Abstract
Background and purpose:
Interactions between the NO system and the cyclooxygenase systems may be important in cardiovascular regulation. Here we measured the effects of acute cyclooxygenase-2 inhibition (with parecoxib), alone and in combination with NOS inhibition (with N G-nitro-L-arginine methyl ester (L-NAME)), on resting cardiovascular variables and on responses to the glucagon-like peptide 1 agonist, exendin-4, which causes regionally-selective vasoconstriction and vasodilatation.
Experimental approach:
Rats were instrumented with flow probes and intravascular catheters to measure regional haemodynamics in the conscious, freely moving state. L-NAME was administered as a primed infusion 180 min after administration of parecoxib or vehicle, and exendin-4 was given 60 min after the onset of L-NAME infusion.
Key results:
Parecoxib had no effect on resting cardiovascular variables or on responses to L-NAME. Exendin-4 caused a pressor response accompanied by tachycardia, mesenteric vasoconstriction and hindquarters vasodilatation. Parecoxib did not affect haemodynamic responses to exendin-4, but L-NAME inhibited its hindquarters vasodilator and tachycardic effects. When combined, L-NAME and parecoxib almost abolished the hindquarters vasodilatation while enhancing the pressor response.
Conclusions and implications:
Cyclooxygenase-2-derived products do not affect basal haemodynamic status in conscious normotensive rats, or influence the NO system acutely. The inhibitory effects of L-NAME on the hindquarters vasodilator and tachycardic effects of exendin-4 are consistent with a previous study that showed those events to be β-adrenoceptor mediated. The additional effect of parecoxib on responses to exendin-4 in the presence of L-NAME, is consistent with other evidence for enhanced involvement of vasodilator prostanoids when NO production is reduced.
Keywords: cyclooxygenase-2, exendin-4, nitric oxide, haemodynamics
Introduction
Cyclooxygenase is the enzyme responsible for the first step in the production of prostanoids from arachidonic acid, which, via different subsequent enzyme pathways, can result in the synthesis of a range of products, some of which are vasodilator (e.g., prostacyclin) and some of which are vasoconstrictor (e.g., thromboxane A2). There are at least two isoforms of cyclooxygenase, namely, cyclooxygenase-1 and cyclooxygenase-2; the former is generally considered to be important under physiological conditions, whereas the latter is readily induced in conditions such as inflammation (for reviews see Vane et al., 1998; Warner and Mitchell, 2004). However, there is also evidence that cyclooxygenase-2 is constitutively expressed (Vane et al., 1998), and cyclooxygenase-2-derived products may influence vascular tone under normal conditions, although whether the cyclooxygenase-2 products are predominantly vasodilator or vasoconstrictor is unclear, and results may depend on the vascular territory studied and the experimental conditions.
For example, administration of arachidonic acid to anaesthetized rats caused a rise in pulmonary vascular resistance and a fall in systemic vascular resistance, both of which were attenuated by administration of a cyclooxygenase-2 inhibitor, suggesting that cyclooxygenase-2 is responsible for the production of vasoconstrictor products in the pulmonary circulation and vasodilator products in the systemic circulation under normal conditions (Baber et al., 2003a). In addition, cyclooxygenase-2 inhibition has been shown by some (Qi et al., 2002), but not by others (Baber et al., 2003b, 2005), to enhance the pressor response to angiotensin II, again suggesting that cyclooxygenase-2-derived products are primarily vasodilator. In line with this, increases in blood pressure have been reported with chronic administration of cyclooxygenase-2 inhibitors in rats (Muscará et al., 2000; Höcherl et al., 2002), although Knight and Johns (2005) recently reported that cyclooxygenase-2 inhibition lowered resting blood pressure in normotensive rats, possibly indicating a vasoconstrictor role for cyclooxygenase-2 products. Beierwaltes (2002) reported no effect of acute cyclooxygenase inhibition on basal blood pressure or renal haemodynamics in anaesthetized rats but to date, the regional vascular effects of acute cyclooxygenase-2 inhibition in conscious rats have not been measured.
There are complex interactions between the cyclooxygenase system and the nitric oxide (NO) system, which may be important in cardiovascular regulation, but, again, the nature of the interaction is unclear. Thus, Bratz and Kanagy (2004) showed that cyclooxygenase-2 inhibition reduced noradrenaline-induced contraction of isolated mesenteric arteries from rats by a mechanism which involved NO, suggesting that cyclooxygenase-2 products inhibited NO synthase (NOS), whereas other studies have shown that prostacyclin analogues can upregulate endothelial NOS (Niwano et al., 2003). Furthermore, NO can influence the cyclooxygenase system by activating prostacyclin synthase, inhibiting thromboxane A2 synthase and potentiating the vascular actions of prostacyclin (for review see Antman et al., 2005).
