Abstract
Background and purpose:
Fluoxetine (Prozac®) is a widely prescribed drug in adults and children, and it has an active metabolite, norfluoxetine, with a prolonged elimination time. Although uncommon, Prozac causes QT interval prolongation and arrhythmias; a patient who took an overdose of Prozac exhibited a prolonged QT interval (QTc 625 msec). We looked for possible mechanisms underlying this clinical finding by analysing the effects of fluoxetine and norfluoxetine on ion channels in vitro.
Experimental approach:
We studied the effects of fluoxetine and norfluoxetine on the electrophysiology and cellular trafficking of hERG K+ and SCN5A Na+ channels heterologously expressed in HEK293 cells.
Key results:
Voltage clamp analyses employing square pulse or ventricular action potential waveform protocols showed that fluoxetine and norfluoxetine caused direct, concentration-dependent, block of hERG current (IhERG). Biochemical studies showed that both compounds also caused concentration-dependent reductions in the trafficking of hERG channel protein into the cell surface membrane. Fluoxetine had no effect on SCN5A channel or HEK293 cell endogenous current. Mutations in the hERG channel drug binding domain reduced fluoxetine block of IhERG but did not alter fluoxetine's effect on hERG channel protein trafficking.
Conclusions and implications:
Our findings show that both fluoxetine and norfluoxetine at similar concentrations selectively reduce IhERG by two mechanisms, (1) direct channel block, and (2) indirectly by disrupting channel protein trafficking. These two effects are not mediated by a single drug binding site. Our findings add complexity to understanding the mechanisms that cause drug-induced long QT syndrome.
Keywords: hERG, arrhythmia, electrophysiology, long-QT syndrome, drug safety
Introduction
Acquired long QT syndrome (LQTS) has become an important liability for clinically available drugs and developmental compounds. The mechanism commonly proposed for drug-induced QT interval prolongation is direct block of human ether-a-go-go-related-gene (hERG) (Kv11.1) K+ channels or its native current, rapidly activating delayed rectifier potassium current (IKr). The drugs bind to a structurally unique receptor domain in the pore-S6 region of the channel to suppress K+ ion permeation (for review, see Sanguinetti and Mitcheson (2005)). Recently, a second mechanism for drug-induced LQTS has emerged, which is the selective disruption of hERG channel protein trafficking to the cell surface membrane. Fewer mature hERG channels reach the surface membrane, thus reducing hERG K+ current (IhERG) or IKr. This indirect mechanism has been shown for only a few uncommonly used drugs, and the drug-binding site mediating this effect is unknown, and the importance of this mechanism is not well understood (for review, see Eckhardt et al., 2005b).
This report was prompted by a patient who ingested an overdose of the widely used drug fluoxetine (Prozac) and developed a prolonged QT interval. Fluoxetine is a selective serotonin reuptake inhibitor prescribed for depression, postpartum depression, premenstrual dysphoric syndrome and bulimia. Its popularity is in part owing to the few reported cardiotoxic side effects compared to tricyclic antidepressants (Upward et al., 1988). However after initial marketing concerns over the safety of fluoxetine have been raised (Pacher and Kecskemeti, 2004), including reports of QT interval prolongation (Varriale, 2001; Curtis et al., 2003), cardiac arrhythmias (Allhoff et al., 2001; Abebe-Campino et al., 2002) and Torsades de Pointes (Appleby et al., 1995; Lherm et al., 2000; Wilting et al., 2006) as well as synergistic effects when used in combination with other drugs (Michalets et al., 1998; Isbister et al., 2004; Nykamp et al., 2005). The principal metabolite, norfluoxetine, is also active as an antidepressant and has a five-to seven-fold longer elimination half-life compared to the parent drug. Newborns metabolize fluoxetine and norfluoxetine more slowly than adults and toxicity with ventricular arrhythmias has been reported in infants of mothers treated with fluoxetine during pregnancy and while breast feeding (Hale et al., 2001; Abebe-Campino et al., 2002; Dubnov et al., 2005). Previously, fluoxetine was reported to block IhERG studied in Xenopus oocytes or Chinese hamster ovary cells with IC50 values of 3.1 or 1.5 μM, respectively (Thomas et al., 2002; Witchel et al., 2002). Norfluoxetine's effect on hERG channels has not been reported previously and nothing is known about possible indirect effects of fluoxetine or norfluoxetine on hERG channel protein trafficking.
We have investigated the electrophysiological properties of fluoxetine and norfluoxetine on hERG K+ and voltage-gated cardiac sodium channel gene (SCN5A) Na+ channels heterologously expressed in a human cell line. We studied hERG and SCN5A channels because they are common targets for drug interaction and proarrhythmia. Both compounds selectively reduced IhERG at similar drug concentrations by two mechanisms, (1) directly by blocking ion permeation, and (2) indirectly by disrupting channel protein trafficking. A preliminary report of this work has appeared (Eckhardt et al., 2005a).
