Abstract
Background and purpose:
The mitochondrial permeability transition pore (mPTP), an energy-dissipating channel activated by calcium, contributes to reperfusion damage by depolarizing the mitochondrial inner membrane potential. As mitochondrial Ca2+ overload is a main inductor of mPTP opening, we examined the effect of Ru360, a selective inhibitor of the mitochondrial calcium uptake system against myocardial damage induced by reperfusion in a rat model.
Experimental approach:
Myocardial reperfusion injury was induced by a 5-min occlusion of the left anterior descending coronary artery, followed by a 5-min reperfusion in anaesthetized open-chest rats. We measured reperfusion-induced arrhythmias and functions indicative of unimpaired mitochondrial integrity to evaluate the effect of Ru360 treatment.
Key results:
Reperfusion elicited a high incidence of arrhythmias, haemodynamic dysfunction and loss of mitochondrial integrity. A bolus intravenous injection of Ru360 (15-50 nmol kg−1), given 30-min before ischaemia, significantly improved the above mentioned variables in the ischaemic/reperfused myocardium. Calcium uptake in isolated mitochondria from Ru360-treated ventricles was partially diminished, suggesting an interaction of this compound with the calcium uniporter.
Conclusions and implications:
We showed that Ru360 treatment abolishes the incidence of arrhythmias and haemodynamic dysfunction elicited by reperfusion in a whole rat model. Ru360 administration partially inhibits calcium uptake, preventing mitochondria from depolarization by the opening of the mPTP. We conclude that myocardial damage could be a consequence of failure of the mitochondrial network to maintain the membrane potential at reperfusion. Hence, it is plausible that Ru360 could be used in reperfusion therapy to prevent the occurrence of arrhythmia.
Keywords: mitochondria, reperfusion injury, calcium uniporter, Ru360, permeability transition pore, arrhythmias, calcium overload
Introduction
Mitochondrial oxidative phosphorylation provides all the energy required for the contractile process. This energy accounts for more than 90% of that required by the myocardium (Mootha et al., 1997). Under pathological conditions such as ischaemia, mitochondrial ATP synthesis is abolished, resulting in severe damage to the integrity of heart cells. At reperfusion, abrupt re-oxygenation causes further cell damage by reactive oxygen-derived species (ROS) (Ferrari et al., 2004). ROS affect the sarcoplasmic reticulum and the sarcolemmal membranes, increasing the cytosolic calcium concentration ([Ca2+]c) (Krause et al., 1989; Dixon et al., 1990) and therefore the mitochondrial calcium concentration ([Ca2+]m) (Miyamae et al., 1996). At high [Ca2+]m, mitochondria undergoes, energy-consuming futile cycles through calcium release and re-uptake, because the proton-driven energy from the respiratory chain is used for cation transport instead of mitochondrial ATP production (Saris and Carafoli, 2005). In addition, mitochondrial calcium overload triggers a nonspecific increase in the inner membrane permeability, which contributes to the uncoupling of oxidative phosphorylation and thereby to a diminished ATP synthesis. Recent findings also indicate that mitochondria undergoing nonspecific membrane permeability changes release intramitochondrial molecules that participate in apoptotic death signalling, that is, cytochrome c, Smac/DIABLO and apotosis-inducing factor (Regula and Kirshenbaum, 2005).
On the other hand, it has been suggested that an early mechanism by which ischaemic preconditioning exerts its beneficial effects in the reperfused heart, is the opening of mitochondrial K+-ATP channels, that dissipate the inner mitochondrial membrane potential and reduce the driving force for Ca2+ influx through the mitochondrial calcium uniporter (mCaU) (Yellon and Downey, 2003; O'Rourke, 2004). Clearly, this molecule has been a critical target for cardioprotective approaches. In this regard, ruthenium red (RR) a classical inhibitor of the mCaU, shows protective effects against reperfusion injury in rat hearts (Ferrari et al., 1982; Carry et al., 1989; Miyamae et al., 1996). However, it has been demonstrated that this compound interacts with many proteins related to the excitation-contraction cycle, altering the contractile response in normal hearts and affecting other excitable tissues (Velasco and Tapia, 2000; Zhou and Bers, 2002).
Recently we demonstrated that Ru360, a RR analogue, exerts specific inhibition of the mCaU, preventing mitochondrial permeability transition pore opening when perfused into isolated heart. We found that [Ca2+]m decreased dramatically in mitochondria obtained from Ru360-treated reperfused hearts, correlating with a partial inhibition of the mCaU (García-Rivas et al., 2005).
Therefore, in this study we explored the ability of this compound to protect against ischaemia–reperfusion damage in an in vivo rat model. We present evidence that Ru360 prevents the post-ischaemic electrical dysfunction induced by reperfusion, by inhibiting calcium overload and the opening of the mitocondrial permeability transition pore (mPTP).
Methods
Animal groups
All procedures and protocols were performed on male Wistar rats, weighing 250–300 g, in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NHI publication No. 85 (23) revised 1996). The rats were randomly divided into three groups: (1) The control group (n=20) underwent identical surgical procedures as the ischaemia/reperfusion group (I/R) group, without coronary artery ligation. (2) The I/R (n=23) received saline solution (0.9%) for 30 min before ischaemia and then was subjected to the reperfusion protocol. (3) The treated group (I/R+Ru360, n=26), received a bolus injection of Ru360 dissolved in saline solution 30 min before artery ligation and then was subjected to the reperfusion protocol.