Against this background, the aims of the present study were to investigate further the interactions between the cyclooxygenase-2 and NO systems in the context of cardiovascular regulatory mechanisms, by measuring the effects of acute cyclooxygenase-2 inhibition on baseline cardiovascular variables and on responses to acute NOS inhibition in normotensive rats. In addition, since several vasoactive peptides exert their effects, at least in part, via endothelium-dependent mechanisms which may involve NO and/or cyclooxygenase products, we considered it important to assess the influence of our experimental interventions on such regional vascular responses. In this context, we chose to look at the glucagon-like peptide (GLP)-1 agonist, exendin-4, because GLP-1 agonists are promising therapeutic agents for the treatment of type-2 diabetes due to their insulinotropic actions (see Knudsen, 2004). We have recently reported haemodynamic effects of exendin-4 in normotensive rats (Gardiner et al., 2006), but it is important to delineate the cardiovascular actions of such agents when endothelial function is impaired, since there is a high occurrence of endothelial abnormalities in the target patient population (Makimattila and Yki-Jarvinen, 2002). The present study, therefore, compared the effects of exendin-4 in the absence and presence of cyclooxygenase-2 and/or NOS inhibition. All experiments were carried out in two different outbred strains of normotensive rat, since there is evidence to suggest strain-dependent differences in the cardiovascular responses to NOS inhibition (Forster et al., 2001). The main findings show that acute cyclooxygenase-2 inhibition does not influence regional vascular tone or responses to NOS inhibition, but augments the inhibitory effect of NOS inhibition on the vasodilator action of exendin-4, resulting in enhancement of its pressor effect.
Methods
Animals
All procedures were carried out with approval of the University of Nottingham Local Ethical Review Committee, under Home Office Project and Personal Licence authority. Adult, male, Sprague–Dawley and Wistar rats, weighing 300–350 g, were obtained from Charles River (UK) and housed in a temperature-controlled (21–23°C) environment with a 12 h light–dark cycle (lights on at 0600) and free access to food (Teklad Global 18% Protein Rodent Diet, Harlan UK) and water.
Surgery
Animals were instrumented for chronic recording of cardiovascular variables as described previously (Gardiner et al., 2006). Surgery was performed in two stages, under general anaesthesia (fentanyl and medetomidine, 300 μg kg−1 of each i.p., supplemented as required), with anaesthetic reversal and provision of analgesia achieved with atipamezole and nalbuphine, respectively (1 mg kg−1 of each s.c.). The first surgical stage involved implantation of miniature pulsed Doppler flow probes around the left renal artery, the superior mesenteric artery and the distal abdominal aorta (to monitor hindquarters haemodynamics). The second surgical stage involved implantation of catheters in the distal abdominal aorta, via the caudal artery, for arterial blood pressure monitoring and the derivation of heart rate, and in the right jugular vein for peptide and drug administration. Up to three separate intravenous (i.v.) catheters were placed in the jugular vein to enable concurrent administration of different compounds. The surgical stages were separated by at least 7 days and the fitness of the animals between stages was certified by the Named Veterinary Surgeon.
Experiments began 24 h after surgery for catheter implantation, with animals fully conscious and unrestrained in home cages, with free access to food and water.
Cardiovascular recordings
A customized, computer-based system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, The Netherlands) connected to a transducer amplifier (Gould (Ohio, USA) model 13-4615-50) and a Doppler flowmeter (Crystal Biotech (Holliston, USA) VF-1 mainframe (pulse repetition frequency 125 kHz) fitted with high velocity (HVPD-20) modules) were used for data capture. Raw data were sampled by HDAS every 2 ms, averaged every cardiac cycle, and stored to disc at 5 s intervals.
Experimental protocol
Experiments were run over 3 days. On Day 1, after a period of at least 60 min baseline recording, rats were given the cyclooxygenase-2 inhibitor, parecoxib sodium (10 mg kg−1 i.v.) or vehicle (isotonic saline, 0.1 ml). Parecoxib sodium is the water-soluble prodrug of valdecoxib, and it has been shown that conversion is rapid, and plasma levels of valdecoxib remain elevated for at least 5 h following bolus i.v. administration to rats (Talley et al., 2000). The effectiveness of the chosen dose has been demonstrated in hyperalgesic models (Padi et al., 2004). Furthermore, it has been shown that i.v. doses of up to 20 mg kg−1 parecoxib are without effect in tests of cyclooxygenase-1 activity (Padi et al., 2004), indicating that at the chosen dose, any effects observed are due to inhibition of cyclooxygenase-2. As a control for NG nitro-L-arginine methyl ester (L-NAME) (see below), saline was administered i.v. as a bolus (0.1 ml) followed by continuous infusion (0.4 ml h−1) 180 min after parecoxib or vehicle, followed 60 min later by a bolus dose of exendin-4 (250 ng kg−1 i.v., Gardiner et al., 2006).
