophage (green) engulfment of apoptotic neutrophils (red), indicated by the arrows. Scale bar represents 50 μm. PMN, polymorphonuclear neutrophil; IR, immunoreactive.
Discussion
The present study demonstrated that AM-36, a Na+ channel blocker and antioxidant, reduces infarction and functional deficits following transient focal cerebral ischaemia. Furthermore, our results show for the first time that AM-36 reduces breakdown of the BBB and modulates the neutrophil inflammatory response by decreasing the ratio of ED-1 positive macrophages and PMN positive neutrophils colocalized cells, as compared to the ET-1 control group, and by reducing neutrophil accumulation in the brain parenchyma.
We have previously shown that the temporal profile of neutrophil infiltration into the brain parenchyma increases acutely (0–72 h) with the progression of the ischaemic injury in a conscious model of stroke (Weston et al., 2006). Therefore, the effects of AM-36 were examined at 48 h post-stroke, when neutrophil infiltration into the ischaemic region was still increasing. Neutrophil infiltration, as assessed by MPO activity and infarct volume measurements, were significantly reduced following AM-36 administration at 48 h after stroke, by 54 and 60%, respectively. The reduction in MPO activity and infarct volume following AM-36 administration compares favourably with other compounds designed to reduce neutrophil infiltration, such as an anti-CD18 monoclonal antibody (WT3) which showed a reduction in MPO activity and infarct volume by 75 and 61%, respectively, using the filament model of MCAo in rats (Matsuo et al., 1994). AM-36 was also shown to afford profound neuroprotection to both the cortex and striatum, which is important as the striatum is considered to be the core of the ischaemic injury. These results, however, did not indicate whether AM-36 directly targets the neutrophil inflammatory response, or whether the observed reductions in neutrophil infiltration were secondary to reductions in infarct volume. Consequently, we utilized the more powerful investigatory tool of double-label immunofluorescence (Weston et al., 2006) to specifically and selectively examine the accumulation of neutrophils (PMN anti-sera) and macrophages (ED-1) within selected cerebral regions of the ischaemic core and penumbra.
In penumbral regions, which included the striatum, forelimb and hindlimb regions of the cortex, motor and parietal cortex, AM-36 protected against neuronal damage and also reduced neutrophil accumulation, with neutrophils confined to the margins of the ischaemic damage. In particular, the hindlimb region of the cortex showed consistent ischaemic damage in ET-1 control rats, whereas AM-36-treated rats that showed no damage in this region. Conversely, the barrel region of the somatosensory cortex, which represents the ischaemic core, demonstrated consistent ischaemic damage in both vehicle- and AM-36-treated rats. Furthermore, no significant difference in infarct volume within the barrel region of the cortex was seen when compared with ET-1 control rats, although AM-36 administration did reduce neutrophil accumulation. This result indicates that AM-36 is able to modulate the accumulation of neutrophils into the brain and that its effects are not merely the result of reductions in infarct size, as seen in the barrel region of the somatosensory cortex. We previously showed that the resolution of neutrophil accumulation in rat brain parenchyma following transient cerebral ischaemia is achieved through phagocytosis of neutrophils by macrophages and that a significant correlation between neutrophil infiltration and infarct formation exists (Weston et al., 2006). AM-36 also significantly reduced the extent of colocalization between ED-1 and PMN positive stained cells, indicating the engulfment of neutrophils by macrophages was also modulated following AM-36 administration.
The cellular basis underlying the neuroprotective effects of AM-36 in modulating the inflammatory response of neutrophils following cerebral ischaemia is unknown. Meszaros et al. (1999) demonstrated that macrophages utilize distinct receptor patterns to recognize and ingest only neutrophils undergoing apoptotic cell death. The reduced colocalization of PMN and ED1 IR cells following AM-36 treatment may indicate that neutrophil apoptosis was impaired, and thus macrophage receptor recognition and engulfment of neutrophils was subsequently limited (Fadok et al., 2001), although additional research would be required to elucidate the importance of this pathway. More specifically, the Na+ channel blocking and antioxidant properties of AM-36, which have previously been shown to inhibit neuronal apoptosis (Callaway et al., 2001), could be involved in modulating the neutrophil inflammatory response.
