Abstract
Background and purpose:
RANTES is an inflammatory chemokine with a critical role in T-lymphocyte activation and proliferation. Its effects are mediated through G protein-coupled heptahelical receptors (GPCRs). We show for the first time that RANTES activates the orphan G protein-coupled receptor 75 (GPR75).
Experimental approach:
To identify a ligand for GPR75 we have used three different and independent methods, namely luciferase assay, bioluminescence assay and IP3 accumulation assay.
Key results:
Treatment of cells expressing GPR75 with subnanomolar concentrations of RANTES led to stimulation of the luciferase activity in a reporter-gene assay, an increase in inositol trisphosphate, and intracellular Ca2+. The latter effect was blocked by the phospholipase-C inhibitor (PLC) U73122 indicating that Gq proteins mediate GPR75 signaling. RANTES enhanced the phosphorylation of AKT and mitogen-activated protein kinase (MAPK) in GPR75-transfected cells and this effect was blocked by the PLC inhibitor U73122 and the phosphatidylinositol 3-kinase (PI3K) inhibitor, wortmannin. The hippocampal cell line HT22, which expresses GPR75 endogenously, but not the other known RANTES receptors, was used to study the effects of RANTES and GPR75 on neuronal survival. Treatment of HT22 cells with RANTES significantly reduced the neurotoxicity of amyloid-β peptides, by activating PLC and PI3K.
Conclusions and implications:
This demonstrate clearly and undoubtedly the ability of RANTES to act on GPR75. Defects in the RANTES/GPR75-signaling pathway may contribute to neuroinflammatory and neurodegenerative processes as observed in Alzheimer's disease.
Keywords: RANTES, GPCR, GPR75, chemokine, HT22 cells
Introduction
G protein-coupled receptors (GPCRs) are the largest family of cell-surface receptors encoded by more than 1000 genes in the human genome (Howard et al., 2001). They regulate numerous physiological and pathophysiological processes in response to extracellular stimuli, for example, hormones, peptides, neuroamines, growth factors, lipids, ions and sensory signals. Identification of novel GPCRs and their endogenous ligands can help to gain insight into different physiological processes and pathophysiological disorders (Howard et al., 2001).
Recently, G protein-coupled receptor 75 (GPR75) was identified as a novel human GPCR: the corresponding gene maps to human chromosome 2p16 and encodes a 540 amino-acids protein (Tarttelin et al., 1999). The entire open-reading frame is present within a single exon, a typical hallmark of GPCRs. Human GPR75 is most closely related to a putative Caenorhabditis elegans neuropeptide Y receptor (24% homology), the rat galanin receptor type 3 (25% homology) and the porcine growth hormone secretagogue receptor type 1b (25% homology) (Tarttelin et al., 1999). This suggests that peptides could be cognate ligands for GPR75.
It has been reported that human GPR75 is predominantly expressed in the retina and in the central nervous system (CNS) (Tarttelin et al., 1999; Sauer et al., 2001). Interestingly, an inherited retinal dystrophy, Doyen's honeycomb retinal dystrophy, colocalizes with GPR75 on chromosome 2p16 (Tarttelin et al., 1999). However, no link between GPR75 and the disease pathology has been found (Tarttelin et al., 1999). Similarly, no direct connection was evident for another retina-associated disease, age-related macular degeneration (Sauer et al., 2001). Further functional studies and identification of the endogenous ligand are required to understand the role of GPR75 in retinal and brain physiology and pathophysiology.
GPCRs serve as receptors for a large family of cytokines called chemokines that play a regulatory role in the inflammatory processes (Cartier et al., 2005). RANTES (regulated upon activation, normal T-cell expressed and secreted), a 68 amino-acid protein, belongs to the CC-chemokine subfamily (it has the systematic name CCL 5), and was originally identified as a T-cell-specific gene (Schall et al., 1988). In addition to its well-established role in the immune system, recent data suggest its involvement in CNS homeostasis, brain inflammation, and ocular and neurodegenerative diseases (Mennicken et al., 1999; Wallece et al., 2004; Cartier et al., 2005).
Here, we report the identification and cloning of a mouse ortholog of the human GPR75 expressed predominantly in mouse brain and heart. In a search for ligands for this receptor, we discovered that RANTES increased inositol trisphosphate (IP3) and intracellular Ca2+ in cells heterogeneously expressing GRP75. It responded via Gq protein-coupled signal-transduction pathways and activated phospholipase-C (PLC). RANTES via GPR75 led to the activation of the antiapoptotic mitogen-activated protein kinase (MAPK) via the PLC/phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway and prevented amyloid-β (Aβ)-induced cell death in the hippocampal cell line, HT22.
Methods
Cells
The human embryonic kidney (HEK), African green monkey kidney (CV-1) and hippocampal (HT22) cell lines were kindly provided by Dr Axel Methner (ZMNH, Hamburg, Germany) and the Chinese hamster ovary (CHO-K1) cells by Dr Jenny Stables (GlaxoSmithKline, Stevenage, UK).