Ruthenium complex synthesis
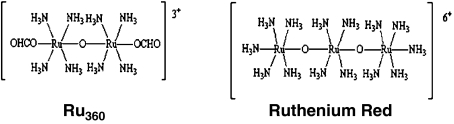
Ru360 (μ-oxo) bis (trans-formatotetramine ruthenium), is a coordination complex that forms a near-linear structure containing two ruthenium atoms linked by an oxygen-bridge and surrounded by amine groups (Figure 1). To synthesize this complex, we followed the procedure described by Ying et al. (1991). The purified preparation was slightly yellowish and exhibited a single λmax at 360 nm. Ru360 was obtained in 0.4 M ammonium formiate buffer, pH 5.5. Ru360 concentration after chemical synthesis was calculated from the molar extinction coefficient of the complex at 360 nm (ɛ=2.6 × 104 M−1 cm−1), as described by several groups including ours (Ying et al., 1991; Matlib et al., 1998; Zazueta et al., 1999). Ammonium formiate buffer alone, diluted in saline solution, was used in some experiments to discard any effect on reperfusion recovery. Commercial RR was purified by the technique described by Luft (1971). This preparation was not contaminated with Ru360. A single absorption peak at 533 nm was observed with distilled water.
Figure 1.
Structures of the oxo-bridged amine dinuclear ruthenium complex: Ru360 and RR. Modified from Ying et al. (1991).
In vivo reperfusion protocols
Rats anaesthetized with sodium pentobarbitone (55 mg kg−1 i.p.) were intubated and air ventilated (10 ml kg−1, 72 breaths min−1) using a rodent respirator (model 683, Harvard Apparatus, Cambridge, MA, USA). The arterial pressure was measured through a cannula inserted into the femoral artery and connected to a hydrostatic pressure transducer. Electrocardiogram (ECG) was monitored by using three platinum electrodes placed at DII standard position. Arterial blood pressure (ABP) and ECG were recorded during the first 30 s of each minute, in a polygraph model 79-D (Grass Instrument Co. Quincy, MA, USA). The femoral vein was cannulated for the administration of the ruthenium complex. A bolus of the ruthenium compound (RR or Ru360) or the corresponding saline solution volume was administered to the rats. The heart was exposed by lateral left thoracotomy. Regional ischaemia was produced by a ligature (6-0 silk) around the left coronary artery, approximately 2 mm from its origin, according to the method of Selye et al. (1960). Artery occlusion was performed by placing a short rigid tube over the vessel and tying both firmly with a silk thread. In I/R and I/R+ Ru360 groups myocardial ischaemia was confirmed by the appearance of regional cyanosis, akinesia or bulging in the epicardium distal to the artery occlusion and ST segment elevation. After 5 min of ischaemia, the silk was removed by cutting it carefully over the tube to restore blood flow to the myocardium. Reperfusion was confirmed by the colour change in the ventricular surface, from cyanosis to hyperaemia and by the onset of ventricular tachycardia (VT). The heart was reperfused for 5 min, in accordance with previous studies, to induce cardiac damage, characterized by a higher incidence of reperfusion-induced VT and ventricular fibrillation (VF) (Manning and Hearse, 1984; Hagar et al., 1991; Arteaga et al., 1992; Bobadilla et al., 2001; Parra et al., 2005). The incidence and time course of arrhythmias were compared between groups and their classification was established in agreement with the Lambeth Convention (Walker et al., 1988).
Rats that developed arrhythmias before the ischaemia or VF immediately after ischaemia were discarded and replaced. Thus, all analyses only represent animals that survived the whole procedure.
Measurements of mitochondrial integrity
After reperfusion, heart tissue from the left ventricle was minced and homogenized in isolation medium, containing (in mM) KCl (125), ethylenediaminetetraacetic acid (1) and N-2-hydroxyl piperazine-N′-2-ethane sulphonic acid (HEPES)-HCl (10), pH 7.3. The mitochondrial fraction was obtained by differential centrifugation, as previously described, by using the protease Nagarse (García-Rivas et al., 2005). Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode (Yellow Springs Instruments, OH, USA). The experiments were carried out in 1.5 ml of assay medium, containing (in mM) KCl (125), HEPES-HCl (10) and KH2PO4-TRIS (3), pH 7.3. State 4 respiration was evaluated in the presence of 10 mM succinate, plus 1 μg ml−1 rotenone. State 3 respiration was measured after addition of 200 μM ADP. Respiratory control index (RC) was calculated as the ratio between state 3 and state 4 rates. ADP/O ratio was calculated as (nmol) of added ADP per (ng) of oxygen consumed during state 3 respiration.
Mitochondrial aconitase activity [E.C.4.2.1.3] was determined spectrophotometrically, by monitoring the disappearance of cis-aconitate at 240 nm (ɛ=3.6 mM−1 cm−1) (Hoerter et al., 2004). One mIU was defined as the amount of enzyme that consumed 1 nmol cis-aconitate min−1. Protein content was measured by the Lowry method (1951).
Measurement of mCaU activity
Mitochondrial calcium uptake was measured with 45CaCl2 (specific activity 1000 cpm nmol−1) using the filtration technique. Briefly, 0.5 mg of mitochondria were incubated in assay medium at the indicated times. Aliquots were withdrawn and filtered through Millipore filters of 0.45 μm pore size. Non-entrapped 45Ca2+ was washed with 0.1 M KCl and the radioactivity retained in the filter was measured in a scintillation counter (Beckman, CA, USA). The assay medium contained (in mM) KCl (125), HEPES-HCl (10), 10 succinate (10), KH2PO4-TRIS (3), ethyleneglycol tetraacetate (0.5), 1 μg ml−1 rotenone and 50 μM free calcium, calculated by using the Chelator program (Th. Schoenmakers, Nijmegen, Netherlands), pH 7.3.
