Abstract
Background and purpose:
Although azelnidipine is used clinically to treat hypertension its effects on its target cells, Ca2+ channels, in smooth muscle have not been elucidated. Therefore, its effects on spontaneous contractions and voltage-dependent L-type Ca2+ channels were investigated in guinea-pig portal vein.
Experimental approach:
The inhibitory potency of azelnidipine on spontaneous contractions in guinea-pig portal vein was compared with those of other dihydropyridine (DHP)-derived Ca antagonists (amlodipine and nifedipine) by recording tension. Also its effects on voltage-dependent nifedipine-sensitive inward Ba2+ currents (I Ba) in smooth muscle cells dispersed from guinea-pig portal vein were investigated by use of a conventional whole-cell patch-clamp technique.
Key results:
Spontaneous contractions in guinea-pig portal vein were reduced by all of the Ca antagonists (azelnidipine, K i=153 nM; amlodipine, K i=16 nM; nifedipine, K i=7 nM). In the whole-cell experiments, azelnidipine inhibited the peak amplitude of I Ba in a concentration- and voltage-dependent manner (-60 mV, K i=282 nM; −90 mV, K i=2 μM) and shifted the steady-state inactivation curve of I Ba to the left at −90 mV by 16 mV. The inhibitory effects of azelnidipine on I Ba persisted after 7 min washout at −60 mV. In contrast, I Ba gradually recovered after being inhibited by amlodipine, but did not return to control levels. Both azelnidipine and amlodipine caused a resting block of I Ba at -90 mV. Only nifedipine appeared to interact competitively with S(-)-Bay K 8644.
Conclusions and implications:
These results suggest that azelnidipine induces long-lasting vascular relaxation by inhibiting voltage-dependent L-type Ca2+ channels in vascular smooth muscle.
Keywords: antihypertensive drugs, Ca antagonists, dihydropyridine-derivatives, L-type Ca2+ channels, vascular smooth muscle
Introduction
Various types of antihypertensive drugs are used clinically to control blood pressure and prevent cerebrovascular and cardiovascular complications, such as stroke, coronary heart disease and heart failure (reviewed by Thijs et al., 2004). Ca antagonists have been widely used for the treatment of hypertension as they reliably induce hypotensive effects with few adverse reactions (Salvetti and Di Venanzio, 1994). Moreover, it has been found that Ca antagonists are more effective than any other class of antihypertensive drugs, especially in the prevention of stroke (Staessen et al., 1997). Thus, a wide variety of Ca antagonists, including short- and long-acting Ca antagonists, have been synthesized for clinical use (reviewed by Romero et al., 2003). However, short-acting Ca antagonists readily cause a rapid reduction of blood pressure, increasing the risk of ischaemic heart disease and reflex tachycardia (Furberg et al., 1995; Psaty et al., 1995). Therefore, long-acting Ca antagonists are mainly used to treat hypertension (Alderman et al., 1997). Azelnidipine ((+)-3-(1-diphenylmethylazetidin-3-yl)-5-isopropyl 2-amino-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridine-dicarboxylate; CS-905, Calblock) is a newly developed long-acting dihydropyridine (DHP)-derivative Ca antagonist (Oizumi et al., 1989). A single oral dose of azelnidipine causes a slow but long-lasting hypotensive effect with little reflex tachycardia (Kuramoto et al., 2003). Azelnidipine has been widely used for the clinical treatment of hypertension (Arita et al., 1999). However, surprisingly, the target Ca2+ channels for azelnidipine in native vascular smooth muscle cells have not yet been investigated. Furthermore, the specific inhibitory mechanisms responsible for the effects of azelnidipine on voltage-dependent Ca2+ channels in native vascular smooth muscle have not been elucidated. Voltage-dependent Ca2+ channels are one of the major Ca2+ influx pathways known to have an important role in the initiation of the contraction of vascular smooth muscle and Ca antagonists selectively suppress Ca2+ influx by blocking voltage-dependent Ca2+ channels in vascular smooth muscle (reviewed by McFadzean and Gibson, 2002).
In the present experiments, therefore, the effects of azelnidipine were firstly studied on the spontaneous contractions of muscle strips prepared from guinea-pig portal vein. Tension measurements were undertaken and the inhibitory potency of azelnidipine was compared with those of other well-known DHP-derivative Ca antagonists (amlodipine and nifedipine). Secondly, the effects of azelnidipine were investigated on voltage-dependent nifedipine-sensitive macroscopic Ba2+ currents (IBa, i.e., L-type Ca2+ channels) in single, freshly dispersed vascular smooth muscle myocytes from guinea-pig portal vein using patch-clamp techniques. Thirdly, the inhibitory potency of azelnidipine on IBa was compared with those of other well-known DHP-derived Ca antagonists (amlodipine and nifedipine).
Methods
Cell dispersion
Guinea-pigs of either sex were stunned, exsanguinated and the portal vein was removed. Briefly, the vascular smooth muscle was isolated and immersed in nominally Ca2+-free solution (mM): Na+ 140, K+ 5, Mg2+ 0.5, Cl− 146, HEPES 10/Tris, titrated to pH 7.35–7.40. Guinea-pig vascular myocytes were freshly isolated under microscope by the gentle tapping method after treatment with collagenase (Type IA, 1–2 mg ml−1; Sigma Chemical K.K., Tokyo, Japan), as described previously (Teramoto and Brading, 1996; Teramoto et al., 1996). Relaxed spindle-shaped cells were isolated and stored at 4°C. The dispersed cells were used within 4–5 h for experiments.
