Abstract
Background and purpose:
Skeletal muscle injury by hypolipidemic drugs is not fully understood. An extensive analysis of the effect of chronic treatment with fluvastatin (5 mgkg-1 and 20 mgkg-1), atorvastatin (10 mgkg-1) and fenofibrate (60 mgkg-1) on rat skeletal muscle was undertaken.
Experimental approach:
Myoglobinemia as sign of muscle damage was measured by enzymatic assay. Histological and immunohistochemical techniques were used to estimate muscle integrity and the presence of aquaporin-4, a protein controlling water homeostasis. Electrophysiological evaluation of muscle Cl- conductance (gCl) and mechanical threshold (MT) for contraction, index of intracellular calcium homeostasis, was performed by the two-intracellular microelectrodes technique.
Key results:
Fluvastatin (20 mgkg-1) increased myoglobinemia. The lower dose of fluvastatin did not modify myoglobinemia, but reduced urinary electrolytes, suggesting direct effects on renal function. Atorvastatin also increased myoglobinemia, with slight effects on urinary parameters. No treatment caused any histological damage to muscle or modification in the number of fibres expressing aquaporin-4. Either fluvastatin (at both doses) or atorvastatin reduced sarcolemma gCl and changed MT. Both statins produced slight effects on total cholesterol, suggesting that the observed modifications occur independently of HMGCoA-reductase inhibition. Fenofibrate increased myoglobinemia and decreased muscle gCl, whereas it did not change the MT, suggesting a different mechanism of action from the statins.
Conclusions and Implications
This study identifies muscle gCl and MT as early targets of drugs action that may contribute to milder symptoms of myotoxicity, such as muscle cramps, while the increase of myoglobinemia is a later phenomenon.
Keywords: atorvastatin, fluvastatin, fenofibrate, in vivo studies, myoglobinemia, creatine kinase, electrophysiology, chloride channel conductance, skeletal muscle, rats
Introduction
Statins, inhibitors of cholesterol biosynthesis, are the preferred drugs for the treatment of hypercholesterolaemia (Assmann et al., 1999), whereas fibrates, agonists of peroxisome proliferator-activated receptors (PPAR), are used to treat hypertriglyceridaemia and to favourably increase high-density lipoprotein (HDL)-cholesterol (Chapman, 2003; Jones and Davidson, 2005). Although generally well tolerated, the most important complications of lipid-lowering therapies are myopathies, ranging from myalgia and muscle cramps to life-threatening rhabdomyolysis, which results from destruction of skeletal muscle and leads to myoglobin excretion in the urine and to renal failure. Muscle toxicity was shown to be dependent on the administration of high doses of statins with increased risk when used concomitantly with fibrates or immunosuppressant agents, a situation frequently found in the clinics (Thompson et al., 2003; Cannon et al., 2004).
How statins can injure skeletal muscle is however still unclear. Several theories have been proposed, including the lowering of ubiquinone levels or the alteration of sarcolemma cholesterol content (owing to inhibition of the mevalonate pathway), or the induction of apoptosis and proteolysis (owing to increased Ca2+ release) (Inoue et al., 2003; Thompson et al., 2003; Sirvent et al., 2005). Our previous studies allowed the identification of cellular targets of statin action, as the resting chloride conductance (gCl), sustained by the voltage-gated chloride channel ClC-1, as well as the structures involved in Ca2+ homeostasis and related to muscle contractility (Pierno et al., 1992, 1995, 1999a). ClC-1 is a muscle-specific channel that controls membrane electrical stability and functional processes such as excitation and contraction (De Luca and Conte Camerino, 1992; Pierno et al., 1999b); indeed reduced ClC-1 function due to mutations in the encoding gene, as in Thomsen and Becker myotonia, or secondary to the administration of a number of drugs, cause severe disorders related to abnormal action potential firing. This leads to involuntary muscle contraction which the patients experience as severe muscle stiffness (Jentsch et al., 2002).
