Abstract
Background and purpose:
It has been postulated that isoflurane, a volatile anaesthetic, produces vasodilatation through activation of ATP-sensitive K+ (KATP) channels. However, there is no direct evidence for the activation of vascular KATP channels by isoflurane. This study was conducted to examine the effect of isoflurane on vascular KATP channels and compare it with that on cardiac KATP channels.
Experimental approach:
Effects of isoflurane on KATP channels were examined in aortic smooth muscle cells and cardiomyocytes of the mouse using patch clamp techniques. Effects of the anaesthetic on the KATP channels with different combinations of the inward rectifier pore subunits (Kir6.1 and Kir6.2) and sulphonylurea receptor subunits (SUR2A and SUR2B) reconstituted in a heterologous expression system were also examined.
Key results:
Isoflurane increased the coronary flow in Langendorff-perfused mouse hearts in a concentration-dependent manner, which was abolished by 10 μM glibenclamide. In enzymically-dissociated aortic smooth muscle cells, isoflurane evoked a glibenclamide-sensitive current (i.e. KATP current). In isolated mouse ventricular cells, however, isoflurane failed to evoke the KATP current unless the KATP current was preactivated by the K+ channel opener pinacidil. Although isoflurane readily activated the Kir6.1/SUR2B channels (vascular type), the volatile anesthetic could not activate the Kir6.2/SUR2A channels (cardiac type) expressed in HEK293 cells. Isoflurane activated a glibenclamide-sensitive current in HEK293 cells expressing Kir6.2/SUR2B channels.
Conclusion and implications:
Isoflurane activates KATP channels in vascular smooth muscle cells and produces coronary vasodilation in mouse hearts. SUR2B may be important for the activation of vascular-type KATP channels by isoflurane.
Keywords: ATP-sensitive K+ channel, isoflurane, coronary vasodilation
Introduction
It has long been known that isoflurane, a volatile anaesthetic, produces vasodilatation (Eger, 1981). As the isoflurane-induced vasodilatation was inhibited by glibenclamide, it has been postulated that activation of ATP-sensitive K+ (KATP) channels in vascular smooth muscle cells is involved in the vasodilatation (Cason et al., 1994; Crystal et al., 1997; Zhou et al., 1998). However, there is no direct evidence for the activation of vascular KATP channels by isoflurane. In terms of effects of isoflurane on cardiac KATP channels, apparently inconsistent results have been reported: Han et al. (1996) reported that isoflurane inhibited the openings of KATP channels in rabbit ventricular cells, whereas Kwok et al. (2002) indicated that isoflurane facilitated the KATP channel openings induced by metabolic blockade or the K+ channel opener pinacidil. Therefore, the first aim of this study was to examine the effect of isoflurane on the KATP channel current in vascular smooth muscle cells and to compare it with the effect on cardiac KATP channel current.
Recently, the KATP channel has been described as a hetero-octamer comprising two subunits: the pore-forming Kir6.x (Kir6.1 or Kir6.2) and the regulatory sulphonylurea receptor SUR (SUR1, SUR2A or SUR2B) (Seino and Miki, 2003). Different combinations of Kir6.x and SUR constitute KATP channels with distinct electrophysiological properties (Inagaki et al., 1995, 1996; Gribble et al., 1997; Yamada et al., 1997). Functional studies using genetically engineered mice lacking various KATP channel subunits from this and other laboratories (Suzuki et al., 2001, 2002; Chutkow et al., 2002; Miki et al., 2002) have indicated that Kir6.2 and SUR2A constitute the cardiac-type KATP channel, whereas Kir6.1 and SUR2B constitute the vascular smooth muscle-type KATP channel. If some difference could be detected between the effects of isoflurane on cardiac and vascular KATP channels, it would be of interest to identify which subunit(s) of the KATP channels were responsible for the different isoflurane effects. Accordingly, the second purpose of this study was to determine the effects of isoflurane on the KATP channels with different combinations of Kir6.x and SUR subunits reconstituted in a heterologous expression system. By doing so, we hoped to gain a greater insight into the molecule with which isoflurane interacts in KATP channels in cardiovascular tissues.
Methods
All experiments were performed according to the regulations of the Animal Research Committee of Chiba University Graduate School of Medicine and the Guide for the Care and Use of Laboratory Animals (NIH publication).