On Day 3, the same protocol was followed as on Day 1, but L-NAME (3 mg kg−1, 3 mg kg−1 h−1) was given in place of the saline. No drugs or peptides were given on the intervening day (Day 2). Groups of eight rats of each strain were used for each condition (total 32 rats).
Data analysis
Data were analysed offline using software (Datview, University of Limburg, Maastricht, The Netherlands) which interfaced with HDAS. The original baseline was taken as the average values over the 15 min period before administration of parecoxib or vehicle. Responses to L-NAME were measured as the average 1 min values immediately before and at 10 min intervals following administration. Between-group comparisons were made on the integrated areas under or over the curves between 0 and 60 min.
Responses to exendin-4 were measured before and at 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, 60, 90, 120, 150 and 180 min post-dosing. Comparisons of the effects of exendin-4 were performed on the integrated areas under or over the curves between 0 and 90 min. Data were analysed using a non-parametric, two-way analysis of variance (Friedman's test; Theodorsson–Norheim, 1987) for within-group comparisons, and Mann–Whitney or Kruskal–Wallis (unpaired) and Wilcoxon's (paired) tests for between-group comparisons. Vascular conductances were calculated from the mean arterial blood pressure (BP) and Doppler shift (flow) data. P⩽0.05 was taken as significant.
Materials
Exendin-4 was purchased from Bachem (St Helens, U.K.), L-NAME was purchased from Sigma (Dorset, UK), parecoxib sodium (N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl]sulphonyl] propanamide sodium) was purchased from Sequoia Research Products (Oxford, UK). Stock solutions of exendin-4 were made up in sterile water for injection, and diluted in sterile saline containing 1% bovine serum albumin. Injection volumes were 0.1 ml.
Fentanyl citrate was from Janssen-Cilag (High Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, Kent, UK); nalbuphine hydrochloride (Nubain) was from Bristol-Myers-Squibb (Hounslow, UK).
Results
Effects of parecoxib
Resting cardiovascular variables and values 180 min after administration of parecoxib or vehicle in Sprague–Dawley and Wistar rats are shown in Table 1.
Table 1.
Cardiovascular variables before and 180 min after administration of vehicle or parecoxib
| Strain | SD | SD | SD | SD | Wistar | Wistar | Wistar | Wistar |
|---|---|---|---|---|---|---|---|---|
| Treatment | Vehicle | Vehicle | Parecoxib | Parecoxib | Vehicle | Vehicle | Parecoxib | Parecoxib |
| Time | 0 min | 180 min | 0 min | 180 min | 0 min | 180 min | 0 min | 180 min |
| Heart rate (beats min−1) | 335±7 | 330±6 | 328±10 | 335±9 | 332±10 | 321±11 | 322±8 | 318±6 |
| BP (mm Hg) | 108±2 | 112±2 | 108±3 | 106±2 | 116±2 | 115±1 | 118±2 | 114±2 |
| RVC (U) | 78±8 | 77±7 | 76±6 | 74±5 | 80±9 | 75±8 | 76±10 | 73±12 |
| MVC (U) | 62±4 | 55±4 | 68±5 | 65±5 | 65±6 | 59±5 | 68±4 | 66±5 |
| HVC (U) | 35±2 | 32±2 | 39±3 | 37±2 | 45±5 | 46±5 | 48±3 | 47±4 |
Abbreiatons: H, hindquarters: R, renal; M, mesenteric; SD, Sprague–Dawley; VC, vascular conductance.
Values are mean±s.e.mean; n=8 in each group.
Units for VC are (kHz mm Hg−1) 103.
Resting arterial blood pressures were higher (P<0.05) in Wistar rats than in Sprague–Dawley rats (see also Gaudreault et al., 2001) but there were no other between-strain differences in cardiovascular variables (Table 1). Immediately following administration of parecoxib there was a transient reduction in mesenteric Doppler shift (approximately 20% change, <2 min duration, data not shown) but neither parecoxib nor vehicle had any sustained effects in either strain of rat (Table 1).
Effects of L-NAME in the absence and presence of parecoxib
Administration of L-NAME caused an increase in blood pressure accompanied by bradycardia and widespread vasoconstriction in both strains of rat. The integrated (0–60 min) increase in blood pressure, bradycardia and fall in hindquarters vascular conductances were significantly (P<0.05, Mann–Whitney U-test) greater in Wistar rats than in Sprague–Dawley rats (Table 2). Pretreatment with parecoxib had no significant effect on responses to L-NAME in either strain of rat (Table 2).
Table 2.