Amplification and cloning of GPR75
Human GPR75 was amplified by polymerase chain reaction (PCR) from human genomic DNA using 5′-CATATGGATC CACGGTGGGGACTGGAA-3′ as forward and 5′-ATCTAAA GCTTATGAACTCAACAGGCCA-3′ as reverse primers. The GPR75-PCR product was inserted into the pGEM-T-easy vector (Promega, Heidelberg, Germany) and subcloned into the BamHII/HindIII sites of the pEGFPN1 vector, bearing the enhanced green fluorescent protein (EGFP) (Promega). A query in the mouse genomic database with the human GPR75 sequence revealed a mouse ortholog of GPR75. A 1623 bp fragment covering the entire open-reading frame of mouse GPR75 was amplified by PCR from the genomic mouse DNA using the primer set 5′-ATGAACACAAGTG CCCCGCTTCAGATGTCC-3′ and 5′-TTAAACAGAGGGGATA GGAATTTGTTTT-3′. The resulting full-length nucleotide sequence of mouse GPR75 was submitted to GenBank (accession number AY253852). The GPR75-PCR product was inserted into the pGEM-T-easy vector (Promega). The correctness of all constructs was verified by sequencing.
Tissue distribution of GPR75 and amplification of CCR1, CCR3 and CCR5 in HT22 cells
A mouse multiple-tissue cDNA panel (Clontech, Heidelberg, Germany) and cDNA from HT22 cells were probed by PCR with the above described mouse-specific forward primer for GPR75. The reverse primer matched the 819–843 nucleotide sequence of AY253852. Analysis of the transcript distribution of CCR1, CCR3 and CCR5 in HT22 cells was performed by amplification of the fragments with following gene-specific primers: for CCR1, a 834 bp fragment was amplified with primers derived from positions 1–25 and 811–834 from the mouse sequence (GenBank accession number U29678); for CCR3, a 798 bp fragment ranged from positions 56–78 to 830–854 of the mouse sequence (GenBank accession number U29677); for CCR5, a 813 bp fragment spanned positions 240–136 and 1031–1053 of the human sequence (GenBank accession number X91492). As templates served cDNAs from mouse HT22 cells was used as a template. Amplification of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) fragment served as a control.
Luciferase reporter-gene assay
CV-1 cells were plated in 96-well white, clear-bottom plates in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Karlsrue, Germany) supplemented with 10% fetal calf serum (FCS) and antibiotics. After 24 h, the cells were transiently transfected with human GPR75 or vector cDNA (as control) and the reporter-gene construct using lipofectamine 2000 (Invitrogen). The reporter contains three multiple-response elements (MREs), a cAMP-response element (CRE) and a serum-response element (SRE) upstream of the luciferase gene (Jiang et al., 2003). The ratio of the receptor or vector to the reporter-gene construct was 4:1. The cells were incubated at 37°C in serum-free OptiMEM 1 (Invitrogen) containing the DNA-lipofectamin 2000 complex. After 4–6 h, the medium was replaced by DMEM, supplemented with 5% FCS and incubated for additional 24 h, followed by incubation for 24 h in a serum-free defined medium supplemented with 5 μg ml−1 insulin, 30 μg ml−1 transferrin, 20 μM ethanolamine, 30 nM sodium selenite, 1 μM sodium pyruvate, 1% non-essential amino acids and 2 mM glutamine in DMEM. Thereafter, the cells were additionally incubated for 5 h at 37°C with various ligands at different concentrations, in the presence or absence of 5 μM forskolin, dissolved in 50 μl serum-free defined medium. To determine the luciferase activity, 25 μl Bright-Glo (Promega) was added, and after 2 min the luminescence signal was measured at 37°C using a TriLux luminescence counter (Perkin-Elmer Life Sciences, Rodgau, Germany).
Aequorin-based bioluminescence assay
The assay was performed as already described (Ignatov et al., 2003). Briefly, CHO-K1 cells were transiently transfected with GPR75 or vector cDNA together with a construct encoding the mitochondrially targeted aequorin as Ca2+ sensor. The cells were seeded in 96-well plates for 24 h, followed by incubation at 37°C in a serum-free OptiMEM 1 medium containing the DNA-lipofectamin 2000 complex. After 4–6 h, the medium was replaced by DMEM-F12, supplemented with 5% FCS and incubated for 24 h, followed by 24 h incubation in serum-free defined medium. To measure the Ca2+-induced luminescence, cells were treated for 4 h with 5 μM coelenterazine as cofactor for aequorin. Ligands were dissolved in serum-free defined medium, added to the cells and the luminescence was read at a luminometer (Berthold Technologies) at 37°C for 15 s.
