Abstract
Background and purpose:
The present study investigated whether calcium-activated K+ channels are involved in acetylcholine-evoked nitric oxide (NO) release and relaxation.
Experimental approach:
Simultaneous measurements of NO concentration and relaxation were performed in rat superior mesenteric artery and endothelial cell membrane potential and intracellular calcium ([Ca2+]i) were measured.
Key results.
A combination of apamin plus charybotoxin, which are, respectively, blockers of small-conductance and of intermediate- and large-conductance Ca2+-activated K channels abolished acetylcholine (10 μM)-evoked hyperpolarization of endothelial cell membrane potential. Acetylcholine-evoked NO release was reduced by 68% in high K+ (80 mM) and by 85% in the presence of apamin plus charybdotoxin. In noradrenaline-contracted arteries, asymmetric dimethylarginine (ADMA), an inhibitor of NO synthase inhibited acetylcholine-evoked NO release and relaxation. However, only further addition of oxyhaemoglobin or apamin plus charybdotoxin eliminated the residual acetylcholine-evoked NO release and relaxation. Removal of extracellular calcium or an inhibitor of calcium influx channels, SKF96365, abolished acetylcholine-evoked increase in NO concentration and [Ca2+]i. Cyclopiazonic acid (CPA, 30 μM), an inhibitor of sarcoplasmic Ca2+-ATPase, caused a sustained NO release in the presence, but only a transient increase in the absence, of extracellular calcium. Incubation with apamin and charybdotoxin did not change acetylcholine or CPA-induced increases in [Ca2+]i, but inhibited the sustained NO release induced by CPA.
Conclusions and Implications:
Acetylcholine increases endothelial cell [Ca2+]i by release of stored calcium and calcium influx resulting in activation of apamin and charybdotoxin-sensitive K channels, hyperpolarization and release of NO in the rat superior mesenteric artery.
Keywords: acetylcholine, endothelium, superior mesenteric artery, K+ channels, nitric oxide
Introduction
Acetylcholine (ACh)-evoked vasodilatation is thought to depend on nitric oxide (NO) synthesized by endothelial NO synthase (NOS), prostanoids as well as a non-NO/non-prostanoid endothelium-derived hyperpolarizing factor (EDHF) (Busse et al., 2002). Several candidates for EDHF have been suggested, including potassium ions (Edwards et al., 1998), products of the cytochrome P450 pathway (Fulton et al., 1992; Bauersachs et al., 1994; Bolz et al., 2000; Fisslthaler et al., 2000), C-type natriuretic peptide (CNP) (Chauhan et al., 2003b) and, controversially, hydrogen peroxide (Matoba et al., 2000; Ellis et al., 2003). Both K+ and CNP are believed to activate the Na+/K+-ATPase and/or inward rectifier K+ channels (KIR) channels situated on the vascular smooth muscle cells (Edwards et al., 1998; Chauhan et al., 2003b). Other studies have attributed agonist-evoked non-NO/non-prostanoid relaxation to communication by myoendothelial gap junctions (Chaytor et al., 1998; Yamamoto et al., 1999; Sandow and Hill, 2000; Coleman et al., 2001; Taylor et al., 2001).
The respective roles of NO and EDHF in hyperpolarization of vascular smooth muscle induced by ACh is unclear. ACh-induced hyperpolarization could not be reproduced by authentic NO or NO-donor drugs in some studies (Garland et al., 1995; Buus et al., 2000), and EDHF-type relaxations in small arteries are not associated with increases in NO (Buus et al., 2000). In contrast, in rabbit carotid artery, measurements of the NO concentration revealed that there was NO release in the presence of an inhibitor of NOS (Cohen et al., 1997) and in rat superior mesenteric arteries the combination of an NOS inhibitor and oxyhaemoglobin abolished the residual NO and persisting relaxation in response to ACh (Simonsen et al., 1999; Stankevicius et al., 2002). Moreover, inhibition of guanylyl cyclase was recently shown to inhibit ACh-induced vasodilatation persisting in the presence of NOS inhibitors in the femoral artery of young piglets (Stoen et al., 2003). Furthermore, NO was able to activate, directly, Ca2+-activated K channels in rabbit aorta (Bolotina et al., 1994), and NOS inhibition blunted both ACh-induced relaxation and hyperpolarization in uterine arteries (Tare et al., 1990). Therefore, in large conduit arteries, the possibility that NO contributes to relaxations attributed to EDHF cannot be excluded.
The inhibition of non-NO/non-prostanoid relaxations by apamin and charybdotoxin, blockers of small and intermediate-conductance Ca2+-activated K+ channels, has been considered a unique characteristic (Zygmunt and Hogestatt, 1996; Edwards et al., 1998; Yamamoto et al., 1999; Buus et al., 2000). ACh-evoked hyperpolarization of the endothelial cell coincides with rises in intracellular calcium ([Ca2+]i) in endothelial cells in intact rat aorta (Carter and Ogden, 1994; Usachev et al., 1995), and patch-clamp experiments and reverse transcriptase-polymerase chain reaction (RT-PCR) have provided evidence for the presence of small-, intermediate- and large-conductance Ca2+-activated K+ channels in endothelial cells of intact arteries (Marchenko and Sage 1996; Kohler et al., 2000; Nilius and Droogmans, 2001). Therefore, it has been suggested that apamin and charybdotoxin may exert their effects mainly via Ca2+-activated K+ channels located on the endothelial cells rather than on smooth muscle cells (Edwards et al., 1998; Beny and Schaad, 2000; Burnham et al., 2002). In the superior mesenteric artery, the combination of apamin and charybdotoxin also inhibited smooth muscle hyperpolarization and ACh relaxation (Chen and Cheung, 1997).
Therefore, we hypothesized in the present study that ACh-evoked increases in NO concentration and relaxation would be preceded by increases in [Ca2+]i in endothelial cells causing activation of Ca2+-activated K+ channels and endothelial NOS. To test this hypothesis, we performed measurements of endothelial membrane potential, simultaneous measurements of NO concentration and relaxation, and measurements of [Ca2+]i in endothelial cells in the absence and the presence of blockers of Ca2+-activated K+ channels .
Materials and methods
Membrane potential measurements
Adult male Wistar rats (12–16 weeks old) were obtained from Møllegaard Breeding Center (Skensved, Denmark). The animals were killed humanely by cervical dislocation and exsanguinated by decapitation. All experimental procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications No. 85-23, revised 1996).
Segments from the proximal part between aorta and the first branch of the superior mesenteric artery were dissected in cold (4°C) physiological salt solution (PSS) and mounted onto two 100-μm wires in a single myograph bath. A ‘U' shape was cut out of the arterial segment at one end to allow direct access to the endothelial cells, as described previously (Simonsen et al., 1999). To test contractility, the segments were stimulated twice with 5 μM noradrenaline (NA), and after washout, the preparations were incubated with an inhibitor of cyclooxygenase, indomethacin (3 μM), throughout the experiment. A dihydraulic micromanipulator (Narishige MW3) was attached to the myograph stage, allowing intracellular potential recordings of the endothelial cells to be made with glass electrodes inserted via the U-shaped cut. The electrode was connected to an amplifier (M-707, World Precision Instruments, Stevenage, UK). The electrodes were prepared on a horizontal puller (Sutter P87, World Precision Instruments) from aluminosilicate glass and had resistances of 40–80 MΩ when filled with 3 M KCl. The criterion for an acceptable membrane potential measurement in endothelial cells was an abrupt change in voltage when penetrating the cell and an abrupt change when the electrode was withdrawn. When the electrode went into the subintimal space a potential change of 10–17 mV was observed and a downward deformation of the preparation was observed when trying to pass the electrode through the internal elastic membrane. In these cases, the electrode was retracted and recordings were not made.