Integrated (0–60 min) cardiovascular responses to L-NAME
| Strain | SD | SD | Wistar | Wistar |
|---|---|---|---|---|
| Treatment | Vehicle | Parecoxib | Vehicle | Parecoxib |
| Heart rate (beats) | −2546±430 | −2495±273 | −3661±219* | −3711±523* |
| BP (mm Hg min) | +1473±165 | +1556±141 | +1928±133* | +1879±146* |
| RVC (% min) | −2051±188 | −2470±246 | −2437±172 | −2664±218 |
| MVC (% min) | −2523±158 | −2924±204 | −2813±94 | −2771±152 |
| HVC (% min) | −2107±174 | −2105±266 | −2596±133* | −2732±162* |
Abbreiatons: H, hindquarters; M, mesenteric, R, renal; VC, vascular conductance.
Values are mean±s.e. mean; n=8 in each group.
P<0.05 vs corresponding Sprague–Dawley (SD) group (Kruskal–Wallis test).
Effects of parecoxib and/or L-NAME on responses to exendin-4
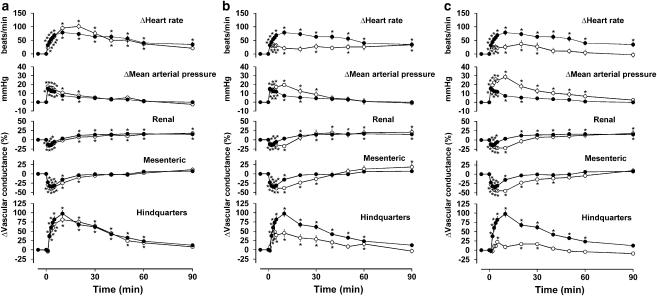
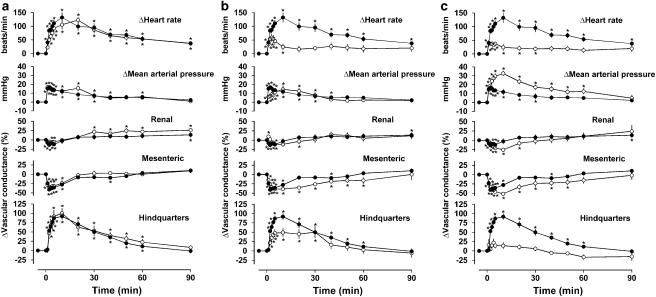
In the presence of vehicle and saline, exendin-4 caused cardiovascular changes as described previously (Gardiner et al., 2006) that is, a tachycardia, rise in blood pressure, mesenteric vasoconstriction and hindquarters vasodilatation; the changes in the renal vascular bed were smaller and less consistent, but generally comprised an initial vasoconstriction followed by vasodilatation (Figures 1 and 2). There were no qualitative or quantitative differences between the integrated (0–90 min) cardiovascular responses to exendin-4 in Sprague–Dawley and Wistar rats (Figures 1 and 2 and Table 3).
Figure 1.
Regional haemodynamic effects of i.v. bolus doses of exendin-4 (250 ng kg−1) in conscious Sprague–Dawley rats (n=8 in each group) in the presence of vehicle (a–c, closed symbols) or parecoxib (a, open symbols), L-NAME (b, open symbols) or parecoxib plus L-NAME (c, open symbols). Values are mean and vertical bars show s.e.m.. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in Table 3.
Figure 2.
Regional haemodynamic effects of i.v. bolus doses of exendin-4 (250 ng kg−1) in conscious Wistar rats (n=8 in each group) in the presence of vehicle (a–c, losed symbols) or parecoxib (a, open symbols), L-NAME (b, open symbols) or parecoxib plus L-NAME (c, open symbols). Values are mean and vertical bars show s.e.m. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in Table 3.
Table 3.
Integrated (0–90 min) cardiovascular responses to exendin-4
| Strain | SD | SD | SD | SD |
|---|---|---|---|---|
| Treatment | Vehicle | Parecoxib | L-NAME | Parecoxib+L-NAME |
| Heart rate (beats) | +4903±687 | +5045±631 | +2665±629a,b | +2000±540a,b |
| BP (mm Hg min) | +463±73 | +533±79 | +756±176 | +1064±198a,b |
| MVC (% min) | −751±193 | −959±215 | −1234±282a,b | −1436±284a,b |
| HVC (% min) | +3958±380 | +3663±376 | +2082±470a,b | +750±167a,b,c |
| Strain | Wistar | Wistar | Wistar | Wistar |
| Treatment | Vehicle | Parecoxib | L-NAME | Parecoxib+L-NAME |
| Heart rate (beats) | +6600±792 | +6217±713 | +2441±660a,b | +2165±497a,b |
| BP (mm Hg min) | +665±128 | +711±130 | +616±130 | +1435±238a,b,c |
| MVC (% min) | −846±154 | −659±173 | −2165±447a,b | −2204±393a,b |
| HVC (% min) | +3250±380 | +3762±376 | +2330±403a,b | +894±177a,b,c |
Abbreviations: H, hindquarters; M, mesenteric; SD, Sprague–Dawley; VC,vascular conductance.
Values are mean±s.e. mean; n=8 in each group.