Methods
hERG and SCN5A channel expression
Stable transfection of hERG1 (KCNH2) wild type (WT) or SCN5A (hH1c) WT complementary DNA (cDNA) into human embryonic kidney cells (HEK293) cells has been described previously (Zhou et al., 1998b; Valdivia et al., 2002). In some experiments, a second hERG WT HEK293 cell line was used. The new cell line differs from the previous HEK293 hERG cell line only in that a bovine growth hormone polyadenylation (poly-A) signal was removed from the pCDNA3 (Invitrogen, Carlsbad, CA, USA) expression vector. This poly-A signal is redundant as the hERG cDNA originally isolated contains a native poly-A signal and tail (Warmke and Ganetzky, 1994). The pCDNA3 poly-A signal was removed through excision by restriction digest at unique sites (Xba1–Bbs1) upstream and downstream of the signal. The single-strand overhangs of the digested vector were filled-in with Klenow polymerase and ligated together to reform the expression vector. The hERG1 WT cDNA was inserted into the BamHI–EcoRI restriction sites of the new vector and native HEK293 cells were stably transfected using Superfect (Qiagen GmbH, Hilden, Germany). The electrophysiological and biochemical properties of both WT hERG HEK293 cells lines were similar.
Two hERG channel engineered mutations, F656A and F656C, were generated using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA), and DNA sequencing confirmed the nucleotide changes. These mutations were transiently transfected into HEK293 cells using SuperFect and studied within 24–48 h as described previously (Zhou et al., 1998b).
Electrophysiology
Membrane currents were recorded using the whole-cell patch clamp technique (Hamill et al., 1981). pCLAMP 8.0 software (Axon Instruments, Union City, CA, USA) was used to generate voltage clamp protocols and acquire data, which were analyzed using pCLAMP 8.0 and Microcal Origin (MicroCal, Northampton, MA, USA) software. For recording IhERG, cells were superfused with Tyrode solution containing (in mM) NaCl 137, KCl 4, CaCl2 1.8, MgCl2 1, glucose 10, N-2-hydroxylpiperazine-N′-2-ethanesulfonic acid (HEPES) 10, pH adjusted to 7.4 with NaOH. Patch pipettes contained (in mM) KCl 130, MgCl2 1, MgATP 5, ethyleneglycoltetraacetate (EGTA) 5, HEPES 10, pH adjusted to 7.2 with KOH and had resistances of 1.5–2 MΩ. For recording Na+ current (INa), cells were superfused with Tyrode solution containing (in mM) NaCl 140, KCl 4.0, CaCl2 1.8, MgCl2 0.75, HEPES 5 and pH adjusted to 7.4 with NaOH. Patch pipettes contained (in mM) CsCl 20, CsF 120, EGTA 2, HEPES 5 and pH adjusted to 7.4 with CsOH, and had resistances of 1–1.5 MΩ. Series resistance compensation was 70–80% and leak subtraction was used for experiments with INa. Membrane currents were recorded using square pulse or ventricular action potential waveform protocols at 23±1 and 37±1°C, respectively.
Western blot analysis
Western blot analysis of hERG protein was performed as described previously (Zhou et al., 1998b). Whole-cell lysates were analyzed by solubilizing HEK293 cells in lysis buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40 (NP-40) buffer and 10% glycerol) containing a protease inhibitor mini-tablet (Roche Diagnostics, Mannheim, Germany) for 2 h at 4°C. The insoluble materials were pelleted at 12 000 r.p.m. for 10 min at 4°C. Protein concentration was measured using the BCA method (Pierce Chemical, Rockford, IL, USA) or Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Soluble lysate proteins were then heated with an equal amount of Laemmli sample buffer containing 5 mM dithiothreitol for 30 min at 37°C. The denatured protein lysates were subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis followed by elecrophoretic transfer onto nitrocellulose membranes. The membranes were incubated with hERG antiserum (1:10 000 dilution) at room temperature overnight, and the antibody was detected as described previously (Zhou et al., 1998b). Each Western blot shows data scanned from one nitrocellulose membrane and reproduced at constant grayscale intensity.
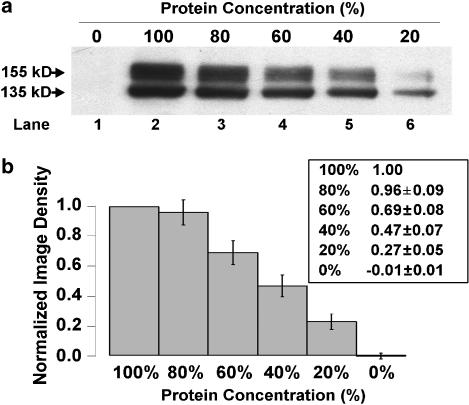
Image density analysis of hERG protein detected on Western blot analysis was performed using a Bio-Rad GS-700 Imaging Densitometer and Molecular Analyst software (Bio-Rad Laboratories). Using this software, a box of constant size was placed around each control and experimental 155 kDa (mature hERG protein) band, as well as an empty lane (background), and pixel intensities were integrated to yield relative density values. Following background subtraction, these values were normalized against the control value on each Western blot. To validate that this densitometry method yielded a graded response with decreasing protein concentration, serial dilutions of hERG WT lysate (100, 80, 60, 40, 20 and 0% protein concentration) were made with NP-40 lysis buffer followed by Western blot analysis. Example Western blot data are given in Figure 1a and show a graded decrease in the intensity of both the 155 and 135 kDa bands with serial dilution. The 155 kDa band densities were normalized to the 100% protein concentration band (lane 2). Figure 1b summarizes the density analysis of 10 Western blots to show a step-wise, nearly linear, decrease in 155 kDa band density with decreasing protein concentration.
Figure 1.