Recording procedure
Patch-clamp experiments were performed at room temperature (21–23°C), as described previously (Teramoto et al., 2005). Junction potentials between bath and pipette solutions were measured with a 3 M KCl reference electrode and were <2 mV, so that correction for these potentials was not necessary. Capacitance noise was kept to a minimum by maintaining the test solution in the electrode as low as possible.
Drugs and solutions
To record voltage-dependent Ba2+ currents (IBa) in whole-cell configuration, pipettes containing a high concentration of caesium were used, the composition of the pipette solutions was (mM): Cs+ 130, tetraethyl ammonium (TEA+) 10, Mg2+ 2, Cl− 144, glucose 5, EGTA 5, ATP 5, HEPES 10/Tris (pH 7.35–7. 40). The bath solution contained (mM): Ba2+ 10, TEA+, 135, Cl− 155, glucose 10, HEPES 10/Tris (pH 7.35–7.40). The bath solution was superfused by gravity throughout the experiments at a rate of 2 ml min−1. All drugs were obtained from Sigma Chemical (Tokyo, Japan). Nifedipine, amlodipine, S(-)-Bay K 8644 and azelnidipine (kindly provided by the Sankyo Pharmaceutical Co. Ltd., Tokyo, Japan) were prepared as 100 mM stock solutions in dimethyl sulphoxide (DMSO). The final concentration of DMSO was <0.3%, and this concentration was shown to have no effect on IBa in guinea-pig portal vein.
Data analysis
The whole-cell current data were low-pass filtered at 1 kHz by an eight pole Bessel filter, sampled at 1 ms and analysed on a computer (PowerMac G4, Tokyo, Japan) by the commercial software ‘MacLab 3.5.6' (ADInstruments Pty Ltd., Castle Hill, Australia). Data are expressed as mean with the standard deviation (s.d.). In order to obtain precise components of IBa, the method for subtraction of the leak and capacitive currents was performed to subtract IBa in the presence of 100 μM Cd2+ from IBa (Teramoto et al., 2005).
The peak amplitude of IBa, elicited by a step pulse to +10 mV from the holding potential just before the application of a drug, was normalized to one. The curves were drawn by fitting the following equation, using the least-squares method:
where Ki, D and nH are the inhibitory dissociation constant, concentration of drug (nM or μM) and Hill coefficient, respectively.
Conditioning pulses of various amplitudes were applied (up to +30 mV, 10 s duration) before application of the test pulse (to +10 mV, 1 s duration) at a holding potential of −90 mV. An interval of 20 ms was allowed between these two pulses to estimate possible contamination by capacitive current. The peak amplitude of IBa evoked by each test pulse was measured before and after application of drugs. The peak amplitude of IBa in the absence and presence of drugs without application of any conditioning pulse was normalized to one. The lines were draw by fitting the data to the following equation using the least-squares method,
where I, Imax, V, Vhalf, k and C are the relative amplitude of IBa (I) observed at various amplitudes of conditioning pulse, that observed (Imax) with a −90 mV conditioning pulse, amplitude of the conditioning pulse (V), conditioning pulse amplitude (Vhalf) which evokes IBa of amplitude half Imax, slope factor (k) and fraction of the non-inactivating component of IBa (C).
Activation curves were derived from the current–voltage relationships. Conductance (G) was calculated from the equation G=IBa/(Em−EBa), where IBa is the peak current elicited by depolarizing test pulses to +40 mV from a holding membrane potential of −60 mV and EBa is the equilibrium potential for Ba2+. Gmax is the maximal Ba2+ conductance (calculated at potentials above +10 mV). Values for G/Gmax were plotted against membrane potential as relative amplitude.
The dissociation constant for drug binding to the channel to produce the inactivated state was estimated from the shift of the voltage-dependent inactivation curve and the concentration–response curve obtained in the resting state by using the following equation (Uehara and Hume, 1985),
where ΔVhalf is the amplitude of the shift of the voltage-dependent inactivation curve, k is a slope factor for the inactivation curve and [D] is the concentration of azelnidipine applied. Kinact and Krest are dissociation constants of azelnidipine for the inactivated and the resting states of voltage-dependent Ca2+ channels, respectively.
Tension measurement
To measure tension in the vascular smooth muscle, modified Krebs solution was used (mM): Na+ 137, K+ 5.9, Mg2+ 1.2, Ca2+ 2.5, Cl− 133.7, HCO3− 15.4, H2PO4− 1.2 and glucose 11.5, which was bubbled with 97% O2 and 3% CO2. Fine strips were prepared as described previously (Teramoto et al., 1996). An initial tension equivalent to 0.5 g weight was applied to each strip. Strips were then allowed to equilibrate for approximately 1.5–2 h while basal vascular tone developed and became stable (37°C). Data were recorded on a Macintosh computer running ‘MacLab 3.5.6' software (ADInstruments Pty Ltd., Castle Hill, Australia). Tension is expressed as mN mg−1 of tissue.
Statistics
Statistical analyses were performed with a paired t-test (two-factor with replication). Changes were considered significant when P<0.01.
