Abstract
Background and purpose:
ATP-sensitive K+ channels (KATP channels) play important roles in regulating the resting membrane potential of detrusor smooth muscle. Actions of ZD0947, a novel KATP channel opener, on both carbachol (CCh)-induced detrusor contractions and membrane currents in human urinary bladder myocytes were investigated.
Experimental approach:
Tension measurements and patch-clamp techniques were utilized to study the effects of ZD0947 in segments of human urinary bladder. Immunohistochemistry was also performed to detect the expression of the sulphonylurea receptor 1 (SUR1) and the SUR2B antigens in human detrusor muscle.
Key results:
ZD0947 (≥0.1 μM) caused a concentration-dependent relaxation of the CCh-induced contraction of human detrusor, which was reversed by glibenclamide. The rank order of the potency to relax the CCh-induced contraction was pinacidil>ZD0947>diazoxide. In conventional whole-cell configuration, ZD0947 (≥1 μM) caused a concentration-dependent inward K+ current which was suppressed by glibenclamide at -60 mV. When 1 mM ATP was included in the pipette solution, application of pinacidil or ZD0947 caused no inward K+ current at -60 mV. Gliclazide (≤1 μM), a selective SUR1 blocker, inhibited the ZD0947-induced currents (K i=4.0 μM) and the diazoxide-induced currents (high-affinity site, K i1=42.4 nM; low-affinity site, K i2=84.5 μM) at -60 mV. Immunohistochemical studies indicated the presence of SUR1 and SUR2B proteins, which are constituents of KATP channels, in the bundles of human detrusor smooth muscle.
Conclusions and Implications:
These results suggest that ZD0947 caused a glibenclamide-sensitive detrusor relaxation through activation of glibenclamide-sensitive KATP channels in human urinary bladder.
Keywords: ATP-sensitive K+ channels, human urinary bladder, K+ channel opener, overactive bladder, SUR1, SUR2, ZD0947
Introduction
The overactive bladder (OAB) is a syndrome based on the presence of the symptoms of urgency, with or without urge incontinence, accompanied with frequency and nocturia in the absence of pathologic or metabolic diseases. Initial treatment of the condition normally involves an integrated approach using both behavioural methods and pharmacotherapy (Andersson, 2004).
Antimuscarinic agents are currently available for the clinical treatment for OAB syndrome. However, antimuscarinic agents are somewhat limited by certain side effects (such as dry mouth, constipation and blurred vision, etc.). Thus, the development of new compounds with novel mechanisms of action for the treatment of OAB is essential. Although several alternative pharmacotherapies have been tried in the clinical field, more urinary bladder-selective agents should be synthesized and developed (Andersson, 2004).
In the smooth muscle cells of the lower urinary tract, K+ channels play an important role in regulating membrane potential and excitability (Brading, 1987; Andersson, 1993). In the condition of detrusor overactivity in which the smooth muscle becomes hyperexcitable, K+ channel openers potently decrease excitability and may be useful in the treatment of OAB (Foster et al., 1989a, 1989b). It is well-known that K+ channel openers hyperpolarize the membrane potential of smooth muscle cells by increasing K+ permeability, followed by a significant detrusor relaxation.
In the urological field, recent molecular identification of ATP-sensitive K+ channels (KATP channels) has renewed interest in the development of detrusor-selective KATP channel openers for the treatment of OAB and urinary incontinence. Several detrusor-selective KATP channel openers, such as YM-934, (Uchida et al., 1994), ZD6169, (Trivedi et al., 1995) and WAY-133537 (Wojdan et al., 1999), have been newly synthesized, targeting glibenclamide-sensitive KATP channels in smooth muscle of the urinary bladder.
ZD0947 (3-[(4S)-5-oxo-2-(trifluoromethyl)-1,4,5,6,7,8-hexahydroquinolin-4-yl]benzonitrile; Abdel-Karim et al., 2002) is a newly developed dihydropyridine (DHP) derivative KATP channel opener. Although the effects of ZD0947 in rats with spinal cord injury and with detrusor hyperreflexia have been reported (Abdel-Karim et al., 2002), the effects of ZD0947 on the muscle tone and the membrane currents have not been investigated as yet in detrusor smooth muscle. Furthermore, there has been no report concerning the effects of KATP channel openers on the glibenclamide-sensitive KATP currents in human detrusor myocytes. Recent studies have revealed that recombinant KATP channels are heteromeric complexes composed of at least two subunits; one from a family of inwardly rectifying K+ channels (Kir6.x) and the other from a family of sulphonylurea receptors (SUR.x) (Inagaki et al., 1995). It is also considered that KATP channel openers interact with SUR.x subunits, and not with Kir6.x subunits of KATP channels (Schwanstecher et al., 1998). However, the nature of the SUR.x proteins in human detrusor KATP channels still remain to be established.