Serial dilution densitometry. (a) Typical Western blot of serial dilutions of hERG protein lysates. (b) Analysis of the normalized 155 kDa band densities of Western blots (n=10). Individual normalized densitometry values (mean±s.e.m.) are given in the table (inset).
Statistical methods
Data are given as mean±s.e.m. Concentration–response relations for block of IhERG by fluoxetine or norfluoxetine were determined using the Hill equation,
where Idrug/Icontrol is fractional block, D is drug concentration, IC50 is drug concentration for 50% block and n is the Hill coefficient (Zhou et al., 1998b). Concentration–response relations for the effect of fluoxetine or norfluoxetine on normalized Western blot density values of the 155 kDa protein band were determined using a modified Hill equation,
where ddrug/dcontrol is fractional reduction in density compared to control, D is drug concentration, dC50 is the drug concentration for a 50% decrease in density and n is the Hill coefficient (see Ficker et al., 2002, 2004). Statistical significance was determined using Student's paired t-test and a P-value of <0.05 was considered significant.
Drugs
Fluoxetine, norfluoxetine, E4031 and cisapride were purchased from Sigma Chemicals (St Louis, MO, USA) or Biomol International LP (Plymouth Meeting, PA, USA). Each was dissolved in distilled water to give a 10 mM stock solution and further dilutions were made in Tyrode solution.
Results
Clinical history
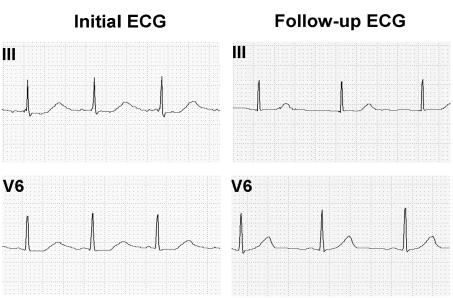
A 19-year-old female with a history of depression, anorexia and bulimia, was admitted to the University of Wisconsin Hospital and Clinics following the intentional overdose of ∼400 mg of Prozac. Her initial ECG showed sinus rhythm of 88 b.p.m. with a prolonged QT interval of 515 ms and a QTc (Bazett method) of 625 ms (Figure 2). There was no family history of cardiac disease, palpitations, syncope, seizure, deafness or sudden death. Her physical examination was unremarkable. Urine toxin screen showed no abnormalities. Serum blood levels of fluoxetine and norfluoxetine were not obtained. A follow-up ECG 3 weeks later showed a QTc of 400 ms (Figure 2). We hypothesized that Prozac caused her LQTS by reducing IhERG.
Figure 2.
ECG tracings (normal standard calibration) of leads III and V6 of the patient with Prozac overdose.
Direct block of IhERG by fluoxetine and norfluoxetine
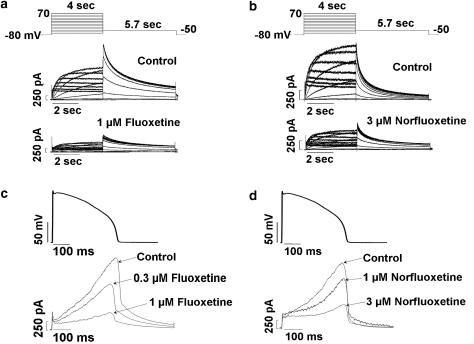
The effects of fluoxetine and norfluoxetine on IhERG in HEK293 cells were studied using two voltage clamp protocols, a square pulse protocol and an action potential waveform protocol. Figure 3 shows these protocols along with typical IhERG traces. For the square pulse protocol, cells were depolarized from a holding potential of −80 to 70 mV in 10 mV increments for 4 s, followed by a step to –50 mV for 5.7 s to record tail IhERG, with the protocol applied every 15 s. Figure 3a shows representative IhERG traces from one cell for control conditions and after 10 min of exposure to fluoxetine (1 μM). Figure 3b shows representative IhERG traces from a different cell for control conditions and following norfluoxetine (3 μM). Both compounds directly reduce IhERG. For the action potential waveform protocol, cells were depolarized from a holding potential of −82 mV to a peak voltage of 42 mV and repolarization was 90% complete (APD90) in 380 ms (Zhou et al., 1998b), with the waveform applied at 5 s intervals. Because of its channel gating properties, IhERG reaches its maximum amplitude during phases 2 and 3 of the ventricular action potential waveform (Zhou et al., 1998b). Fluoxetine (0.3 and 1 μM, Figure 3c) and norfluoxetine (1 and 3 μM, Figure 3d) caused a concentration-dependent reduction in IhERG. Using these voltage clamp protocols, block of IhERG was determined for fluoxetine (0.1, 0.3, 1, 3 and 10 μM, n=5–6 cells at each concentration) or norfluoxetine (0.3, 1, 3, 10 and 30 μM, n=3–5 cells at each concentration). Block of IhERG developed rapidly and was reversible following drug washout (data not shown). Peak tail IhERG (square pulse protocol, voltage step from 70 to −50 mV) or the maximum outward IhERG (action potential waveform protocol) at steady-state block for each fluoxetine or norfluoxetine concentration was normalized to its respective control IhERG value obtained before drug exposure. The averaged normalized IhERG values at each drug concentration were fitted to the Hill equation. The IC50 value for fluoxetine was 0.7±0.1 μM with a slope factor of 1.0±0.2 obtained using the square pulse protocol and 0.7±0.1 μM with a slope factor of 1.4±0.2 obtained with the action potential waveform protocol. The IC50 values for norfluoxetine were 2.3±0.2 μM with a slope factor of 1.3±0.1 and 2.5±0.1 μM with a slope factor of 1.3±0.1 for the two different protocols, respectively. Thus, the two voltage clamp protocols generated nearly identical concentration–response relations, suggesting that both compounds rapidly achieve steady-state block following cell depolarization.