Determination of Ru360 concentrations in blood and myocardial tissue
Anaesthetized control rats (not subjected to the I/R protocol) under assisted respiration were treated with the protective dose of Ru360 (50 nmol kg−1) then, at the indicated times blood aliquots (500 μl) were obtained from the left ventricular cavity, before the hearts were removed from the rat. The hearts were mounted in a Langendorff apparatus as previously described and washed for 10 min with cold Krebs–Henseleit Buffer (García-Rivas et al., 2005). Then, the hearts were lyophilized and digested using Suprapure HNO3, HCl and 30% H2O2 (6:2:1) (Merck Darmstandt, Germany). For each inductively coupled plasma optical emission spectroscopy (ICP-OES) determination, 1 g of cardiac dry tissue was required (three or four different hearts).
Ruthenium content was analysed by ICP-OES at 240.272 nm single wavelength in a Simultaneous Optima 4300 DV apparatus (Perkin Elmer, CT, USA). Ruthenium standards (4–15 μg l−1) were prepared from primary pure standards (1000 μg l−1) (Perkin Elmer, CT, USA). For each sample group, independent calibration curves and blanks were prepared. No spectral interferences were detected at 240.272 nm. Ru360 content was calculated on the basis of its reported molecular weight, that is, 550.8 g mol−1.
Data expression and analysis
Data are expressed as the mean±s.e. Statistical analysis was by Student's t-test. The data for heart rate, blood pressure, duration time of arrhythmias, mitochondrial activities and calcium transport were compared between the control, I/R and I/R+Ru360 groups. A P-value of <0.05 was considered statistically significant.
Results
Effect of Ru360 on functional recovery of rat hearts after ischaemia
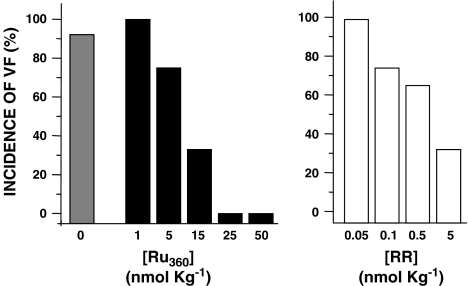
A striking feature in myocardial reperfusion is a considerable increase in the appearance of cardiac arrhythmias (Tsuchihashi and Curtis, 1991; del Monte et al., 2004). Particularly, VF has been used as a criterion of potential lethal damage induced by reperfusion injury (Roh et al., 2005). In this context, we found that I/R rats showed a 78% incidence of VF, whereas Ru360 administration gradually diminished this incidence, until VF disappeared at doses between 25 and 50 nmol kg−1 (Figure 2). Further experiments with Ru360 were performed using a dose of 50 nmol kg−1. Next, we compared the effectiveness of this compound against RR, a related and widely used inhibitor of calcium uptake, with known cardioprotective properties in different models (Ferrari et al., 1982; Carry et al., 1989; Miyamae et al., 1996). As observed in Figure 2, RR treatment decreased the incidence of VF and enhanced myocardial recovery only at higher doses (5 μmol kg−1), in accordance with previous findings (Carry et al., 1989).
Figure 2.
Dose-dependent protective effect of ruthenium complexes on the incidence of VF in reperfused rat hearts. Incidence of VF at the fifth minute of reperfusion. The shaded column represents the values of I/R rats; solid columns represent the values from rats treated with Ru360 (I/R+Ru360). Open columns represent the values from rats treated with RR. n=20, for I/R and I/R+Ru360 rats. For RR treatment, n=3.
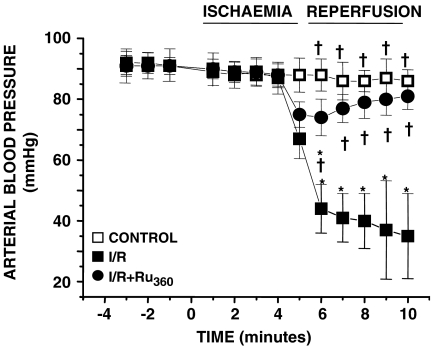
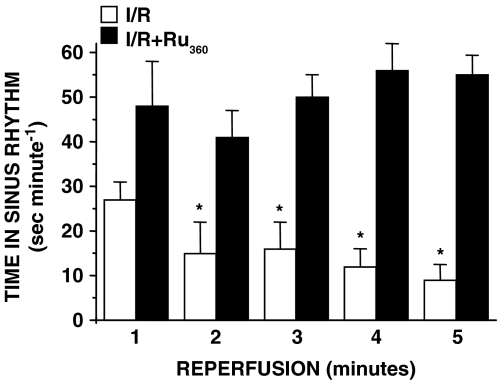
Figure 3 shows the temporal ABP in Ru360-treated rats. Negative numbers represent the last 3 min of the 30-min period after drug injection and before artery ligation. We show these time points to demonstrate that Ru360 did not elicit haemodynamic or arrhythmic effects, at least during the 30 min before the ischaemia. In I/R and I/R+ Ru360 groups a discrete drop in the ABP was observed during ischaemia. However, in I/R rats the ABP decreased approximately 60% during the reperfusion, whereas in I/R+Ru360 rats, the ABP was maintained. Diminished ABP correlated with an increase in heart rate in I/R rats, indicating reperfusion-induced haemodynamic dysfunctions. There was no significant difference in the total number of arrhythmias that occurred during ischaemia between I/R and Ru360-treated rats (data not shown). However, to determine the impact of Ru360-treatment on the development of reperfusion-induced cardiac electrical abnormalities, we analysed the ventricular arrhythmias in I/R and I/R+Ru360 rats; normal beats, VT and VF were studied. In the I/R+Ru360 rats the incidence of arrhythmias was significantly modified and the sinus rhythm was recovered at the first minute of reperfusion. At 5 min of reperfusion normal beats reached about 90% of the total beats per minute, in contrast to the results in I/R rats in which normal beats represented 15% of the total number of beats (Figure 4). Notably, after 3 min of reperfusion, VT and VF were totally absent in I/R+Ru360 rats.