In the present study, we have firstly investigated the effects of ZD0947 on the muscle tone of isolated human detrusor smooth muscle by use of tension measurement. Secondly, we have studied the effects of ZD0947 on the membrane currents in human detrusor myocytes using whole-cell recordings. Thirdly, we have localized the SUR1 (Aguilar-Bryan et al., 1995) and SUR2B (Isomoto et al., 1996) proteins, by immunohistochemical methods, in the smooth muscle bundles of human urinary bladder.
Methods
Tension measurement and data analysis
Small segments of human detrusor was obtained from patients with a stable urinary bladder who were generally undergoing cystectomy for bladder cancer after informed patient consent and approved of ethical consent of the Kyushu University Faculty of Medicine Ethical Committee (Fukuoka, Japan) had been obtained. A segment of detrusor was excised and quickly transferred into modified physiological salt solution. For isometric tension recording, fine strips (1 × 1 × 3 mm) were prepared as described previously (Tomoda et al., 2005). Modified Krebs solution was used (mM): Na+ 137, K+ 5.9, Mg2+ 1.2, Ca2+ 2.5, Cl− 133.7, HCO3− 15.4, H2PO4− 1.2 and glucose 11.5 which was bubbled with 97% O2 and 3% CO2 (pH 7.35–7.45 at 37°C). An initial tension equivalent to 0.5 g weight was applied to each human detrusor strip, which was then allowed to equilibrate for approximately 1–1.5 h until the basal urethral tone became stable (36–37°C). Data were recorded on a Macintosh computer (Macintosh G4, Apple Computer Japan, Tokyo, Japan), through ‘MacLab 3.5.6' (ADInstruments Pty Ltd, Castle Hill, Australia). The tension was expressed as mN mg−1 of tissue.
Cell preparation and patch-clamp experiment recording procedure
We used freshly dispersed single detrusor myocytes prepared from human urinary bladder as described previously (Tomoda et al., 2005). We employed the smooth cell dispersion method previously described (the gentle tapping method; Teramoto and Brading, 1996). The set-up of the patch-clamp experimental system used was essentially the same as described previously (Tomoda et al., 2005). All experiments were performed at room temperature (21–23°C).
Data analysis
The whole-cell current data were low-pass filtered at 500 Hz (−3 dB) by an 8 pole Bessel filter (3611 multifunction filter, NF Electronic Instruments, Yokohama, Japan), sampled at 1 ms and analysed on a computer (Macintosh G4, Apple Computer Japan, Tokyo, Japan) by use of the commercial software ‘Mac Lab 3.5.6' (ADInstruments Pty Ltd, Castle Hill, Australia).
The curve was drawn by fitting the equation using the least-squares method:
 |
where I is the mean amplitude of the muscle contraction in the presence of KATP channel openers, Ic is the mean amplitude of carbachol (CCh)-induced contraction in control (in the absence of KATP channel openers), Ki, D and h are apparent dissociation constant, concentration of KATP channel openers (μM) and the Hill coefficient, respectively (Figure 3d). In Figure 6b, I is the mean amplitude of the diazoxide-induced KATP current in the presence of gliclazide, Ic is the mean amplitude of the diazoxide-induced KATP current in control (in the absence of gliclazide), Ki, D and h are the inhibitory dissociation constant, concentration of gliclazide (μM) and Hill's coefficient, respectively.
Figure 3.
Relaxing effects of KATP channel openers on the 300 nM CCh-induced contraction of human detrusor strips. The dashed line indicates the stabilized muscle tone level and the line indicates the zero tone level. (a) The effects of cumulative addition of ZD0947 and after recovery, 10 μM pinacidil. (b, c) The effects of cumulative addition of pinacidil and diazoxide on the CCh-induced contraction. (d) Relationships between the relative value of KATP channel opener-induced relaxation and the concentration of KATP channel opener. The peak amplitude of the 10 μM pinacidil-induced relaxation was normalized as 1.0. The following values were used for the curve fitting: pinacidil, Ki=0.8 μM, h=1.2 (n=4); ZD0947, Ki=3.5 μM, h=1.2 (n=4); diazoxide, Ki=51.3 μM, h=1.2 (n=4). Each symbol indicates mean with s.d. shown by vertical lines. Some of s.d. bars are less than the size of the symbols.
Figure 6.
