Abstract
Chemokines and their receptors play a central role in the trafficking of leukocytes within the body, a process which is amenable to antagonism by small molecules and which holds promise as a treatment for clinically important diseases. In the issue of the British Journal of Pharmacology accompanying this commentary, Ignatov and colleagues describe an unexpected role for the chemokine RANTES/CCL5, namely an ability to signal via the orphan G protein-coupled receptor named GPR75. This receptor bears little homology to other chemokine receptors, most strikingly within the putative intracellular domains, with the third loop and C-terminal tail dwarfing those of other known chemokine receptors. This most likely accounts for the atypical pertussis toxin-insensitive signalling induced by RANTES. Intriguingly, this signalling is neuro-protective, inducing the survival of a hippocampal cell line following insult with the neurotoxic amyloid-beta peptide. Since this peptide is implicated in the pathogenesis of Alzheimer's disease, it may be that exploitation of this signalling pathway presents itself as a future therapeutic treatment.
Keywords: chemokine, chemokine receptor, inflammation, central nervous system, Alzheimer's disease
Chemokines or chemotactic cytokines are small, basic proteins with a key role in development, haematopoesis and immunity. Chemokines exert their functions by binding to G protein-coupled receptors (GPCRs) and are best known for their ability to recruit leukocytes bearing the appropriate chemokine receptor on their cell surface. Inappropriate or excessive release of chemokines has been implicated in the inflammatory processes of several clinically important diseases such as arthritis and asthma (Charo and Ransohoff, 2006). This, coupled with the discovery that human immunodeficiency virus-1 utilizes chemokine receptors as cofactors for cellular entry, has fuelled much research in this field and the development of potent, well-tolerated, small molecule antagonists of chemokine receptors is a goal for several pharmaceutical companies. There is room for optimism, as approximately half of all currently described drugs are estimated to target GPCRs.
In this issue of the British Journal of Pharmacology, Ignatov et al. (2006) assign the chemokine RANTES (an acronym for the unwieldy full name Regulated upon Activation, Normal T cell Expressed and presumably Secreted)/CCL5 as a functional ligand of the murine orphan receptor GPR75, following a screen of biological fluids and peptides. RANTES was initially identified as being produced by T cells upon stimulation (Schall et al., 1988) and allegedly owes its name more to a Mexican B-movie character than to its production by leukocytes (Cohen, 1996). Although RANTES is one of the more promiscuous varieties of chemokine, binding to the receptors CCR1, CCR3 and CCR5 and also the ‘silent' non-signalling chemokine receptors D6 and DARC, its activity at GPR75 comes as somewhat of a surprise, as it shares only 12–16% amino acid identity with other chemokine receptors.
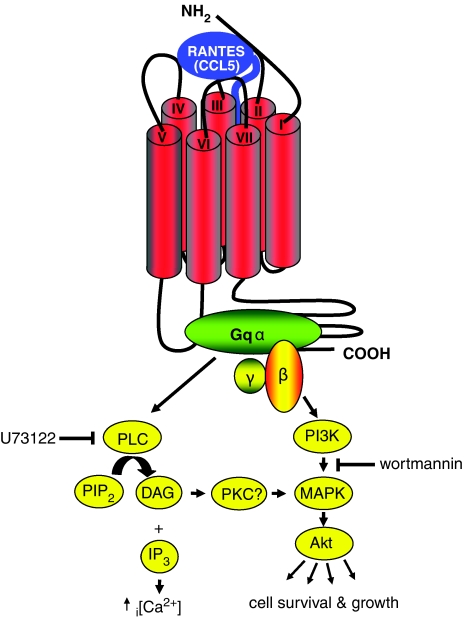
At an estimated 540 amino acids, GPR75, is almost 200 amino acids longer than its brethren, with a putative third intracellular loop of 92 residues and an enormous C-terminal tail of 169 residues, dwarfing the 50 or so amino acids observed at this position within other chemokine receptors (Figure 1). These intracellular differences may well account for the reported preferential Gqα coupling of GPR75 rather than the pertussis toxin-sensitive Gαi, a hallmark of chemokine receptor signalling. Also absent from the chemokine receptor ‘identikit' picture is the highly acidic N-terminus believed to bind the basic chemokine, and the structurally important aspartate/arginine/tyrosine (DRY) motif of transmembrane helix 3. Whilst a limited screen of five chemokines identified RANTES and the related chemokine macrophage inflammatory protein (MIP)-1α/CCL3 as ligands for GPR75, it would be worthwhile extending this search to examine possible interactions with other members of the chemokine family.
Figure 1.