Figure 3.
Effect of Ru360 on ABP in reperfused rats. Time course analysis of ABP in control, I/R and I/R+Ru360 (50 nmol kg−1) rats. Values are the mean of at least 25 different experiments±s.e. *P⩽0.05, significantly different vs control and †P⩽0.05 vs I/R.
Figure 4.
Electric cardiac profile of I/R+Ru360 rats. Analysis of the duration of sinus rhythm during reperfusion. Open columns represent I/R and solid columns correspond to Ru360-treated rats. Values are the mean of at least 25 different experiments±s.e. *P⩽0.05, significantly different vs Ru360-treated rats.
The effect of Ru360 on cardiac mitochondrial integrity after reperfusion
A growing body of experimental evidence supports the idea that mitochondria contribute to cardiac dysfunction and myocyte injury in the pathophysiology of ischaemia–reperfusion (Lesnefsky et al., 2001). Hence, we investigated cardiac mitochondria integrity of Ru360-treated rats subjected to reperfusion injury. Mitochondrial respiratory activity was measured in the presence of succinate as substrate (Table 1). Mitochondria from I/R ventricles exhibited a 45% reduction in state 3 respiration rate, compared to control mitochondria, whereas respiratory rates in mitochondria obtained from I/R+Ru360 ventricles did not show any change. There was no significant difference between the two groups in state 4 respiration rates. RC, an indicator of the mitochondrial electron transport coupling to ADP phosphorylation, was calculated to determine mitochondrial integrity. RC value of control mitochondria was 6.0±0.8, whereas in mitochondria isolated from reperfused ventricles, this value diminished to 3.5±0.6. In contrast, reperfusion did not affect the RC in I/R+Ru360 ventricles. The ADP/O indexes for mitochondria isolated from I/R and from I/R+Ru360 ventricles were 0.73±0.3 and 1.4±0.5, respectively; this difference was statistically significant (P⩽0.05). ADP/O values for control and I/R+Ru360 mitochondria did not change.
Table 1.
Respiratory activity in mitochondria isolated from control, I/R and IR+Ru360 rat ventricles
| Control | I/R | I/R+Ru360 | |
|---|---|---|---|
| State 3 (nmol O min−1 mg−1) | 372±34† (n=5) | 205±18* (n=4) | 358±45† (n=4) |
| State 4 (nmol O min−1 mg−1) | 61±13 (n=5) | 48±8 (n=4) | 63±16 (n=4) |
| ADP/O | 1.32±0.4† (n=5) | 0.73±0.3* (n=4) | 1.4±0.5† (n=4) |
| RC | 6.0±0.8† (n=5) | 3.5±0.6* (n=4) | 5.6±0.7† (n=4) |
Abbreviation: I/R, ischaemia/reperfusion group; I/R+Ru360, Ru360-treated group; RC, respiratory control.
Mitochondrial respiratory activity was determined in a standard buffer. Values are the mean±s.e.
P⩽0.05 significantly different vs control
P⩽0.05 vs I/R.
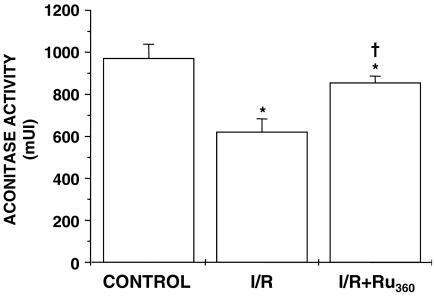
It is well known that the oxidative damage produced during reperfusion affects mitochondrial integrity, affecting important enzymatic activities, so we measured mitochondrial aconitase activity as evidence of such damage. Aconitase activity is inversely proportional to the amount of O2−• produced during oxidative stress (Hoerter et al., 2004). In I/R mitochondria, aconitase activity decreased significantly (35%) as compared to control mitochondria. Interestingly, aconitase activity was protected against oxidative damage in I/R+Ru360 mitochondria (Figure 5). We determined that Ru360 has no ROS-scavenger properties by assessing thiobarbituric acid reactive substances content in control mitochondria subjected to oxidative stress, as previously described (García et al., 2005) (data not shown).
Figure 5.
Effect of Ru360 treatment on the mitochondrial aconitase activity of coronary artery-ligated rat hearts. Mitochondrial aconitase activity was measured in mitochondria obtained from control, I/R and I/R+Ru360 (50 nmol kg−1) hearts. Values given are the mean of at least four different experiments±s.e. *P⩽0.05, significantly different vs control and †P⩽0.05 vs I/R.