Importantly, the action of statins on skeletal muscle is related to their physicochemical characteristics. We have shown that the lipophilic simvastatin impairs muscle function by reducing gCl and thus increasing sarcolemma excitability in rat skeletal muscle after 2 months chronic treatment; an effect observed also after acute application to muscle fibres in vitro (Pierno et al., 1995). Moreover, chronic treatment with simvastatin shifts the mechanical threshold for contraction (MT), an index of the excitation–contraction coupling mechanism, toward more negative potentials (Pierno et al., 1999a). By contrast, the highly hydrophilic pravastatin has no effect on the above parameters, even at levels 100-fold higher than therapeutic doses (Pierno et al., 1995, 1999a).
Thus, we proposed that the risk of myopathy is much higher with lipophilic statins because of their ability to enter muscle cells and to alter membrane structure. Strikingly, also clofibrate and some of its metabolites when applied in vitro or administered in vivo are able to significantly reduce the resting gCl in rat skeletal muscle fibers, most probably through the direct block of ClC-1 channels, thereby producing a myotonic-like state (Conte Camerino et al., 1984). Fluvastatin, atorvastatin and fenofibrate, are today widely prescribed for their higher potency and safety profile with respect to the older generation drugs (Bernini et al., 2001). Nevertheless, the most potent cerivastatin was withdrawn from the market because of the occurrence of fatal cases of rhabdomyolysis (Staffa et al., 2002). These compounds show different pharmacokinetics that may influence toxicity on skeletal muscle, thus the evaluation of the recent powerful and lipophilic drugs, such as fluvastatin, atorvastatin and fenofibrate, on skeletal muscle function is relevant for their clinical handling.
With this aim we have undertaken a large analysis of the effects of a chronic administration to rats with these compounds on the electrophysiological and contractile parameters described above and on several biomarkers of muscle toxicity. In particular, we have monitored vital parameters of treated animals. The state of skeletal muscle was examined by histological analysis, and by measuring the blood levels of myoglobin, lactate dehydrogenase (LDH) enzyme, creatine kinase (CK) and lipids. Moreover, we have taken into account changes in aquaporin-4 (AQP-4), the water channel responsible for water regulation in fast-twitch muscles which may have a role in determining oedema and muscle damage (Frigeri et al., 2004). Finally, renal toxicity was evaluated by measuring myoglobinuria and urinary electrolytes. Resting gCl and excitability of rat skeletal muscle represent the early target of statin and fibrate action. These modifications may be responsible for the mild symptoms, such as muscle cramps. By increasing the doses administered, other muscle modifications (with release of myoglobin and CK in plasma) take place that may contribute to the most dramatic side effects, such as rhabdomyolysis.
Methods
Animals and dosing
Male Wistar rats (Charles River Laboratories, Italy), initially weighing 300–350 g were used. The animals were housed individually in appropriate cages and fed with approximately 30 g day−1 of a commercial rodent chow (Charles River, 4RF21) and tap water ad libitum. Rooms were maintained at constant temperature (22–24°C) and exposed to a light cycle of 12 h day−1 (0800–2000 hours). The animals were subdivided in six experimental groups as follows: the first group (10 rats) was chronically treated with 20 mg kg−1 day−1 of fluvastatin, the second (10 rats) with 5 mg kg−1 day−1 of fluvastatin, the third (13 rats) with 10 mg kg−1 day−1 of atorvastatin, the fourth (13 rats) with 60 mg kg−1 day−1 of fenofibrate, the fifth (10 rats) only with the vehicle (aqueous methylcellulose, CMC) used to dissolve the drugs and the sixth (10 rats) was a untreated control group. Fluvastatin, atorvastatin and fenofibrate dissolved in CMC (0.5%) suspension were administered to the different groups of animals orally via an oesophageal cannula, once a day, for 2 months. For each rat, the weight related dose was formulated so that the maximal volume of drug-containing suspension was 1 ml. The results obtained from the two control groups were similar, so we have combined them and show the results as a single control value.