The Na+ channel blockers, including TTX, have been shown to inhibit ATP depletion following cerebral ischaemia (Gleitz et al., 1996). Inflammatory mediators such as adenosine, which has been shown to increase and prolong neutrophil activation through adenosine A2A receptor activation (Yu et al., 2004), are produced as ATP is hydrolysed and depleted following MCAo (Rudolphi et al., 1992). By maintaining ionic homeostasis and thus preventing ATP depletion (Gleitz et al., 1996), through Na+ channel blocking action, AM-36 could inhibit the formation of pro-inflammatory mediators thereby limiting neutrophil activation. Indeed, AM-36 exhibits potent Na+ channel blocking activity, both in vitro and in vivo, which is comparable to that of tetrodotoxin (TTX) and sipatrigine, and has been shown to inhibit excess activation of slow-inactivating Na+ channels (Callaway et al., 2004). In vitro studies using cultured cerebellar granule cells demonstrated that AM-36 completely inhibited cell death caused by exposure to the Na+ channel opener veratridine, with this effect being more potent and effective than other Na+ channel blockers such as sipatrigine and dibucaine (Callaway et al., 2001; Callaway et al., 2004). Additionally, these Na+ blocking properties could directly act on neutrophils to inhibit adherence to post-capillary venules as well as inhibit neutrophil activation, as similar effects have been demonstrated in studies using the Na+ channel blocker lidocaine. Lidocaine was shown to inhibit neutrophil adherence (Scott et al., 1993), the production of superoxide anions (Peck et al., 1985; Vitola et al., 1997) and MPO secretion from azurophilic granules (Fittschen and Henson, 1991) resulting in decreased infarct formation in the myocardium. Reductions in neutrophil accumulation and MPO activity demonstrated in the present study following AM-36 treatment were more potent than those described for lidocaine (Vitola et al., 1997; Lan et al., 2004), and may indicate the dual properties of AM-36 are more efficacious.
ROS have also been shown to affect neutrophil activation and infiltration following cerebral ischaemia by inducing the activation of transcription factors such as nuclear factor kappa B, which increases the expression of adhesion molecules leading to increased neutrophil infiltration (Howard et al., 1998; Christman et al., 2000). Previous studies using antioxidants, such as BN 80933, have shown reduced neutrophil activation and infiltration (Ding-Zhou et al., 2003). AM-36 has also been demonstrated to possess antioxidant properties (Callaway et al., 2003). In rat brain homogenates, AM-36 was found to inhibit lipid peroxidation, with its activity comparing favourably to that of known antioxidants such as BHT, Trolox and LY231617 (Jarrott et al., 1999). Further, Callaway et al. (2003) showed a reduction in ROS metabolites in the striatum of rats with AM-36 treatment following cerebral ischaemia, demonstrating this drug has direct redox effects within the brain parenchyma. Hence, AM-36 could act directly on neutrophils to prevent the respiratory burst (Nathan, 2006) and reduce the release of ROS following ischaemia-reperfusion in a similar way to propofol (Mikawa et al., 1998), or indirectly by reducing ROS-induced neuronal damage and oxidative stress (Chan, 2001) and subsequent neutrophil infiltration.