Effect of Ru360 on mCaU activity
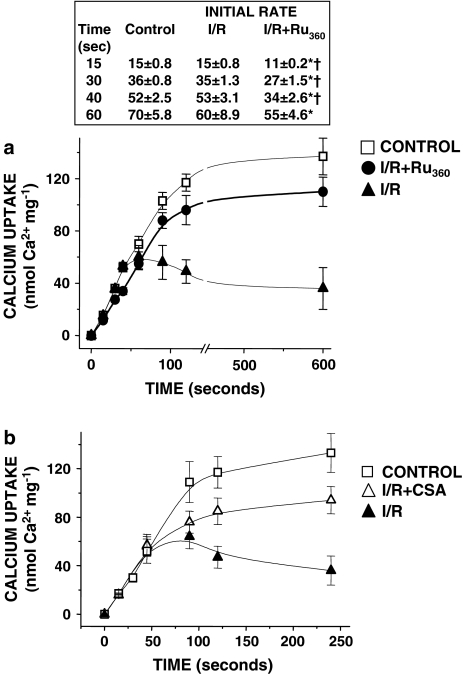
To assess the effect of Ru360 on the mCaU activity, we investigated calcium uptake in heart mitochondria from control, I/R and Ru360-treated rats. Linear fitting of initial uptake velocities showed a slower calcium influx in mitochondria isolated from Ru360-treated hearts compared with control and I/R mitochondria, suggesting an interaction between Ru360 and its mitochondrial target. A longer time course analysis showed that in mitochondria from I/R ventricles, calcium uptake was followed by a rapid release, probably due to the opening of the mPTP, whereas calcium was maintained inside mitochondria isolated from control and Ru360-treated rats (Figure 6a). In parallel experiments, we measured mitochondrial membrane potential in mitochondria from each group. As expected, I/R mitochondria developed lower membrane potentials than control or Ru360-treated mitochondria. Calcium addition induced a transitory mitochondrial membrane depolarization in control and I/R+Ru360 mitochondria, whereas in I/R mitochondria it promoted an irreversible drop in the transmembrane potential, indicating the opening of the mPTP (data not shown).
Figure 6.
Ru360 treatment diminishes the initial mitochondrial calcium uptake rate. (a) Time course analysis of mitochondrial calcium uptake. In mitochondria from control, I/R and I/R+Ru360 (50 nmol kg−1) rat hearts. The insert shows the statistical analysis of initial calcium influx rate (nmol Ca2+ mg−1). *P⩽0.05 significantly different vs control and †P⩽0.05 vs I/R. (b) CSA inhibits the mPTP in mitochondria from I/R hearts. Calcium transport was measured in isolated heart mitochondria from I/R rats in the presence of 1 μM CSA and without CSA. The data represent the mean of at least five different hearts (a) and four experiments (b)±s.e.
Calcium release in I/R mitochondria was prevented by the addition of cyclosporine A (CSA) in the assay medium (Figure 6b). Under this condition, calcium accumulation increased by 60%.
Ru360 accumulation in heart tissue
To quantify Ru360 accumulation in blood and heart tissue, we measured total ruthenium content 4 and 30 min after Ru360 administration. Ruthenium content, 4 min after Ru360 administration was 0.41±0.03 μg ml−1 (n=4) in blood; this value diminished to 0.32±0.01 μg ml−1 (n=4), 26 min later. In the cardiac tissue, ruthenium was undetectable at early administration times (4 min), but at 30 min, ruthenium content increased to 1±0.35 μg g−1 dry tissue (n=3). Similar to previous measurements of protein content and distribution in myocardial cells (Idell-Wenger et al., 1978; Vinnakota and Bassingthwaighte, 2004), we calculated a concentration of 2.1±0.45 pmol Ru360 mg−1 of protein of myocardial tissue.
Discussion and conclusions
Calcium homeostasis undergoes fluctuations in balance during reperfusion, largely owing to the release of calcium from intracellular stores, particularly from the sarcoplasmic reticulum (Krause et al., 1989; Temsah et al., 1999). Experimental observations of calcium signal transmission between endoplasmic reticulum and mitochondria suggest the existence of a stable mitochondria–reticulum interaction, where mitochondria could accumulate a large fraction of the calcium released through the ryanodine receptor and the IP3 receptor (Hajnoczky et al., 2000). According to this hypothesis, confocal image analysis provides evidence of high calcium concentration microdomains, susceptible to being sensed by mitochondria (Filippin et al., 2003).
As calcium accumulation in mitochondria has been proposed to play a key role in triggering cellular damage in the reperfused heart (Miyata et al., 1992; Miyamae et al., 1996; García-Rivas et al., 2005), we suggest that interventions reducing mitochondrial calcium overload would prevent mPTP opening and hence, membrane potential depolarization, matrix swelling and abolition of ATP synthesis, events that concur with the incidence of arrhythmias and haemodynamic dysfunction elicited by reperfusion in a whole-rat model.
The compound used in this study is a mCaU inhibitor, which permeates slowly into the cell, and specifically inhibits mitochondrial calcium uptake in intact cardiomyocytes and in isolated heart. Matlib et al. (1998), showed that 1 μM 103Ru360 was taken up by myocardial cells and accumulated in the cytosol in a biphasic manner. A rapid accumulation phase was observed, possibly related to binding to the cell surface, whereas a second slow phase could be due to intracellular accumulation. They calculated a final concentration after the slow phase of 3 pmol 103Ru360/106 cells. At this concentration total inhibition of calcium uptake into mitochondria was observed in situ, in single voltage-clamped myocytes. Experiments from our group in isolated hearts, showed that free mitochondrial matrix calcium from Ru360-treated hearts is diminished as compared to [Ca2+]m in control hearts, indicating that Ru360 targets the mCaU (García-Rivas et al., 2005). Also, a quite recent report suggests the involvement of the mCaU in cardioprotection; Ru360 (10 μM) treatment of isolated hearts provides cardioprotective effects and the mitochondria obtained from those hearts are resistant to calcium-induced swelling (Zhang et al., 2006). With regard to the permeation properties of this poli-charged compound and its ability to reach the mitochondrial membranes, policationic copper-based antineoplastic drugs have been demonstrated to affect mitochondrial metabolism when perfused into the isolated heart (Hernández-Esquivel et al., 2006).