IP3 determination
Human embryonic kidney (HEK293) cells were grown in DMEM supplemented with 7.5% FCS, 0.5 mM glutamine, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin. To measure IP3 production, HEK293 cells were transiently transfected using the Effecten reagent (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions and grown in 24-well plates. The cells were metabolically labeled for 24 h with 4 μCi ml−1 of myo-[3H]inositol (Amersham Biosciences, Freiburg, Germany). They were stimulated for 30 min with various concentrations of chemokines in the presence of 10 mM LiCl. IP3 content was determined by scintillation counting after methanol extraction, separation on a Dowex AG1-X8 column (Bio-Rad, Marnes-La-Coqutte, France) and elution with 1 M ammonium formate, as described previously (Robert et al., 2005).
Phosphorylation of Akt and MAPK
CV-1 cells, transfected with vector or GPR75, were grown in six-well plates overnight, then incubated with 1 nM RANTES for 0, 1, 5, 10 and 15 min with or without prior incubation with various inhibitors. The cells were washed with phosphate-buffered saline, collected in buffer preheated to 95°C (5 mM Na2HPO4, 2% sodium dodecyl sulfate, 0.1 M dithiothreitol, 5% beta-mercaptoethanol, 10% glycerol and 0.05% bromophenol blue) and incubated for 5 min at 95°C. The cells were ultrasonicated and the proteins in the homogenates separated by polyacrylamide gel electrophoresis. After semidry blotting, the membranes were immunodetected with monoclonal antibodies against phosphorylated MAPK (1:2000) or phopshorylated Akt kinase (1:2000). Following incubation with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:2000) (New England Biolabs, Frankfurt, Germany), the detection was accomplished with the SuperSignal peroxidase substrate (Perbio Science, Bonn, Germany). The same membranes were stripped and reprobed with a polyclonal antibody against total MAPK (1:1000) (New England Biolabs) and with a monoclonal antibody against acetylated tubulin (1:1000) (Sigma-Aldrich, Taufkirchen, Germany).
MTT viability assay
HT22 cells, transfected with GPR75 or vector alone, were grown in 96-well plates for 24 h. Cells were pretreated with 1 nM of different chemokines for 1 h, before addition of 20 μM Aβ1–42, Aβ25–35, or as control the Aβ peptide with a reverse amino-acid sequence Aβ42–1. Aβ peptides were dissolved in 0.1 N HCl to obtain a 1 mM stock solution, briefly heated to 95°C, neutralized with NaOH, and aliquots were stored frozen until use. The cells were incubated for 24 h with the Aβ peptides before treatment for 2 h in the dark at 37°C with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Following incubation with a lysis buffer, the cell viability was determined by measuring the absorbance at 560 nm.
Data analysis
The results are expressed as means of six determinations±s.d. Statistical analysis was carried out using Student's t-test for paired observations. When three or more means were compared, analysis of variance was applied using the Prism program (Graph Pad Software, San Diego, CA, USA). Curve fittings were performed with the Prism program.
Ligands and signal-transduction inhibitors
RANTES (CCL 5), macrophage inflammatory protein 1α (MIP1α; CCL 3), macrophage inflammatory protein 1β (MIP1β; CCL 4), monocyte chemoattractant protein 3 (MCP3; CCL 7), stromal cell-derived factor-1 (SDF-1; CXCL 12) and Aβ peptides were obtained from Bachem (Weil am Rein, Germany), U73122 from Merck (Darmstadt, Germany) and SDF-1, wortmannin and PTX from Sigma-Aldrich. Stock solutions were prepared as recommended by the manufacturers and were stored at −20°C.
Results
Cloning and tissue-expression profile of mouse GPR75
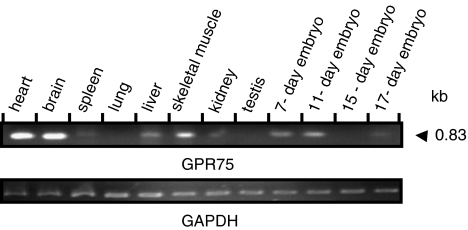
Comparison of the nucleotide sequence of the intron less human GPR75 with the mouse genomic database allowed construction of the PCR primers and amplification of mouse GPR75. Sequence analysis revealed an open-reading frame of 1623 nucleotides corresponding to a protein of 540 amino acids (GenBank accession number AY253852). It shared 87% identity at the amino-acid level with human GPR75. Mouse GPR75 was found to map to chromosome 11A4. Tissue distribution of mouse GPR75 was analyzed by PCR on a cDNA panel representing 12 different tissues from adult mouse and from whole mouse embryos (Figure 1). The highest level of expression was observed in the brain and heart. Lower levels of GPR75 expression were detected in skeletal muscles and in 7- and 15-day-old embryos. Very low abundance of expression was observed in the liver, kidney and 17-day-old embryo.
Figure 1.
Expression analysis of mouse GPR75 mRNA. The expression of GPR75 mRNA in different adult mouse tissues and 7-, 11-, 15- and 17-day-old embryos was assessed by PCR using a multiple-tissue cDNA panel and GPR75-specific primers that amplify a product of 843 bp (upper panel). GAPDH served as loading control (bottom panel).