Figure 3.
Effect of fluoxetine and norfluoxetine on IhERG. (a and b) Square pulse protocol along with families of IhERG traces for control conditions and after 1 μM fluoxetine or 3 μM norfluoxetine. (c and d) Action potential waveform protocol along with IhERG traces for control conditions, after 0.3 and 1 μM fluoxetine or 1 and 3 μM norfluoxetine.
Disruption of hERG protein trafficking
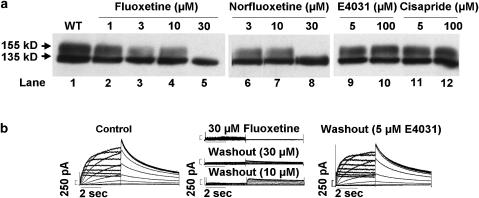
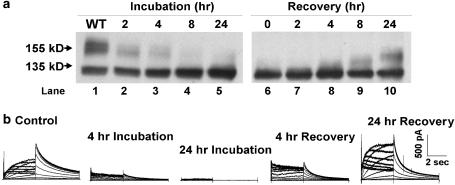
We studied the effect of fluoxetine and norfluoxetine on hERG channel protein trafficking. hERG channel biogenesis involves complex co- and post-translational protein processing through the secretory pathway (Zhou et al., 1998a, 1998b; Jones et al., 2004), and its disruption is an important mechanism in congenital type-2 LQTS (see Delisle et al., 2004; Anderson et al., 2006). Cells expressing hERG WT channel protein were incubated with fluoxetine or norfluoxetine for 24 h as described previously (Zhou et al., 1999). Cells were then subjected to Western blot analysis. Figure 4a shows representative results. WT channels (lane 1) show protein bands at ∼135 kDa (immature, core glycosylated protein in the endoplasmic reticulum) and at ∼155 kDa (mature, complexly glycosylated channel protein in the surface membrane). The intensity of the 155 kDa protein band decreased with increasing cell culture concentrations of fluoxetine (1, 3 and 10 μM; lanes 2–4) or norfluoxetine (3 and 10 μM; lanes 6 and 7), whereas the 135 kDa protein band remained intact, and at 30 μM fluoxetine (lane 5) or 30 μM norfluoxetine (lane 8) only the 135 kDa protein band was detected. Similar findings of a concentration-dependent decrease in the 155 kDa band were obtained in six Western blot analyses with fluoxetine (1, 3, 10 and 30 μM, 4–6 blots at each concentration) and in four Western blot analyses with norfluoxetine (1, 3, 10 and 30 μM, 2–4 blots at each concentration). Two additional drugs, E4031 and cisapride (5 or 100 μM), which block hERG channels with even higher affinities (IC50 values 7.7 and 4.3–6.5 nM, respectively (Mohammad et al., 1997; Zhou et al., 1998b; Anson et al., 2004)) were studied similarly. Western blot analysis detected both the 135 and 155 kDa protein bands with E4031 (lanes 9 and 10) and cisapride (lanes 11 and 12). These bands were similar in intensity to control conditions (lane 1) and the same results were obtained in five additional Western blots. Thus, E4031 and cisapride at concentrations >10 000-fold above their IC50 values for hERG channel block had no effect on the trafficking of the channel protein.
Figure 4.
(a) Western blot analysis of hERG WT channel protein with increasing concentrations of fluoxetine, norfluoxetine, E4031 and cisapride. (b) IhERG traces (square pulse protocol, see Figure 3a) for control conditions (left panel), following 24 h incubation without drug washout or after 1 h drug-free conditions for 10 or 30 μM fluoxetine (middle panels), and IhERG 1 h following washout of 5 μM E4031 (right panel).
We confirmed for fluoxetine that disruption of trafficking of the 155 kDa protein band abolished IhERG. Representative IhERG traces are shown in Figure 4b. For control conditions (left panel) the peak tail IhERG was 103.4±4.4 pA/pF for the voltage step from 70 to −50 mV (n=4 cells). The middle panel shows that IhERG was virtually absent when the cells were incubated for 24 h and studied in 30 μM fluoxetine (peak tail IhERG=2.7±1.5 pA/pF, n=4 cells), conditions that both disrupt trafficking and block the channel. Fluoxetine (30 μM) washout for 1 h resulted in little additional IhERG (peak tail IhERG=11.8±3.8 pA/pF, n=4 cells). Incubation for 24 h in 10 μM fluoxetine, which incompletely disrupts hERG protein trafficking (Figure 4a, lane 4), followed by drug washout for 1 h resulted in a larger peak tail IhERG (35.2±5.6 pA/pF, n=4 cells, P<0.05 compared to peak tail IhERG after culture in and washout of 30 μM fluoxetine). The right panel shows IhERG traces recorded after incubation of cells in 5 μM E4031 for 24 h followed by 1 h of drug washout. Peak tail IhERG was 100.2±21.5 pA/pF (n=4 cells), similar to control (P>0.05). These data confirm electrophysiologically the biochemical finding that fluoxetine disrupts hERG protein trafficking whereas the higher affinity IhERG blocking drug E4031 did not.