In the present study, we showed that Ru360 treatment suppressed arrhythmias and haemodynamic dysfunction elicited by reperfusion, and prevented mPTP opening, by a mechanism possibly related to the diminution of mitochondrial calcium overload (Figure 6). Although many mechanisms have been proposed to explain the development of reperfusion arrhythmias, calcium overload is one of the main factors promoting its generation. High intracellular calcium induces electrical effects on the action potential (AP), such as inward currents and delayed after depolarizations in the pacemaker cells, which lead to VT and VF (Bers, 2002). It has also been pointed out, that there is a direct connection between loss of mitochondrial function and alterations in the cellular AP (O'Rourke, 2000). Evidence for the involvement of post-ischaemic electrical dysfunction and mitochondrial bioenergetics has been obtained from experiments with inhibitors of the mitochondrial benzodiazepine receptor (a putative component of the mPTP), which block depolarization of the mitochondrial membrane potential and prevent reperfusion arrhythmias (Akar et al., 2005). These findings, together with our own results indicate that I/R-related arrhythmias could be, in part, a consequence of the failure of the mitochondrial network to maintain the membrane potential at reperfusion as a consequence of mPTP opening, induced by calcium overload.
A subpopulation of mitochondria that undergoes irreversible mPTP opening, would be totally disrupted and would not be recovered in the mitochondrial pellet. This would account for the mitochondrial yields always being lower in reperfused hearts than in control- or drug-treated hearts. The ‘surviving' mitochondria recovered from reperfused hearts showed a higher sensitivity to mitochondrial calcium overload, as shown in Figure 6. This increased sensitivity, compared to control- and drug-treated mitochondria, could be a reflection of their inability to regenerate the membrane potential, possibly due to loss of adenine nucleotides or to oxidative stress damage to the mitochondrial respiratory complexes. As the I/R mitochondria undergo more permeability transitions, the more the effect becomes additive, mainly reflecting calcium extrusion. This mechanism is in agreement with that proposed for the propagation of permeability changes, where the local liberation of calcium from mitochondria triggers propagating waves of calcium-induced Ca2+ release in the entire mitochondrial network. (Pacher and Hajnoczky, 2001). The finding that CSA addition to I/R mitochondria (panel b in the same figure) reversed this effect, indicates that the major release pathway involved is the mPTP. Mitochondria from drug-treated hearts did not show such sensitivity and were able to accumulate more calcium than I/R mitochondria, but as the mitochondrial uptake pathway remained partially blocked, calcium accumulation was diminished.
We detected 2.1 pmol Ru360 accumulated per mg of protein of myocardial tissue in drug-treated hearts by using ICP-OES, this concentration is in the low range of KD values reported for in vitro mCaU inhibition (Ying et al., 1991; Matlib et al., 1998; Zazueta et al., 1999), and should only produce a partial inhibition of the mCaU. In this respect, it is not always possible to compare data obtained from in vitro studies with those from in vivo models. It has been demonstrated that many factors are critical when correlating the true intrinsic potency of a drug or inhibitor in vivo with in vitro determinations; among others are the concentration of the inhibitor at the site of its metabolic activity, tissue specificity and drug metabolism (Prueksaritanont et al., 1997; Schmider et al., 1999).
However, the assumption that Ru360 reaches the mitochondrial membranes has been supported by results from our own group that demonstrated that Ru360 actually gets inside the mitochondria when perfused into isolated hearts. In that study, we perfused increasing concentrations of this compound and observed a dose-dependent response inhibition of the mitochondrial calcium uptake (García-Rivas et al., 2005).
Other drugs, such as diazoxide (Wang et al., 2001) and RR (Ferrari et al., 1982; Carry et al., 1989; Miyamae et al., 1996) have been used as modulators of the mitochondrial calcium content. Diazoxide is a mitochondrial K+-ATP channel opener, that exerts an impressive recovery from the effects of reperfusion in isolated hearts, when used at low concentrations (30–100 μM) (Garlid et al., 1997; Wang et al., 2001; Hausenloy et al., 2004). Whereas, in a whole-rat model, this compound only partially protects at concentrations up to 625 μM (Fryer et al., 2000). RR also exerts protection in isolated hearts at 0.025–10 μM (Ferrari et al., 1982; Miyamae et al., 1996), but in a whole-rat model it shows a protective effect at doses close to 30 μM (Carry et al., 1989). At these concentrations both compounds show collateral effects, not only in the heart, but in other organs (Balazs et al., 1975; Belmar et al., 1995; Silvani et al., 2004). Mitochondrial integrity can also be maintained after reperfusion by inhibiting the opening of the mPTP. In this context, a wide variety of molecules that inhibit this mega-channel, including CSA, have been used as protectors against reperfusion injury (Arteaga et al., 1992; Duchen et al., 1993). Other examples are sanglifehrin A (Clarke et al., 2002) and more recently NIM811 (Argaud et al., 2005) and octylguanidine (Parra et al., 2005). However, we proposed that prevention from calcium overload, instead of closing the mPTP could be a more effective strategy for the prevention of reperfusion injury, as ROS production in mitochondria appears to be mediated by an increase in [Ca2+]m. The results obtained from measuring the activity of aconitase, a mitochondrial marker of oxidative stress, further support this idea. I/R mitochondria showed a diminution of mitochondrial aconitase activity and such inactivation was partially abolished by Ru360-treatment, indicating that calcium accumulation into the mitochondrial matrix increases ROS production, an effect that has also been observed by other groups using RR (Petrosillo et al., 2004; Votyakova and Reynolds, 2005). Although the exact mechanism by which [Ca2+]m induces ROS production is not clear (Brookes et al., 2004), one possible explanation is that calcium induces mitochondrial membrane depolarization, enhancing ROS production (Cadenas and Boveris, 1980; Turrens, 1997). Another possibility is that Ca2+-binding to cardiolipin molecules dissociates cytochrome c from the inner membrane, inhibiting the respiratory complex III (ubiquinol cytochrome c oxidoreductase) and increasing ROS generation in the ubiquinone cycle (Grijalba et al., 1999; Petrosillo et al., 2004).