The RANTES/CCL5 signalling cascade mediated via the novel chemokine receptor, GPR75. Inhibition of observed signalling by the use of wortmannin and U73122 is also shown. Although the involvement of PKC in this pathway has not been directly demonstrated, activation of PKC has been previously reported to enhance the survival of the HT22 murine hippocampal cell line (Maher, 2001). Note the large third intracellular loop linking transmembrane helices V and VI and also the long C-terminus extending from transmembrane helix VII. These regions of GPR75 are anticipated to interact with the heterotrimeric G protein and may account for the atypical signalling described by Ignatov et al. (2006).
So what role does this additional RANTES receptor play in vivo? Unlike other chemokine receptors, the expression of human GPR75 at the messenger RNA (mRNA) level is reportedly absent in the ‘traditional' locations of chemokine receptors, such as leukocytes, thymus and spleen, instead being restricted to the brain, spinal cord and retinal pigment epithelium. Its initial description in the literature came from analysis of a region of chromosome 2p16 believed to be involved in the inheritance of Doyne's honeycomb retinal dystrophy (DHRD) (Tarttelin et al., 1999) and an independent study also identified the GPR75 transcript from a screen of retinal complementary DNA (cDNA) libraries (Sauer et al., 2001). Despite the identification of a handful of polymorphisms within the GPR75 gene, no direct association with either a phenotype at the DHRD locus (Tarttelin et al., 1999) or age-related macular degeneration (Sauer et al., 2001) has been reported.
Although the bulk of the signalling data obtained by Ignatov et al. (2006) employed GPR75 transfectants, the authors were also able to demonstrate expression of GPR75 mRNA in the murine hippocampal cell line, HT22. These cells are susceptible to the neurotoxic amyloid-β peptide, mimicking the cell death observed in Alzheimer's disease (AD). Pretreatment of cultures with RANTES led to increased HT22 cell viability in the presence of the amyloid-β peptide, which although not directly attributable to GPR75, could be enhanced by co-transfection with GPR75 cDNA. This suggests that GPR75 mediates the RANTES-induced cell survival, which appears to be mediated by phosphatidylinositol 3-kinase and phospholipase C as also observed with GPR75 transfectants. It would be worthwhile underscoring the role of GPR75 in RANTES signalling perhaps by knockdown of GPR75 transcripts in the HT22 cell line or by the development of blocking antibodies. In addition, it would be interesting to see if such neuro-protective effects are also observed in human cells expressing GPR75 and if expression of GPR75 extends to other non-neuronal cells of the CNS, such as microglia and astrocytes.
It is noteworthy that both RANTES and MIP-1α have been previously shown to be produced by astrocytes in vitro following stimulation with amyloid-β peptide (Johnstone et al., 1999; Smits et al., 2002), which concurs with clinical studies reporting the expression of the MIP-1α/RANTES receptor CCR5 on microglia associated with amyloid deposits within the brain of AD patients (Xia et al., 1998). At a first glance, induction of RANTES and MIP-1α in AD would be expected to result in the exacerbation of disease by the recruitment of proinflammatory cells such as monocytes and microglia. However, the study by Ignatov et al. (2006) suggests that the proinflammatory roles of either chemokine may be tempered by the neuro-protective effects they can mediate via GPR75. It is to be hoped that this unexpected signalling pathway may be exploited as a future therapy for the treatment of AD.
Abbreviations
- AD
Alzheimer's disease
- DARC
Duffy antigen receptor for chemokines
- DHRD
Doyne's honeycomb retinal dystrophy
- GPCRs
G protein-coupled receptors
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
References
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Cohen P. Hooked on HIV – What's the connection between a 1980s film character and the cutting edge of AIDS research? New Scientist. 1996;2060:24. [Google Scholar]
- Ignatov A, Robert J, Gregory-Evans C, Schaller HC.RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75 Br J Pharmacol 2006149490–497.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer CG, White K, Stohr H, Grimm T, Hutchinson A, Bernstein PS, et al. Evaluation of the G protein coupled receptor-75 (GPR75) in age related macular degeneration. Br J Ophthalmol. 2001;85:969–975. doi: 10.1136/bjo.85.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- Smits HA, Rijsmus A, Van Loon JH, Wat JW, Verhoef J, Boven LA, et al. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J Neuroimmunol. 2002;127:160–168. doi: 10.1016/s0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- Tarttelin EE, Kirschner LS, Bellingham J, Baffi J, Taymans SE, Gregory-Evans K, et al. Cloning and characterization of a novel orphan G-protein-coupled receptor localized to human chromosome 2p16. Biochem Biophys Res Commun. 1999;260:174–180. doi: 10.1006/bbrc.1999.0753. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains. Am J Pathol. 1998;153:31–37. doi: 10.1016/s0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]