Inhibitory effects of gliclazide on the ZD0947- and the diazoxide-induced inward membrane currents at −60 mV in nystatin-perforated patch configuration. (a, b) Cumulative application of gliclazide caused a concentration-dependent inhibition of the ZD0947-induced currents. The dashed line indicates zero current. (c) Relationship between relative inhibition of KATP channel openers-induced current and the concentration of gliclazide. The following values were used for the curve fitting: 100 μM ZD0947, Ki=4.0 μM, h=0.9; 500 μM diazoxide, Ki1=42.4 nM, h1=0.8; Ki2=84.5 μM, h2=1.2. Each symbol indicates the mean of four observations with ±s.d. shown by vertical lines. Some of s.d. bars are less than the size of the symbols.
For curve fitting of the biphasic effects of gliclazide on the diazoxide-induced currents, following equation was used:
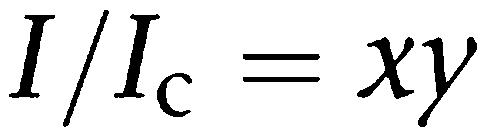 |
where I and Ic are same as above, x is a term describing the high-affinity site and y a term describing the low-affinity site.
 |
where [D] is the concentration of ZD0947 (μM), Ki1, Ki2 are the ZD0947 concentrations at which relaxant effect is half maximum at high- and low-affinity sites, respectively; h1, h2 are the Hill coefficients for the high- and low-affinity sites, respectively; and L is the fractional current amplitude remaining when the high-affinity sites are fully occupied.
Solutions
For whole-cell recording, the following solutions were used: 140 mM K+ solution containing (mM): Na+ 5, K+ 140, Mg2+ 1.2, Ca2+ 2, glucose 5, Cl− 151.4, N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10, titrated to pH 7.35–7.40 with Tris base (sometimes 60 mM K+ solution was used; Na+ 85, K+ 60, Mg2+ 1.2, Ca2+ 2, glucose 5, Cl− 151.4, HEPES 10/Tris); high potassium pipette solution containing (mM): K+ 140, Cl− 140, glucose 5, ethylene glycol-bis-(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 5, HEPES 10 and ATP 0.1 (sometimes 1 mM ATP was included)/Tris (pH 7.35–7.40). The bath solution was superfused by gravity throughout the experiments at a rate of 2 ml min−1.
Immunohistochemical studies
Tissue samples from human urinary bladder were embedded in optimal cutting temperature (OCT) compound (Tissues-Tek, SAKURA, Tokyo, Japan) in disposable plastic plates and rapidly frozen in hexane surrounded by liquid nitrogen. Frozen sections were cut with a cryostat (CM3050S, Leica, Tokyo, Japan) at a thickness of 6 μm and mounted on silane-precoated glass slides, then allowed to air dry at room temperature for approximately 30 min. Sections were fixed in cold acetone for 5 min and washed thoroughly in phosphate-buffered saline (PBS) before staining. The tissue sections were treated in 5% normal mouse serum (Santa Cruz Biotechnology, CA, USA), 3% bovine serum albumin (BSA, GIBCO, NY, USA) and 0.2% Triton-X 100 (Amersham Biosciences, Uppsala, Sweden) in PBS for 1 h to avoid nonspecific staining, and then primary antibodies were applied to sections at 4°C overnight at the following dilutions with 1.5% normal mouse serum and 1% BSA in PBS: purified goat anti-SUR1 antibody (sc-5789; Santa Cruz Biotechnology, CA, USA), 1:200; goat anti-SUR2B antibody (sc-5793; Santa Cruz Biotechnology, CA, USA), 1:200. As a negative control, the primary antibody was absorbed with the peptide against which it was made (blocking peptide). Following washing, three times (5 min duration) in PBS, sections were incubated with phycoerythrin (PE)-conjugated mouse anti-goat IgG (sc-3752; Santa Cruz Biotechnology, CA, USA) diluted to 1:50 in PBS with 1.5% normal mouse serum and 1% BSA, and fluorescein isothiocyanate (FITC)-conjugated mouse anti-goat IgG (sc-2356; Santa Cruz Biotechnology, CA, USA) diluted to 1:50 for 1 h at room temperature under dark conditions. Sections were then washed for three times (5 min duration) in PBS. Coverslips were mounted onto slides by use of fluorescence mounting medium and slides viewed by fluorescent microscopy (Biozero BZ-8000, KEYENCE, Osaka, Japan).
Statistical analysis
Statistical analyses were performed with analysis of variance (ANOVA) test (two-factor with replication). Changes were considered significant at P<0.01 (*). Data are expressed as mean with the standard deviation (s.d.).