Renal vascular responses have not been included since they were generally small and inconsistent (see Results).
P<0.05 vs vehicle
P<0.05 vs parecoxib
P<0.05 vs L-NAME (Kruskal–Wallis test).
Pretreatment with parecoxib alone had no significant effect on responses to exendin-4 in either strain of rat (Figures 1a and 2a and Table 3). Pretreatment with L-NAME alone reduced the tachycardic and hindquarters vasodilator effects of exendin-4, but the integrated fall in mesenteric vascular conductance was enhanced due to a prolongation of effect, rather than an increase in the maximum response (Figures 1b and 2b and Table 3). In the presence of parecoxib together with L-NAME, the hindquarters vasodilator effect of exendin-4 was almost abolished and the pressor response was substantially enhanced (Figures 1 and 2 and Table 3). The effects of L-NAME in the absence and presence of parecoxib on responses to exendin-4 were similar in both strains of rat.
Discussion and conclusions
Understanding the cardiovascular consequences of cyclooxygenase-2 inhibition is of relevance because of the recent concern surrounding the cardiovascular risk associated with the use of cyclooxygenase-2 inhibitors, although the explanation for the increased risk is not entirely clear and may be related to more chronic conditions (see Antman et al., 2005, Grosser et al., 2006). Nevertheless, since cyclooxygenase-2 is a major source of systemic prostacyclin biosynthesis in healthy humans (McAdam et al., 1999), it is not unreasonable to hypothesize that acute cyclooxygenase-2 inhibition may have cardiovascular effects. Thus, the first aim of the present study was to examine possible interactions between the cyclooxygenase-2 system and the NO system with respect to baseline cardiovascular status.
There are reports of pressor effects of chronic cyclooxygenase-2 inhibition (Muscará et al., 2000; Höcherl et al., 2002), although others have found no effect on blood pressure (Qi et al., 2002), or even an hypotensive response (Knight and Johns, 2005). Acute administration of cyclooxygenase-2 inhibitors is generally reported to be without effect on blood pressure (e.g., Leach et al., 1998; Beierwaltes, 2002; Qi et al., 2002; Bulut et al., 2003; Widlansky et al., 2003; Sivarajah et al., 2005; Adami et al., 2006). However, we speculated it was feasible that, in the absence of any effect on blood pressure, there could have been opposing changes in conductance in different vascular beds, or subtle changes confined to a single vascular bed, as demonstrated by Qi et al. (2002). They showed an effect of cyclooxygenase-2 inhibition on renal medullary blood flow under conditions where there was no effect on blood pressure, albeit in anaesthetized mice. However, it was clear from our data that acute inhibition of cyclooxygenase-2 with parecoxib had no effect on any cardiovascular variable measured, suggesting that, under basal conditions, cyclooxygenase-2-derived prostanoids do not affect regional vascular tone in conscious, normotensive, Sprague–Dawley or Wistar rats.
In the light of the various interactions between the cyclooxygenase-2 system and the NO system (see Introduction), we next tested the hypothesis that the cardiovascular effects of acute NOS inhibition would be influenced by pretreatment with a cyclooxygenase-2 inhibitor. Others have shown that chronic (3 week) treatment with the cyclooxygenase-2 inhibitor, celecoxib, caused a slight, but not significant, upregulation of endothelial NOS and improved endothelium-dependent relaxation in salt-induced hypertension; interestingly the same effects were not seen with a different cyclooxygenase-2 inhibitor, namely, rofecoxib (Hermann et al., 2003). Furthermore, it has been shown that impaired flow-mediated vasodilatation in patients with essential hypertension is improved as soon as 3 h after an oral dose of parecoxib (Widlansky et al., 2003), although whether or not this involved any interaction with the NO system is unknown. In addition, in vitro studies have shown that inhibition of cyclooxygenase-2 caused a marked attenuation of noradrenaline-induced contraction of preparations of superior mesenteric arteries of rats by a mechanism involving NO (Bratz and Kanagy, 2004). However, our results clearly show no effect of pretreatment with parecoxib on the cardiovascular responses to L-NAME, suggesting that, under these acute conditions, inhibition of cyclooxygenase-2 is not associated with either upregulation or downregulation of NOS activity in vivo.
We used two different outbred strains of rat, as differences in cardiovascular responses to NOS inhibition have been reported in different strains of rat (Forster et al., 2001), and even in the same strain of rat from different commercial suppliers (Pollock and Rekito, 1998). In the present study it was shown that Wistar rats, which had modestly elevated resting BPs relative to Sprague–Dawley rats, also tended to show larger responses to L-NAME although the differences were not great. This finding is consistent with the studies of Pollock and Rekito (1998) and Forster et al. (2001) in which it was also observed that the rat strain with the higher resting blood pressure showed the larger response to L-NAME, suggesting increased activity of the NO system in response to the high blood pressure. In contrast, it has been reported that, within a particular rat strain, the hypertensive response to NOS inhibition is inversely related to the resting BP (Sorrentino and Pinto, 1997; Pollock and Rekito, 1998), indicating a relative deficiency in NO may be causally associated with the high blood pressure.