The doses of drugs were chosen on the basis of data present in the literature. In humans, the doses needed to decrease low-density lipoprotein (LDL)-cholesterol by 30% are 10 mg day−1 (0.15 mg kg−1 day−1) for atorvastatin and 20 mg day−1 (0.30 mg kg−1 day−1) for fluvastatin (Jones et al., 1998; Stein, 2003; Psaty et al., 2004). For both drugs the highest dose used in therapy is 1 mg kg−1 day−1. As pharmacokinetic studies in rodents indicate that greater statin doses are required to achieve similar effective concentrations (Youssef et al., 2002), we first used the dose of 10 mg kg−1 day−1 for atorvastatin and 20 mg kg−1 day−1 for fluvastatin (10-fold and 20-fold higher than the highest clinical dose, respectively). However, the high dose of fluvastatin appeared to be highly toxic for the animals then we decided to lower the dose to 5 mg kg−1 day−1. For fenofibrate we tested a dose 20-fold higher than the therapeutic one because it was described as the safest among fibrates (Jones and Davidson, 2005).
In vivo studies
All experiments were conducted in accordance with the Italian Guidelines for the use of laboratory animals, which conform with the European Community Directive published in 1986 (86/609/EEC). During all the treatment the rats were periodically weighed and the increment with respect to the beginning (time 0) measured weekly. The performance of skeletal muscle system was evaluated by testing daily in each rat the righting reflex (the ability of the rat to straighten itself on four legs when turned on its back), alteration of which represents a sign of myotonic disorder.
At the end of the chronic treatment rats were anaesthetized with urethane (1.2 g kg−1, i.p.) to allow the dissection of muscles and other organs, then the animals were killed with urethane overdose.
Plasma and urine biochemical analysis
Blood was collected by cardiac puncture, soon after death, in ethylenediaminetetracetic acid (EDTA) rinsed tubes and then centrifuged at 600 g for 10 min at 15°C. The plasma was separated and stored at −20°C until assay. The analysis of plasma biochemical parameters was performed on randomly selected plasma samples. CK determination was performed by standard spectrophotometric analysis by using diagnostic kit (Sigma Aldrich, Milan, Italy) (De Luca et al., 2005). The other parameters (myoglobin, LDH, plasma creatinine, plasma K+, azotemia) were assayed by using standard techniques. Azotemia is a measure of the total nitrogen present in the blood, which originates mainly from urea. HDL-cholesterol level was evaluated after precipitation of apo B-containing lipoproteins, as already described (Chiesa et al., 1998). The urine produced in 24 h was collected and the volume measured (diuresis) at the end of the treatment from treated and control rats. The urine content of electrolytes, creatinine, protein and myoglobin was measured by using standard techniques.
Necropsy and tissue collection
Necropsies were limited to gross examination and collection of different muscles (extensor digitorum longus (EDL), tibialis anterior (TA), soleus) and organs (heart, kidney, liver). After dissection from control and treated animals, the different organs were weighed; their weight was normalized with respect to the body weight of the animal and expressed as mg g−1 (mg of tissue/g of body weight).
Muscle histological analysis
The fast-twitch TA muscle was dissected from five randomly selected controls and five randomly selected treated rats from each group. Muscles were rinsed in normal physiological solution and immediately fixed in 10% buffered formalin solution. After fixation, muscle samples were routinely processed for paraffin embedding, sectioned at 5 μm, and stained with haematoxilin-eosin. From each muscle a transverse and a longitudinal section were obtained and examined. Transverse sections had an area of about 20 mm2 and allowed the examination of at least 15 000 muscular fibres, while longitudinal sections had an area of about 30 mm2 and allowed the examination of at least 500 muscular fibres. The histopathological features analysed were: vacuolization, swelling, hypercellularity (presence of peripheral nuclei), homogeneity of the cytoplasm and the possible presence of necrotic fibres.
Fast-MHC and AQP-4 determination by immunofluorescence
Fast myosin heavy chain (MHC) and AQP-4 determination was performed by immunofluorescence staining as described elsewhere (Frigeri et al., 2001; Pierno et al., 2002). Briefly, TA muscles from control and treated rats were dissected and rapidly frozen in isopentane cooled with liquid nitrogen. Cryostat cross-sections (5 μm) were incubated with affinity purified rabbit AQP-4 antibodies and with type II (Sigma, St Louis, MO, USA) MHC mouse monoclonal antibodies (Frigeri et al., 2001) for 1 h at room temperature. After washing, sections were incubated for 1 h with CY3-coupled goat anti-rabbit and FITC-coupled goat anti-mouse antibodies. Sections were examined with a Leica DMRXA photomicroscope equipped for epifluorescence, and digital images were obtained with a cooled CCD camera (Princeton Instruments, Princeton, NJ, USA). For fibre quantification, the analysis has been performed on 18 randomly chosen different areas of three different sections of the same muscle. The number of examined animals was five control rats and five of each treatment. The MHC type II and AQP-4 positive fibres were counted and the stained cells expressed as a percentage of the total cells on the photograph.