In conclusion, our results indicate that Ru360 increases the functional recovery of hearts subjected to ischaemia–reperfusion and maintains the mitocondrial integrity when perfused into a whole rat. The mechanism by which this compound prevented the damage could be related to the partial inhibition of the mitochondrial calcium transport. In this respect, it would be very interesting to explore the potential of Ru360 as an alternative novel drug for use in reperfusion therapy.
Acknowledgments
We thank Dr Liliana Saldívar and Q Nadia Munguía (Departamento de Química Analítica de la Facultad de Química, UNAM) for helpful advice and expert technical assistance with ICP-OES. This work was partially supported by CONACyT Grant 46456-M to CZ. This work was submitted in partial fulfilment of the requirements for the PhD degree of Gerardo de Jesús García-Rivas for the Doctorate in Biomedical Sciences of the Universidad Nacional Autónoma de México.
Abbreviations
- ABP
arterial blood pressure
- AP
action potential
- [Ca2+]c
cytosolic calcium concentration
- [Ca2+]m
mitochondrial calcium concentration
- CSA
cyclosporine A
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- I/R
ischaemia/reperfusion group
- I/R+Ru360
Ru360-treated group
- mCaU
mitochondrial calcium uniporter
- mPTP
mitochondrial permeability transition pore
- RC
respiratory control
- ROS
reactive oxygen-derived species
- RR
ruthenium red
- Ru360
oxygen-bridged dinuclear ruthenium amine complex
- VF
ventricular fibrillation
- VT
ventricular tachycardia.
Conflict of Interest
The authors state no conflict of interest.
References
- Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischaemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Arteaga D, Odor A, Lopez RM, Contreras G, Pichardo J, García E, et al. Impairment by cyclosporin A of reperfusion-induced arrhythmias. Life Sci. 1992;51:1127–1134. doi: 10.1016/0024-3205(92)90514-p. [DOI] [PubMed] [Google Scholar]
- Balazs T, Herman EH, Earl FL, Wolff FW. Cardiotoxicity studies with diazoxide, reserpine, guanethidine, and combinations of diazoxide and propranolol in dogs. Toxicol Appl Pharmacol. 1975;33:498–504. doi: 10.1016/0041-008x(75)90075-7. [DOI] [PubMed] [Google Scholar]
- Belmar E, Garcia-Ugalde G, Tapia R. Motor alterations and neuronal damage induced by intracerebral administration of Ruthenium red: effect of NMDA receptor antagonists and other anticonvulsant drugs. Mol Chem Neuropathol. 1995;26:285–299. doi: 10.1007/BF02815144. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–17. [PubMed] [Google Scholar]
- Bobadilla I, Franco M, Cruz D, Zamora J, Robles SG, Chavez E. Hypothyroidism provides resistance to reperfusion injury following myocardium ischaemia. Int J Biochem Cell Biol. 2001;33:499–506. doi: 10.1016/s1357-2725(01)00016-4. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J. 1980;188:31–37. doi: 10.1042/bj1880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carry MM, Mrak RE, Murphy ML, Peng CF, Straub KD, Fody EP. Reperfusion injury in ischaemic myocardium: protective effects of ruthenium red and of nitroprusside. Am J Cardiovasc Pathol. 1989;2:335–344. [PubMed] [Google Scholar]
- Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- Del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischaemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon IM, Kaneko M, Hata T, Panagia V, Dhalla NS. Alterations in cardiac membrane Ca2+ transport during oxidative stress. Mol Cell Biochem. 1990;99:125–133. doi: 10.1007/BF00230342. [DOI] [PubMed] [Google Scholar]
- Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- Ferrari R, di Lisa F, Raddino R, Visioli O. The effects of ruthenium red on mitochondrial function during post-ischaemic reperfusion. J Mol Cell Cardiol. 1982;14:737–740. doi: 10.1016/0022-2828(82)90186-9. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Guardigli G, Mele D, Percoco GF, Ceconi C, Curello S. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10:1699–1711. doi: 10.2174/1381612043384718. [DOI] [PubMed] [Google Scholar]
- Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischaemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- García N, García JJ, Correa F, Chávez E. The permeability transition pore as a pathway for the release of mitochondrial DNA. Life Sci. 2005;76:2873–2880. doi: 10.1016/j.lfs.2004.12.012. [DOI] [PubMed] [Google Scholar]
- García-Rivas GJ, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitocondrial calcium uniporter by oxo-bridged dinuclear ruthenium amine complex (Ru360), prevents from irreversible injury in post-ischaemic rat heart. FEBS J. 2005;272:3477–3488. doi: 10.1111/j.1742-4658.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Grijalba M, Vercesi A, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulation generation of reactive oxygen species by the respiratory chain. Biochemistry. 1999;38:13279–13287. doi: 10.1021/bi9828674. [DOI] [PubMed] [Google Scholar]