RANTES and MIP1α stimulate the luciferase activity in a reporter-gene assay via GPR75
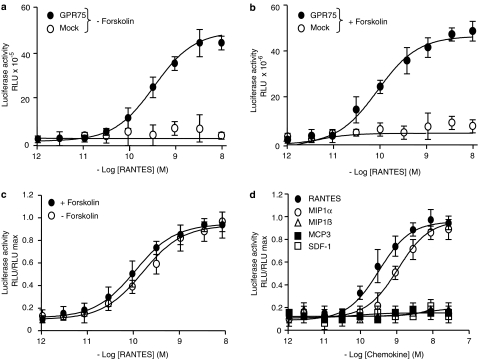
To identify the endogenous ligand(s) for different GPCRs, a variety of peptides and peptide-enriched tissue extracts were tested in a luciferase-reporter assay (Jiang et al., 2003). For this purpose CV-1 cells were co-transfected with plasmids bearing the GPCR of interest and the luciferase reporter gene. As control served an empty vector or another GPCRs. Stimulation of GPCRs with a cognate ligand should result in an increase (Gs, Gq) or a decrease (Gi) of the forskolin-induced luminescence signals (Jiang et al., 2003). The transfection efficiency of CV-1 cells, transiently expressing GPR75 was in the range of 25–30% as assessed by GPR75-EGFP fluorescence. We found that RANTES increased the basal luciferase activity (Figure 2a) as well as the forskolin-induced luciferase activity (Figure 2b) in cells transiently transfected with the GPR75, suggesting a coupling of GPR75 to Gq- or Gs-proteins. No response to RANTES was observed in cells transfected with the EGFP vector alone (Figures 2a and b). The comparison of the dose–response curves, obtained by using the ratio between the luminescence of the individual samples and by maximal stimulation, revealed quite similar half-maximal effective concentrations (EC50) of 0.11 and 0.26 nM in the presence and absence of forskolin, respectively (Figure 2c). A typical hallmark of the chemokine receptors is their promiscuity (Cartier et al., 2005), therefore other peptides belonging to CC- and CXC-chemokine subfamily were also tested for their effect on the luciferase activity in GPR75-transfected cells. In addition to RANTES, only MIP1α reacted with GPR75 (Figure 2d), albeit with lower affinity (EC50=1 nM). MIP1β, MCP3 and SDF-1 had no effect on the stimulation of the luciferase activity (Figure 2d).
Figure 2.
GPR75-mediated stimulation of luciferase expression by RANTES. CV-1 cells were transiently transfected with either GPR75 or empty vector (Mock) and the MRE/SRE/CRE-reporter construct. 48 h later, cells were stimulated for 5 h with different concentrations of RANTES in the absence (a), or presence (b) of 5 μM forskolin. The luciferase activity is expressed in relative light units (RLU). (c) The stimulation of the luciferase activity given as a ratio between the luminescence measured for the individual samples in RLU and the maximal stimulation (RLU max). The calculated EC50 values were: 0.11 and 0.16 nM. (d) Cells were stimulated for 5 h with 5 μM forskolin and with various concentrations of different chemokines. Note that in addition to RANTES, only MIP1α increased the luciferase activity over the forskolin-stimulated background response with an EC50 value of 1 nM. Data are expressed as mean values±s.d.
RANTES stimulates Ca2+ mobilization in CHO-K1 cells expressing GPR75
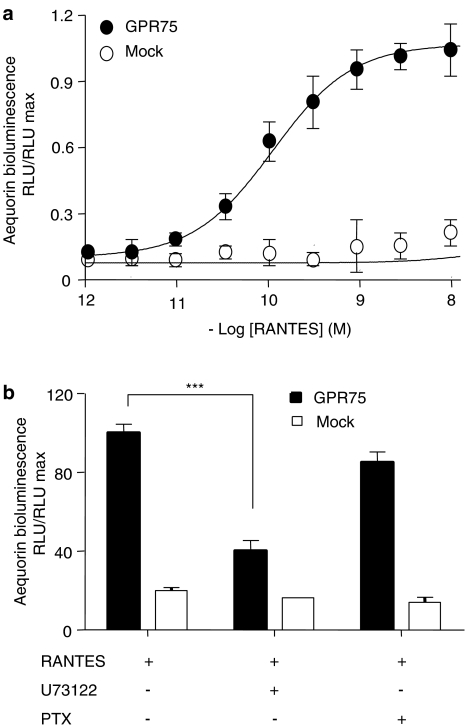
The luciferase activity can be increased both over Gq- or Gs-dependent pathways (Jiang et al., 2003). With the next set of experiments, we aimed to determine the natural coupling of the GPR75 receptor to intracellular signaling pathways. CHO cells were transiently co-transfected with constructs coding for GPR75 or vector and with the bioluminescent Ca2+ sensor apoaequorin. This assay had proven to be useful for ligand identification as described earlier (Ignatov et al., 2003). GPR75 transfection efficiencies were in the range of 30–40%. RANTES was able to stimulate Ca2+-induced bioluminescence response in GPR75-transfected cells, but not in vector-transfected cells (Figure 3a). No effect was observed after activation with other chemokines including MIP1α. The RANTES-induced Ca2+ mobilization was dose dependent, and the dose–response curve revealed an EC50 of 0.12 nM (Figure 3a). It indicates that GPR75 utilizes endogenous G proteins to stimulate PLC.