Recordings were obtained in the presence of indomethacin (3 μM), and in the presence of indomethacin and the combination of apamin (500 nM) and charybdotoxin (100 nM) or barium (30 μM) and ouabain (100 μM).
Simultaneous measurements of NO concentration and force
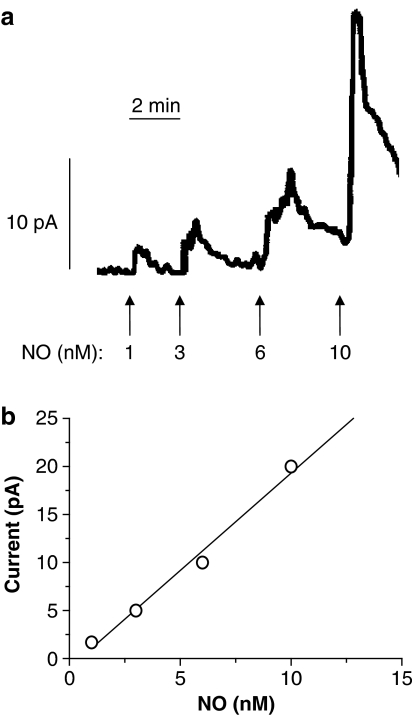
For simultaneous measurement of force and NO concentration, an NO-sensitive microelectrode (ISONOP3020, World Precision Instruments) with a diameter of 30–50 μm was first calibrated by use of NO solution and then introduced into the lumen of the artery mounted in the myograph, as described previously (Simonsen et al., 1999). The microsensors responded with increases in current to nanomolar concentrations of NO (Figure 1a), and the output current of the microsensors correlated linearly with the concentration of NO (Figure 1b). The sensitivity of different microsensors varied between 0.15 and 2.20 nM pA−1 with averages of 0.59±0.15 nM pA−1 for 20 different microsensors. In some experiments, calibration of the electrode was performed before and after the experimental protocol and the sensitivity remained unchanged (n=5 electrodes). To test selectivity of the electrodes, a lack of response to sodium nitrite up to 10 μM was taken as evidence for an intact coating of the electrode. NA is oxidized on carbon fibres, where coating is damaged, and electrodes were discarded if NA (0.5–1 μM) in the absence of vascular tissues increased electrode current.
Figure 1.
(a) Representative recordings of NO microsensor calibration performed in PSS at 37°C with constant stirring. The vertical bars below the trace indicate injection of increasing concentrations of NO. The horizontal bar indicates time. (b) Linear regression analysis of the relationship between amount of NO added and current output of the microsensor for one electrode tip (ISONOP3020), r2=0.9878, 1 pA=0.49 nM.
To investigate the role of NO, simultaneous measurements of force and NO concentration was performed in the absence and the presence of increasing concentrations of an inhibitor of NOS, NG,NG-asymmetric dimethyl-L-arginine (ADMA, 0.1–1 mM), a scavenger of free NO, oxyhaemoglobin (10 μM) and the combination of ADMA and oxyhaemoglobin.
Treatments blocking K+ channels and also causing inhibition of the ACh-induced relaxations were examined to clarify whether it was an effect on the endothelial or smooth muscle cells. The segments were contracted with NA (0.5 μM) and the simultaneous changes in force and increases in NO concentration were measured when ACh (10 μM) was added. To evaluate the effect on ACh relaxation and NO release of raising the K+ concentration (50 and 80 mM), the bath solution was changed. This caused an abrupt change in current and when the contraction reached plateau and the NO-sensitive electrode current had stabilized, ACh was added. In case of the K+ channel blockers, responses were obtained for ACh (10 μM) in the absence and the presence of barium chloride (BaCl2) (30 μM), charybdotoxin (100 nM), apamin (0.5 μM), the combination of apamin and charybdotoxin or apamin plus charybdotoxin and ADMA (300 μM).
Measurements of endothelial cell calcium
The superior mesenteric artery was everted (intimal surface outside lumen) as described earlier for porcine coronary segments (Tanko et al., 1999), and mounted in myograph baths as described above. As described previously (Jensen et al., 1992), the myograph was mounted on an inverted microscope equipped for dual excitation wavelength fluorescence microfluorimetry with a neofluar objective × 10 (Carl Zeiss, Göttingen, Germany). At 10-s intervals, the vessel was briefly illuminated with 340/380 nm light, and emitted light collected through a 500–530 nm bandpass filter connected to a photomultiplier. Emission intensities at the two excitation wavelenghts (F340 and F380) and force were sampled at intervals of 0.1–10 s as required using commercial software (Felix Software version 1.41, Photon Technology International, Monmouth Junction, NJ, USA). Changes in fluorescence were expressed as the ratio of F340/F380.
To load the endothelial cell layer selectively, the experiments were performed at room temperature. The everted vessel was initially incubated in PSS containing 6.5 μM FURA-2-AM, 0.5% dimethylsulphoxide and 0.1% Cremophor EL for 2 h. The preparation was washed and relaxation responses were obtained for ACh (10 μM) and cyclopiazonic acid (30 μM) in NA (0.5 μM)-contracted segments in the absence and the presence of the combination of apamin (0.5 μM) and charybdotoxin (0.1 μM). Endothelium-denuded everted preparations were also mounted.
To investigate whether incubation with FURA-2 leads to loading of the smooth muscle layer at room temperature, vessels were mounted in normal configuration and incubated in PSS containing 6.5 μM FURA-2-AM, 0.5% dimethylsulphoxide and 0.1% Cremophor EL for 2 h. To check, other preparations were mounted in normal configuration at 37°C and were loaded with FURA-2-AM. In endothelium-intact segments mounted in normal configuration and loaded with FURA-2 at room temperature, there was no measurable FURA-2 signal (n=5), but at 37°C the smooth muscle layer was loaded and NA caused a pronounced increase in smooth muscle calcium, which was lowered by the addition of ACh (data not shown).
Statistics
The mechanical responses of the vessels were measured as force and expressed as active wall tension, ΔT, which is the increase in measured force, ΔF, divided by twice the segment length. By using a computer programme (GraphPad, Institute for Scientific Information, San Diego, CA, USA), the concentration–response curves were fitted to the classical Hill equation, as described earlier (Simonsen et al., 1997). Sensitivity to the agonists is expressed in terms of pD2=−log (EC50), EC50 being the concentration (M) of agonist required to give half-maximal relaxation.
The results are expressed as means±s.e.m., and the response curves presented on a semilogarithmic scale. Differences between means were analysed using either one-way analysis of variance followed by a Bonferroni t-test, Student's t-test or paired t-test as appropriate; P<0.05 was considered significant.