In the final part of the study, we assessed the effects of parecoxib and L-NAME, alone and in combination, on the cardiovascular responses to the GLP-1 receptor agonist, exendin-4. We have recently described pressor and mesenteric vasoconstrictor actions of i.v. exendin-4, together with hindquarters vasodilatation and tachycardia and, showed a major involvement of β-adrenoceptors in the hindquarters vasodilator and tachycardic effects (Gardiner et al., 2006). Our previous study was in Sprague–Dawley rats and here we show that the effects of exendin-4 are very similar in Wistar rats. The relatively long duration of the cardiovascular effects of exendin-4 are notable in the context of the short plasma half-life (less than 18 min) of the peptide following intravenous administration in rats (Parkes et al., 2001), indicating prolonged receptor binding and/or activation of secondary mechanisms.
We were not surprised that we found no effect of parecoxib alone on the responses to exendin-4, since we know of no evidence implicating prostanoids in any of the reported effects of this family of ‘incretin mimetics' (Drucker, 2001; Knudsen, 2004). Moreover, the inhibitory effects of L-NAME alone on the hindquarters vasodilator responses to exendin-4 were not entirely unexpected, given that, in vitro, the relaxant effect of GLP-1 in pulmonary arteries has been shown to be NO-dependent (Golpon et al., 2001), although in femoral arteries the relaxation is reportedly endothelium-independent (Nyström et al., 2005). In vivo, short-term infusion of GLP-1 increases flow-mediated vasodilatation in patients with type-2 diabetes mellitus, possibly through an effect on NOS (Nyström et al., 2004). However, a more likely explanation of our findings relates to the fact that there is now good evidence for an involvement of NO in β-adrenoceptor-mediated vasodilatation in vivo (Gardiner et al., 1991; see Ritter et al. (2006) for review). As we have shown complete inhibition of the hindquarters vasodilator effect of exendin-4 by propranolol (Gardiner et al., 2006), it is likely that the effect of L-NAME was due to inhibition of β-adrenoceptor-mediated hindquarters vasodilatation.
Treatment with L-NAME not only inhibited the hindquarters vasodilator effect of exendin-4, but also caused a substantial reduction in its tachycardic effect. These findings are consistent with previous results of ours (Gardiner et al., 1991), and others (Reid et al., 1994, Schmetterer et al., 1999), showing that NOS inhibition reduces the tachycardic response to β-adrenoceptor agonists. As we demonstrated previously that the adrenal medulla was the source of the catecholamines involved in the β-adrenoceptor-mediated effects of exendin-4 (Gardiner et al., 2006), it is feasible that some of the effect of L-NAME may have been to reduce adrenal medullary catecholamine secretion. However, recent evidence suggests that NOS inhibition increases rather than decreases adrenomedullary catecholamine release (Vicente et al., 2005).
An additional effect of L-NAME was to prolong the vasoconstrictor action of exendin-4, suggesting that, normally, release of NO acts to oppose that effect. However, a similar effect of L-NAME has been seen on vasoconstrictor responses to angiotensin II (Gardiner et al., unpublished observations), suggesting that such an action is not unique to exendin-4.
In the light of the absence of effect of parecoxib alone on the responses to exendin-4 (see above), the most unexpected finding in the present study was the additional effect on responses to exendin-4 when parecoxib was given in combination with L-NAME. Under those conditions, there was almost complete inhibition of the hindquarters vasodilatation and the pressor effect of exendin-4 was increased substantially, probably due to the loss of the buffering effect of the hindquarters vasodilatation (Gardiner et al., 2006). As parecoxib is the water-soluble prodrug of valdecoxib, one possible interpretation of these findings is that NO in some way influences the bioactivation of parecoxib such that, in the absence of NO (i.e., in the presence of L-NAME), the extent of cyclooxygenase inhibition is more effective. However, we feel this is an unlikely explanation since the chosen dose (10 mg kg−1) is maximally effective in several models of inflammation (Padi et al., 2004), yet parecoxib given alone clearly had no effect on responses to exendin-4.
These findings indicate that, in spite of a lack of an apparent involvement of cyclooxygenase-2 in controlling cardiovascular status in the presence of L-NAME, cyclooxygenase-2-derived products do contribute to the hindquarters vasodilator response to exendin-4 under these conditions, where the latter effect is due to adrenal medullary adrenaline release (Gardiner et al., 2006).