- Hagar JM, Hale SL, Kloner RA. Effect of preconditioning ischaemia on reperfusion arrhythmias after coronary artery occlusion and reperfusion in the rat. Circ Res. 1991;68:61–68. doi: 10.1161/01.res.68.1.61. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529:69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- Hernández-Esquivel L, Marin-Hernandez A, Pavon N, Carvajal K, Moreno-Sanchez R. Cardiotoxicity of copper-based antineoplastic drugs casiopeinas is related to inhibition of energy metabolism. Toxicol Appl Pharmacol. 2006;212:79–88. doi: 10.1016/j.taap.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, et al. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischaemic–reperfusion damage. Circulation. 2004;110:528–533. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger JA, Grotyohann LW, Neely JR. Coenzyme A and carnitine distribution in normal and ischaemic hearts. J Biol Chem. 1978;253:4310–4318. [PubMed] [Google Scholar]
- Krause SM, Jacobus WE, Becker LC. Alterations in cardiac sarcoplasmic reticulum calcium transport in the postischaemic “stunned” myocardium. Circ Res. 1989;65:526–530. doi: 10.1161/01.res.65.2.526. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischaemia–reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luft JH. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971;171:347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Manning AS, Hearse DJ. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984;16:497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischaemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am J Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- Miyata H, Lakatta EG, Stern MD, Silverman HS. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992;71:605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Arai AE, Balaban RS. Maximum oxidative phosphorylation capacity of the mammalian heart. Am J Physiol. 1997;272:H769–H775. doi: 10.1152/ajpheart.1997.272.2.H769. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol. 2000;529:23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra E, Cruz D, Garcia G, Zazueta C, Correa F, Garcia N, et al. Myocardial protective effect of octylguanidine against the damage induced by ischaemia reperfusion in rat heart. Mol Cell Biochem. 2005;269:19–26. doi: 10.1007/s11010-005-2989-0. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Gorham L, Ma B, Liu L, Yu X, Zhao J, et al. In vitro metabolism of simvastatin in humans. Identification of metabolizing enzymes and effect of the drug on haepatic P450s. Drug Metab Dispos. 1997;25:1191–1199. [PubMed] [Google Scholar]
- Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol. 2005;38:3–13. doi: 10.1016/j.yjmcc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Roh HY, Jung IS, Park JW, Yun YP, Yi KY, Yoo SE, et al. Cardioprotective effects of [5-(2-methyl-5-fluorophenyl)furan-2-[carbonyl]guanidine (KR-32568) in an anesthetized rat model of ischaemia and reperfusion heart injury. Pharmacology. 2005;75:37–44. doi: 10.1159/000086192. [DOI] [PubMed] [Google Scholar]
- Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry. 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Schmider J, von Moltke L, Shader R, Harmatz J, Greenblatt D. Extrapolating in vitro data on drug metabolism to in vivo pharmacokinetics: evaluation of the pharmacokinetic interaction between amitriptyline and fluoxetine. Drug Metab Rev. 1999;31:545–560. doi: 10.1081/dmr-100101935. [DOI] [PubMed] [Google Scholar]
- Selye H, Bajusz E, Grasso S, Mendell P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Silvani P, Camporesi A, Mandelli A, Wolfler A, Salvo I. A case of severe diazoxide toxicity. Paediatr Anaesth. 2004;14:607–609. doi: 10.1111/j.1460-9592.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischaemic-reperfused rat heart. Am J Physiol. 1999;277:H584–H594. doi: 10.1152/ajpheart.1999.277.2.H584. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi K, Curtis MJ. Influence of tedisamil on the initiation and maintenance of ventricular fibrillation: chemical defibrillation by Ito blockade. J Cardiovasc Pharmacol. 1991;18:445–456. doi: 10.1097/00005344-199109000-00018. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- Velasco I, Tapia R. Alterations of intracellular calcium homeostasis and mitochondrial function are involved in ruthenium red neurotoxicity in primary cortical cultures. J Neurosci Res. 2000;60:543–551. doi: 10.1002/(SICI)1097-4547(20000515)60:4<543::AID-JNR13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004;286:H1742–H1749. doi: 10.1152/ajpheart.00478.2003. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho SA, Figueredo V, et al. Preconditioning limits mitochondrial Ca(2+) during ischaemia in rat hearts: role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2321–H2328. doi: 10.1152/ajpheart.2001.280.5.H2321. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Ying WL, Emerson J, Clarke MJ, Sanadi DR. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry. 1991;30:4949–4952. doi: 10.1021/bi00234a016. [DOI] [PubMed] [Google Scholar]
- Zazueta C, Sosa-Torres ME, Correa F, Garza-Ortiz A. Inhibitory properties of ruthenium amine complexes on mitochondrial calcium uptake. J Bioenerg Biomembr. 1999;31:551–557. doi: 10.1023/a:1005464927366. [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Gao Q, Cao CM, Bruce IC, Xia Q. Involvement of the mitochondrial calcium uniporter in cardioprotection by ischaemic preconditioning. Life Sci. 2006;78:738–745. doi: 10.1016/j.lfs.2005.05.076. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Bers DM. Time course of action of antagonists of mitochondrial Ca uptake in intact ventricular myocytes. Pflugers Arch. 2002;445:132–138. doi: 10.1007/s00424-002-0909-7. [DOI] [PubMed] [Google Scholar]