Neutrophil appearance in the microvasculature following cerebral ischaemia has also been shown to increase the permeability of the BBB via the release of superoxide anion (Fagan et al., 2004). It is well established that ROS can disrupt vascular integrity and increase microvascular permeability by injuring endothelial cells (Chan et al., 1984; Nelson et al., 1992; Gasche et al., 2001). Previous studies utilizing antioxidant therapies have shown that decreases in ROS with following cerebral ischaemia can attenuate BBB disruption (Kim et al., 2001; Ding-Zhou et al., 2003). Examination of BBB integrity following ET-1-induced MCAo in this study, revealed a similar time course of Evans Blue extravasation to that found by Belayev et al. (1996) following transient MCAo in rats, with BBB permeability increasing from 3 to 48 h within the ipsilateral hemisphere, where it peaks, before decreasing at 72 h. The degree of Evans Blue extravasation into brain tissue, in the present study, was also significantly higher in the ipsilateral hemisphere compared to the contralateral hemisphere in all rats following ET-1-induced MCAo.
Treatment with AM-36 reduced BBB breakdown, seen by the reduced extravasation of Evans Blue into the brain parenchyma. This effect could in part be mediated through the antioxidant effect of AM-36 on superoxide anions and hydroxyl radicals, which are released from neutrophils and contribute to BBB opening (Nelson et al., 1992). Similar reductions in neutrophil accumulation and BBB breakdown following transient cerebral ischaemia have been seen using the compound BN 80933, which combines neuronal nitric oxide synthase inhibition and antioxidant properties (Ding-Zhou et al., 2003). By preserving the integrity of the BBB, AM-36 may also be inhibiting neutrophil adherence and subsequently decreasing trans-endothelial migration of neutrophils (Ainsworth et al., 1996). Interestingly, accumulation of neutrophils into the brain parenchyma closely correlates with the area stained by Evans Blue, in both AM-36 and control rats, adding further support to the hypothesis that AM-36 alters neutrophil adherence at the BBB and subsequently limits infiltration. Alternatively, AM-36 may indirectly affect neutrophils by decreasing inflammatory mediators and oxidative stress through antioxidant actions.
As increased permeability of the BBB and neutrophil infiltration have been correlated with ischaemic damage and neurological deficits (Albayrak et al., 1997; Weston et al., 2006), it was therefore considered important to assess if AM-36 had a neuroprotective effect on behavioural outcomes. Thus, we utilized the cylinder test to investigate limb use asymmetries in the forelimbs. The cylinder test showed that rats had no preference for either limb when rearing or landing pre-ischaemia. Following MCAo, rats showed an increased reliance on the ipsilateral (unimpaired) forelimb on rearing and for exploratory movements along the cylinder wall indicating limb use asymmetry, which is consistent with the results observed by Schallert et al. (2000) at acute times after stroke induction. Conversely, rats showed an increased preference for the contralateral (impaired) forelimb when landing. Rats treated with AM-36 only showed significant limb use asymmetries when rearing at 24 h, with no significant forelimb preference observed at 48 h when rearing, or while landing at either 24 or 48 h as compared with sham-occluded rats. Thus, AM-36 reduced forelimb deficits in rats following stroke, as seen by the absence of limb use asymmetries in the cylinder test when compared to vehicle-treated rats.
In contrast to the cylinder test, the grid-walking test is an effective method for assessment of motor coordination and functional recovery in all four limbs following cerebral ischaemia (Rogers et al., 1997). Both vehicle and AM-36-treated rats showed increased numbers of errors associated with the contralateral forelimb and hindlimb, which is consistent with other studies (Soblosky et al., 1996; Rogers et al., 1997). AM-36 administration significantly reduced the number of forelimb and hindlimb errors compared with vehicle-treated rats at 48 h. Thus AM-36 was able to reduce functional deficits and improve motor coordination in both the forelimbs and hindlimbs, a finding not previously reported. Furthermore, the pronounced protection afforded by AM-36 was associated with a decrease in the infarct volume in both cortical and subcortical regions involved in motor coordination; including the striatum, forelimb and hindlimb regions of the cortex, motor and parietal cortex. Previous studies have shown that reductions or prevention of infarct formation in these regions of the cortex can be correlated with improved performance in behavioural paradigms (Rogers et al., 1997; Virley et al., 2000).