Materials
ZD0947 (kindly provided by the AstraZeneca Pharmaceuticals, Wilmington, DE, USA) was prepared daily as 100 mM stock solutions in dimethylsulphoxide (DMSO). The final concentration of DMSO was less than 0.3%, not affecting membrane currents. The rest of the chemicals were purchased from Sigma (Sigma Chemical KK, Tokyo, Japan).
Results
The effects of ZD0947 on the CCh-induced contraction in human detrusor
Application of CCh (⩾30 nM, 7 min duration) caused a concentration-dependent contraction in the strips of human detrusor (Figure 1a). In the presence of 100 nM ZD0947, the CCh (⩽300 nM)-induced contraction was inhibited although the 1 μM CCh-induced detrusor contraction was not affected by 100 nM ZD0947. Similarly, 1 μM ZD0947 caused little effect on 1 μM CCh-induced detrusor contraction. In the presence of 10 μM ZD0947, the CCh-induced contractions were inhibited. Figure 1b summarizes the effects of ZD0947 on the CCh-induced contractions in human detrusor strips; the contractions induced by 1 μM CCh, in the absence of ZD0947, were normalized to a value of 1.0. The reduction produced by ZD0947 (10 μM) in the 300 nM CCh-induced contraction was reversed by additional application of 1 μM glibenclamide to the control level (Figure 2, n=4). Application of 300 nM CCh caused a phasic contraction following a tonic contraction in human detrusor. After the muscle tone level of the CCh-induced tonic contraction became stable, cumulative application of ZD0947 (0.1–10 μM) produced a concentration-dependent relaxation of the CCh-induced contraction (Figure 3a). After washing out of the drug, the detrusor tone recovered to the control level. Subsequently, 10 μM pinacidil was applied in order to obtain the maximum relaxation in the detrusor strips. Similar experiments were performed to use different types of KATP channel openers (pinacidil, Figure 3b; diazoxide, Figure 3c). Figure 3d shows the concentration–response curves for pinacidil (Ki=0.8 μM), ZD0947 (Ki=3.5 μM) and diazoxide (Ki=51.3 μM), expressed relative to the maximum relaxation to 10 μM pinacidil.
Figure 1.
Effects of ZD0947 on CCh-induced contraction of human detrusor strips. (a) CCh-induced contraction in the absence (control) and presence of ZD0947. (b) Effects of ZD0947 on the peak amplitude of CCh-induced contraction of human detrusor strips, when the peak amplitude of 1 μM CCh-induced contraction in the absence of ZD0947 is normalized as unity. Each symbol indicates mean with s.d. shown by vertical lines. Some of s.d. bars are less than the size of the symbols.
Figure 2.
Effects of both ZD0947 and glibenclamide on the CCh-induced contraction of human detrusor strips. The dashed line indicates the zero tone level. (a) CCh (300 nM, 4 min application) caused a contraction. In the presence of 10 μM ZD0947, the CCh-induced contraction was suppressed. After treatment with 1 μM glibenclamide, the CCh-induced contraction recovered to the control level, even in the presence of ZD0947. The results illustrated were obtained from a single smooth muscle strip. (b) Relative amplitude of the CCh-induced contraction under the indicated conditions. The amplitude of the CCh-induced contraction was normalized as 1.0 (Control, open column). The solid column indicates the relative amplitude of 10 μM ZD0947-induced relaxation. The hatched column represents the relative amplitude of the CCh-induced contraction in the presence of ZD0947. Asterisks indicate a statistically significant difference, demonstrated using a paired t-test (P<0.01). Each column represents the relative mean value with +s.d. (n=4).
Effects of ZD0947 on membrane currents in human urinary bladder myocytes
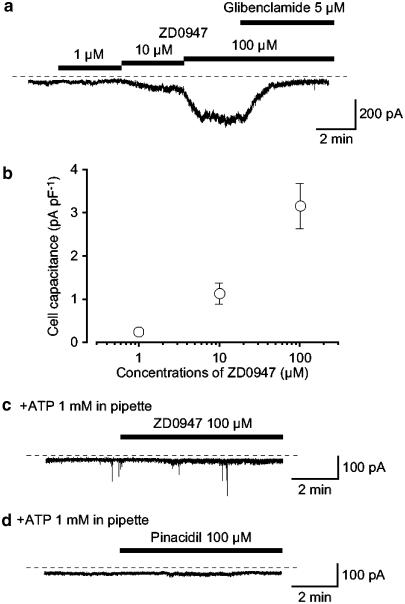
In order to study further the actions of ZD0947 on human detrusor, the membrane currents were recorded from dispersed human detrusor smooth muscle myocytes by use of a conventional whole-cell configuration. Cumulative application of ZD0947 (1–100 μM) caused a significant inward current in a concentration-dependent manner at a holding potential of −60 mV (Figure 4a and b). As shown in Figure 4a, the peak amplitude of the 100 μM ZD0947-induced inward current was inhibited by 5 μM glibenclamide. When 1 mM ATP was included in the pipette solution, little appreciable inward current was evoked by application of ZD0947 after the conventional whole-cell configuration had become established (Figure 4c). Similar results were observed on application of 100 μM pinacidil (Figure 4d). These results suggest that the ZD0947-induced inward current seems to possess not only a glibenclamide-sensitivity but also an intracellular ATP-sensitivity in human detrusor myocytes.