We tested whether E4031 (5 μM) could prevent fluoxetine or norfluoxetine-mediated disruption of hERG protein trafficking, as E4031 has been shown to cause ‘pharmacological rescue' of some LQT2 trafficking-defective channels (Zhou et al., 1999). Cells expressing hERG WT channels were incubated for 24 h with (1) 5 μM E4031, (2) 5 μM E4031+30 μM fluoxetine or (3) 5 μM E4031+30 μM norfluoxetine, and were then subjected to Western blot analysis. E4031 did not prevent fluoxetine or norfluoxetine-mediated disruption of the 155 kDa protein band (n=2 blots, data not shown), whereas E4031 alone had no effect (see Figure 4a, lane 9).
Time course of disruption and recovery of hERG protein trafficking
Drug-induced alteration in hERG channel protein biogenesis should develop and recover gradually. Western blot analysis (Figure 5a) shows the time-dependent loss of the mature hERG protein band with cell incubation in 30 μM fluoxetine and its recovery following drug washout from the culture medium. Similar findings were obtained in four additional Western blot analyses. Patch clamp recordings (Figure 5b) show the time-dependent loss of IhERG with cell incubation in 30 μM fluoxetine (followed by 1 h of culture in drug-free conditions) and its recovery following drug washout from the culture medium. Peak tail IhERG densities (voltage step from 70 to −50 mV, n=4–7 cells at each time) normalized to the control data showed a 58.1% reduction at 4 h and a 94.2% reduction at 24 h of fluoxetine incubation. Following 4 and 24 h of washout of the drug from the culture medium, normalized peak tail IhERG densities increased to 57.8 and 137.8% of control.
Figure 5.
(a) Western blot analysis of the time-dependence of the development and recovery of fluoxetine-induced disruption of hERG protein trafficking. (b) Families of IhERG traces for control conditions, and at different times of incubation in 30 μM fluoxetine, and after removal of fluoxetine from the culture medium.
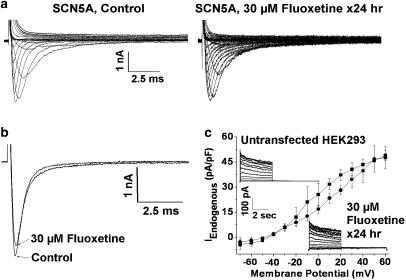
Fluoxetine does not alter SCN5A Na+ or HEK293 cell endogenous currents
To understand whether fluoxetine is selective for hERG channels, or whether it can affect similarly other ion channels, we tested its effects on INa. Cells expressing SCN5A channels were depolarized from a holding potential of −140 mV to between −120 to 80 mV in 10 mV increments for 24 ms. Figure 6a shows representative families of INa traces recorded for control conditions and after 24 h of culture in 30 μM fluoxetine (followed by 1 h of culture in drug-free conditions). Peak inward INa for control conditions was −230±79 pA/pF (n=6 cells) and following 24 h culture in fluoxetine was −280±50 pA/pF (n=6 cells, P>0.05 compared to control). We also tested whether fluoxetine directly blocked INa. As shown in Figure 6b, INa was recorded for control conditions and following 30 μM fluoxetine treatment for 10 min (voltage step −140 to −20 mV). Peak inward INa for control conditions was −194±18 pA/pF and in fluoxetine it was −171±15 pA/pF (n=5 cells, P>0.05 compared to control). Thus, fluoxetine did not significantly alter INa. We also studied the effect of fluoxetine on the small amplitude endogenous current found in untransfected HEK293 cells. Representative families of current traces are shown in the insets in Figure 6c (voltage clamp protocol as in Figure 3a). Endogenous current (IEndogenous) was measured at the end of depolarizing steps to between –70 and 60 mV for cells in control conditions (n=3 cells) and following 24 h incubation in 30 μM fluoxetine (followed by 1 h of culture in drug-free conditions, n=3 cells), and are plotted in Figure 6c. Cell culture in fluoxetine for 24 h had minimal effect on IEndogenous.
Figure 6.
(a) Families of INa recorded for control conditions and following 24 h incubation in 30 μM fluoxetine. (b) INa recordings for control conditions and after 10 min superfusion of 30 μM fluoxetine. (c) I–V plots of IEndogenous for control conditions and after 24 h incubation in 30 μM fluoxetine. Insets show representative families of current traces.