In vitro functional study using Langendorff-perfused hearts
C57BL/6 mice were purchased and used in this study. Mice were anaesthetized with urethane (1.5 mg g−1 body weight, intraperitoneally (i.p.)) and heparinized (0.1 U g−1 body weight, intravenously (i.v.)). Hearts were rapidly excised and connected to the perfusion cannula via the aorta, as described previously (Suzuki et al., 2001). Retrograde perfusion was maintained at a constant pressure of 80 cm H2O with a modified Krebs–Henseleit solution containing (in mM): NaCl 119, KCl 4.8, KH2PO4 1.2, MgSO4 1.2, CaCl2 1.8, glucose 10, NaHCO3 24.9. The perfusate was equilibrated with 95% O2 and 5% CO2 (pH 7.4, 37°C). Coronary flow was monitored continuously using an ultrasonic flow probe (Transonic Systems, Ithaca, NY, USA). Hearts were paced at a constant rate (480 beats/min) through a bipolar platinum electrode attached to the right atrium using an electronic stimulator (Nihon Kohden, Tokyo, Japan). After a 10-min stabilization period, the control perfusing solution was changed to one that was bubbled with various concentrations of isoflurane or others containing isoflurane and 10 μM glibenclamide.
Cell culture and transfection
Human embryonic kidney (HEK)293 cells (American Type Culture Collection, Rockville, MD, USA) were grown in Dulbecco's modified Eagle's Medium (Sigma-Aldrich Japan, Tokyo, Japan) supplemented with 10% foetal bovine serum (Invitrogen Corp., Carlsbad, CA, USA) and 100 U ml−1 penicillin G and 100 μg ml−1 streptomycin (Sigma-Aldrich Japan, Tokyo, Japan), and maintained at 37°C in a humidified atmosphere with 95% air and 5% CO2. Transient transfection was performed using lipofectAMINE Plus reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer's instructions. HEK293 cells were seeded on glass coverslips and co-transfected with the expression vector pCMV containing rat SUR2A or rat SUR2B and the pCMV containing rat Kir6.1 or human Kir6.2 (these plasmids were kindly provided by Dr S Seino, Kobe University) at a molar plasmid ratio of 1:1. The amounts of vector per 35 mm dish were as follows: 0.9 μg plasmid containing SUR2A or SUR2B and 0.6 μg plasmid containing Kir6.1 or Kir6.2. In general, pEGFP-C1 vector (Clontech, Mountain View, CA, USA), encoding for green fluorescent protein, was added for easy identification of transfected cells; the procedure did not affect the electrophysiological properties. Cells were allowed to express transfected DNA for 48 h and were then used for electrophysiological experiments.
Electrophysiology
Vascular smooth muscle cells
Single smooth muscle cells were enzymically isolated from the adult mouse aorta. The thoracic aorta was isolated from anaesthetized mice and cleaned of fat and connective tissues. The aorta was incubated in Hanks' solution containing 0.4% of collagenase for 40 min at 37.0°C. The composition of the Hanks' solution was (in mM): NaCl 137, KCl 5.4, NaH2PO4 0.168, KH2PO4 0.44, glucose 5.6 and NaHCO3 4.17. The solution was aerated with a mixture of 95% O2 and 5% CO2, and pH of the solution was maintained at 7.2–7.4. The tissue was gently agitated with a glass pipette.
Membrane currents were recorded at room temperature using the whole-cell patch-clamp technique, as described previously (Suzuki et al., 2001). The composition of the extracellular high-K solution was (in mM): NaCl 2.9, KCl 140, CaCl2 2.2, MgCl2 1.2, glucose 14, HEPES-KOH buffer 10 (pH 7.4), and that of the pipette solution was (in mM): KCl 140, MgCl2 4, K2-ATP 1, EGTA 10 and HEPES-KOH buffer 10 (pH 7.2).