Figure 3.
RANTES stimulates the Ca2+-induced bioluminescence in GPR75-transfected CHO-K1 cells. (a) CHO-K1 cells were transiently co-transfected with GPR75 or empty vector and apoaequorin as Ca2+ sensor. Cells were treated with different concentrations of RANTES, and the Ca2+-induced bioluminescence was measured at 469 nm and is expressed as RLU/RLU max. The dose–response curve yielded an EC50 value of 0.12 nM. (b) CHO-K1 cells transiently co-transfected with apoaequorin and GPR75 or empty vector were pretreated with 50 ng ml−1 PTX for 18 h, with 5 μM PLC inhibitor U73122 for 10 min, before the response to RANTES was measured as Ca2+-induced luminescence. The RANTES-induced Ca2+ increase was significantly inhibited by U73122 compared to untreated controls, whereas PTX had no effect (***P<0.001). Data are expressed as mean values±s.d.
Moreover, the RANTES-induced bioluminescence in GPR75-transfected cells was completely blocked by preincubation of the cells with the PLC-specific inhibitor, U73122, whereas PTX did not show a significant reduction (Figure 3b).
GPR75 induces IP3 formation in HEK293 cells
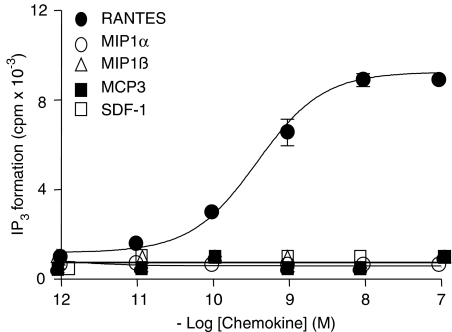
To confirm the Gq-protein coupling of GPR75, we measured the production of IP3 in GPR75-transfected HEK293 cells after stimulation with various concentrations of different chemokines. RANTES enhanced IP3 formation in GPR75-transfected cells in a dose-dependent manner with an EC50 value of 0.3 nM (Figure 4), which was in the same order of magnitude as the values obtained in the luciferase-reporter and the aequorin-based bioluminescence assays. Activation with MIP1α, MIP1β, MIP3 and SDF-1 led to no detectable change in the IP3 production.
Figure 4.
RANTES stimulates IP3 formation in GPR75-transfected HEK293 cells. HEK293 cells were transiently transfected with GPR75. Cells were treated with various concentrations of RANTES, MIP1α, MIP1β, MCP3 and SDF-1 for 30 min, and the production of IP3 was measured in extracts from intact HEK293 cells. The dose–response curve yielded an EC50 value of 0.3 nM for RANTES-induced production of IP3. Data are expressed as mean values±s.d.
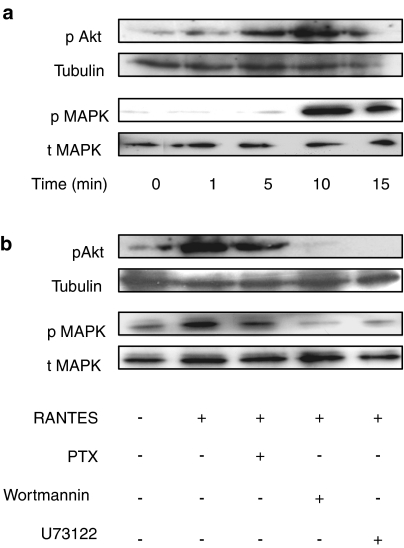
RANTES activates phosphorylation of Akt and MAPK in GPR75-transfected CV-1 cells
Activation of Akt and MAPK is crucial for promoting cell proliferation and cell survival, both typical chemokine-induced processes (Cartier et al., 2005). To determine if this is the case, we investigated the influence of GPR75 and RANTES on the activation of Akt and MAPK. CV-1 cells transiently transfected with either GPR75 or empty vector were incubated with 1 nM RANTES at various times. The phosphorylation of Akt and MAPK was detected using specific antibodies for phosphorylated Akt and phosphorylated p44/p42 MAPK. RANTES induced a sustained phosphorylation of Akt and MAPK in GPR75-transfected cells (Figure 5a), but not in vector-transfected cells (data not shown). The phosphorylation of Akt was evident after 5 min, whereas the MAPK phosphorylation required 10 min. The levels of total MAPK and tubulin remained constant (Figure 5a). To identify the signaling pathway involved in the RANTES-induced Akt and MAPK phosphorylation, we preincubated the CV-1 cells transiently transfected with GPR75, with wortmannin, U73122 and PTX, before addition of RANTES. Wortmannin and U73122 abolished the RANTES-induced phosphorylation of Akt and MAPK, whereas PTX had no effect (Figure 5b).