In vitro electrophysiological experiments
The in vitro electrophysiological recordings started after 2 months of treatment. The EDL muscles were dissected from treated and control animals. The preparations were immediately placed in a 25 ml muscle bath, maintained at 30°C and perfused with normal and/or chloride-free physiological solution (Bryant and Conte Camerino, 1991). By means of standard two-intracellular-microelectrode technique, RMP, cable parameters, component conductances and excitability characteristics of treated and untreated muscle fibres were measured in current-clamp mode. Cable parameters, in both normal and chloride-free solutions, were calculated from the electrotonic potential elicited by square-wave hyperpolarizing current pulse (100 ms duration) injected by the current electrode. The membrane voltage responses were monitored by the voltage electrode at two distances from the current electrode. The current pulse generation, the acquisition of the voltage records and the calculation of the fibre constants were performed in real-time under computer control as described elsewhere (Bryant and Conte Camerino, 1991). From the experimentally determined values of input resistance, space constants and time constant and from an assumed Ri of 125 Ω cm, dcalc, Rm and Cm were calculated (De Luca et al., 1994). The reciprocal of Rm from each fibre in normal physiological solution was assumed to be gm and the same parameter measured in chloride-free solution was considered to be the potassium conductance (gK). The mean gCl was estimated as the mean gm minus the mean gK (De Luca et al., 1994).
The excitability characteristics of the sampled fibres were determined by recording the intracellular membrane potential response to a square-wave constant (100 ms) current pulse. In each fibre, the membrane potential was set by a steady holding current to −80 mV before passing the depolarizing pulses. By increasing the amplitude of the pulse we were able to elicit first a single action potential, from which the AP (amplitude of action potential, in mV), Ith (threshold current, in nA) and Lat (latency of action potential, delay from the beginning of the current pulse to the onset of an action potential at threshold, in ms) were calculated. By further increasing current intensity in the same fibre a train of action potentials (N spikes) was then generated (De Luca et al., 1994).
The MT for contraction was determined using a two-microelectrode point voltage clamp method in the presence of 3 μM tetrodotoxin, as described previously (Dulhunty, 1982; Heiny et al., 1990; De Luca and Conte Camerino, 1992; Pierno et al., 2003). A voltage sensing electrode (3 M KCl) and a current passing electrode (2 M potassium citrate) were inserted within 50 μm of each other into a randomly selected superficial fibre. The holding potential was set at −90 mV. Depolarizing current pulses of increasing duration (5–500 ms) were given repetitively at a rate of 0.3 Hz, while the impaled fibres were viewed continuously with a stereomicroscope (× 100 magnification). As a standard protocol, the command pulse duration was set sequentially to each of the following values: 500, 50, 5, 200, 20, 100 and 10 ms. The command voltage was increased until contraction was just visible and the threshold membrane potential at this point was read from a digital sample-and-hold voltmeter. The mean threshold membrane potential V (mV)±s.e.m. (n fibres) was plotted as a function of the pulse duration t (ms) and the relationship was fitted to the equation where H is the holding potential (−90 mV), R (mV) is the rheobase voltage and τR (ms) is the time constant to reach R. The MT values were expressed as the fitted R parameter along with the s.e. that was determined from the variance–covariance matrix in the nonlinear least-squares fitting algorithm. We estimated the uncertainty of any single measurement for a given fibre to be 1–2 mV. The measurements were performed for all experimental conditions in an identical manner, with about the same length of time involved in each determination. This methodology allows detection of a functional change of excitation–contraction coupling. The measure of MT is independent of gCl reduction (De Luca et al., 1996) but can be due to an increase of cytosolic calcium (De Luca et al., 2005; Fraysse et al., 2003; Pierno et al., 2003) and/or to a modification of the cellular structures dealing with calcium handling (i.e. RyR, DHP receptor, calcium binding proteins).