Adult ventricular cells
Single ventricular cells of the adult mouse heart were isolated by conventional enzymic digestion (Sakamoto et al., 1998). Whole-cell membrane currents were recorded at 36.0°C by the patch-clamp method, as described previously (Suzuki et al., 2001). The composition of the pipette solution was (in mM): KCl 20, MgCl2 1, K-aspartate 110, K2-ATP 1, phosphocreatine-K2 1, CaCl2 1.41, EGTA 10 and HEPES 5 (pCa 8.0, pH 7.4). The external solution used was HEPES-Tyrode solution containing (in mM): NaCl 143, KCl 5.4, CaCl2 1.8, MgCl2 0.5, NaHPO4 0.33, glucose 5.5 and HEPES 5 (pH 7.4). A liquid junction potential between the internal solution and the bath solution of −8 mV was corrected. A ramp-pulse protocol was used to record the quasi-steady-state membrane current, as described previously (Sakamoto et al., 1998). The membrane potential was held at −40 mV and depolarized first to +50 mV at a rate of 1.2 mV ms−1. It was then repolarized or hyperpolarized to −100 mV with a slope of −1.2 mV ms−1, during which time the changes in the membrane current was automatically plotted against the membrane potential. The current–voltage relation was measured during the repolarized or hyperpolarized phase and the current level at 0 mV was obtained. The ramp voltage pulses were applied at appropriate timing.
Transfected HEK cells
The ionic currents through the channels expressed in the HEK293 cells were recorded in the whole-cell configuration of the patch-clamp technique. The membrane potential was held at −40 mV and the same ramp-pulse protocol as that in the experiments using mouse ventricular cells was used to record the quasi-steady-state membrane current. The current level at 0 mV was measured and the current density was calculated by normalization with the membrane capacitance. The pipette solution and the extracellular solution were the same as those used for the recording of the membrane currents in vascular smooth muscle cells.
Statistics
All data are presented as mean±s.e.m. Statistical analyses of the data were performed using two-way analysis of variance (ANOVA) combined with Fisher's post hoc test. Probability values less than 0.05 were considered significant.
Drugs
The following drugs were used: isoflurane (Merck, Osaka, Japan); glibenclamide (Sigma-Aldrich Japan, Tokyo, Japan); pinacidil (Sigma-Aldrich Japan, Tokyo, Japan); and theophylline (Wako, Osaka, Japan). Glibenclamide was dissolved in dimethyl sulphoxide (final concentration of the solvent was less than 0.1%) and pinacidil was dissolved in 0.1 N HCl as stock solutions. Isoflurane was introduced into the oxygen through Isoflurane Vapour (ACOMA I type MKIII, Tokyo, Japan) and dissolved in the external solution. From the data of a previous study in which isoflurane was vaporized into a physiological solution (Ozaki et al., 1990), the concentration of isoflurane in the solution dissolved at 4% would be expected to be around 1.36 mM.
Results
Effects of isoflurane on coronary flow in isolated mouse hearts
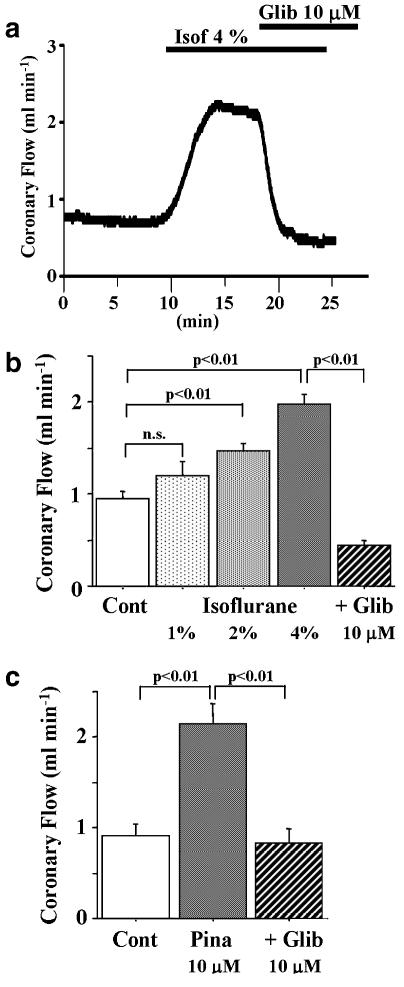
Representative changes of coronary flow after isoflurane alone and co-application of glibenclamide are shown in Figure 1. Isoflurane increased the coronary flow in a concentration-dependent manner. The coronary flow decreased below the control level after co-application of glibenclamide. Isoflurane dissolved at a concentration of 4% doubled the coronary flow and this effect was abolished by the addition of 10 μM glibenclamide. The K+ channel opener, pinacidil (10 μM), also doubled coronary flow and this was also antagonized by the addition of glibenclamide.