Importantly, AM-36 was shown to be neuroprotective following delayed administration in this model, and it should be emphasized that while recovery in this study was only investigated up to 48 h post-ischaemia, sustained protective effects have been demonstrated in more chronic studies conducted in our laboratory (unpublished observations). Thus, the profound neuroprotection and functional recovery with this drug is a permanent effect and not merely the result of spontaneous recovery that has been observed in previous studies (Schallert et al., 2003).
In conclusion, the present data confirms the effectiveness of AM-36 in reducing infarct size and functional deficits following transient cerebral ischaemia. More importantly, this data demonstrates that AM-36 can reduce the neutrophil inflammatory response and BBB breakdown after focal stroke. The mechanism by which AM-36 exerts this action is unknown, but it is likely that AM-36 reduces disruption to the BBB, thus inhibiting trans-endothelial migration of neutrophils through a combination of its properties. The present study adds support to a pathogenic role of neutrophils following cerebral ischaemia and provides the basis for additional investigation into development of drugs to limit neutrophil entry into the brain parenchyma.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Program Grant # 236805). Dr Y Ishizuka was the recipient of a fellowship from the Mitsubishi Pharma Research Foundation.
Abbreviations
- AM-36
[1-(2-(4-chloro-phenyl)-2-hydroxy)-ethyl-4-(3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl) methylpiperazine di-tartrate]
- BW619C89
[4-amino-2-(4-methyl-1-piperazinyl)-5-(2,3,5-trichlorophenyl) pyrimidine]
- BBB
blood brain barrier
- BHT
3,5-dibutyl-4-hydroxy-toluene
- BN 80933
(S)-N-[4-[4-[(3,4-Dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)carbonyl]-1-piperazinyl]phenyl]-2-thiophenecarboximid-amide
- ET-1
endothelin-1
- ICAM-1
intracellular adhesion molecule-1
- IR
immunoreactive
- LY231617
2,6-bis-(1,1-Dimethylethyl)-4-[[(1-ethyl)amino]methyl]phenol, Hydrochloride
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
- MPO
myeloperoxidase
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NGS
normal goat serum
- PMN
polymorphonuclear neutrophil
- ROS
reactive oxygen species
- TTX
tetrodotoxin
Conflicts of interest
The authors state no conflict of interest.
References
- Ainsworth TM, Lynam EB, Sklar LA.Neutrophil function in inflammation and infection Cellular and Molecular Pathogenesis 1996Lippincott-Raven Publishers: Philadelphia; 37–55.In: Sirica AE (Ed.) [Google Scholar]
- Albayrak S, Zhao Q, Siesjo BK, Smith ML. Effect of transient focal ischemia on blood-brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol (Berlin) 1997;94:158–163. doi: 10.1007/s004010050688. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18 Suppl 2:s18–s22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Croci N, Plotkine M, Margaill I. Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia-reperfusion in rats. Brain Res. 2003;987:32–38. doi: 10.1016/s0006-8993(03)03224-4. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Beart PM, Jarrott B, Giardina SF. Incorporation of sodium channel blocking and free radical scavenging activities into a single drug, AM-36, results in profound inhibition of neuronal apoptosis. Br J Pharmacol. 2001;132:1691–1698. doi: 10.1038/sj.bjp.0704018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JK, Castillo-Melendez M, Giardina SF, Krstew EK, Beart PM, Jarrott B. Sodium channel blocking activity of AM-36 and sipatrigine (BW619C89): in vitro and in vivo evidence. Neuropharmacology. 2004;47:146–155. doi: 10.1016/j.neuropharm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Knight MJ, Watkins DJ, Beart PM, Jarrott B. Delayed treatment with AM-36, a novel neuroprotective agent, reduces neuronal damage after endothelin-1-induced middle cerebral artery occlusion in conscious rats. Stroke. 1999;30:2704–2712. doi: 10.1161/01.str.30.12.2704. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Knight MJ, Watkins DJ, Beart PM, Jarrott B, Delaney PM. A novel, rapid, computerized method for quantitation of neuronal damage in a rat model of stroke. J Neurosci Methods. 2000;102:53–60. doi: 10.1016/s0165-0270(00)00278-8. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Lawrence AJ, Jarrott B. AM-36, a novel neuroprotective agent, profoundly reduces reactive oxygen species formation and dopamine release in the striatum of conscious rats after endothelin-1-induced middle cerebral artery occlusion. Neuropharmacology. 2003;44:787–800. doi: 10.1016/s0028-3908(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PH, Schmidley JW, Fishman RA, Longar SM. Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology. 1984;34:315–320. doi: 10.1212/wnl.34.3.315. [DOI] [PubMed] [Google Scholar]
- Christman JW, Blackwell TS, Juurlink BH. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol. 2000;10:153–162. doi: 10.1111/j.1750-3639.2000.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JA. Editorial comment. Delayed treatment with AM-36, a novel neuroprotective agent, reduces neuronal damage after endothelin-1-induced middle cerebral artery occlusion in conscious rats. Stroke. 1999;30:2712. doi: 10.1161/01.str.30.12.2704. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Ding-Zhou L, Marchand-Verrecchia C, Palmier B, Croci N, Chabrier PE, Plotkine M, et al. Neuroprotective effects of (S)-N-[4-[4-[(3,4-Dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)carbonyl]-1-piperazinyl]phenyl]-2-thiophenecarboximid-amide (BN 80933), an inhibitor of neuronal nitric-oxide synthase and an antioxidant, in model of transient focal cerebral ischemia in mice. J Pharmacol Exp Ther. 2003;306:588–594. doi: 10.1124/jpet.103.051490. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Dean RL, 3rd, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct. Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Kent TA. Superoxide anion production during reperfusion is reduced by an antineutrophil antibody after prolonged cerebral ischemia. Free Radic Biol Med. 1999;26:355–361. doi: 10.1016/s0891-5849(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- Fittschen C, Henson PM. Selective secretion of azurophil granule contents induced by monovalent cation ionophores in human neutrophils: evidence for direct ionophore effects on the granule membrane. J Leukoc Biol. 1991;50:517–528. doi: 10.1002/jlb.50.5.517. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Gleitz J, Tosch C, Beile A, Peters T. The protective action of tetrodotoxin and (+/−)-kavain on anaerobic glycolysis, ATP content and intracellular Na+ and Ca2+ of anoxic brain vesicles. Neuropharmacology. 1996;35:1743–1752. doi: 10.1016/s0028-3908(96)00106-2. [DOI] [PubMed] [Google Scholar]
- Hayward NJ, Elliott PJ, Sawyer SD, Bronson RT, Bartus RT. Lack of evidence for neutrophil participation during infarct formation following focal cerebral ischemia in the rat. Exp Neurol. 1996;139:188–202. doi: 10.1006/exnr.1996.0093. [DOI] [PubMed] [Google Scholar]
- Howard EF, Chen Q, Cheng C, Carroll JE, Hess D. NF-kappa B is activated and ICAM-1 gene expression is upregulated during reoxygenation of human brain endothelial cells. Neurosci Lett. 1998;248:199–203. doi: 10.1016/s0304-3940(98)00239-0. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bone marrow spawns brain killers. Nat Med. 2004;10:1044–1045. doi: 10.1038/nm1004-1044. [DOI] [PubMed] [Google Scholar]
- Jarrott B, Callaway JK, Jackson WR, Beart PM. Development of a novel arylalkylpiperazine compound (AM-36) as a hybrid neuroprotective drug. Drug Dev Res. 1999;46:261–267. [Google Scholar]