Drugs
ACh HCl, apamin, ADMA, BaCl2, charybdotoxin, CPA, 1-ethyl-2-benzimidazolinone (1-EBIO), glibenclamide, iberiotoxin, indomethacin, (−)-NA HCl, potassium nitrite, pyrogallol, sodium nitrite (NaNO2), potassium iodide (Sigma-Aldrich, Vallenbæk, Denmark) and S-nitroso-N-acetylpenicillamine (SNAP) (GEA Ltd, Copenhagen, Denmark). FURA-2-AM was obtained from Molecular Probes (Eugene, OR, USA). Stock solution of indomethacin (10 mM) was prepared in equimolar Na2CO3 solution with pH adjusted to 7.4 and diluted in PSS before use. CPA was dissolved in dimethylsulphoxide and further diluted in distilled water. Other drugs were prepared in distilled water, which in the case of SNAP had been deoxygenated with nitrogen.
Results
ACh-evoked hyperpolarization
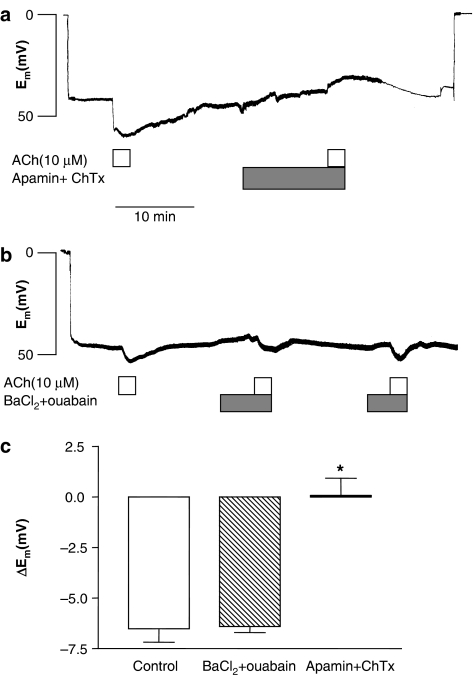
The resting endothelial cell membrane potential was −42.5±2.3 mV (n=26). Superfusion with ACh (10 μM) evoked a hyperpolarization that showed a rapid transient followed by sustained phase. Peak hyperpolarization induced by ACh (10 μM) was 6.6±0.8 mV (n=10) (Figure 2). Incubation with a combination of apamin (0.5 μM) and charybdotoxin (0.1 μM) did not change the resting membrane potential, but it abolished ACh-elicited hyperpolarization (n=6) (Figure 2a and c). The addition of BaCl2 (30 μM) and ouabain (100 μM) in combination depolarized the membrane potential 3.0±1.4 mV (n=3), but it did not affect ACh hyperpolarization (Figure 2b and c).
Figure 2.
Original recordings of endothelial cell membrane potential in two arterial segments showing that (a) ACh (10 μM)-induced hyperpolarization is abolished in the presence of apamin (500 nM) and charybdotoxin (100 nM), whereas (b) ACh induces hyperpolarization in the absence and the presence of BaCl2 (30 μM) and ouabain (100 μM). (c) Average of ACh-evoked hyperpolarization in the absence and in the presence of the combination of BaCl2 (30 μM) and ouabain (100 μM) or apamin (500 nM) and charybdotoxin (100 nM). The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from three to seven animals. Significant differences evaluated by one-way analysis of variance (ANOVA) followed by unpaired t-test: *P<0.05 versus control.
Effect of ADMA and oxyhaemoglobin on ACh-evoked NO concentration and relaxation
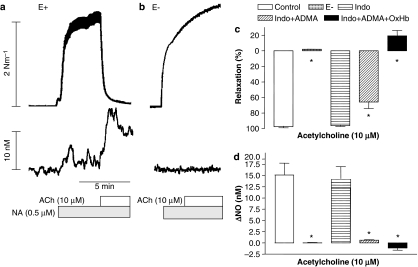
In rat superior mesenteric artery segments with intact endothelium, contractions evoked by NA were associated with an increase in NO concentration (3.0±0.9 nM; n=14). Addition of ACh caused a further marked increase in the NO concentration (15.3±1.8 nM) and relaxed the artery (97.6±1.3%) (Figure 3a). Repeated activation with NA and ACh revealed that the increases in NO concentration and relaxation were reproducible (n=6, data not shown). In preparations without endothelium, the contraction induced by NA was increased (6.37±0.66 nM−1, n=4, P<0.05), and there was no change in NO concentration in response to NA or ACh (n= 4) (Figure 3b).
Figure 3.
Oxyhaemoglobin reverses relaxations and increases in NO concentration persisting in the presence of an NOS inhibitor. Original trace showing recording of simultaneous changes in tension (upper traces) and NO concentration (lower traces) in a segment of rat superior mesenteric artery (a) with endothelium and (b) after mechanical removal of the endothelium. (c and d) Average increases in NO concentration and relaxations induced by ACh (10 μM) in NA (0.5 μM)-contracted preparations in the absence and the presence of indomethacin (3 μM), indomethacin and ADMA (300 μM), and indomethacin, ADMA plus oxyhaemoglobin (OxHb, 10 μM). The points are means±s.e.m. of arteries from four to five animals. Significant differences evaluated by one-way ANOVA followed by unpaired t-test: *P<0.05 versus control.
Incubation with the cyclooxygenase inhibitor, indomethacin (3 μM), induced a transient contractile response corresponding to 20–25% of the response to 0.5 μM NA, but the NA contraction (data not shown) as well as responses to ACh were unchanged in the presence of indomethacin (n=10) (Figure 3c and d). In the presence of indomethacin, incubation with an inhibitor of NOS, ADMA (0.1–1 mM) reduced, but did not eliminate, ACh-evoked increases in NO concentration and relaxation. Further addition of oxyhaemoglobin abolished the persisting increases in NO concentration and relaxation (Figure 3c and d).
Effect of Ca2+-activated K+ channel blockers on ACh-evoked NO concentration and relaxation
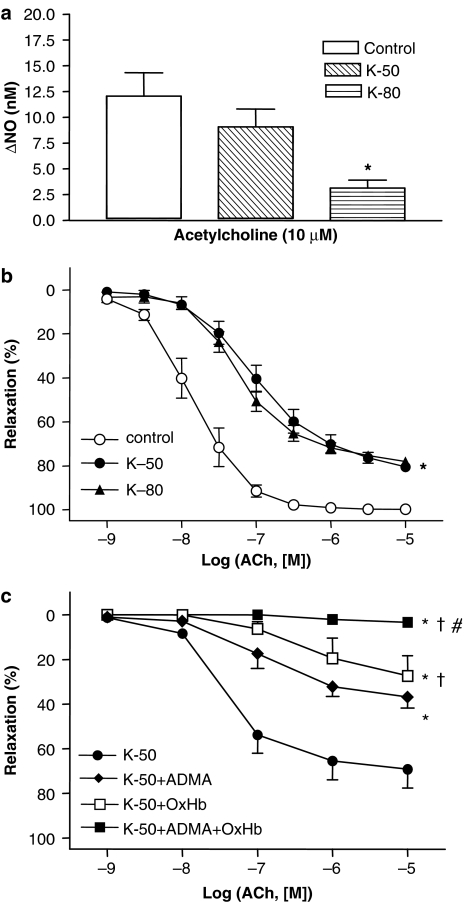
In segments contracted by 50 mM K+, ACh (10 μM) caused less relaxation than in NA-stimulated arteries; ACh-induced increases in NO concentration were reduced under these conditions, but this was not statistically significant (Figure 4a and b). Increasing the K+ concentration to 80 mM reduced both the maximal relaxation and the increase in NO concentration in response to ACh (Figure 4a and b), despite similar pre-contraction. Incubation with ADMA (300 μM) or oxyhaemoglobin (10 μM) inhibited ACh relaxation in preparations contracted by 50 mM K+, but only the combination of ADMA plus oxyhaemoglobin abolished ACh relaxation (Figure 4c).