Results
Effects of azelnidipine and other dihydropyridine-derivative Ca antagonists on the spontaneous contractions of guinea-pig portal vein
As shown in Figure 1a, the muscle strips of guinea-pig portal vein produced spontaneous contractions with a range of amplitudes and frequencies. Application of azelnidipine (⩾10 nM, 20 min duration) gradually inhibited the amplitudes but not the frequency of spontaneous contractions (n=5). Cumulative doses of azelnidipine caused a concentration-dependent inhibition of the spontaneous contractions. The integrated area of the spontaneous contractions was normalized to one just before the application of azelnidipine (i.e. control, 3 min duration) and a relative value for the spontaneous contractions was measured for each concentration of azelnidipine. Similarly, nifedipine (⩾1 nM, 20 min duration) and amlodipine (⩾10 nM, 20 min duration) were cumulatively applied. Figure 1b summarizes the concentration-dependent inhibitory effect of several dihydropyridine (DHP)-derivative Ca antagonists on the relative value of the spontaneous contractions (azelnidipine, Ki=153 nM; amlodipine, Ki=16 nM; nifedipine, Ki=7 nM).
Figure 1.
Electrophysiological properties of IBa in guinea-pig portal vein
As the mechanism of the spontaneous contractions in guinea-pig portal vein involves the activation of voltage-dependent Ca2+ channels (Teramoto et al., 1996), the electrophysiological properties of Ca2+ inward currents in guinea-pig portal vein myocytes were characterized using whole-cell recordings. In the present experiments, Ba2+ (10 mM) was used as a charge carrier in the bath solution and to isolate voltage-dependent inward currents through Ca2+ channels, other Ca2+-activated mechanisms (such as Ca2+-activated K+ currents, Ca2+-activated Cl− currents etc.) were inhibited, by filling the pipette with a Cs+-TEA+ solution containing 5 mM EGTA. Depolarizing step pulses (10 mV increments from −40 to +40 mV for 1 s duration, every 20 s) were applied from a holding potential of −90 mV by use of patch-clamp techniques. As shown in Figure 2a, at potentials more positive than −30 mV a IBa was evoked; this reached a peak and then gradually decayed. The amplitude of this current was voltage-dependent; the maximum peak amplitude was observed at approximately +10 mV and then was reduced at more positive potentials (Figure 2b). Figure 2b shows the current–voltage relationships of IBa obtained at −40 and −90 mV (n=5). When the holding potential was shifted to −40 mV in the same myocytes, both the peak amplitude and the amplitude at the end of the command pulse were smaller, but the time course of the current decay was identical at both holding potentials (Figure 2a). Similar results were obtained in four other cells.
Figure 2.
Inward IBa recorded by application of depolarizing pulses at two different holding potentials (−40 and −90 mV) in guinea-pig portal vein. Whole-cell recording, pipette contained Cs+-TEA+ solution plus 5 mM EGTA and the bath solution contained 10 mM Ba2+ and 135 mM TEA+. (a) (i) IBa at each indicated depolarizing potential from both holding potentials superimposed. (ii) IBa from (i) scaled to match their peak amplitudes and superimposed. (b) Current–voltage relationships of IBa obtained at −40 and −90 mV. Each symbol indicates the mean of five observations with ±s.d. shown by vertical lines. The curves were drawn by eye.
Inhibitory effects of azelnidipine on IBa
In the conventional whole-cell experiments, the IBa evoked by a depolarizing pulse to +10 mV from a holding potential of −60 mV increased slightly in amplitude; the peak currents reached steady state approximately 4 min after the rupture of the membrane patch. This peak value was then maintained for at least 35 min (the peak amplitude of IBa at 35 min being 98±2%, n=15) when test depolarization pulses (1 s duration) were applied at 20 s intervals. Consequently, all experiments were performed within 35 min after the peak amplitude of IBa, evoked by a depolarizing pulse from a holding potential of −60 mV, had become stable.
Figure 3a shows the time course of the effect of azelnidipine (300 nM and 1 μM, applied cumulatively) on IBa evoked by a depolarizing pulse to +10 from −60 mV. The depolarizing pulses were applied every 20 s. Azelnidipine (300 nM) reduced the peak amplitude of IBa. On removal of azelnidipine, the peak amplitude of IBa did not return to the control level. Subsequent application of a high concentration of azelnidipine (1 μM) further inhibited the peak amplitude of IBa and 10 μM nifedipine suppressed IBa further to zero.
Figure 3.
Effects of azelnidipine and nifedipine on IBa in guinea-pig portal vein. (a) The time course of the effect of azelnidipine and nifedipine on the peak amplitude of IBa evoked by repetitive depolarizing pulses to +10 mV from a holding potential of −60 mV. Time 0 indicates the time when 300 nM azelnidipine was applied to the bath. (b) Original current traces before (control, (i)) and after application of azelnidipine (300 nM, (ii); 1 μM, (iv)) as indicated in (a). (v) indicates a current trace just before application of 10 μM nifedipine.
To investigate the long-lasting inhibitory effects of azelnidipine on IBa further, two different concentrations of azelnidipine were applied to different myocytes. Figure 4a shows the time course of the effects of azelnidipine (100 and 300 nM) on IBa evoked by a depolarizing pulse to +10 mV from a holding potential of −60 mV. Azelnidipine gradually reduced the peak amplitude of IBa, but it became stable within a few min (100 nM, 0.67±0.03, n=6; 300 nM, 0.49±0.06, n=6). On removal of azelnidipine, the azelnidipine-induced inhibitory effects on IBa still remained even after 7 min of washout (Figure 4b). Subsequent application of nifedipine (10 μM) suppressed IBa even more. In Figure 4c, the time course of the effects of amlodipine (3 and 10 nM) on IBa evoked by a depolarizing pulse to +10 mV from −60 mV is also shown. When 3 nM amlodipine was removed, the amlodipine-induced inhibitory effects did not persist and IBa gradually recovered after 7 min of washout, but the peak amplitude of IBa did not return to the control level (0.86±0.06, n=4, Figure 4d).