Figure 4.
Concentration–response relationships of the ZD0947-induced inward current. (a) A cumulative application of ZD0947 caused a concentration-dependent inward current. The dashed line indicates the zero current level. (b) Relationship between the current density of inward currents (pA pF−1) and concentrations of ZD0947 (μM). The amplitudes of the ZD0947-induced currents were measured from the current level in the presence of 5 μM glibenclamide. Each symbol indicates mean and ±s.d. of four observations. (c, d) When 1 mM ATP was included in the pipette solution, neither ZD0947 (c) nor pinacidil (d) caused a significant inward current. The dashed line indicates the zero current level.
To make a rough estimate of the ion selectivity and the reversal potential of the ZD0947-induced glibenclamide-sensitive current, voltage ramps were applied and the extracellular K+ concentration ([K+]o) was changed by iso-osmotic substitution of Na+. Figure 5a shows the experimental protocol. ZD0947 (10 μM) caused a sustained inward current in 140 mM K+ solution and then the voltage protocol was performed. In the presence of ZD0947 (control), current–voltage relationships were obtained by the application of four ramp pulses in solutions containing 140 mM K+ solution followed by 60 mM K+ solution. When [K+]o was decreased from 140 to 60 mM, the ZD0947-induced membrane current at −60 mV was markedly decreased. Figure 5b shows the average of the four ramp currents before and during application of 5 μM glibenclamide for the cell shown in Figure 5a when ZD0947 was present. In each [K+]o condition, the net membrane current inhibited by 5 μM glibenclamide was obtained by subtracting the averaged control current from the mean ZD0947-induced current. The current demonstrated an inwardly rectifying property. The reversal potential of the ZD0947-induced glibenclamide-sensitive membrane current in this cell was 4.4 mV in 140 mM K+ solution and −21.6 mV in 60 mM K+ solution (Figure 5c). Mean values from four cells were 2.8±3.0 mV (n=4) for 140 mM K+ solution and −23.5±5.5 mV (n=4) for 60 mM K+ solution. These values were close to the theoretical potassium equilibrium potential (EK) in each [K+]o condition (140 mM K+; EK=0 mV, 60 mM K+; EK=−21 mV). These results suggest that in human detrusor myocytes, the ZD0947-induced glibenclamide-sensitive membrane currents are carried mainly by potassium ions.
Figure 5.
Measurement of the ZD0947-induced membrane current. (a) Ramp currents induced by the four ramp potential pulses (see inset) applied every 15 s before and during application of 10 μM ZD0947. In the presence of ZD0947, the ramp membrane currents were obtained in either the 140 mM K+ solution or the 60 mM K+ solution. Glibenclamide suppressed the ZD0947-induced membrane current. The vertical deflections indicate ramp currents. The dashed line indicates the zero current level. (b) The mean ramp membrane currents (the falling phase of the ramp pulse) on an expanded time scale in several conditions. Each symbol is the same as in (a). (c) Net membrane current was obtained by subtraction of the two ramp membrane currents (shown in (b)) recorded before and during application of ZD0947 in each [K+]o condition.
The effects of gliclazide on KATP channel opener-induced currents
In order to further investigate whether the SUR1 subunit may be involved in the ZD0947-induced glibenclamide-sensitive membrane currents in these cells, gliclazide, a specific SUR1 blocker (Gribble and Ashcroft, 2000), was utilized. The nystatin-perforated patch configuration was utilized to maintain the basal amplitude of KATP channel opener-induced inward currents at a holding potential of −60 mV. As shown in Figure 6a, gliclazide (0.1–0.3 μM) showed little effect on the ZD0947-induced currents. When the concentrations of gliclazide were increased (⩽1 μM), the ZD0947-induced membrane current at −60 mV was inhibited in a concentration-dependent manner (Ki=4.0 μM) and gliclazide (100 μM) completely suppressed the ZD0947-induced current at −60 mV (Figure 6b). In contrast, 100 nM gliclazide reduced the current amplitude induced by 500 μM diazoxide by approximately 10% (to 0.89±0.05 times the initial current, n=4, P<0.01) and higher concentrations of gliclazide inhibited the diazoxide-induced current in a concentration-dependent manner, demonstrating two inhibitory sites: a high-affinity site (Ki1=42.4 nM) and a low-affinity site (Ki2=84.5 μM) in Figure 6c.