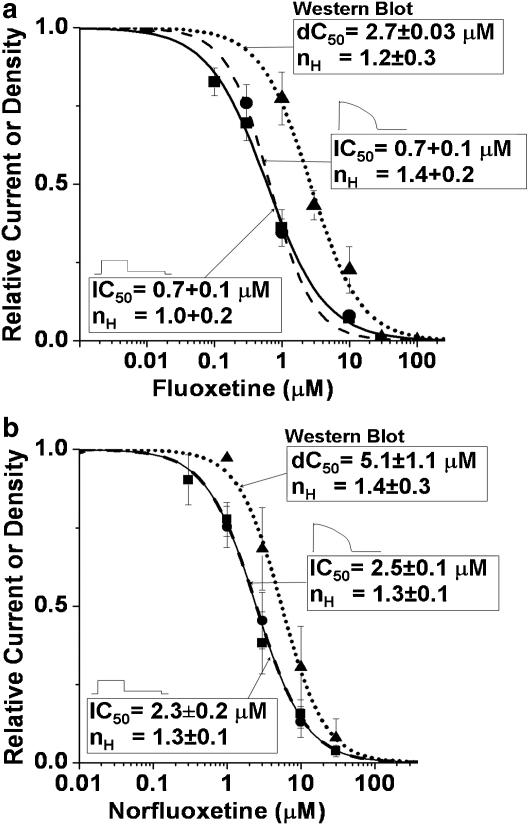
Concentration–response relations: block of IhERG versus disruption of hERG protein trafficking
The concentration–response relations for block of peak tail IhERG by fluoxetine and norfluoxetine are shown in Figure 7a and b, respectively, determined with the square pulse and action potential waveform protocols (Figure 3a and b). Block was concentration-dependent, had similar IC50 values, and the slope factors were close to unity, consistent with drug interaction at a single site. Figure 7a and b also show the concentration–response relations for fluoxetine and norfluoxetine derived from normalized Western blot image density measurements of the 155 kDa band. When fitted to the Hill equation, the dC50 values for disruption of the mature hERG protein band were 2.7±0.03 and 5.1±1.1 μM, with slope factors of 1.2±0.3 and 1.4±0.3, respectively. These values are close to those obtained for direct block of IhERG.
Figure 7.
(a and b) Concentration–response relations for fluoxetine and norfluoxetine, obtained using square pulse protocol, action potential waveform protocol and from normalized Western blot image density measurements fitted to Hill equation (see text for detail).
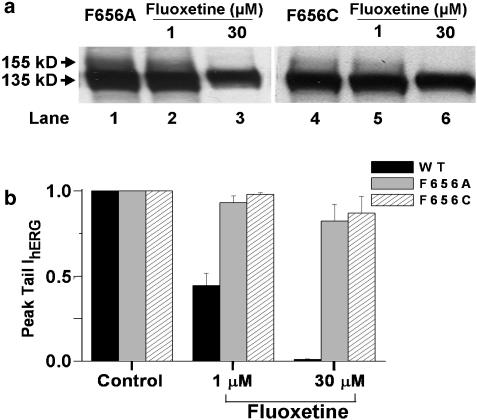
One explanation for the similar drug sensitivities and slope values is that fluoxetine and norfluoxetine bind to a common site that mediates both drug-induced hERG channel block and disruption of its protein trafficking. In the hERG channel, aromatic residues of the S6 domain (Y652 and F656) form part of a drug-binding site for high-affinity channel block (Lees-Miller et al., 2000; Mitcheson et al., 2000). We used site-directed mutagenesis to substitute the phenylalanine at position 656 with alanine (F656A) or cysteine (F656C), and tested whether these engineered mutations altered fluoxetine-induced drug block and disruption of protein trafficking. Figure 8a shows Western blot analysis of the effect of fluoxetine. Cells were transiently transfected with the F656A and F656C channel mutations. For control conditions, F656A (lane 1) or F656C (lane 4) showed both the 135 and 155 kDa protein bands, similar to hERG WT protein (see Figure 3a, lane 1). Incubation with 1 μM fluoxetine for 24 h (lanes 2 and 5) showed both the 135 and 155 kDa protein bands, whereas, incubation with 30 μM fluoxetine for 24 h resulted in the disappearance of the 155 kDa protein bands (lanes 3 and 6). Similar results were obtained with three additional Western blot analyses. Figure 8b shows plots of normalized peak tail IhERG recorded from cells transiently transfected with hERG WT, F656A and F656C constructs. Cells were depolarized from a holding potential of −80 to 20 mV for 4 s, followed by a repolarizing step to −120 mV for 5.7 s to record tail current with the protocol applied every 15 s. Following control recordings, perfusion was switched to Tyrode solution containing 1 or 30 μM fluoxetine. Steady-state block of peak tail IhERG was recorded and normalized to the control value. With the F656A mutation, 1 or 30 μM fluoxetine reduced peak tail IhERG by only 7.0±4.2% (n=4 cells) and 17.8±9.0% (n=4 cells), respectively. With the F656C mutation, 1 or 30 μM fluoxetine reduced peak tail IhERG by 2.0±1.0% (n=4 cells) and 13.1±9.0% (n=4 cells), respectively. With hERG WT channels, 1 or 30 μM fluoxetine reduced IhERG by 56.0±7.0% (n=5 cells) and 99.0±4.0% (n=6 cells), respectively. These data show that engineered mutations (F656A and F656C) in the hERG drug-binding domain markedly reduced channel sensitivity to block by fluoxetine, but did not alter fluoxetine-mediated disruption of hERG channel protein trafficking.
Figure 8.
(a) Western blot analysis of F656A and F656C mutations for control conditions and following 24 h incubation with 1 and 30 μM fluoxetine. (b) Plots of normalized peak tail IhERG (square pulse protocol) for WT, and the F656A and F656C mutations, for control conditions and following 1 or 30 μM fluoxetine exposure.