Figure 1.
Changes of coronary flow after isoflurane and pinacidil in Langendorff-perfused mouse hearts. (a) A representative increase in coronary flow after isoflurane (Isof) bubbled at a concentration of 4%. The increase in coronary flow was antagonized by 10 μM glibenclamide (Glib). Summarized data of peak coronary flow after isoflurane and pinacidil (Pina) are indicated in (b) and (c), respectively. Values are expressed as mean±s.e.m. of 6–8 preparations. Statistical analyses of the data were conducted using two-way ANOVA combined with Fisher's post hoc test.
Since it has been suggested that some of the activating effect of isoflurane on KATP channels is mediated by adenosine receptor stimulation (Gassmayr et al., 2003), effects of isoflurane on the coronary flow were examined in the presence of theophylline, a non-selective adenosine receptor antagonist. Even in the presence of 100 μM theophylline, isoflurane increased coronary flow from 1.15±0.20 to 2.09±0.09 ml min−1 (P<0.01, n=5).
Effects of isoflurane on KATP current in vascular smooth muscle cells
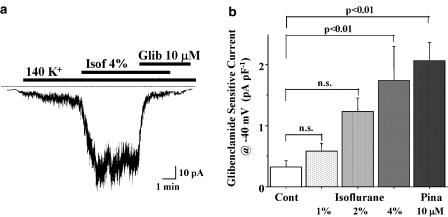
Effects of isoflurane and pinacidil on the whole-cell membrane current were examined in isolated aortic smooth muscle cells. Pinacidil (10 μM) activated a glibenclamide-sensitive inward current in smooth muscle cells held at −40 mV in the high K+ solution, as described previously (Suzuki et al., 2001). As shown in Figure 2, isoflurane, dissolved at a concentration of 4%, also activated a glibenclamide-sensitive inward current in aortic smooth muscle cells increasing almost six-fold the density of the glibenclamide-sensitive inward current. The isoflurane-induced increase in the inward current at −40 mV was concentration-dependent and the current was readily blocked by 10 μM glibenclamide.
Figure 2.
Isoflurane-induced current in mouse vascular smooth muscle cells. Representative change of the inward current after isoflurane (Isof) bubbled at a concentration of 4% is shown in (a). The inward current was sensitive to glibenclamide (Glib). The density of the glibenclamide-sensitive inward current after isoflurane and pinacidil (Pina) in aortic smooth muscle cells held at −40 mV are indicated in (b). Values are expressed as mean±s.e.m. of 5–12 cells. Statistical analyses of the data were conducted using two-way ANOVA combined with Fisher's post hoc test.
Effects of isoflurane on KATP current in ventricular cells
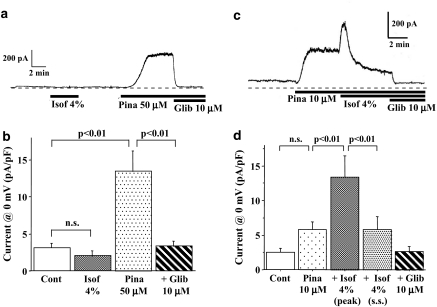
Effects of isoflurane and pinacidil on the whole-cell membrane current recorded using a ramp-pulse protocol were examined in mouse ventricular cells. Isoflurane dissolved at a concentration of 4% hardly affected or slightly decreased the quasi-steady-state membrane current (Figure 3a and b). However, pinacidil (50 μM) increased the steady-state current at 0 mV from 3.4±0.7 to 14.2±3.1 pA pF−1 (P<0.01, n=8), which was readily blocked by 10 μM glibenclamide. Thus, pinacidil but not isoflurane activated the glibenclamide-sensitive KATP current in mouse ventricular myocytes.
Figure 3.