Figure 5.
RANTES induces the phosphorylation of Akt and MAPK in GPR75-transfected CV-1 cells via the PLC/PI3K/Akt signaling pathway. (a) CV-1 cells were transiently transfected with GPR75 and 48 h later incubated with 1 nM RANTES for the indicated times. Whole-cell extracts were analyzed by Western blotting using antibodies against phosphorylated MAPK (pMAPK), phosphorylated Akt (pAkt), total MAPK (tMAPK) and acetylated tubulin. Note that the levels of total MAPK and tubulin remained constant. The blot is a representative of three different experiments. (b) GPR75-transfected CV-1 cells were pretreated with 50 ng ml−1 PTX for 18 h, with 5 μM PLC inhibitor U73122 for 10 min, and with 0.5 μM PI3K inhibitor wortmannin for 1 h, before1 nM RANTES was added for 10 min. The blot is representative of two experiments.
GPR75 mediates the RANTES-induced neuroprotection against Aβ toxicity
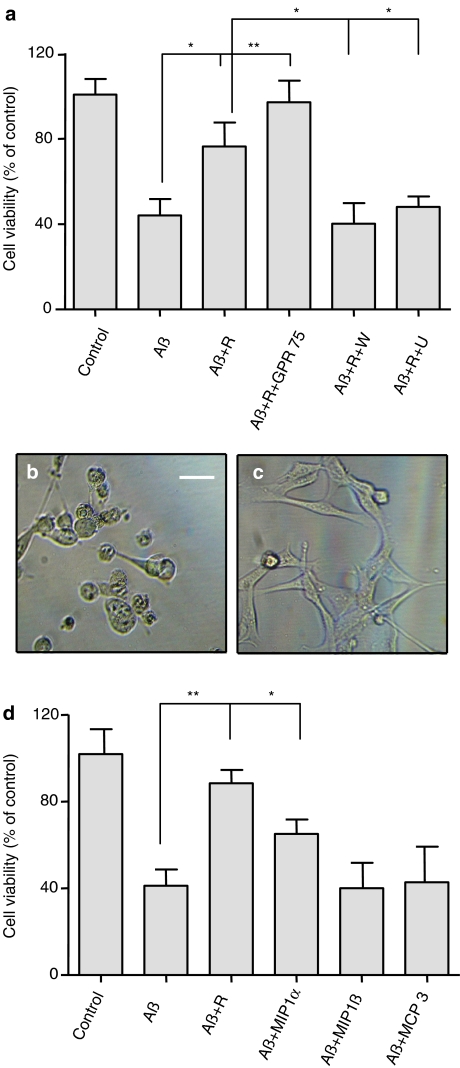
The hippocampus is a primary target for neuronal degeneration in patients with Alzheimer's disease. GPR75 has been found to be expressed in this region (Tarttelin et al., 1999; Sauer et al., 2001). We next asked, whether RANTES and GPR75 can influence the Aβ-induced cell death in the mouse hippocampal cell line HT22 with endogenous GPR75 (Figure 6). Approximately 60% of HT22 cells died 24 h after Aβ1−42 exposure (Figure 6). Comparable results were obtained with Aβ25−35, which has been shown to be as neurotoxic as full-length Aβ1−42 (Varadarajan et al., 2001). The Aβ peptide with a reverse amino-acid sequence (Aβ42−1) had no effect (Figure 6). Pretreatment of the cells with RANTES before the exposure to 20 μM Aβ25−35 led to a significant increase in cell viability compared to the untreated cells (Figure 7a). Phase-contrast microscopy confirmed the prevention of cell death by preincubation with RANTES (compare Figures 7b and c). Dying cells were characterized by condensed cell bodies and retracted cellular protrusions (Figure 7b). Additionally, transient transfection with GPR75 stabilized the HT22 cells and further increased their survival efficiency (Figure 7a). Interestingly, wortmannin and U73122 completely abrogated the RANTES-induced survival effect (Figure 7a). In addition to RANTES, only MIP1α inhibited the Aβ toxicity, albeit with a lower efficiency (Figure 7d). As observed with RANTES, MIP1α-stimulated protection was abolished by wortmannin and U73122.
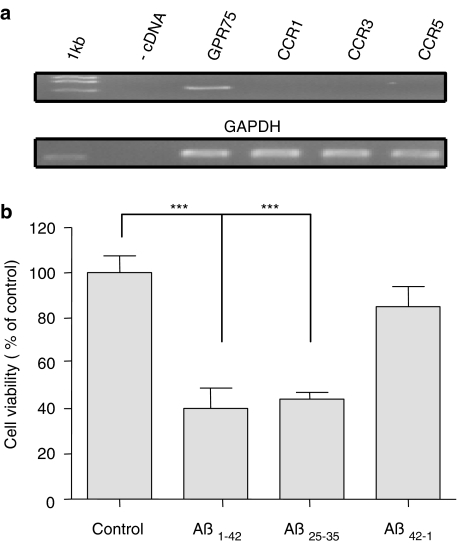
Figure 6.