Discussion
Drug-induced QT interval prolongation occurs with many types of cardiovascular and non-cardiovascular drugs (Roden, 2004). The mechanism proposed for most has been direct block of IKr by drug binding to specific amino-acid residues in the pore-S6 region of hERG channels (Lees-Miller et al., 2000; Mitcheson et al., 2000). A second mechanism recently proposed is disruption of post-translational hERG channel protein processing to reduce channel density in the surface membrane. This indirect mechanism was first reported for the anti-cancer drug arsenic trioxide (Ficker et al., 2004), however, direct block of IhERG by arsenic trioxide has also been reported (Drolet et al., 2004). The antiprotozoal agent pentamidine also disrupts hERG channel protein trafficking (Cordes et al., 2005; Kuryshev et al., 2005). These investigators showed that culturing cells in pentamidine selectively reduced expression of hERG (but not KCNQ1, Kv1.5 and Kv4.3) K+ channels, and caused action potential prolongation in guinea pig ventricular myocytes. Direct block of IhERG occurred only at much higher (∼250-fold) pentamidine concentrations (Cordes et al., 2005). Recently, celastrol, a plant extract, was also reported to inhibit the hERG channel biogenesis at concentrations below those that block directly IhERG (Sun et al., 2006).
The present report is the first to show that both fluoxetine and norfluoxetine reduce IhERG at similar drug concentrations, and this is caused by both direct channel block and indirectly by disruption on channel protein trafficking. This supports the idea that both mechanisms may contribute to LQTS. Furthermore, block of IhERG and disruption of hERG protein trafficking was reversible, and fluoxetine did not alter INa and IEndogenous in HEK293 cells. These findings support the conclusion that fluoxetine and norfluoxetine mediated the reduction in IhERG and disruption of hERG channel trafficking is by a non-apoptotic process that appears to be selective for hERG channels, at least with respect to SCN5A and endogenous current channels. We did not study, however, other ion channels or IKr in native heart cells.
Drug binding to a pore-S6 domain of the hERG channel (Lees-Miller et al., 2000; Mitcheson et al., 2000) is thought to mediate direct block of IhERG, whereas the drug-binding site for the selective disruption of hERG channel protein trafficking is unknown. We studied whether the F656A and F656C engineered mutations in the drug-binding domain could alter similarly the drug sensitivity of both the direct and indirect mechanisms. These mutations reduced the sensitivity of IhERG to drug block by fluoxetine, but did not alter the sensitivity to drug-induced disruption of hERG channel protein trafficking. We conclude that direct block of IhERG by fluoxetine is mediated by the pore-S6 drug-binding domain, whereas fluoxetine-induced disruption of hERG channel protein trafficking is mediated by drug binding to a different site on the channel or to a different protein in the secretory pathway.
Despite its widespread use, reports linking QT interval prolongation and cardiac arrhythmias to fluoxetine have been uncommon. The therapeutic steady-state serum concentrations of fluoxetine and norfluoxetine found in humans have been reported to be 0.14–1.36 and 0.22–1.86 μM, respectively (Orsulak et al., 1988), and both compounds are highly protein bound which should reduce free drug levels. Our concentration–response relations suggest that, under normal conditions, serum drug levels are likely to have minimal effects on IhERG and its protein trafficking. However, several clinical settings could potentiate the effect of fluoxetine and norfluoxetine on hERG channels to prolong action potentials and the QT interval. These include drug overdose, underlying ion channel diseases (e.g., congenital LQTS), concomitant administration of other QT prolonging drugs, structural heart disease, hypokalemia, altered hepatic drug metabolism via P450 2D6 (e.g., infants, cirrhosis, etc) or conditions that lower serum protein concentrations.
In summary, fluoxetine and its metabolite, norfluoxetine, act to selectively reduce IhERG by direct and indirect mechanisms. There are several clinical implications to these findings: First, fluoxetine is a widely used antidepressant drug with Prozac having been the sixth best-selling drug worldwide in 2000 before expiration of its patent (http://www.imshealth.com), and in 1999 it was the fourth most commonly prescribed drug in the United States with a potential for QT interval prolongation (Curtis et al., 2003). Thus, disruption of hERG channel protein trafficking may be a more commonly occurring mechanism contributing to acquired LQTS than was previously understood. Second, direct block of IhERG develops rapidly, whereas disruption of protein trafficking develops slowly, thus the development of drug-induced QT interval prolongation in vivo could have different time courses based upon its underlying mechanism. Finally, in vitro drug safety testing to assess hERG channel block usually employs acute screening protocols, which may be inadequate to detect the indirect mechanism where hERG channel density is reduced slowly over time.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL60723 (CTJ), R01 HL71092 (JCM) and T32 HL07936 (LLE), and an American Heart Association Scientist Development Grant (BPD).