Immunohistochemical localization of SUR1 and SUR2B proteins in human detrusor
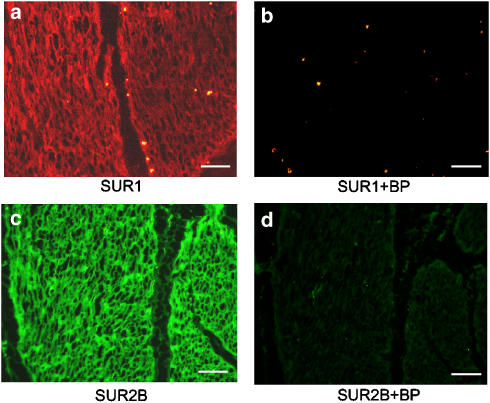
To localize the KATP channels characterized above, immunohistochemistry was performed to detect the expression of the SUR1 and the SUR2B proteins (Figure 7). As shown in Figure 7, both SUR1 and SUR2B immunoreactivity is clearly visible in the smooth muscle bundles of human detrusor (Figure 7a and c).
Figure 7.
Fluorescent images of immunoreactivity for SUR1 and SUR2B in the human detrusor bundles. (a, b) SUR1 immunoreactivity and negative control (in the presence of the blocking peptide (BP)). (c, d) SUR2B immunoreactivity and negative control (in the presence of the BP). Note that the scale bar (white line) represents 100 μm in each picture.
Discussion
In the present experiments, we have demonstrated that ZD0947 induces a glibenclamide-sensitive K+ current, which was inhibited by endogenous ATP in human detrusor myocytes.
Target K+ channels for ZD0947 in human urinary bladder
A glibenclamide-sensitive, cromakalim-induced, hyperpolarization has been recorded in human urinary bladder myocytes by use of the current-clamp technique (Wammack et al., 1994). They reported that neither rat nor guinea-pig is a suitable model to investigate the pharmacological and clinical potential of KATP channel openers since significant species differences for the effects of KATP channel openers are present in the urinary bladder. It is clear that human detrusor is the most suitable tissue to study the effects of KATP channel openers on the activity of KATP channels in terms of eventual use in urology (Wammack et al., 1994). However, for the last decade, little attention has been paid to species differences when the effects of KATP channel openers were investigated.
In the present experiments, we have demonstrated that ZD0947-induced concentration-dependent human detrusor relaxation is reversibly suppressed by glibenclamide, and that ZD0947-induced concentration-dependent K+ current was inhibited by glibenclamide and also intracellular ATP in human detrusor myocytes. Given this, the ZD0947-induced detrusor relaxation is likely to be evoked through the activation of the glibenclamide-sensitive KATP channels in human detrusor myocytes.
Possible existence of SURs in human urinary smooth muscle
In functional expression experiments, pharmacological and electrophysiological studies have indicated that Kir6.2/SUR1 represents the pancreatic β-cell KATP channel, that Kir6.2/SUR2A is thought to represent the cardiac KATP channel, whereas Kir6.1/SUR2B represents the smooth muscle-type KATP channel (Babenko et al., 1998). It is generally considered that SURs are responsible not only for sulphonylurea compound-sensitivity but also for interaction with KATP channel openers (Schwanstecher et al., 1998).
In detrusor smooth muscle, the molecular composition of SURs assessed by reverse transcriptase–polymerase chain reaction (RT–PCR) analysis has revealed the presence of mRNA transcripts for SUR1 and SUR2B (guinea-pig, Gopalakrishnan et al., 1999; pig and human, Buckner et al., 2000). However, Gopalakrishnan et al. (1999) concluded that SUR2B but not SUR1 was solely expressed as a SUR protein for regulating the activity of KATP channels in guinea-pig urinary bladder, since there was much less sensitivity to diazoxide in comparison to that of pancreatic β-cells and no high-affinity binding site for [3H]glyburide (Gopalakrishnan et al., 1999; Buckner et al., 2000). However, no functional study has yet been undertaken of human urinary bladder smooth muscle KATP channels to establish the possible involvement of SUR1 subunits.