Effects of isoflurane on the membrane current in mouse ventricular myocytes. Actual traces of the holding current at −40 mV are depicted in (a) and (c). Current densities at 0 mV, measured by a ramp-pulse protocol (a voltage change from +50 to −100 mV at a rate of 1.2 mV ms−1), are summarized in (b) and (d). Isoflurane (Isof) alone failed to activate a glibenclamide (Glib)-sensitive outward current (panels a and b). However, isoflurane transiently activated the KATP current when it was preactivated by a low concentration of pinacidil (Pina) (panels c and d). The densities of the outward current at 0 mV after pinacidil, pinacidil+isoflurane and pinacidil+isoflurane+glibenclamide are summarized in panel d. The peak and steady state (s.s.) current densities after the addition of isoflurane are shown. Values are expressed as mean±s.e.m. of 8–10 cells. Statistical analyses of the data were conducted using two-way ANOVA combined with Fisher's post hoc test.
Isoflurane was reported to sensitize the cardiac KATP channel to pinacidil in guinea-pig ventricular myocytes (Gassmayr et al., 2003). In their study, pretreatment with isoflurane produced a higher density of the pinacidil-induced KATP current than that induced by pinacidil alone in guinea-pig ventricular cells. In this study, we also examined effects of isoflurane on the KATP current pre-activated by pinacidil in mouse ventricular cells. Pinacidil at a concentration of 10 μM slightly increased the outward current, as shown in Figure 3c. Addition of isoflurane transiently increased and then suppressed the glibenclamide-sensitive outward current. When isoflurane (4%) and pinacidil (10 μM) were introduced simultaneously, the steady-state current at 0 mV was not different from control values.
Effects of isoflurane on recombinant KATP channels in HEK293 cells
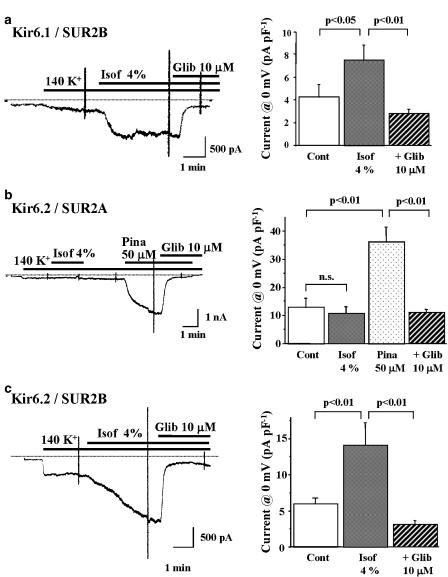
In order to gain a greater insight into the molecular target for isoflurane, we examined effects of isoflurane on recombinant KATP channels expressed in HEK293 cells using patch-clamp techniques. HEK293 cells were co-transfected with a Kir6.1 or Kir6.2 subunit in combination with either of SUR2A or SUR2B. Effects of isoflurane on Kir6.1/SUR2B channels, representing smooth muscle-type KATP channels, are shown in Figure 4a. Isoflurane dissolved at a concentration of 4% increased an inward current, which was sensitive to 10 μM glibenclamide. Effects of isoflurane on Kir6.2/SUR2A channels, representing cardiac-type KATP channels, were also examined. Isoflurane did not increase the inward current in HEK293 cells transfected with Kir6.2 and SUR2A. After washout of isoflurane, however, pinacidil (50 μM) activated the inward current that was readily abolished by the addition of 10 μM glibenclamide (Figure 4b). Since isoflurane activated Kir6.1/SUR2B channels but not Kir6.2/SUR2A channels, we examined the effect of the volatile anaesthetic on Kir6.2/SUR2B channels (Figure 4c). Isoflurane significantly increased the inward current in HEK293 cells transfected with Kir6.2 and SUR2B, although the time course of the current activation was relatively slow.
Figure 4.
Effects of isoflurane on recombinant KATP channels expressed in HEK293 cells. Representative changes of Kir6.1/SUR2B, Kir6.2/SUR2A and Kir6.2/SUR2B channel currents after isoflurane (Isof), pinacidil (Pina) and glibenclamide (Glib) and the summarized data are shown in (a–c), respectively. The membrane potential was held at −40 mV and the same ramp-pulse protocol as that in Figure 3 was used to record the quasi-steady-state membrane current. The current level at 0 mV was measured and then the current density was calculated. Values are expressed as mean±s.e.m. of nine cells. Statistical analyses of the data were conducted using two-way ANOVA combined with Fisher's post hoc test.