Figure 4.
Effects of azelnidipine (100 and 300 nM), amlodipine (3 and 10 nM) and nifedipine (10 μM) on IBa in guinea-pig portal vein. (a) The time course of the effects of application of azelnidipine (100 and 300 nM) and nifedipine on the peak amplitude of IBa evoked by repetitive depolarizing pulses to +10 mV from a holding potential of −60 mV. Time 0 indicates the time at which azelnidipine was applied to the bath. (b) Inhibitory effect of azelnidipine on IBa in the presence and absence (after 7 min washout) of each concentration of azelnidipine. Each column shows the mean of 6 observations with +s.d. shown by vertical lines. (c) The time course of the effects of application of amlodipine (3 and 10 nM) and nifedipine on the peak amplitude of IBa evoked by repetitive depolarizing pulses to +10 mV from a holding potential of −60 mV. Time 0 indicates the time at which amlodipine was applied to the bath. (d) Inhibitory effect of amlodipine on IBa in the presence and absence (after 7 min washout) of each concentration of azelnidipine. Each column shows the mean of four observations with +s.d. shown by vertical lines. Asterisk indicates a statistically significant difference, demonstrated using a paired t-test (*P<0.01).
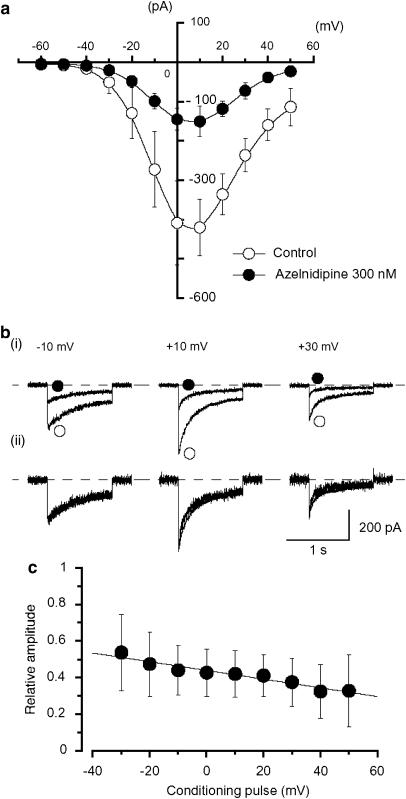
Figure 5 shows the relative peak amplitude of IBa evoked by depolarizing pulses to +10 mV from two different holding potentials (−60 and −90 mV) applied every 20 s, plotted against concentration of azelnidipine. Azelnidipine inhibited the peak amplitude of IBa in a concentration-dependent manner (−60 mV, Ki=282 nM; −90 mV, Ki=2 μM). In order to compare the inhibitory potency of azelnidipine with the potencies of the other DHP-derived Ca antagonists, amlodipine and nifedipine were applied in a similar manner (Figure 5). Both nifedipine and amlodipine also reduced the peak amplitude of IBa in a concentration- and voltage-dependent manner (amlodipine, −60 mV, Ki=15 nM; −90 mV, Ki=446 nM; nifedipine, −60 mV, Ki=10 nM; −90 mV, Ki=241 nM). Azelnidipine inhibited the peak amplitude of IBa evoked by depolarizing pulses (1 s duration) from a holding potential of −60 mV at levels more positive than −30 mV. Figure 6a shows the current–voltage relationships of IBa in the absence and presence of 300 nM azelnidipine; the inhibition appeared to be voltage-dependent (Figure 6c, n=5).
Figure 5.
Effects of Ca antagonists on the peak amplitude of IBa in guinea-pig portal vein. Whole-cell recording, pipette solution Cs+-TEA+ solution containing 5 mM EGTA and bath solution 10 mM Ba2+ containing 135 mM TEA+. (a) Relationships between relative inhibition of the peak amplitude of IBa and the concentration of Ca antagonists at two holding potentials (−60 and −90 mV) in guinea-pig portal vein. The following values were used for the fitted curve: azelnidipine, −60 mV, Ki=282 nM, nH=0.8; −90 mV, Ki=2 μM, nH=0.8; amlodipine, −60 mV, Ki=15 nM, nH=0.8; −90 mV, Ki=446 nM, nH=0.8; nifedipine, −60 mV, Ki=10 nM, nH=0.8; −90 mV, Ki=241 nM, nH=0.8. Each symbol indicates the mean of 4–10 observation with ±s.d. shown by vertical lines. Some of the s.d. bars are smaller than the symbol. (b) The current traces in the absence and presence of azelnidipine (300 nM) when the membrane potential was held at −60 mV. (c) The current traces in the absence and presence of azelnidipine (3 μM) at a holding potential of −90 mV.
Figure 6.
Effects of azelnidipine on IBa at −60 mV in guinea-pig portal vein. (a) Current–voltage relationships obtained in the absence (control) or presence of 300 nM azelnidipine. Each symbol indicates the mean of five observations with ±s.d. shown by vertical lines. The curves were drawn by eye. (b) (i) Original current traces before (control) and after application of 300 nM azelnidipine at the indicated pulse potentials. (ii) IBa from (i) scaled to match their peak amplitudes and superimposed. (c) Relationship between the test potential and relative value of IBa inhibited by 300 nM azelnidipine, expressed as a fraction of the peak amplitude of IBa evoked by a range of depolarizing pulses in the absence of azelnidipine. Each symbol indicates the mean of seven observations with ±s.d. shown by vertical lines. The line was draw by eye.