Both SUR1 and SUR2B appear to share responsiveness to diazoxide, whereas SUR2A shows no response to this compound (Babenko et al., 1998). On the other hand, pinacidil activates KATP channels through SUR2 (SUR2A and SUR2B) but not through SUR1 (Babenko et al., 1998). So, in the present experiments, we utilized diazoxide as an agonist for SUR1 and pinacidil as a selective agonist for SUR2, but not for SUR1. We have demonstrated that both diazoxide and pinacidil caused a concentration-dependent relaxation in human detrusor, suggesting the presence of SUR1 and SUR2, although the potency of the pinacidil-induced relaxation was much effective than that of diazoxide. The same order of the potency was observed in other smooth muscles (rat portal vein and urinary bladder, Edwards et al., 1991).
At concentrations of less than 1 μM, gliclazide is generally believed to exhibit selective inhibitory effects on SUR1 but not SUR2A or SUR2B in recombinant KATP channels. Only much higher concentrations of gliclazide (⩾3 μM) directly block the channel pore region in KATP channels (Gribble and Ashcroft, 2000). In the present experiments, we have used gliclazide (<1 μM) as an antagonist for SUR1 but not for SUR2 (SUR2A and SUR2B). The diazoxide-induced KATP currents were significantly inhibited by gliclazide (⩾1 μM) although the gliclazide (1 μM)-sensitive component was less than 20% of the diazoxide-induced KATP currents.
Further, we have directly demonstrated that not only SUR1 but also SUR2B immunoreactivity is clearly visible in human detrusor smooth muscle bundles. These results suggest that SUR1 may be expressed as well as SUR2B in human urinary bladder.
In the present experiments, gliclazide inhibits the diazoxide- but not the ZD0947-induced currents at low concentrations (between 10 and 100 nM) although, at higher concentrations (⩾10 μM), this agent did suppress the ZD0947-induced currents. Moreover, in mouse pancreatic β-cells, the activity of pancreatic KATP channels, which consist of a combination of Kir6.2 and SUR1 proteins (Inagaki et al., 1995) was evoked by diazoxide but not by ZD0947 using the cell-attached mode (Teramoto, unpublished observation). These results suggest that ZD0947 may activate KATP channels mainly through binding to SUR2B in human detrusor myocytes. Further biological studies (such as expression studies) will cast light upon the effects of ZD0947 on the activity of KATP channels.
Pharmacological implications of KATP channels in human urinary bladder
Since the synthesis of KATP channel openers and their introduction to the urological field, their potential use for the treatment of urgency and frequency of micturition has been investigated (Andersson, 1993). Increase in membrane stability is one of the most useful targets for the treatment of OAB since involuntary detrusor activity results from increased sensitivity to depolarizing stimuli. It is generally thought that KATP channel openers will result in hyperpolarization of detrusor smooth muscle, which leads to a reduced Ca2+ influx through the closing of voltage-dependent Ca2+ influx pathways (such as voltage-dependent Ca2+ channels, nonselective cation channels, etc.). Modulation of KATP channels allows the contribution of native KATP channels to be finely tuned, so regulating the contractility of smooth muscle (Teramoto, 2006).
In the urological field, several KATP channel openers have been developed with the aim of reducing the frequency of micturition with no cardiovascular side effects (Howe et al., 1995) and inhibit urinary bladder hyperactivity without changing either blood pressure or heart rate (Wojdan et al., 1999). For this, it is essential that the actions of KATP channel openers on detrusor smooth muscle are more selective than those on other smooth muscles (such as vascular and urethral smooth muscles).
Abdel-Karim et al. (2002) reported that ZD0947 shows greater apparent selectivity for urinary bladder mediated effects than for cardiovascular mediated actions in rat. As the therapeutic utility of KATP channel openers in the treatment of OAB may be limited by hypotension as a result of insufficient selectivity in vivo for urinary bladder in comparison with vascular smooth muscle, it is necessary that the effects of KATP channel openers on human KATP channels are beneficial in vivo. Further studies will be required to investigate the selectivity of ZD0947 for the urinary bladder, in comparison to its effects on human vascular smooth muscle.
In conclusion, we have demonstrated that ZD0947 causes a detrusor relaxation through activation of glibenclamide-sensitive KATP channels in human urinary bladder.
Acknowledgments
We thank Professor Alison F Brading (University, Department of Pharmacology, Oxford, UK) for her helpful discussion and critical reading of the manuscript. This work was supported by both a Grant-in-Aid for Scientific Research (B)-(2) from the Japanese Society for the Promotion of Science (Noriyoshi Teramoto, Grant Number 16390067) and a Grant-in-Aid for Exploratory Research from the Ministry of Education and Culture of Japan (Noriyoshi Teramoto, Grant Number 17659075). We thank Mr Hiroshi Fujii for his excellent help with histological experiments.