Figure 4.
Raising the extracellular K+ concentration inhibits ACh-evoked increases in NO concentration. Average simultaneous increases in (a) NO concentration and (b) relaxations induced by ACh in rat superior mesenteric arterial segments contracted with either NA (6.7±0.8 Nm−1, n=6), 50 mM K+ (K-50, 6.0±0.4 Nm −1, n=6) or 80 mM K+ (K-80, 8.1±0.7 Nm−1, n=4). (c) Concentration–response curves for ACh in arterial segments contracted with 50 mM K+ in the absence and the presence of ADMA (300 μM), oxyhaemoglobin (OxHb, 10 μM) and ADMA plus OxHb. The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from five to seven animals. *P<0.05 versus control, †P<0.05 versus ADMA and #P<0.05 versus OxHb.
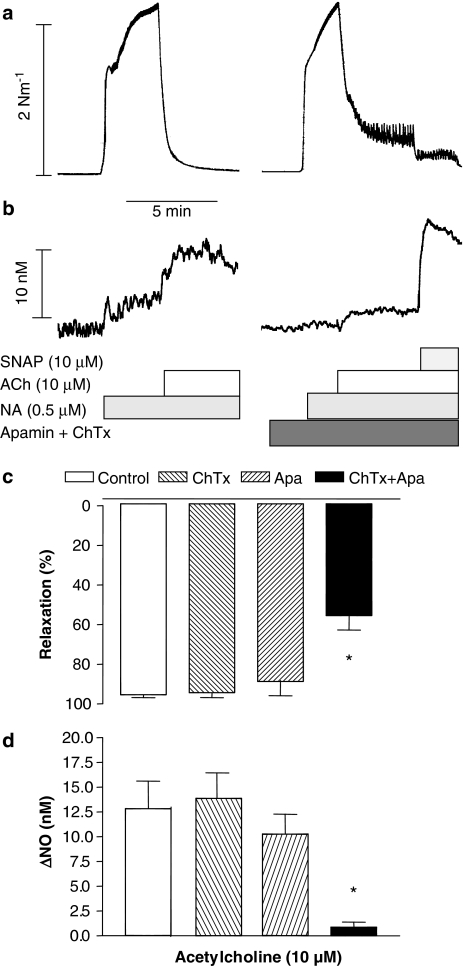
Incubation with either the blocker of small-conductance Ca2+-activated K channels, apamin (0.5 μM), the blocker of intermediate- and large-conductance Ca2+-activated K channels, charybdotoxin (0.1 μM), or the blocker of large-conductance Ca2+-activated K channels, iberiotoxin (10 nM) did not cause changes in resting tension or concentration–response curves for ACh (Table 1). Charybdotoxin (0.1 μM) or apamin (0.5 μM) applied alone changed neither the increases in NO concentration nor the relaxations evoked by ACh (Figure 5).
Table 1.
Concentration–response curves for acetylcholine in the rat superior mesenteric artery
| n | NA (Nm−1) |
Acetylcholine |
||
|---|---|---|---|---|
| pD2 | Maximum relaxation (%) | |||
| Control | 5 | 2.6±0.6 | 7.45±0.42 | 99±1 |
| Apamin | 6 | 3.2±0.6 | 8.11±0.26 | 98±1 |
| Control | 7 | 4.0±0.4 | 7.68±0.16 | 99±2 |
| ChTx | 7 | 4.6±0.2 | 7.80±0.45 | 99±1 |
| Control | 6 | 4.0±0.3 | 7.28±0.17 | 99±1 |
| IbTx | 6 | 4.7±0.4 | 7.36±0.17 | 94±5 |
| Control | 6 | 4.3±0.3 | 7.33±0.30 | 98±1 |
| Apamin+IbTx | 6 | 4.4±0.1 | 7.41±0.09 | 98±1 |
| Control | 6 | 4.2±0.5 | 8.64±0.23 | 98±1 |
| Apamin+ChTx | 6 | 4.6±0.2 | 7.05±0.02* | 69±8* |
Abbreviation: NA, noradrenaline.
Concentration–response curves were obtained in the absence or the presence of apamin (0.5 μM) charybdotoxin (ChTx, 0.1 μM), and iberiotoxin (IbTx, 0.1 μM), or the combination. Values are means±s.e.m., n, number of vessels, pD2=−log (EC50), where EC50 is the concentration of acetylcholine required to produce half-maximal relaxation. Significantly different point evaluated by paired t-test:
P<0.05 versus control.
Figure 5.
Effect of the combination of apamin and charybdotoxin on ACh-evoked relaxations and NO concentrations. Original trace recordings showing simultaneous changes in tension (a) and NO concentration (b) in a vascular segment of the rat superior mesenteric artery. Contraction was induced by NA and a first control relaxation and increase in NO concentration was obtained for ACh, and a second response in the presence of apamin (500 nM) and charybdotoxin (100 nM). Finally, the NO donor, SNAP, was added. Average increases in (d) NO concentration and (c) relaxations induced by ACh in the absence or the presence of both apamin and charybdotoxin. The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from six animals. *P<0.05 versus control.
In contrast, the combination of apamin (0.5 μM) and charybdotoxin (0.1 μM) significantly reduced relaxation induced by ACh in NA-activated arteries (Table 1). Apamin and charybdotoxin did not affect contraction induced by 50 mM K+ (4.8±0.7 nM−1, n=4 versus4.6±0.1 nM−1, n=4), and there was no effect of the combination of these K+ channel blockers on the relaxations induced by ACh in 50 mM K+-activated preparations. ACh relaxed 50 mM K+-contracted preparations with pD2-values and maximal relaxations of 7.08±0.11 and 79±8% (n=4), respectively, in the absence, and 7.30±0.28 and 80±8% (n=4), respectively, in the presence of apamin and charybdotoxin. In the presence of apamin and charybdotoxin, the increase in NO concentration and relaxation evoked by ACh was reduced (Figure 5b and c). Charybdotoxin and apamin did not significantly affect the increase in NO concentration owing to the NO donor, SNAP (10 μM) (18.1±2.0 nM versus 16.2±6.8 nM, n=5).