This voltage-dependency was investigated before and after application of azelnidipine using the experimental protocol shown in Figure 7a (conditioning pulse duration, 10 s; test pulse duration, 1 s; holding membrane potential, −90 mV). In the absence of azelnidipine (control), inactivation of IBa occurred with depolarizing pulses more positive than −50 mV from −90 mV in the test pulse. After application of azelnidipine (approximately 7 min later), the voltage-dependent inactivation curve in the same cells was significantly shifted to the left (16 mV). In Figure 7, the activation curves obtained from the current–voltage relationships in Figure 6, fitted to the Boltzmann equation, are also shown. Azelnidipine (300 nM) caused little shift of the activation curve (the 50% activation potentials; −8 mV (control; −8.2±0.4 mV, n=7) versus −7 mV (azelnidipine; −7.3±0.3 mV, n=7)). Figure 7b shows the current traces of the test pulses, at the potentials indicated, in the absence and presence of 300 nM azelnidipine. In the presence of azelnidipine, the peak-matched traces at the conditioning pulse potentials indicated are also shown (Figure 7b); the peak amplitude of IBa evoked by the test pulse with no conditioning pulse was superimposed to that in the absence of azelnidipine (i.e. control).
Figure 7.
Effects of azelnidipine on the voltage-dependent activation and inactivation of IBa in guinea-pig portal vein. (a) Steady-state inactivation curves, obtained in the absence (control) and presence of azelnidipine, were fitted to the Boltzmann equation. Peak current values were used. The steady-state inactivation curve was obtained using the double-pulse protocol (see Methods and inset). The current measured during the test pulse is plotted against membrane potential and expressed as relative amplitude. The steady-state inactivation curves in the absence or presence of azelnidipine were drawn using the following values: control, Imax=1.0, Vhalf=−34 mV, k=8 and C=0.04; azelnidipine, 300 nM, Imax=1.0, Vhalf=−50 mV, k=9 and C=0.01. Each symbol indicates the mean of six observations with ±s.d. shown by vertical lines. Some of the s.d. bars are smaller than the symbol. Asterisk indicates a statistically significant difference, demonstrated using a paired t-test (*P<0.01). Activation curves were obtained from the current–voltage relationships of Figure 6, fitting to the Boltzmann equation (see Methods). The activation curves in the absence or presence of azelnidipine were drawn using the following values: control, Imax=1.0, Vhalf=−8 mV, k=7 and C=0.01; azelnidipine, 300 nM, Imax=1.0, Vhalf=−7 mV, k=7 and C=0.02. Each symbol indicates the mean of seven observations with ±s.d. shown by vertical lines. Some of the s.d. bars are smaller than the symbol. (b) Original current traces before (control, (i)) and after application of 300 nM azelnidipine (ii) at the indicated conditioning pulse potentials. (iii) The inward Ba2+ currents from (ii) scaled to match the peak amplitude of the current with no conditioning pulse in the absence of azelnidipine (i.e. control) were shown.
As shown in Figure 8, when a depolarizing pulse was applied from −90 mV to +10 mV after an interval of 4 min in the presence of azelnidipine (1 μM), the size of the peak amplitude of IBa was reduced (0.89±0.05, n=6), compared with that observed before application of azelnidipine (i.e. control). Similar results were obtained after an interval of 4 min in the presence of amlodipine (300 nM) at −90 mV (0.8±0.05, n=5).
Figure 8.
The effects of azelnidipine (1 μM) and amlodipine (300 nM) on IBa in guinea-pig portal vein. No pulses were applied for the initial 4 min after application of the drug at a holding potential of −90 mV. Each symbol shows the mean value of the peak amplitude of IBa evoked by depolarizing pulses (see inset) delivered after this 4 min period. The peak amplitude of IBa just before application of the drug was normalized to one (control).
The effects of azelnidipine on the peak amplitude of IBa in the presence of S(-)-Bay K 8644
In order to obtain further information regarding the binding site(s) for azelnidipine, S(-)-Bay K 8644, a well-known dihydropyridine-derived L-type Ca2+ channel agonist, was utilized. The inhibitory potency of the Ca antagonists, nifedipine and azelnidipine, were compared in the absence and presence of S(-)-Bay K 8644. When the peak current amplitude just before application of S(-)-Bay K 8644 was taken as one, S(-)-Bay K 8644 (0.5 μM, control) greatly enhanced IBa (2.1±0.4, n=26; Figure 9a and b). The addition of azelnidipine (300 nM) caused inhibitory effects on IBa (0.51±0.05, n=6; Figure 9a and b) which were close to those observed in the absence of S(-)-Bay K 8644 (0.45±0.10, n=12; Ki=282 nM, i.e. Figure 5a); a small shift of the concentration-response curve to azelnidipine occurred in the presence of S(-)-Bay K 8644 (Ki=353 nM; Figure 9c and d). In contrast, the concentration–response curve to nifedipine was shifted to the right in the presence of S(-)-Bay K 8644 (Ki=4.1 μM; Figure 9c and d) in comparison with that observed in Figure 5a (Ki=10 nM), in an apparently competitive manner.
Figure 9.