- Kim GW, Lewen A, Copin J, Watson BD, Chan PH. The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood-brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience. 2001;105:1007–1018. doi: 10.1016/s0306-4522(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Lan W, Harmon D, Wang JH, Ghori K, Shorten G, Redmond P. The effect of lidocaine on in vitro neutrophil and endothelial adhesion molecule expression induced by plasma obtained during tourniquet-induced ischaemia and reperfusion. Eur J Anaesthesiol. 2004;21:892–897. doi: 10.1017/s0265021504000249. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Swan JH, Eisenthal D, Dopson M, Nobbs M. BW619C89, a glutamate release inhibitor, protects against focal cerebral ischemic damage. Stroke. 1993;24:1063–1067. doi: 10.1161/01.str.24.7.1063. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab. 1995;15:941–947. doi: 10.1038/jcbfm.1995.119. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994;656:344–352. doi: 10.1016/0006-8993(94)91478-8. [DOI] [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage phagocytosis of wound neutrophils. J Leukoc Biol. 1999;65:35–42. doi: 10.1002/jlb.65.1.35. [DOI] [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, et al. Propofol inhibits human neutrophil functions. Anesth Analg. 1998;87:695–700. doi: 10.1097/00000539-199809000-00039. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nelson CW, Wei EP, Povlishock JT, Kontos HA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am J Physiol. 1992;263:H1356–1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Shigeno T, Balarsky AM, Ford I, McCulloch J, Teasdale GM, et al. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987;50:402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1986Academic Press: San Diego, CA; 2nd edn [Google Scholar]
- Peck SL, Johnston RB, Jr, Horwitz LD. Reduced neutrophil superoxide anion release after prolonged infusions of lidocaine. J Pharmacol Exp Ther. 1985;235:418–422. [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB.Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat Stroke 1997282060–2065.discussion 2066 [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992;4:346–369. [PubMed] [Google Scholar]
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT, Fleming SM. Experimental focal ischemic injury: behavior-brain interactions and issues of animal handling and housing. ILAR J. 2003;44:130–143. doi: 10.1093/ilar.44.2.130. [DOI] [PubMed] [Google Scholar]
- Scott BD, Shasby DM, Tomanek RJ, Kieso RA, Seabold JE, Ponto JA, et al. Lidocaine and dextran sulfate inhibit leukocyte accumulation but not postischemic contractile dysfunction in a canine model. Am Heart J. 1993;125:1002–1011. doi: 10.1016/0002-8703(93)90107-k. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1993;13:865–871. doi: 10.1038/jcbfm.1993.108. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Matthews MA, Davidson JF, Tabor SL, Carey ME. Traumatic brain injury of the forelimb and hindlimb sensorimotor areas in the rat: physiological, histological and behavioral correlates. Behav Brain Res. 1996;79:79–92. doi: 10.1016/0166-4328(95)00264-2. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- van der Goes A, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, et al. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- Virley D, Beech JS, Smart SC, Williams SC, Hodges H, Hunter AJ. A temporal MRI assessment of neuropathology after transient middle cerebral artery occlusion in the rat: correlations with behavior. J Cereb Blood Flow Metab. 2000;20:563–582. doi: 10.1097/00004647-200003000-00015. [DOI] [PubMed] [Google Scholar]
- Vitola JV, Forman MB, Holsinger JP, Atkinson JB, Murray JJ. Reduction of myocardial infarct size in rabbits and inhibition of activation of rabbit and human neutrophils by lidocaine. Am Heart J. 1997;133:315–322. doi: 10.1016/s0002-8703(97)70226-6. [DOI] [PubMed] [Google Scholar]
- Weston RM, Jarrott B, Jones NM, Callaway JK.Inflammatory cell infiltration following endothelin-1-induced cerebral ischemia: Histochemical and myeloperoxidase correlation with temporal changes in brain injury J Cereb Blood Flow Metab 2006 10.1038/sj.jcbfm.9600324E-pub ahead of print: 31 May 2006doi [DOI] [PubMed]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]