Aβ peptides reduce cell viability in HT22 cells. (a) HT22 cells express GPR75 endogenously but not CCR1, CCR3 and CCR5 chemokine receptors (upper panel). GAPDH served as a loading control (lower panel). cDNA represents the negative control. The experiment is representative of three detections. (b) HT22 cells respond to a 24 h treatment with Aβ peptides by cell death, as assessed by the MTT assay. Note that no difference was observed between 20 μM Aβ1−42 and Aβ25−35, whereas Aβ42−1 had no effect. Data are expressed as mean values±s.d.
Figure 7.
RANTES protects HT22 cells from cell death induced by Aβ peptide. (a) HT22 cells were preincubated for 1 h with and without 1 nM RANTES (R), before addition of 20 μM Aβ1−42 (Aβ). After 24 h, cell viability was determined using the MTT assay. The effect of RANTES on cell survival was significant compared to cells treated with Aβ alone (*P<0.05). Additional transient transfection with GPR75 increased the protective effect of RANTES (**P<0.01). The protective effect of RANTES was significantly inhibited by pretreatment of HT22 cells for 1 h with 0.5 μM wortmannin (W) or for 10 min with 5 μM U73122 (U), before RANTES was added (*P<0.05). (b and c) HT22 cells were seeded in six-well plates for 24 h. They were incubated for 24 h with 20 μM Aβ1−42 without (b) or with (c) preincubation with 1 nM RANTES for 1 h and visualized by phase-contrast microscopy. Scale bar – 50 μm. (d) After pretreatment for 1 h with 1 nM of the indicated chemokines, HT22 cells were incubated with 20 μM Aβ25−35, and after 24 h the cell viability was determined by the MTT assay. Only RANTES and, to a lesser extent, MIP1α had a significant neuroprotective effect (**P<0.01 and *P<0.05, respectively). The data in (a) and (d) are expressed as mean values±s.d.
Next, to determine the specificity of the GPR75 effect, we analyzed the abundance of the other three known RANTES receptors in HT22 cells using PCR. GPR75 message was readily amplified by 30 cycles (Figure 6a), whereas the amplification of CCR1, CCR3 and CCR5 failed even after 60 cycles.
Discussion
GPR75 contains most of the characteristic features of GPCRs, namely seven transmembrane spanning domains, N-glycosylation sites in the N-terminus, and numerous serine and threonine phosphorylation sites in the C-terminus. Comparison of amino sequence, however, showed less than 25% homology to other GPCRs. Among various peptides and peptide-enriched extracts we tested, only the chemokine RANTES (CCL 5) turned out to stimulate the orphan GPR75. RANTES treatment of cells expressing GPR75 heterologously led to an increase in luciferase activity in a reporter-gene assay and stimulated Ca2+ mobilization and IP3 formation in respective assays with EC50 values in subnanomolar range. So far 19 chemokine receptors have been identified (Cartier et al., 2005). They share about 30–50% sequence homology, show common structural features and can be activated by multiple chemokines (Cartier et al., 2005). From the whole spectrum of chemokines tested with GPR75, only MIPα stimulated the luciferase activity, albeit with lower efficacy (EC50=1 nM) compared to RANTES. GPR75 possesses neither homology to the chemokine-receptor family nor promiscuity regarding ligands, which distinguish it as a new type of chemokine receptor.
RANTES is expressed in various normal and diseased tissues, for example, skeletal muscle, myocytes, kidney, retina and brain (Crane et al., 1998; Bajetto et al., 2001; Hirata et al., 2003; Wallece et al., 2004). The highest expression of human GPR75 has been detected in CNS (Tarttelin et al., 1999; Sauer et al., 2001), whereas our results show that the mouse GPR75 is present in cDNA from adult mouse brain, heart, skeletal muscle, kidney and in cDNA from 7- and 11-day-old mouse embryos (Figure 1). This expression pattern overlaps partially with the expression of RANTES. Therefore, it is tempting to speculate that RANTES and GPR75 may play an important role in the physiology and pathophysiology of these tissues. The expression of GPR75 in the developing embryo is suggestive of an essential role for organogenesis. However, a more detailed study by in situ hybridization and using knockout strains is needed to assess the function of GPR75 signaling in multiple embryogenic events.
The hippocampus is one of the areas affected in patients with Alzheimer's disease, where the histopathology is manifested by aggregation of the Aβ peptide and recruiting of several other cellular proteins (Cummings et al., 1998). The role of chemokines in the etiology and pathophysiology of Alzheimer's disease is not well understood. It has been proposed that Aβ is able to stimulate chemokine production in astrocytes and oligodendrocytes and the accompanying neuroinflammatory effect induces neuronal cell death (Bajetto et al., 2001; Cartier et al., 2005). It was of particular interest to investigate a possible role of GPR75 in Aβ-stimulated cell death. In the hippocampal cell line, HT22, which expresses GPR75 endogenously, RANTES reduced the cell death induced by Aβ1−42. Transient transfection with GPR75 enhanced the neuroprotective effect of RANTES. Other chemokines – MIP1β, MCP3 – that activate the known RANTES receptors CCR1, CCR3 and CCR5 (Cartier et al., 2005), were inactive, suggesting that the effects of RANTES are exclusively executed through the GPR75 in HT22 cells. It should be noted that the soluble form of Aβ is less toxic than the aggregated form.