There is evidence for cross-talk between the NO and cyclooxygenase systems from both in vitro and in vivo experiments (see Introduction). Of relevance to the present findings, it has been shown that inhibition of NOS (i) increases shear-induced prostacyclin release from cultured endothelial cells (Osanai et al., 2000), (ii) increases prostacyclin production in human isolated saphenous vein preparations (Barker et al., 1996), and (iii) unmasks cyclooxygenase-2-dependent renal vasodilatation in anaesthetized rats (Beierwaltes, 2002). Furthermore, flow-induced vasodilatation is preserved in endothelial NOS knockout mice due to an increased contribution from vasodilator prostanoids (Sun et al., 1999). Also, in man, combined inhibition of NOS and cyclooxygenase have been shown to augment sympathetic vasoconstriction in contracting skeletal muscle, when neither NOS inhibition nor cyclooxygenase inhibition alone was effective (Dinenno and Joyner, 2004). Thus, it is suggested that there is normally a suppressant action of NO on prostacyclin production, which is cGMP-dependent, possibly involving GTP-binding proteins (Osanai et al., 2000), such that in the presence of reduced NO production, prostacyclin release is enhanced. From the present experiments it is feasible that in the presence of L-NAME, enhanced cyclooxygenase-2-dependent prostacyclin production contributes to the vasodilator effect of exendin-4.
Whatever the underlying mechanisms, in the context of the possible clinical relevance of these findings (see Introduction), it may be that the propensity of GLP-1 receptor agonists, such as exendin-4, to increase systemic arterial blood pressure could be enhanced in the presence of vascular dysfunction which impairs both cyclooxygenase-2 and NO-mediated mechanisms.
Abbreviations
- BP
mean arterial blood pressure
- GLP
glucagon-like peptide
- HDAS
haemodynamic data acquisition system
- L-NAME
NG nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
Conflict of interest
The authors state no conflict of interest.
References
- Adami M, Coppelli G, Guaita E, Pozzoli C, Menozzi A, Giovannini E, et al. Effects of cyclooxygenase-1 and -2 inhibition on gastric acid secretion and cardiovascular functions in rats. Pharmacology. 2006;76:84–92. doi: 10.1159/000089834. [DOI] [PubMed] [Google Scholar]
- Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- Baber SR, Champion HC, Bivalacqua TJ, Hyman AL, Kadowitz PJ. Role of cyclooxygenase-2 in the generation of vasoactive prostanoids in the rat pulmonary and systemic vascular beds. Circulation. 2003a;108:896–901. doi: 10.1161/01.CIR.0000084536.87322.BB. [DOI] [PubMed] [Google Scholar]
- Baber SR, Deng W, Rodriguez J, Master RG, Bivalacqua TJ, Hyman AL, et al. Vasoactive prostanoids are generated from arachidonic acid by COX-1 and COX-2 in the mouse. Am J Physiol. 2005;289:H1476–H1487. doi: 10.1152/ajpheart.00195.2005. [DOI] [PubMed] [Google Scholar]
- Baber SR, Hyman AL, Kadowitz PJ. Role of COX-1 and -2 in prostanoid generation and modulation of angiotensin II responses. Am J Physiol. 2003b;285:H2399–H2410. doi: 10.1152/ajpheart.00294.2003. [DOI] [PubMed] [Google Scholar]
- Barker JE, Bakhle YS, Anderson J, Treasure T, Piper PJ. Reciprocal inhibition of nitric oxide and prostacyclin synthesis inhuman saphenous vein. Br J Pharmacol. 1996;118:643–648. doi: 10.1111/j.1476-5381.1996.tb15449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierwaltes WH. Cyclooxygenase-2 products compensate for inhibition of nitric oxide regulation of renal perfusion. Am J Physiol. 2002;283:F68–F72. doi: 10.1152/ajprenal.00364.2001. [DOI] [PubMed] [Google Scholar]
- Bratz IN, Kanagy NL. Nitric oxide synthase-inhibition is associated with altered endothelial cyclooxygenase function. Am J Physiol. 2004;287:H2394–H2401. doi: 10.1152/ajpheart.00628.2004. [DOI] [PubMed] [Google Scholar]
- Bulut D, Liaghat S, Hanefeld C, Koll R, Miebach T, Mügge A. Selective cyclo-oxygenase-2 inhibition with parecoxib acutely impairs endothelium-dependent vasodilatation in patients with essential hypertension. J Hypertens. 2003;21:1663–1667. doi: 10.1097/00004872-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- Forster J, Beebe P, Wang H, Wood JG. The effect of nitric oxide inhibition on blood pressure depends on rat strain. J Surg Res. 2001;96:218–223. doi: 10.1006/jsre.2001.6087. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Bennett T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to adrenaline or BRL 38227 in conscious rats. Br J Pharmacol. 1991;104:731–737. doi: 10.1111/j.1476-5381.1991.tb12496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- Gaudreault N, Santuré M, Pitre M, Nadeau A, Marette A, Bachelard H. Effects of insulin on regional blood flow and glucose uptake in Wistar and Sprague–Dawley rats. Metabolism. 2001;50:65–73. doi: 10.1053/meta.2001.18569. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann M, Camici G, Fratton A, Hurlimann D, Tanner FC, Hellermann JP, et al. Differential effects of selective cyclooxygenase-2 inhibitors on endothelial function in salt-induced hypertension. Circulation. 2003;108:2308–2311. doi: 10.1161/01.CIR.0000101683.30157.0B. [DOI] [PubMed] [Google Scholar]