The effects of azelnidipine on IBa in the presence of 0.5 μM S(-)-Bay K 8644. (a) The time course of the effects of application of 300 nM azelnidipine and 10 μM nifedipine on the peak amplitude of IBa evoked by repetitive depolarizing pulses to +10 mV from a holding potential of −60 mV. Time 0 indicates the time at which S(-)-Bay K 8644 was applied to the bath. (b) Inhibitory effects of azelnidipine on IBa in the presence of S(-)-Bay K 8644. Original current traces before (control, (i)) and after (ii) application of S(-)-Bay K 8644, as indicated in (a). (iii) Azelnidipine in the presence of S(-)-Bay K 8644 and (iv) nifedipine in (a). (c) Relationships between the relative inhibition of the peak amplitude of IBa and the concentration of Ca antagonists at −60 mV in guinea-pig portal vein. The following values were used for the fitted curve: azelnidipine, Ki=353 nM, nH=0.8; nifedipine, Ki=4.1 μM, nH=0.8. Each symbol indicates the mean of 4–7 observations with ±s.d. shown by vertical lines. Some of the s.d. bars are smaller than the symbol. The curves with the broken line (azelnidipine and nifedipine) were taken from Figure 5a, respectively. (d) (i) The current traces in the absence and presence of azelnidipine (300 nM) and with the addition of S(-)-Bay K 8644. (ii) The current traces in the absence and presence of nifedipine (10 nM) with the addition of S(-)-Bay K 8644.
Discussion
The present study provides the first direct electrophysiological and functional evidence that azelnidipine inhibits L-type Ca2+ channels in native vascular smooth muscle.
The rank order of potency of DHP-derived Ca antagonists
To date, the comparative potencies of DHP-derived Ca antagonists have been examined by studying their relaxant effects on either excess [K+]o- or agonist (acetylcholine, phenylephrine, etc.)-induced contractions in vascular smooth muscles. However, it is well documented that excess [K+]o activates voltage-dependent mechanisms, Ca2+-activated mechanisms due to Ca2+ entry (Ca2+-induced Ca2+ release mechanisms, Ca2+-activated K+ channels, Ca2+-activated Cl− channels, etc.) and that acetylcholine also activates stimulatory mechanisms (such as non-selective cation channels, muscarinic receptor-modulated pathway, etc., Kuriyama et al., 1995). Therefore, it is relatively difficult to estimate the individual effects of DHP-derived Ca antagonists on the contractile mechanisms in the presence of either excess [K+]o or agonists. Moreover, the comparative studies have not been performed at normal membrane potential and under unstimulated conditions. In the absence of agonists, spontaneous contractions are observed in portal vein but not arteries. Moreover, the peak amplitude of IBa through voltage-dependent Ca2+ channels in resistant arterial myocytes (such as mesenteric artery) is 50–100 pA (Ohya et al., 1997), much smaller than that observed in portal vein (400–500 pA; Teramoto et al., 1996). Thus, in the present experiments, the portal vein was used to investigate the effects of azelnidipine on IBa. In guinea-pig portal vein, it has been shown that spontaneous contractions are suppressed by subsequent application of not only calciseptine, a selective L-type Ca2+ channel blocking peptide, but also by nifedipine, although (±)-Bay K 8644, a L-type Ca2+ channel agonist, enhanced the amplitude of the spontaneous contractions (Teramoto et al., 1996). Teramoto et al. (1996) suggested that the spontaneous contractions are related to the activation of voltage-dependent Ca2+ channels in guinea-pig portal vein. In the present experiments, we evaluated the rank order of potency of DHP-derived Ca antagonists on fresh intact tissue, in the absence of excess [K+]o or agonists, by recording changes in tension. The rank order of potency was found to be nifedipine>amlodipine>azelnidipine.
Properties of voltage-dependent Ca2+ channels in the smooth muscle cells of guinea-pig portal vein
In the present study, IBa studied in guinea-pig portal vein probably flow through L-type voltage-dependent Ca2+ channels. This is supported by the following electrophysiological and pharmacological observations; (1) there was no hump or second peak at a less positive membrane potential in the current–voltage relationships obtained by whole-cell recordings. (2) When the holding membrane potential was changed from −40 to −90 mV, the threshold potential for IBa did not shift. (3) There was no difference in the inactivated decay of IBa recorded at two different holding membrane potentials (−40 and −90 mV). (4) Application of nifedipine, one of the most common DHP-derived Ca antagonists, suppressed the amplitude of IBa at −60 mV. Taken together, these results suggest that the L-type Ca2+ channel is probably the only type of voltage-dependent Ca2+ channel present in guinea-pig portal vein under the present experimental conditions. Several other smooth muscles may also possess only a single type of voltage-dependent Ca2+ channel (reviewed by Bolton et al., 1999).
Target channels for azelnidipine in native vascular smooth muscle
From the measurement of tension changes, we found that azelnidipine caused a concentration-dependent inhibition of spontaneous contractions (Ki=153 nM). In patch-clamp experiments, azelnidipine, similar to nifedipine, suppressed IBa and demonstrated a similar inhibitory potency (Ki=282 nM) at −60 mV. Furthermore, we demonstrated that the rank order of potency of the compounds at inhibiting the amplitude of IBa was nifedipine>amlodipine>azelnidipine, consistent with the observed changes in tension produced by these compounds. Thus, it is conceivable that azelnidipine reduces spontaneous contractions mainly through inhibition of L-type voltage-dependent Ca2+ channels in guinea-pig portal vein.