Statistical analysis
The data are expressed as mean±s.e.m. The estimates for s.e.m. and number of fibres (n) of gCl were obtained from the variance of gm and gK, assuming no covariance, and from the number of fibres sampled for gm and gK, respectively (Bryant and Conte Camerino, 1991). The estimates of s.e.m. and n of normalized gCl values were obtained as described by Green and Margerison (1978). One-way analysis of variance (ANOVA) was used to evaluate multiple statistical differences between groups followed by Bonferroni's t-test for allowing a better evaluation of intra- and intergroup variability. Comparison of means from each treated rat and the related control group was evaluated by unpaired Student's t-test.
Solutions and drugs
The normal physiological solution had the following composition (in mM): NaCl 148, KCl 4.5, CaCl2 2.0, MgCl2 1.0, NaHCO3 12.0, NaH2PO4 0.44 and glucose 5.55. The chloride-free solution was prepared by equimolar replacement of methylsulphate salts for NaCl and KCl and nitrate salts for CaCl2 and MgCl2. Both solutions were continuously gassed with 95% O2 and 5% CO2 (De Luca et al., 1994). To suppress spontaneous contraction of muscle preparations, 1 μM tetrodotoxin was added to the chloride-free solutions. The pH of all the solutions used was carefully maintained between 7.2 and 7.3 during each experiment.
Commercially available fluvastatin (Lescol, Novartis), atorvastatin (Torvast, Pfizer) and fenofibrate (Lipsin, Caber) in CMC (0.5%) suspension were used for the chronic dosing of rats in vivo.
Results
In vivo studies
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on animal health
Rats chronically treated with high doses of fluvastatin (20 mg kg−1 day−1) showed adverse physical signs, such as a decrease of food consumption and a significant decrease in body weight (Table 1). This effect was obvious as early as 3–4 weeks of treatment and two out of 10 rats showed hindlimb paralysis and a slowing of the righting reflex. These two animals died before the end of the treatment. The remaining rats were examined for histological, biochemical and electrophysiological parameters at the end of the treatment. On the contrary, rats treated with the lower dose of fluvastatin (5 mg kg−1 day−1) as well as those given atorvastatin (10 mg kg−1 day−1) or fenofibrate (60 mg kg−1 day−1) showed normal body weight gain and appeared in good health, similarly to control rats. In all these animals, the righting reflex was normal during the whole treatment.
Table 1.
Effects of 2 months of chronic treatment with atorvastatin, fluvastatin and fenofibrate on rat body and tissue weight
| Treatment | n rats | % Change body weight | Tibialis muscle (mg g−1) | Soleus muscle (mg g−1) | Heart (mg g−1) | Kidney right (mg g−1) | Kidney left (mg g−1) | Liver (mg g−1) |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 42.4±10 | 1.95±0.1 | 0.56±0.03 | 2.75±0.10 | 3.28±0.15 | 3.24±0.10 | 31±0.9 |
| Atorvastatin | 10 | 43.3±6.5 | 2.16±0.04 | 0.50±0.04 | 2.90±0.05 | 3.47±0.15 | 3.30±0.15 | 32±1.2 |
| 10 mg kg−1 | *P<0.025 | |||||||
| Fluvastatin | 10 | 40.6±8.0 | 2.02±0.05 | 0.50±0.02 | 2.90±0.07 | 3.29±0.17 | 3.21±0.11 | 30±1.1 |
| 5 mg kg−1 | ||||||||
| Fluvastatin | 7 | −15.4±9.0 | 1.57±0.20 | 0.58±0.04 | 3.38±0.14 | 4.12±0.12 | 4.15±0.20 | 33±1.9 |
| 20 mg kg−1 | *P<0.001 | *P<0.005 | *P<0.001 | *P<0.005 | *P<0.001 | |||
| Fenofibrate | 10 | 40.2±4.5 | 1.99±0.04 | 0.52±0.04 | 3.03±0.08 | 3.58±0.30 | 3.81±0.09 | 58±2.6 |
| 60 mg kg−1 | *P<0.02 | *P<0.005 | *P<0.001 | |||||
| ANOVA | F=8.58 | F=5.13 | F=1.01 | F=6.35 | F=2.52 | F=9.54 | F=54 | |
| §P<0.001 | §P<0.02 | NS | §P<0.001 | NS | §P<0.001 | §P<0.001 |
Abbreviation: ANOVA, analysis of variance.