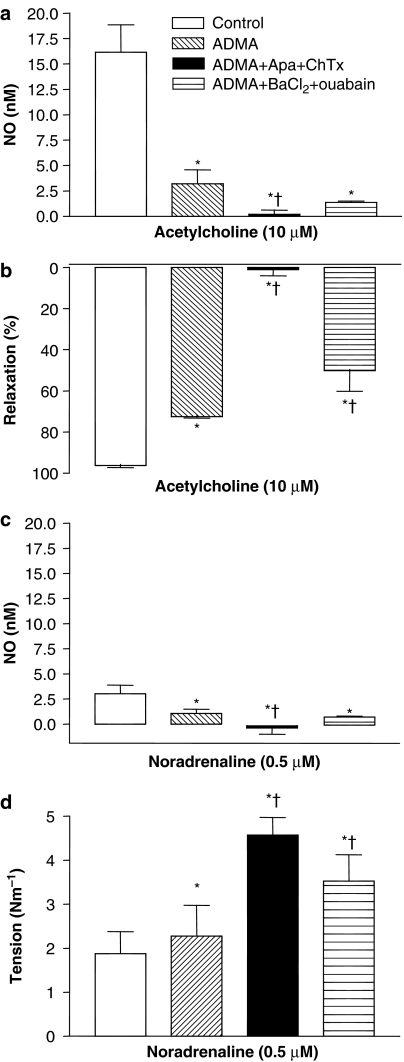
In the presence of ADMA and indomethacin, the combination of apamin and charybdotoxin reduced ACh-induced increases in NO concentration further and abolished relaxation, whereas addition of the combination of barium and ouabain further reduced relaxation (Figure 6a and b).
Figure 6.
Effect of the endogenous NOS inhibitor, ADMA, and the combination of apamin and charybdotoxin. Average increases in (a) NO concentration and (b) relaxations induced by ACh in the absence or the presence of ADMA (300 μM), ADMA, apamin (500 nM) and charybdotoxin (100 nM), and ADMA, BaCl2 (30 μM) and ouabain (100 μM). (c and d) Effect of these treatments on NA-evoked NO concentration and contraction. The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from five to six animals. *P<0.05 versus control and †P<0.05 versus ADMA.
Incubation with indomethacin and ADMA markedly reduced the increases in NO concentration induced by NA and increased contraction (Figure 6c and d), and the increases in NO were further reduced and contraction enhanced by the combination of ADMA, apamin and charybdotoxin (Figure 6), whereas the combination of ADMA, barium and ouabain further increased NA contraction compared to ADMA alone.
A putative opener of small- and intermediate-conductance Ca2+-activated K+ channels (Syme et al., 2000), 1-EBIO (10 μM), increased NO concentration by 3.9±0.9 nM (n=6), and further addition of oxyhaemoglobin (10 μM) reversed 1-EBIO-evoked increases in NO concentration and lowered the NO concentration (4.0±1.1 nM) below baseline levels (n=3).
Role of extracellular calcium for the release of NO in rat superior mesenteric artery
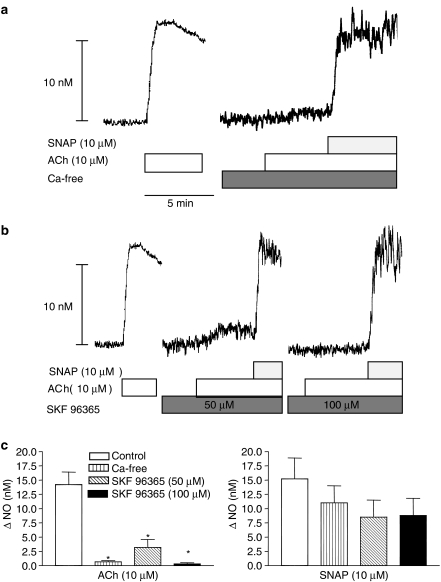
ACh added at resting tension in the presence of extracellular calcium increased NO concentration, but after removal of extracellular calcium, the response was abolished and not even a transient increase in NO was detected (Figure 7a). SNAP-evoked increases in NO concentration were unaltered in calcium-free solution (Figure 7a). Incubation with a blocker of calcium influx channels, SKF 96365 (100 μM), inhibited the concentration-dependent, ACh-evoked increases in NO concentration, whereas SNAP-evoked increases in NO concentration were unaltered (Figure 7b and c).
Figure 7.
Role of extracellular calcium for the release of NO in rat superior mesenteric artery. Original recordings of four different vascular segments from two animals showing (a) increase in NO concentration to ACh in the presence and the absence of extracellular calcium, and (b) in the absence and the presence of the inhibitor of the store-operated calcium channels, SKF 96365. (c) Average of increases in NO concentration to ACh in the presence and the absence of Ca2+, and SKF 96365. The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from six animals. *P<0.05 versus control, Student's t-test.
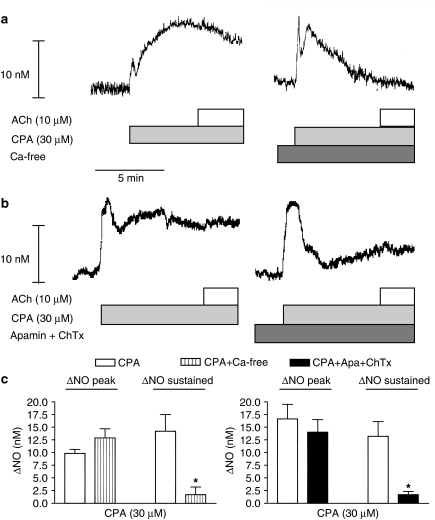
An inhibitor of sarcoplasmic reticulum calcium ATPase, CPA (30 μM), induced a sustained increase in NO concentration in the presence, and a transient increase in NO concentration in the absence, of extracellular calcium (Figure 8a). In the presence of CPA, ACh did not increase the NO concentration further (Figure 8b and c). In the presence of the combination of apamin and charybdotoxin, CPA only evoked a transient increase in NO concentration (Figure 8).
Figure 8.
Effect of apamin and charybdotoxin on CPA-induced increase in NO on the rat superior mesenteric artery. Original recordings of two different vascular segments showing increase in NO concentration, measured with the same microsensor, to CPA (a) in the presence and the absence of extracellular calcium, and (b) in the absence and the presence of apamin (500 nM) and charybdotoxin (100 nM). The ACh was added at the height of the CPA-induced increase in NO concentration. (c) Average of increases in NO concentration to CPA in the absence and the presence of extracellular calcium, and apamin plus charybdotoxin. The experiments were performed in the presence of 3 μM indomethacin. The points are means±s.e.m. of arteries from four to six animals. *P<0.05 versus control, Student's t-test.
Effect of Ca2+-activated K channel blockers on endothelial cell calcium in the rat superior mesenteric artery
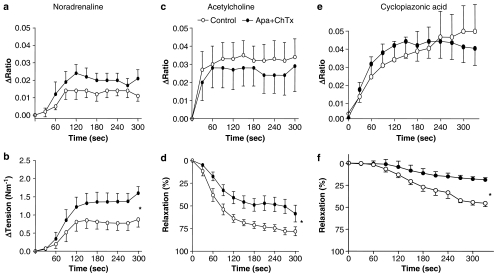
In endothelium-denuded, everted segments of the superior mesenteric artery kept at room temperature, there was no measurable FURA-2 signal (n=3). In preparations with endothelium, increases in endothelial cell [Ca2+]i evoked by ACh (10 μM) were reproducible increasing FURA-2 ratio with, respectively, 0.035±0.012 and 0.038±0.015 (n=4) in a first and second stimulation. ACh induced a sustained increase in endothelial cell [Ca2+]i in the presence (Figure 9a), but only caused a transient increase in [Ca2+]i in the absence, of extracellular calcium (n=2, data not shown). Incubation with nifedipine (1 μM) did not change ACh- and CPA-induced increases in [Ca2+]i, but the combination of nifedipine and SKF96365 (100 μM) abolished the sustained increases in [Ca2+]i induced by ACh- and CPA-evoked increases (n=4–6, data not shown). Incubation with apamin and charybdotoxin did not alter changes in [Ca2+]i evoked by NA, ACh and CPA, but inhibited relaxations induced by ACh and CPA (Figure 9b and c).