In the present experiments, S(-)-Bay K 8644, a DHP derivative that is an L-type Ca2+ channel agonist, affected the nifedipine-induced inhibitory effects on the peak amplitude of IBa, whereas S(-)-Bay K 8644 caused little or no shift of the azelnidipine-induced inhibitory effects on IBa. These results suggest that nifedipine, but not azelnidipine, interacts competitively with S(-)-Bay K 8644. In binding studies (Janis et al., 1984), it has been shown that Bay K 8644 competes for the same binding sites as nitrendipine (a DHP derivative). One possible explanation for the discrepancy between such binding studies and our patch-clamp experiments is that azelnidipine seems to possess multiple binding affinities to voltage-dependent L-type Ca2+ channels. In radioligand-binding studies, it was found that azelnidipine possessed a high affinity for DHP-binding sites (IC50=3 nM) in comparison with those of other binding sites in L-type Ca2+ channels (phenylalkylamine-binding site, IC50=4 μM; benzothiazepine-binding site, IC50=8 μM; Koike et al., 2002). Recent molecular biological studies have detected specific DHP-binding sites in amino-acid residues of α1C (reviewed by Striessnig et al., 1998). Thus it is possible that azelnidipine binds to both the S6 segments in repeats III and IV (IIIS6 and IVS6) and the IIIS5-S6 linker of α1C in a non-specific manner, even though azelnidipine has a high affinity for the IIIS5-S6 linker (i.e. DHP-binding sites). Further studies are needed to elucidate the nature of specific functional binding sites for azelnidipine in voltage-dependent L-type Ca2+ channels in vascular smooth muscle.
Kinetic studies on the actions of azelnidipine on IBa
When the holding potential was elevated to −60 mV from −90 mV, voltage-dependent inhibition by azelnidipine was observed and the concentration–response curve was shifted to the left. The voltage-dependent inactivation curve was also shifted to the left after application of 300 nM azelnidipine. These results suggest that the voltage-dependent inhibitory actions of azelnidipine occur at the inactivated state of voltage-dependent Ca2+ channels in guinea-pig portal vein (voltage-dependent block).
The same amplitude of IBa was produced by application of depolarizing pulses to +10 mV from potentials of −90 mV or more negative, suggesting that all of the voltage-dependent Ca2+ channels at these potentials may be in the resting state. It has been reported that several DHP derivatives commonly cause a potent resting state block on L-type Ca2+ channels (Bolton et al., 1999). Similarly, both azelnidipine and amlodipine had an inhibitory effect on the peak amplitude of IBa evoked by a depolarizing pulse to +10 from −90 mV. These results suggest that azelnidipine may inhibit IBa in a voltage-independent manner (resting state block). In the present experiments, the Krest value was estimated to be 2.2 μM from the concentration–response curve at a holding potential of −90 mV. When the value of ΔVhalf was obtained from the results using 10 s conditioning pulses, the estimated Kinact value was 9.5 nM (see Methods). Given this, we suggest that azelnidipine may bind to the inactivated state with approximately 230 times higher affinity than to the resting state in guinea-pig portal vein.
The implication of the long-lasting effects of azelnidipine in hypertension treatment
The long-lasting azelnidipine-induced reduction of spontaneous contractions was observed by recording tension after washout of azelnidipine. Similarly, on removal of azelnidipine, the long-lasting inhibitory effects of azelnidipine on IBa were also observed in patch-clamp experiments. Koike et al. (2002) reported that azelnidipine still remained in the vascular smooth muscle walls even after washout of the compound. It has been reported that the hydrophobicity of DHP-derivatives is closely related to the limiting rate for diffusion in the lipid bilayer of cell membrane (Rhodes et al., 1985). As azelnidipine is a potent hydrophobic compound (log partition (log P) coefficient; 4.4 at pH 9 and log P measured by HPCL technique (log PHPLC) coefficient, 5.2; personal communication from the Sankyo Pharmaceutical Co. Ltd.), azelnidipine is likely not only to bind to the high affinity sites in voltage-dependent Ca2+ channels but also to remain in the lipid bilayer of the smooth muscle membrane. These long-lasting inhibitory actions of azelnidipine on voltage-dependent Ca2+ channels may be of clinical benefit in the control of blood pressure for long periods.
Recently, it has been found that azelnidipine possesses several other actions in addition to its Ca antagonistic effects. Namely, azelnidipine has a potent anti-oxidative effect in human cultured arterial endothelial cells (Shinomiya et al., 2004). It appears to have a protective role in the development and progression of atherosclerosis through these anti-oxidative properties (Yamagishi et al., 2004). Moreover, azelnidipine attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells (Tanaka et al., 2004). Thus, taken together, these results indicate that azelnidipine is likely to be classified into a new category of Ca antagonists that have various unique pharmacological actions.
In conclusion, we demonstrated that azelnidipine causes a long-lasting vascular relaxation through persistant inhibition of L-type Ca2+ channels in guinea-pig portal vein.
Acknowledgments
We thank Dr Frank R Edwards (Division of Neuroscience, John Curtin School of Medical Research, Australia National University, Canberra, Australia) for helpful discussion and critical reading of the manuscript. This work was supported by both a Grant-in-Aid for Scientific Research (B)-(2) from the Japanese Society for the Promotion of Science (Noriyoshi Teramoto, Grant Number 16390067) and a Grant-in-Aid for Exploratory Research (Noriyoshi Teramoto, Grant Number 17659075) from the Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- azelnidipine
(+)-3-(1-diphenylmethylazetidin-3-yl)-5-isopropyl 2-amino-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridine-dicarboxylate
- Bay K 8644
methyl 1,4-dihydro-2,6-dimethyl-3-nitro-4-(2-trrifluoromethylphenyl)-pyridine-5-carboxylate
- DHP
dihydropyridine
- DMSO
dimethyl sulphoxide
- IBa
voltage-dependent nifedipine-sensitive inward Ba2+ currents
- log P
log partition coefficient
- log PHPLC
log partition coefficient measured by HPCL technique
- PSS
physiological salt solution
- TEA+
tetraethyl ammonium
Conflict of interest
The authors state no conflict of interest.