Discussion and conclusions
In this study, isoflurane increased coronary flow in isolated mouse hearts, which is consistent with coronary vasodilation observed in hearts of other species including humans (Reiz et al., 1983). As the isoflurane-induced increase of coronary flow was abolished by co-administration of glibenclamide, the involvement of activated KATP channel in this coronary vasodilatation seemed likely. We have provided direct evidence that isoflurane can activate the KATP current in isolated vascular smooth muscle cells. As far as we know, this is the first report showing direct evidence for the activation of KATP current by volatile anaesthetics in vascular smooth muscle cells.
In terms of effects of isoflurane on cardiac KATP channels, inconsistent results have been reported. Han et al. (1996) reported that isoflurane inhibited the openings of KATP channels in inside-out patches of rabbit ventricular cells. However, it was reported that isoflurane potentiated the KATP channel current induced by the K+ channel opener pinacidil or metabolic inhibition in guinea-pig ventricular cells (Fujimoto et al., 2002; Kwok et al., 2002). In addition, Gassmayr et al. (2003) demonstrated that pretreatment with isoflurane increased the density of the KATP current induced by pinacidil in guinea-pig ventricular cells. In this study, isoflurane per se failed to activate the KATP current in mouse ventricular cells. However, isoflurane transiently enhanced the KATP current and then inhibited the current when the KATP current was pre-activated by pinacidil. When both isoflurane and pinacidil were applied simultaneously in mouse ventricular cells, pinacidil failed to activate the KATP current. Thus, the effects of isoflurane on cardiac KATP channels are undoubtedly complex. Isoflurane has inhibiting as well as activating effects on cardiac KATP channels and the isoflurane effect appears to be dependent on the experimental conditions, which might explain the inconsistencies in previous reports (Han et al., 1996; Kwok et al., 2002).
The mechanism(s) by which isoflurane produced dual effects on cardiac KATP channels could not be clarified from this study. It was reported that isoflurane reduced the openings of KATP channels at normal pH, but facilitated them at reduced pH in inside-out patches of guinea-pig ventricular cells (Stadnicka and Bosnjak, 2003). In addition, the isoflurane-induced sensitization of the cardiac KATP channel to pinacidil was ascribed to generation of reactive oxygen species and/or activation of protein kinase C (An et al., 2004; Marinovic et al., 2005). As anionic phospholipids such as phosphatidylinositol 4,5-diphosphate are known to modulate KATP channel activity (Baukrowitz et al., 1998; Shyng and Nichols, 1998), isoflurane might affect cardiac KATP channels through the changes of membrane phospholipids. One possibility is that isoflurane might directly inhibit cardiac KATP channels and indirectly activate them through changes in membrane composition or second messengers. However, this is entirely speculative and further experimentation is needed to prove it. In this context, halothane, another volatile anaesthetic, inhibited the KATP current induced by metabolic inhibition, but did not affect the current that evoked by pinacidil in guinea-pig ventricular cells (Kwok et al., 2002), although the same anaesthetic evoked coronary vasodilation probably through the activation of KATP channels (Crystal et al., 1997). Thus, it appears that volatile anaesthetics including isoflurane do not easily activate cardiac KATP channels under basal conditions, although isoflurane can activate the channels under certain conditions.
In this study, isoflurane activated the vascular-type Kir6.1/SUR2B channels but not the cardiac-type Kir6.2/SUR2A channels expressed in HEK293 cells, which is consistent with the results of the experiments using mouse perfused heart and vascular smooth muscle cells. Isoflurane also activated Kir6.2/SUR2B channels, although the time course of K+ channel activation was relatively slow. Therefore, SUR2B may be important for the activation of KATP channels by isoflurane.