Akt and MAPK phosphorylation was stimulated by RANTES in CV-1 cells transfected with GPR75. The activation of Akt and MAPK is known to promote cell survival and growth (Marinissen and Gutkind 2001; Maceyka et al., 2002; Li et al., 2003), and may, therefore, explain the antiapoptotic action of RANTES on HT22 cells. Aβ-induced cell death and the stimulation of Akt and MAPK phosphorylation were inhibited by wortmannin and U73122, suggesting a role for PI3K and PLC in the RANTES/GPR75-induced neuroprotection. Although RANTES increased the phosphorylation of both kinases with a similar pattern, with a maximal effect at 10 min, the fact that Akt phosphorylation preceded MAPK phosphorylation is indicative of a PI3K/Akt-coupled phosphorylation of MAPK.
In summary, we report the characterization of a new kind of chemokine receptor, GPR75. The chemokine RANTES was identified as cognate ligand for the GPR75 and it prevented Aβ-induced cell death in the hippocampal cell line, HT22, via activation of the pro-survival signaling pathway PLC/PI3K/Akt/MAPK. This finding offers a unique approach for unraveling the molecular mechanisms of GPR75 and RANTES in brain physiology and pathophysiology and suggests a new potential target for limiting Aβ-stimulated neuronal loss.
Acknowledgments
We thank Dr Axel Methner for kindly providing the luciferase gene-reporter construct and Simon Hempel for help with the figures.
Abbreviations
- Aβ
amyloid-β
- CRE
cAMP-response element
- CV-1
African green monkey kidney cells
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPCRs
G protein-coupled receptors
- GPR75
G protein-coupled receptor 75
- HEK293
human embryonic kidney cells
- IP3
inositol trisphosphate
- MAPK
mitogen-activated protein kinase
- MCP3
monocyte chemoattractant protein 3
- MIP1α
macrophage inflammatory protein 1α
- MIP1β
macrophage inflammatory protein 1β
- MRE
multiple-response element
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase-C
- PTX
pertussis toxin
- RANTES
regulated upon activation, normal T-cell expressed and secreted
- SDF-1
stromal cell-derived factor-1
- SRE
serum-response element
Conflict of interest
The authors state no conflict of interest.
References
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinl. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Crane IJ, Kuppner MC, McKillop-Smith S, Knott RM, Forrester JV. Cytokine regulation of RANTES production by human retinal pigment epithelial cells. Cell Immunol. 1998;184:37–44. doi: 10.1006/cimm.1997.1235. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS.Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities Neurology 199851S2–S17.(Discussion S65–S17) [DOI] [PubMed] [Google Scholar]
- Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, et al. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003;163:203–215. doi: 10.1016/S0002-9440(10)63644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AD, McAllister G, Feighner SD, Liu Q, Nargund RP, van der Ploeg LH, et al. Orphan G protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Lintzel J, Hermans-Borgmeyer I, Kreienkamp HJ, Joost P, Thomsen S, et al. Role of the G protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23:907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Chen G, Zeng X, Ouyang K, Hu Y. Generation of a bioactive neuropeptide in a cell-free system. Anal Biochem. 2003;316:34–40. doi: 10.1016/s0003-2697(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Gonzales I, Meinkoth JL, Fieeld J, Kazanietz MG, Li Y, et al. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J Biol Chem. 2003;278:9585–9591. doi: 10.1074/jbc.M213244200. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstein S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Maki R, De Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- Robert J, Auzan C, Ventura MA, Clauser E. Mechanisms of cell-surface rerouting of an ER-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J Biol Chem. 2005;280:42198–42206. doi: 10.1074/jbc.M510180200. [DOI] [PubMed] [Google Scholar]
- Sauer CG, White K, Stohr H, Grimm T, Hutchinson A, Bernstein PS, et al. Evaluation of the G protein coupled receptor-75 (GPR75) in age related macular degeneration. Br J Ophthalmol. 2001;85:969–975. doi: 10.1136/bjo.85.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- Tarttelin EE, Kirshner LS, Bellingham J, Baffi J, Taymans SE, Gregory-Evans K, et al. Cloning and characterization of a novel orphan G-protein-coupled receptor localized to human chromosome 2p16. Biochem Biophys Res Commun. 1999;260:174–180. doi: 10.1006/bbrc.1999.0753. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Kanaski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's A beta (1–42) and A beta (25–35) J Am Chem Soc. 2001;123:5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- Wallece GR, Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Prog Retin Eye Res. 2004;23:435–448. doi: 10.1016/j.preteyeres.2004.04.004. [DOI] [PubMed] [Google Scholar]