References
- Alderman MH, Cohen H, Roque R, Madhavan S. Effect of long-acting and short-acting calcium antagonists on cardiovascular outcomes in hypertensive patients. Lancet. 1997;349:594–598. doi: 10.1016/S0140-6736(96)08359-6. [DOI] [PubMed] [Google Scholar]
- Arita M, Hashizume T, Tanigawa K, Yamamoto H, Nishio I. A new Ca-antagonist, azelnidipine, reduced blood pressure during exercise without augmentation of sympathetic nervous system in essential hypertension: a randomized, double-blind, placebo-controlled trial. J Cardiovasc Pharmacol. 1999;33:186–192. doi: 10.1097/00005344-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circ. 1995;92:1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- Janis RA, Sarmiento JG, Maurer SC, Bolger GT, Triggle DJ. Characteristics of the binding of [3 H]nitrendipine to rabbit ventricular membranes: modification by other Ca2+ channel antagonists and by the Ca2+ channel agonist Bay K 8644. J Pharmacol Exp Ther. 1984;231:8–15. [PubMed] [Google Scholar]
- Koike H, Kimura T, Kawasaki T, Sada T, Ikeda T, Sanbuissho A, et al. Azelnidipine, a long-acting calcium channel blocker with slow onset and high vascular affinity. Ann Report Sankyo Res Lab. 2002;54:1–62. [Google Scholar]
- Kuramoto K, Ichikawa S, Hirai A, Kanada S, Nakachi T, Ogihara T. Azelnidipine and amlodipine: a comparison of their pharmacokinetics and effects on ambulatory blood pressure. Hypertens Res. 2003;26:201–208. doi: 10.1291/hypres.26.201. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y, Adachi N, Setoguchi M, Abe I, Fujishima M. Effects of CP-060S on membrane channels in vascular smooth muscle cells from guinea pig. Eur J Pharmacol. 1997;330:93–99. doi: 10.1016/s0014-2999(97)00173-8. [DOI] [PubMed] [Google Scholar]
- Oizumi K, Nishino H, Koike H, Sada T, Miyamoto M, Kimura T. Antihypertensive effects of CS-905, a novel dihydropyridine Ca2+ channel blocker. Jpn J Pharmacol. 1989;51:57–64. doi: 10.1254/jjp.51.57. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- Rhodes DG, Sarmiento JG, Herbette LG. Kinetics of binding of membrane-active drugs to receptor sites: diffusion-limited rates for a membrane bilayer approach of 1, 4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985;27:612–623. [PubMed] [Google Scholar]
- Romero M, Sanchez I, Pujol MD. New advances in the field of calcium channel antagonists: cardiovascular effects and structure-activity relationships. Curr Med Chem Cardiovasc Hematol Agents. 2003;1:113–141. doi: 10.2174/1568016033477487. [DOI] [PubMed] [Google Scholar]
- Salvetti A, Di Venanzio L. Clinical pharmacology of long-acting calcium antagonists: what relevance for therapeutic effects. J Cardiovasc Pharmacol. 1994;23 Suppl 5:S31–S34. doi: 10.1097/00005344-199423005-00007. [DOI] [PubMed] [Google Scholar]
- Shinomiya K, Mizushige K, Fukunaga M, Masugata H, Ohmori K, Kohno M, et al. Antioxidant effect of a new calcium antagonist, azelnidipine, in cultured human arterial endothelial cells. J Int Med Res. 2004;32:170–175. doi: 10.1177/147323000403200210. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Byttebier G, Buntinx F, Celis H, O'Brien ET, Fagard R. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. Ambulatory blood pressure monitoring and treatment of hypertension investigators. JAMA. 1997;278:1065–1072. [PubMed] [Google Scholar]
- Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, et al. Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol. 2004;15:2320–2333. doi: 10.1097/01.ASN.0000138287.46849.82. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Brading AF. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br J Pharmacol. 1996;118:635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto N, Ogata R, Okabe K, Kameyama A, Kameyama M, Watanabe TX, et al. Effects of calciseptine on unitary barium channel currents in the guinea-pig portal vein. Pflügers Arch. 1996;432:462–470. doi: 10.1007/s004240050158. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Tomoda T, Ito Y. Mefenamic acid as a novel activator of L-type voltage-dependent Ca2+ channels in smooth muscle cells from pig proximal urethra. Br J Pharmacol. 2005;144:919–925. doi: 10.1038/sj.bjp.0706051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L, Den Hond E, Nawrot T, Staessen JA. Prevalence, pathophysiology and treatment of isolated systolic hypertension in the elderly. Expert Rev Cardiovasc Ther. 2004;2:761–769. doi: 10.1586/14779072.2.5.761. [DOI] [PubMed] [Google Scholar]
- Uehara A, Hume JR. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985;85:621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Inagaki Y, Nakamura K, Imaizumi T. Azelnidipine, a newly developed long-acting calcium antagonist, inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endothelial cells through its anti-oxidative properties. J Cardiovasc Pharmacol. 2004;43:724–730. doi: 10.1097/00005344-200405000-00016. [DOI] [PubMed] [Google Scholar]