At the present time, it is difficult to decide whether the isoflurane effect on KATP channels is due to direct interaction with the KATP channel subunits or to secondary changes of intracellular messengers. SUR2A and SUR2B differ by only 42 amino acids in the C-terminus, caused by alternative splicing (Isomoto et al., 1996). The molecular mechanism by which these 42 C-terminal amino acids determine the pharmacological characteristics of SUR2A and SUR2B is not fully understood. Recently, it has been demonstrated that SUR2B shows greater affinity to K+ channel openers compared with SUR2A, which is related to a difference in Mg-nucleotide handling between these two SUR2 isoforms (Reimann et al., 2000). In addition, several studies have suggested that interaction of nucleotide-binding domains (NBD1 and NBD2) with ATP and ADP and subsequent dimerization may allosterically regulate pore openings (Yamada and Kurachi, 2004; Yamada et al., 2004). They have suggested that nucleotide-bound NBD1 and NBD2 more strongly promote conformational changes in SUR2B than SUR2A (Yamada and Kurachi, 2005). Isoflurane might facilitate the dimerization of NBDs more efficiently in SUR2B than SUR2A. Further experiments are required to clarify the precise mechanism by which isoflurane activates KATP channels via interaction with SUR2B.
Isoflurane was reported to produce a cardioprotective effect, a phenomena known as cardiac preconditioning, in experimental animals (Kersten et al., 1997; Ismaeil et al., 1999). It has been postulated that cardiomyocytes have two distinct types of KATP channels, that is, sarcolemmal KATP channels and mitochondrial KATP channels, and mitochondrial KATP channels play an important role in cardiac pre-conditioning, although the molecular identity of mitochondrial KATP channels remains unclarified (O'Rourke, 2004). It has been demonstrated that isoflurane activates mitochondrial KATP channels, as shown by flavoprotein oxidation (Kohro et al., 2001) and enhancement of diazoxide-induced flavoprotein oxidation by isoflurane (Zaugg et al., 2002). Such anaesthetic preconditioning through the activation of mitochondrial KATP channels may lead to more favourable outcomes in patients undergoing coronary artery bypass graft surgery (Zaugg et al., 2003a, 2003b). In addition to the protective effect of isoflurane on cardiomyocytes, the coronary vasodilatation induced by isoflurane, as observed in this study, may improve the perioperative cardiovascular outcome in patients at high risk of cardiovascular complications.
In summary, this study has demonstrated that isoflurane activates KATP channels in vascular smooth muscle cells, thereby producing coronary vasodilation and that the SUR2B subunit is important for the isoflurane-induced activation of vascular-type KATP channels.
Acknowledgments
We thank Dr S Seino (Kobe University) for providing us the cDNAs of rat SUR2A, rat SUR2B, human Kir6.2 and rat Kir6.1. We also thank Y Reien and I Sakashita for excellent technical and secretarial assistance. This work was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from K Watanabe Research Foundation.
Abbreviations
- HEK
human embryonic kidney
- KATP channel
ATP-sensitive K+ channel
- NBD
nucleotide-binding domain
- SUR
sulphonylurea receptor
Conflict of interest
The authors state no conflict of interest.
References
- An J, Standnicka A, Kwok WM, Bosnjak ZJ. Contribution of reactive oxygen species to isoflurane-induced sensitization of cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel to pinacidil. Anesthesiology. 2004;100:575–580. doi: 10.1097/00000542-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Cason BA, Shubayev I, Hickey RF. Blockade of adenosine triphosphate-sensitive potassium channels eliminates isoflurane-induced coronary artery vasodilation. Anesthesiology. 1994;81:1245–1255. doi: 10.1097/00000542-199411000-00019. [DOI] [PubMed] [Google Scholar]
- Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal GJ, Gurevicius J, Salem MR, Zhou X. Role of adenosine triphosphate-sensitive potassium channels in coronary vasodilation by halothane, isoflurane, and enflurane. Anesthesiology. 1997;86:448–458. doi: 10.1097/00000542-199702000-00020. [DOI] [PubMed] [Google Scholar]