Figure 9.
Simultaneous measurements of endothelial cell calcium and tension in the rat superior mesenteric artery. NA-evoked increases (a) in endothelial cell calcium and (b) tension were, respectively, decreased and enhanced in the presence of the combination of apamin (500 nM) and charybdotoxin (100 nM). Addition of (c and d) ACh (10 μM) and (e and f) CPA (30 μM) increased endothelial cell calcium and caused relaxation. Apamin plus charybdotoxin did not change endothelial cell calcium (c and e), but decreased ACh and CPA-evoked relaxation (d and f). Changes in FURA-2 fluorescence is expressed as increases in ratio (Δ ratio) of emission intensities at the two excitation wavelengths (340 versus 380 nm). Basal fluorescence intensity was 0.828±0.010 and 0.832±0.013 (n=8), respectively, in the absence and the presence of apamin plus charybdotoxin. The results are means±s.e.m. of arteries from eight animals. The measurements were paired. Significant differences between curves were evaluated by comparison of the area under the curve followed by a paired t-test: *P<0.05 versus control.
Effect of Ca2+-activated K channel blockers on NO donor, SNAP-evoked relaxation
In arteries without endothelium, sensitivity to SNAP was similar in preparations contracted with 50 mM K+ or with NA, whereas maximal relaxation was reduced (Table 2). In NA-contracted arteries, SNAP relaxations were unchanged in the presence of the combination of apamin and charybdotoxin, whereas concentration–response curves for NO were shifted to the right. The combination of barium and ouabain did not change concentration–response curves for SNAP and NO (Table 2).
Table 2.
Concentration–response curves for SNAP and NO in the rat superior mesenteric artery without endothelium
| n |
SNAP |
NO |
|||
|---|---|---|---|---|---|
| pD2 | Maximal relaxation (%) | pD2 | Maximal relaxation (%) | ||
| Control | 5 | 6.72±0.26 | 91±3 | — | — |
| 50 mM K+ | 6 | 6.31±0.42 | 77±1* | — | — |
| Control | 7 | 6.27±0.07 | 91±2 | 6.13±0.04 | 93±1 |
| Apamin+ChTx | 7 | 6.22±0.08 | 87±2 | 5.94±0.07* | 89±2 |
| Control | 6 | 6.43±0.20 | 98±1 | 6.44±0.20 | 100±0 |
| Ba+Ouabain | 6 | 6.78±0.21 | 97±3 | 6.71±0.30 | 99±1 |
Abbreviations: NO, nitric oxide; SNAP, S-nitroso-N-acetylpenicillamine.
Concentration–response curves were obtained in the absence or the presence of potassium, noradrenaline (0.5 μM, control) and the combination of apamin (0.5 μM) and charybdotoxin (ChTx, 0.1 μM), or BaCl2 (30 μM) and ouabain (100 μM). Values are means±s.e.m., n, number of vessels, pD2=−log (EC50), where EC50 is the concentration of SNAP or NO required to produce half-maximal relaxation. Significantly different point evaluated by paired t-test:
P<0.05 versus control.
Discussion
This study provides the first direct evidence showing endothelial Ca2+-activated K+ channels are involved in ACh-evoked NO release. We have shown that in intact arteries ACh increases [Ca2+]i, hyperpolarizes the endothelial cell layer and leads to the release of NO from endothelial cells. Moreover, ACh-induced NO release is inhibited by depolarization with high K+. Inhibition of relaxation by the combination of the Ca2+-activated K+ channel blockers, apamin and charybdotoxin, has been considered a unique characteristic for EDHF relaxation (Zygmunt and Hogestatt 1996; Edwards et al., 1998). However, our findings suggest that Ca2+-activated K+ channels in endothelial cells, either directly or indirectly, are also involved in ACh-evoked NO release in rat superior mesenteric artery.
Role of NO in ACh-evoked vasodilatation in rat superior mesenteric artery
In contrast to mesenteric small arteries, where ACh induces maximal relaxations in the presence of an inhibitor of NOS and oxyhaemoglobin, in the rat superior mesenteric artery, inhibition of the NO-L-arginine pathway almost abolished the relaxations induced by ACh (Hwa et al., 1994; Van de Voorde and Vanheel, 1997; Simonsen et al., 1999). In the present study, incubation with the endogenous NOS inhibitor, ADMA, lowered ACh-evoked NO concentration and relaxation to levels similar to NG-nitro-L-arginine (L-NOARG) (Simonsen et al., 1999), but in contrast to L-NOARG it did not have the inconvenience of increasing basal NO levels. Both in the presence of L-NOARG (Simonsen et al., 1999) and in the presence of a maximal concentration of ADMA in the present study, ACh increased the concentration of NO. Although simultaneous measurements showed increases in NO concentration and relaxation induced by ACh are temporally related, the relationship between increases in endogenous NO concentration and relaxation seems exponential rather than linear (Simonsen et al., 1999). Therefore, these results suggest that residual NO contributes to the ACh relaxation observed in the presence of indomethacin and NOS inhibitors. Both incomplete inhibition of NOS and stores of NO in the vascular wall have been suggested to play a role for residual NO-mediated vasorelaxation (Cohen et al., 1997; Andrews et al., 2003; Chauhan et al., 2003a).
In the presence of NOS inhibition, addition of the NO scavenger, oxyhaemoglobin, abolished ACh relaxation, in agreement with previous studies in rat superior mesenteric artery (Simonsen et al., 1999; Stankevicius et al., 2002). In addition to NO, other radicals such as hydrogen peroxide and peroxynitrite can interact with oxyhaemoglobin (Farias-Eisner et al., 1996; Romero et al., 2003), and in rat aorta and main pulmonary artery, haemoglobin also inhibited the ACh-induced relaxation, but it did not change the transient smooth muscle hyperpolarization or increases in marked Rb+ efflux (Chen et al., 1988). However, oxyhaemoglobin in the present study also lowered the ACh-evoked NO concentration further compared to a maximal inhibitory concentration of ADMA. Therefore, the present results do not exclude the presence of an EDHF or myoendothelial gap junction communication, which modulates ACh-induced NO-mediated relaxations in rat superior mesenteric artery.
Involvement of Ca2+-activated K+ channels in ACh-induced NO release and relaxation
Increases in endothelial cell Ca2+ and membrane hyperpolarization are coupled to release of endothelium-derived factors. This is supported by the observations that raising extracellular K+ concentration depolarizes endothelial cells, limits Ca2+ entry (Wang and van Breemen 1999) and reduces release of histamine-induced NO release in cultured endothelial cells (Lantoine et al., 1998). In the present study, increasing the extracellular K+ concentration reduced ACh relaxation, but hardly changed SNAP relaxation, suggesting that depolarization led to a reduced release of NO. This is consistent with the observation that ACh-evoked increases in NO concentration were also decreased in the presence of high extracellular K+ concentration. The depolarization induced by increasing the extracellular K+ concentration was found to limit ACh-elicited Ca 2+ entry through non-selective cation channels in the endothelial cell membrane (Wang and van Breemen 1999). Whether this is due to a direct effect of membrane potential on Ca2+ entry pathways or a reduced driving force for Ca2+ entry is unclear.