Abbreviations
- hERG
human ether-a-go-go-related-gene
- HEK293 cells
human embryonic kidney cells
- IhERG
hERG K+ current
- IKr
rapidly activating delayed rectifier potassium current
- INa
SCN5A Na+ current
- LQTS
long QT syndrome
- SCN5A
voltage-gated cardiac sodium channel gene
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Abebe-Campino G, Offer D, Stahl B, Merlob P. Cardiac arrhythmia in a newborn infant associated with fluoxetine use during pregnancy. Ann Pharmacother. 2002;36:533–534. doi: 10.1345/aph.10255. [DOI] [PubMed] [Google Scholar]
- Allhoff T, Bender S, Banger M, Gastpar M, Sack S, Erbel R, et al. Atrial arrhythmia in a woman treated with fluoxetine: is there a causal relationship? Ann Emerg Med. 2001;37:116–117. doi: 10.1067/mem.2001.111869. [DOI] [PubMed] [Google Scholar]
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- Anson BD, Ackerman MJ, Tester DJ, Will ML, Delisle BP, Anderson CL, et al. Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol. 2004;286:H2434–H2441. doi: 10.1152/ajpheart.00891.2003. [DOI] [PubMed] [Google Scholar]
- Appleby M, Mbewu A, Clarke B. Fluoxetine and ventricular torsade – is there a link? Int J Cardiol. 1995;49:178–180. doi: 10.1016/0167-5273(94)02270-s. [DOI] [PubMed] [Google Scholar]
- Cordes JS, Sun Z, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LH, Ostbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114:135–141. doi: 10.1016/s0002-9343(02)01455-9. [DOI] [PubMed] [Google Scholar]
- Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004;94:1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea. [DOI] [PubMed] [Google Scholar]
- Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109:26–29. doi: 10.1161/01.CIR.0000109484.00668.CE. [DOI] [PubMed] [Google Scholar]
- Dubnov G, Fogelman R, Merlob P. Prolonged QT interval in an infant of a fluoxetine treated mother. Arch Dis Child. 2005;90:972–973. doi: 10.1136/adc.2004.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt L, January CT, Makielski JC, Rajamani S. KCNH2 channel block by the antidepresant drug fluoxetine and its metabolite norfluoxetine. Biophys J. 2005a;88:283a. [Google Scholar]
- Eckhardt LL, Rajamani S, January CT. Protein trafficking abnormalities: a new mechanism in drug-induced long QT syndrome. Br J Pharmacol. 2005b;145:3–4. doi: 10.1038/sj.bjp.0706143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- Hale TW, Shum S, Grossberg M. Fluoxetine toxicity in a breastfed infant. Clin Pediatr (Phila) 2001;40:681–684. doi: 10.1177/000992280104001207. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42:277–285. doi: 10.1081/clt-120037428. [DOI] [PubMed] [Google Scholar]
- Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004;279:44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- Lees-Miller JP, Duan Y, Teng GQ, Duff HJ. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol. 2000;57:367–374. [PubMed] [Google Scholar]
- Lherm T, Lottin F, Larbi D, Bray M, Legall C, Caen D. Torsade de pointes after poisoning with fluoxetine alone. Presse Med. 2000;29:306–307. [PubMed] [Google Scholar]
- Michalets EL, Smith LK, Van Tassel ED. Torsade de pointes resulting from the addition of droperidol to an existing cytochrome P450 drug interaction. Ann Pharmacother. 1998;32:761–765. doi: 10.1345/aph.17351. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S, Zhou Z, Gong Q, January CT. Blockage of the HERG human cardiac K+ channel by the gastrointestinal prokinetic agent cisapride. Am J Physiol. 1997;273:H2534–H2538. doi: 10.1152/ajpheart.1997.273.5.H2534. [DOI] [PubMed] [Google Scholar]
- Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 2005;39:543–546. doi: 10.1345/aph.1E513. [DOI] [PubMed] [Google Scholar]
- Orsulak PJ, Kenney JT, Debus JR, Crowley G, Wittman PD. Determination of the antidepressant fluoxetine and its metabolite norfluoxetine in serum by reversed-phase HPLC with ultraviolet detection. Clin Chem. 1988;34:1875–1878. [PubMed] [Google Scholar]
- Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Mitcheson JS. Predicting drug-hERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol Sci. 2005;26:119–124. doi: 10.1016/j.tips.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Sun H, Liu X, Xiong Q, Shikano S, Li M. Chronic inhibition of cardiac Kir2.1 and HERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J Biol Chem. 2006;281:5877–5884. doi: 10.1074/jbc.M600072200. [DOI] [PubMed] [Google Scholar]
- Thomas D, Gut B, Wendt-Nordahl G, Kiehn J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther. 2002;300:543–548. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- Upward JW, Edwards JG, Goldie A, Waller DG. Comparative effects of fluoxetine and amitriptyline on cardiac function. Br J Clin Pharmacol. 1988;26:399–402. doi: 10.1111/j.1365-2125.1988.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia CR, Ackerman MJ, Tester DJ, Wada T, McCormack J, Ye B, et al. A novel SCN5A arrhythmia mutation, M1766L, with expression defect rescued by mexiletine. Cardiovasc Res. 2002;55:279–289. doi: 10.1016/s0008-6363(02)00445-5. [DOI] [PubMed] [Google Scholar]
- Varriale P. Fluoxetine (Prozac) as a cause of QT prolongation. Arch Intern Med. 2001;161:612. doi: 10.1001/archinte.161.4.612. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting I, Smals OM, Holwerda NJ, Meyboom RH, de Bruin ML, Egberts TC. QTc prolongation and torsades de pointes in an elderly woman taking fluoxetine. Am J Psychiatry. 2006;163:325. doi: 10.1176/appi.ajp.163.2.325. [DOI] [PubMed] [Google Scholar]
- Witchel HJ, Pabbathi VK, Hofmann G, Paul AA, Hancox JC. Inhibitory actions of the selective serotonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett. 2002;512:59–66. doi: 10.1016/s0014-5793(01)03320-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem. 1998a;273:21061–21066. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, et al. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998b;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]