- Eger EI. Isoflurane: a review. Anesthesiology. 1981;55:559–576. doi: 10.1097/00000542-198111000-00014. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Bosnjak ZJ, Kwok WN. Isoflurane-induced facilitation of the cardiac sarcolemmal KATP channel. Anesthesiology. 2002;97:57–65. doi: 10.1097/00000542-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Gassmayr S, Stadnicka A, Suzuki A, Kwok WN, Bosnjak ZJ. Isoflurane sensitizes the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel to pinacidil. Anesthesiology. 2003;98:114–120. doi: 10.1097/00000542-200301000-00020. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Ashfield R, Ammla C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. J Physiol (London) 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kim E, Ho WK, Earm YE. Effects of volatile anesthetic isoflurane on ATP-sensitive K+ channels in rabbit ventricular myocytes. Biochem Biophys Res Commun. 1996;229:852–856. doi: 10.1006/bbrc.1996.1891. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Wang CZ, Agular-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Ismaeil MS, Trachenko I, Gamperl AK, Hickey RF, Cason BA. Mechanisms of isoflurane-induced myocardial preconditioning in rabbits. Anesthesiology. 1999;90:812–821. doi: 10.1097/00000542-199903000-00024. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of KATP channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Kohro S, Hogan QH, Nakane Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–1440. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- Kwok WN, Martinelli AT, Fujimoto K, Suzuki A, Stadnicka A, Bosnjak ZJ. Differential modulation of the cardiac adenosine triphosphate-sensitive potassium channel by isoflurane and halothane. Anesthesiology. 2002;97:50–56. doi: 10.1097/00000542-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C-δ-mediated mechanism. Anesthesiology. 2005;103:540–547. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S, Nakaya H, Gotoh Y, Azuma M, Kemmotsu O, Kanno M. Effects of isoflurane on conduction velocity and maximum rate of rise of action potential upstroke in guinea pig papillary muscles. Anesth Analg. 1990;70:618–623. doi: 10.1213/00000539-199006000-00007. [DOI] [PubMed] [Google Scholar]
- Reimann F, Gribble FM, Ashcroft FM. Differential response to KATP channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol Pharmacol. 2000;58:1318–1325. doi: 10.1124/mol.58.6.1318. [DOI] [PubMed] [Google Scholar]
- Reiz S, Balfors E, Sorensen MB, Ariola S, Jr, Friedman A, Truedsson H. Isoflurane: a powerful coronary vasodilator in patients with coronary artery disease. Anesthesiology. 1983;59:91–97. doi: 10.1097/00000542-198308000-00004. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Uemura H, Hara Y, Saito T, Masuda Y, Nakaya H. Bradykinin B2-receptor-mediated modulation of membrane currents in guinea-pig cardiomyocytes. Br J Pharmacol. 1998;125:283–292. doi: 10.1038/sj.bjp.0702060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Shyng S, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Stadnicka A, Bosnjak ZJ. Isoflurane decreases ATP sensitivity of guinea pig cardiac sarcolemmal KATP channel at reduced intracellular pH. Anesthesiology. 2003;98:396–403. doi: 10.1097/00000542-200302000-00020. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ishii M, Hibino H, Kurachi Y. Mutation in nucleotide-binding domains of sulfonylurea receptor 2 evokes Na-ATP-dependent activation of ATP-sensitive K+ channels: Implication for dimerization of nucleotide-binding domains to induce channel opening. Mol Pharmacol. 2004;66:807–816. doi: 10.1124/mol.104.002717. [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, et al. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol (London) 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kurachi Y. The nucleotide-binding domains of sulfonylurea receptor 2A and 2B play different functional roles in nicorandil-induced activation of ATP-sensitive K+ channels. Mol Pharmacol. 2004;65:1198–1207. doi: 10.1124/mol.65.5.1198. [DOI] [PubMed] [Google Scholar]
- Yamada M, Kurachi Y. A functional role of the C-terminal 42 amino acids of SUR2A and SUR2B in the physiology and pharmacology of cardiovascular ATP-sensitive K+ channels. J Mol Cell Cardiol. 2005;39:1–6. doi: 10.1016/j.yjmcc.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Garcia C, Pasch T, Scaub MC. Anaesthetics and cardiac preconditioning. Part II. Clinical implications. Br J Anaesth. 2003a;91:566–576. doi: 10.1093/bja/aeg206. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial KATP channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003b;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- Zhou X, Abboud W, Manabat NC, Salem MR, Crystal GJ. Isoflurane-induced dilation of porcine coronary arterioles is mediated by ATP-sensitive potassium channels. Anesthesiology. 1998;89:182–189. doi: 10.1097/00000542-199807000-00025. [DOI] [PubMed] [Google Scholar]