In endothelial cells, three different classes of Ca2+-activated K+ channels have been identified: apamin-sensitive small-conductance (∼10 pS) channels; charybdotoxin-sensitive intermediate-conductance (20–80 pS) channels; and iberiotoxin-sensitive large-conductance (>100 pS) channels (Nilius and Droogmans 2001). Furthermore, the three different Ca2+-activated K channels have also been identified in intact arterial preparations by use of RT-PCR and patch clamp (Marchenko and Sage 1996; Kohler et al., 2000). Used singly, none of the drugs selective for the Ca2+-activated K channels affected ACh-elicited NO release and relaxation or SNAP-induced relaxation in the rat superior mesenteric artery. However, previously a combination of charybdotoxin and apamin has been reported to abolish ACh relaxation in arterial segments (Chen and Cheung, 1997; Edwards et al., 1998; Quignard et al., 1999; Buus et al., 2000) and, in the present study, the combination of charybdotoxin and apamin inhibited ACh relaxation in NA-contracted preparations. Apamin and charybdotoxin did not inhibit ACh relaxation in K+ contracted preparations. These results suggest that the effect is specific and that intermediate- and small-conductance Ca2+-activated K+ channels are involved in the endothelium-dependent relaxations induced by ACh in the rat superior mesenteric artery.
It remains to be established whether or not Ca2+-activated K channels are directly involved in the release of NO or if modulation of NO release takes place through a mechanism secondary to EDHF. Of the EDHF candidates, epoxyeicosatrienoic acids were shown to modulate endothelial calcium channels (Graier et al., 1995), carbon monoxide was reported to both increase or reduce release of NO in the vasculature (Thorup et al., 1999; Ishikawa et al., 2005) and there is a considerable cross-talk between the C natriuretic peptide and NO pathways (see Scotland et al., 2005). However, in the present study the NO release and oxyhaemoglobin-sensitive relaxation induced by ACh and persisting in the presence of ADMA and indomethacin is inhibited by the combination of apamin and charybdotoxin. Moreover, a putative opener of small- and intermediate-conductance Ca2+-activated K+ channels, 1-EBIO (Syme et al., 2000), induces oxyhaemoglobin-sensitive increases in the NO concentration in the present study, and NO release induced by NA in the presence of ADMA is abolished by the combination of apamin and charybdotoxin (Figure 7). These findings suggest the apamin and charybdotoxin-sensitive channels in the endothelial cell layer are directly involved in ACh-evoked NO release, although a modulation of NO release by inhibition of an EDHF mechanism cannot be wholly excluded.
Involvement of endothelial cell calcium in ACh-evoked NO formation
Ca2+-activated K+ channels of small- and intermediate-conductance are activated by [Ca2+]i via constitutively bound calmodulin (Xia et al., 1998; Fanger et al., 1999), which acts as an accessory subunit that is also essential for cell surface expression of small-conductance channels (Lee et al., 2003). In the present study, both NA and ACh increased endothelial [Ca2+]i, providing the basis for both activation of NOS and of Ca2+-activated K channels.
The endoplasmatic reticulum plays a pivotal role for removal of calcium from the cytoplasm, and CPA, an inhibitor of endoplasmatic Ca2+-ATPase, increased intracellular calcium and NO as well as inhibiting the ACh-evoked increase in NO concentration. The lack of ACh-induced increase in NO concentration in the absence of extracellular calcium or by blocking calcium influx by use of SKF96356 suggests that both release of calcium from the endoplasmatic reticulum and calcium influx through a calcium channel in the membrane play a role in ACh-evoked increase in calcium and NO formation, although we cannot exclude the contribution of endoplasmatic calcium release is overestimated, since CPA-evoked opening of store-operated channels will also contribute to inhibition of the ACh response. Recent studies have demonstrated that agonist-induced calcium influx takes place through activation of transient receptor potential channels (TRP) in endothelial cells (Nilius and Droogmans 2001).
Opening K+ channels will draw the membrane potential towards the equilibrium potential for K+ and result in hyperpolarization. In endothelial cells, hyperpolarization is thought to increase the driving force for influx of Ca2+ via voltage-independent Ca2+ channels and thereby prolongs and strengthens the activating Ca2+ signal (Nilius and Droogmans 2001). However, there was no evidence in these studies that the combination of apamin plus charybdotoxin inhibited the sustained increase in endothelial [Ca2+]i. These findings agree with other studies showing the combination of apamin and charybdotoxin does not change the sustained increase in endothelial cell [Ca2+]i induced by ACh in mesenteric arteries (Ghisdal and Morel 2001; McSherry et al., 2005), but contrast with numerous studies of isolated or cultured endothelial cells, where inhibition of Ca2+-activated K+ channels is associated with a decrease in [Ca2+]i (see Nilius and Droogmans, 2001). Whether this is simply due to effects of membrane potential on driving force for Ca2+ or whether increased Ca2+ channel opening, as has been reported for TRP channels (Nilius et al., 2005), is involved remains unclear. An important difference in intact versus cultured cells is that buffering of calcium and cell-to-cell communication are changed. Therefore, in intact preparations it is possible that small changes in [Ca2+]i might not be apparent if, as in the present study, only global [Ca2+]i is measured. Interestingly, in the presence of inhibition of endoplasmatic Ca2+-ATPase with CPA, incubation with apamin and charybdotoxin did inhibit the sustained increase in NO concentration. Therefore, it is likely the Ca2+-activated K+ channels are pivotal for increases in NO concentration governed by influx of extracellular calcium in the rat superior mesenteric artery. However, other alternative explanations such as effects of membrane potential on superoxide production (Sohn et al., 2000), or L-arginine uptake (Ogonowski et al., 2000) warrant further investigation.
In summary, the present study shows that ACh increases endothelial cell [Ca2+]i. This leads to the activation of apamin- and charybdotoxin-sensitive K channels, hyperpolarization and release of NO. Release and bioavailability of NO is decreased in cardiovascular disease and further studies should address whether activation of intermediate-conductance Ca2+-activated K+ channels is beneficial in cardiovascular disease.
Acknowledgments
We thank Hanne Jakobsen, Heidi Knudsen and Kristina Litvin for technical assistance. This study was supported by grants from the Danish Heart Association (04-10-B116-A304-22197), Danish Research Council and Aarhus University Research Foundation.
Abbreviations
- ADMA
NG,NG-asymmetric dimethyl-L-arginine
- AUC
area under curve
- ChTx
charybdotoxin
- CPA
cyclopiazonic acid
- EDHF
endothelium-derived hyperpolarizing factor
- EDTA
ethylenediaminetetraacetic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- PSS
physiological salt solution
- SNAP
S-nitroso-N-acetylpenicillamine
Conflict of interest
The authors state no conflict of interest.