Abbreviations
- BP
blocking peptide
- BSA
bovine serum albumin
- CCh
carbachol
- DHP
dihydropyridine
- DMSO
dimethylsulphoxide
- EK
theoretical equilibrium potential of K+
- FITC
fluorescein isothiocyanate
- KATP channels
ATP-sensitive K+ channels
- Kir
inwardly rectifying K+ channel
- NDP
nucleoside diphosphate
- OAB
overactive bladder
- OCT
optimal cutting temperature
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- RT–PCR
reverse transcriptase–polymerase chain reaction
- SUR
sulphonylurea receptor
- ZD0947
3-[(4S)-5-oxo-2-(trifluoromethyl)-1,4,5,6,7,8-hexahydroquinolin-4-yl]benzonitrile
Conflict of interest
The authors state no conflict of interest.
References
- Abdel-Karim AM, Bialecki RA, Elhilali MM. Effects of ZD6169 and ZD0947, 2 potassium adenosine triphosphate channel openers, on bladder function of spinalized rats. J Urol. 2002;168:837–842. [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urol. 2004;63:32–41. doi: 10.1016/j.urology.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/KIR6.x, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Brading AF.Physiology of bladder smooth muscle The Physiology of The Lower Urinary Tract 1987Springer-Verlag: Berlin; 161–191.In: Torrens M, Morrison JFB (eds) [Google Scholar]
- Buckner SA, Milicic I, Daza A, Davis-Taber R, Scott VE, Sullivan JP, et al. Pharmacological and molecular analysis of ATP-sensitive K+ channels in the pig and human detrusor. Eur J Pharmacol. 2000;400:287–295. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- Edwards G, Henshaw M, Miller M, Weston AH. Comparison of the effects of several potassium-channel openers on rat bladder and rat portal vein in vitro. Br J Pharmacol. 1991;102:679–680. doi: 10.1111/j.1476-5381.1991.tb12233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CD, Fujii K, Kingdon J, Brading AF. The effect of cromakalim on the smooth muscle of guinea-pig urinary bladder. Br J Pharmacol. 1989a;97:281–291. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CD, Speakman MJ, Fujii K, Brading AF. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989b;63:284–294. doi: 10.1111/j.1464-410x.1989.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Whiteaker KL, Molinari EJ, Davis-Taber R, Scott VE, Shieh CC, et al. Characterization of the ATP-sensitive potassium channels (KATP) expressed in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther. 1999;289:551–558. [PubMed] [Google Scholar]
- Gribble FM, Ashcroft FM. Sulfonylurea sensitivity of adenosine triphosphate-sensitive potassium channels from beta cells and extrapancreatic tissues. Metabolism. 2000;49:3–6. [PubMed] [Google Scholar]
- Howe BB, Halterman TJ, Yochim CL, Do ML, Pettinger SJ, Stow RB, et al. ZENECA ZD6169: a novel KATP channel opener with in vivo selectivity for urinary bladder. J Pharmacol Exp Ther. 1995;274:884–890. [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Schwanstecher M, Sieverding C, Dörschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, et al. Potassium channel openers require ATP to bind and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–624. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto N, Brading AF. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br J Pharmacol. 1996;118:635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda T, Aishima M, Takano N, Nakano T, Seki N, Yonemitsu Y, et al. The effects of flavoxate hydrochloride on voltage-dependent L-type Ca2+ currents in human urinary bladder. Br J Pharmacol. 2005;146:25–32. doi: 10.1038/sj.bjp.0706284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S, Stetz SL, Potter-Lee L, McConville M, Li JH, Empfield J, et al. K-channel opening activity of ZD6169 and its analogs: effect on 86Rb efflux and 3H-P1075 binding in bladder smooth muscle. Pharmacol. 1995;50:388–397. doi: 10.1159/000139308. [DOI] [PubMed] [Google Scholar]
- Uchida W, Masuda N, Shirai Y, Shibasaki K, Satoh N, Takenada T. The role of extracellular Ca2+ in carbachol-induced tonic contraction of the pig detrusor smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:398–402. doi: 10.1007/BF00178958. [DOI] [PubMed] [Google Scholar]
- Wammack R, Johnel U, Nawrath H, Hohenfellner R. Mechanical and electrophysiological effects of cromakalim on the human urinary bladder. Eur Urol. 1994;26:176–181. doi: 10.1159/000475371. [DOI] [PubMed] [Google Scholar]
- Wojdan A, Freeden C, Woods M, Oshiro G, Spinelli W, Colatsky TJ, et al. Comparison of the potassium channel openers, WAY-133537, ZD6169, and celikalim on isolated bladder tissue and in vivo bladder instability in rat. J Pharmacol Exp Ther. 1999;289:1410–1418. [PubMed] [Google Scholar]