- Höcherl K, Endemann D, Kammerl MC, Grobecker HF, Kurtz A. Cyclo-oxygenase-2 inhibition increases blood pressure in rats. Br J Pharmacol. 2002;136:1117–1126. doi: 10.1038/sj.bjp.0704821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S, Johns EJ. Effect of COX inhibitors and NO on renal hemodynamics following ischemia-reperfusion injury in normotensive and hypertensive rats. Am J Physiol. 2005;289:F1072–F1077. doi: 10.1152/ajprenal.00430.2004. [DOI] [PubMed] [Google Scholar]
- Knudsen LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–4134. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- Leach M, Hamilton LC, Olbrich A, Wray GM, Thiemermann C. Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br J Pharmacol. 1998;124:586–592. doi: 10.1038/sj.bjp.0701869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimattila S, Yki-Jarvinen H. Endothelial dysfunction in human diabetes. Curr Diabetes Rep. 2002;2:26–36. doi: 10.1007/s11892-002-0054-x. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscará MN, Vergnolle N, Lovren F, Triggle CR, Elliott SN, Asfaha S, et al. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br J Pharmacol. 2000;129:1423–1430. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells. Close link between PGI2 signal and NO pathways. Circ Res. 2003;93:523–530. doi: 10.1161/01.RES.0000091336.55487.F7. [DOI] [PubMed] [Google Scholar]
- Nyström T, Gonon AT, Sjöholm Å, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, et al. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol. 2000;278:H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- Padi SSV, Jain NK, Singh S, Kulkarni SK. Pharmacological profile of parecoxib: a novel, potent injectable selective cyclooxygenase-2 inhibitor. Eur J Pharmacol. 2004;491:69–76. doi: 10.1016/j.ejphar.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, et al. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–267. [Google Scholar]
- Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol. 1998;275:R1719–R1723. doi: 10.1152/ajpregu.1998.275.5.R1719. [DOI] [PubMed] [Google Scholar]
- Qi Z, Hao C-M, Langenbach RI, Breyer RM, Redha R, Morrow JD, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IA, Bui H, Chou L. Role of nitric oxide in the renin and heart rate response to β-adrenergic stimulation. Hypertension. 1994;23 Suppl 1:I-49–I-53. doi: 10.1161/01.hyp.23.1_suppl.i49. [DOI] [PubMed] [Google Scholar]
- Ritter JM, Ferro A, Chowienczyk PJ. Relation between beta-adrenoceptor stimulation and nitric oxide synthesis in vascular control. Eur J Clin Pharmacol. 2006;62 suppl:109–113. [Google Scholar]
- Schmetterer L, Dallinger S, Polak K, Eicher H-G, Wolzt M. Systemic NO synthase inhibition blunts the chronotropic, but not the inotropic response to isoprenaline in man. Nitric Oxide. 1999;3:209–215. doi: 10.1006/niox.1999.0223. [DOI] [PubMed] [Google Scholar]
- Sivarajah A, McDonald MC, Thiemermann C. The cardioprotective effects of preconditioning with endotoxin, but not ischemia, are abolished by a peroxisome proliferator-activated receptor-γ antagonist. J Pharmacol Exp Ther. 2005;313:896–901. doi: 10.1124/jpet.104.080598. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Pinto A. The increase in blood pressure induced by inhibition of nitric oxide in anesthetised Wistar rats is inversely related to basal blood pressure value. J Cardiovasc Pharmacol. 1997;29:599–604. doi: 10.1097/00005344-199705000-00006. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, et al. N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl]sulfonyl]propanamide, sodium salt, parecoxib sodium: a potent and selective inhibitor of COX-2 for parenteral administration. J Med Chem. 2000;43:1661–1663. doi: 10.1021/jm000069h. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vicente S, Figueroa S, Pérez-Rodríguez R, González MP, Oset-Gasque MJ. Nitric oxide donors induce calcium-mobilisation from internal stores but do not stimulate catecholamine secretion by bovine chromaffin cells in resting conditions. Cell Calcium. 2005;37:163–172. doi: 10.1016/j.ceca.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Price DT, Gokce N, Eberhardt RT, Duffy SJ, Holbrook M, et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension. 2003;42:310–315. doi: 10.1161/01.HYP.0000084603.93510.28. [DOI] [PubMed] [Google Scholar]