References
- Andrews KL, McGuire JJ, Triggle CR. A photosensitive vascular smooth muscle store of nitric oxide in mouse aorta: no dependence on expression of endothelial nitric oxide synthase. Br J Pharmacol. 2003;138:932–940. doi: 10.1038/sj.bjp.0705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauersachs J, Hecker M, Busse R. Display of the characteristics of endothelium-derived hyperpolarizing factor by a cytochrome P450-derived arachidonic acid metabolite in the coronary microcirculation. Br J Pharmacol. 1994;113:1548–1553. doi: 10.1111/j.1476-5381.1994.tb17172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny JL, Schaad O. An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. Br J Pharmacol. 2000;131:965–973. doi: 10.1038/sj.bjp.0703658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bolz SS, Fisslthaler B, Pieperhoff S, De Wit C, Fleming I, Busse R, et al. Antisense oligonucleotides against cytochrome P450 2C8 attenuate EDHF-mediated Ca(2+) changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. doi: 10.1096/fasebj.14.2.255. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)- activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflugers Arch. 1994;428:476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Rahman A, Nilsson H, Clapp L, MacAllister R, Ahluwalia A. NO contributes to EDHF-like responses in rat small arteries: a role for NO stores. Cardiovasc Res. 2003a;57:207–216. doi: 10.1016/s0008-6363(02)00611-9. [DOI] [PubMed] [Google Scholar]
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Nat Acad Sci USA. 2003b;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508 (Part 2):561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cheung DW. Effect of K(+)-channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. Am J Physiol. 1997;272:H2306–H2312. doi: 10.1152/ajpheart.1997.272.5.H2306. [DOI] [PubMed] [Google Scholar]
- Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and Edrf from rat-blood vessels. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Plane F, Najibi S, Huk I, Malinski T, Garland CJ. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc Nat Acad Sci USA. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Ellis E, Pannirselvam M, Anderson TJ, Triggle CR. Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br J Pharmacol. 2003;140:1193–1200. doi: 10.1038/sj.bjp.0705549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou JM, et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance K–Ca channel, IKCa1. J Biol Chem. 1999;274:5746–5754. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- Farias-Eisner R, Chaudhuri G, Aeberhard E, Fukuto JM. The chemistry and tumoricidal activity of nitric oxide hydrogen peroxide and the implications to cell resistance susceptibility. J Biol Chem. 1996;271:6144–6151. doi: 10.1074/jbc.271.11.6144. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Hinsch N, Chataigneau T, Popp R, Kiss L, Busse R, et al. Nifedipine increases cytochrome P4502C expression and endothelium- derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension. 2000;36:270–275. doi: 10.1161/01.hyp.36.2.270. [DOI] [PubMed] [Google Scholar]
- Fulton D, McGiff JC, Quilley J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br J Pharmacol. 1992;107:722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization – a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Ghisdal P, Morel N. Cellular target of voltage and calcium-dependent K+ channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br J Pharmacol. 2001;134:1021–1028. doi: 10.1038/sj.bjp.0704348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graier WF, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995;482 (Part 2):259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, et al. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–e114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Mulvany MJ, Aalkjaer C. Endogenous and exogenous agonist-induced changes in the coupling between [Ca2+]i and force in rat resistance arteries. Pflugers Arch. 1992;420:536–543. doi: 10.1007/BF00374630. [DOI] [PubMed] [Google Scholar]
- Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- Lantoine F, Iouzalen L, Devynck MA, Millanvoye-van Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem J. 1998;330 (Part 2):695–699. doi: 10.1042/bj3300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin – cell surface expression and gating. J Biol Chem. 2003;278:25940–25946. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol. 1996;492 (Part 1):53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38:23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogonowski AA, Kaesemeyer WH, Jin L, Ganapathy V, Leibach FH, Caldwell RW. Effects of NO donors and synthase agonists on endothelial cell uptake of L-Arg and superoxide production. Am J Physiol Cell Physiol. 2000;278:C136–C143. doi: 10.1152/ajpcell.2000.278.1.C136. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Feletou M, Thollon C, Vilaine JP, Duhault J, Vanhoutte PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br J Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N, Radi R, Linares E, Augusto O, Detweiler CD, Mason RP, et al. Reaction of human hemoglobin with peroxynitrite isomerization to nitrate and secondary formation of protein radicals. J Biol Chem. 2003;278:44049–44057. doi: 10.1074/jbc.M305895200. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Ahluwalia A, Hobbs AJ. C-type natniuretic peptide in vascular physiology and disease. Pharmacol Ther. 2005;105:85–93. doi: 10.1016/j.pharmthera.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Garcia-Sacristan A, Prieto D. Apamin-sensitive K+ channels involved in the inhibition of acetylcholine-induced contractions in lamb coronary small arteries. Eur J Pharmacol. 1997;329:153–163. [PubMed] [Google Scholar]
- Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol. 1999;516 (Part 1):271–282. doi: 10.1111/j.1469-7793.1999.271aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HY, Keller M, Gloe T, Morawietz H, Rueckschloss U, Pohl U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J Biol Chem. 2000;275:18745–18750. doi: 10.1074/jbc.M000026200. [DOI] [PubMed] [Google Scholar]
- Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens. 2002;20:1571–1579. doi: 10.1097/00004872-200208000-00020. [DOI] [PubMed] [Google Scholar]
- Stoen R, Lossius K, Karlsson JOG. Acetylcholine-induced vasodilation may depend entirely upon NO in the femoral artery of young piglets. Br J Pharmacol. 2003;138:39–46. doi: 10.1038/sj.bjp.0705001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- Tanko IB, Mikkelsen EO, Simonsen U. A new experimental approach in endothelium-dependent pharmacological investigations on isolated porcine coronary arteries mounted for impedance planimetry. Br J Pharmacol. 1999;128:165–173. doi: 10.1038/sj.bjp.0702752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ. Hyperpolarization and relaxation of arterial smooth-muscle caused by nitric-oxide derived from the endothelium. Nature. 1990;346:69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor HJ, Chaytor AT, Edwards DH, Griffith TM. Gap junction-dependent increases in smooth muscle cAMP underpin the EDHF phenomenon in rabbit arteries. Biochem Biophys Res Commun. 2001;283:583–589. doi: 10.1006/bbrc.2001.4791. [DOI] [PubMed] [Google Scholar]
- Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol Ren Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Marchenko SM, Sage SO. Cytosolic calcium concentration in resting and stimulated endothelium of excised intact rat aorta. J Physiol. 1995;489 (Part 2):309–317. doi: 10.1113/jphysiol.1995.sp021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Voorde V, Vanheel B. Influence of cytochrome P-450 inhibitors on endothelium-dependent nitro-L-arginine-resistant relaxation and cromakalim-induced relaxation in rat mesenteric arteries. Cardiovas Pharmacol. 1997;29:827–832. doi: 10.1097/00005344-199706000-00018. [DOI] [PubMed] [Google Scholar]
- Wang X, van Breemen C. Depolarization-mediated inhibition of Ca(2+) entry in endothelial cells. Am J Physiol. 1999;277:H1498–H1504. doi: 10.1152/ajpheart.1999.277.4.H1498. [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Imaeda K, Suzuki H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J Physiol. 1999;514:505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]