Each value shown in the Table is the mean±s.e.m. from n rats as indicated. The percent change (− decrease, +increase) in body weight was calculated from the mean of the weight of the animals at the end of the treatment (after 2 months), relative to the initial weight, at the beginning of the treatment. The weight of the tissues is expressed as in mg tissue per g body weight. These tissues were chosen as they can be a target of statin and fibrate action.
Significantly different by ANOVA test
Significantly different with respect to control (by Bonferroni's t-test).
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on the lipid profile of rat plasma
A slight and dose-dependent reduction of the mean value of total plasma cholesterol was observed in six and seven animals treated with fluvastatin at 5 mg kg−1 day−1 (12%) and 20 mg kg−1 day−1 (20%), respectively, with respect to the mean of seven control rats (data not shown). Interestingly, although atorvastatin did not modify total cholesterol, this drug induced a significant increase of HDL-cholesterol from 0.14±0.02 mg ml−1 of plasma to 0.22±0.01 mg ml−1 (P<0.01 by Bonferroni's t-test). HDL cholesterol was not modified in all the other groups of treated animals (not shown). Fenofibrate, as expected, significantly lowered the plasma triglycerides from 1.08±0.18 to 0.72±0.08 mg ml−1 (P<0.025 by Bonferroni's t-test) and slightly reduced cholesterol level as reported in the literature (Chapman, 2003).
Ex vivo studies
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on the weight of muscles and other organs
After killing, several organs from treated and control rats were dissected and weighed. The most important modifications were observed in rats treated with the highest dose of fluvastatin (20 mg kg−1 day−1). The absolute and relative weight of the TA muscle were significantly reduced in these animals, but not in the slow-twitch soleus muscle. Moreover, a significant increase of the weight of heart and kidney was found (Table 1). These effects were dose-dependent since fluvastatin at the minor dose tested did not induce any modification in the weight of the organs (Table 1). Also, the rats treated with fenofibrate showed remarkable modification in the weight of the heart, left kidney and liver (Table 1). On the contrary atorvastatin did not modify any of these parameters, apart from an hypertrophy of TA muscle (Table 1).
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on biochemical parameters of rat plasma and urine
The plasma and urinary biochemical parameters were monitored to look for signs of drug-related muscle and/or renal damage (Tables 2 and 3). We investigated the possible release of myoglobin, LDH, CK or creatinine from injured myocytes into the bloodstream, as well as measuring azotemia and plasma K+. Fluvastatin at the higher dose produced a significant increase of myoglobinemia, plasma CK and azotemia, while no effect was found at the lower dose (Table 2). In the plasma of atorvastatin treated rats we found a significant increase of myoglobin, LDH and CK as signs of marked muscle involvement (Table 2). Fenofibrate also produced a significant increase of myoglobinemia and LDH in the plasma of treated animals (Table 2).
Table 2.
Effects of chronic treatment with atorvastatin, fluvastatin and fenofibrate on biochemical parameters in rat plasma
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Plasma parameter | Control | Atorvastatin 10 mg kg−1 | Fluvastatin 5 mg kg−1 | Fluvastatin 20 mg kg−1 | Fenofibrate 60 mg kg−1 | ANOVA |
| Myoglobin | 0.14±0.02 | 0.23±0.01 | 0.16±0.02 | 0.28±0.05 | 0.23±0.03 | F=4.27 |
| (ng ml−1) | *P<0.02 | *P<0.005 | *P<0.025 | §P<0.01 | ||
| LDH | 587±76 | 1255±202 | 550±77 | 915±151 | 1065±295 | F=4.08 |
| (mU ml−1) | *P<0.005 | *P<0.025 | §P<0.01 | |||
| CK | 1238±217 | 2118±202 | 1468±40 | 1795±189 | 1517±437 | F=2.31 |
| (mU ml−1) | *P<0.005 | *P<0.05 | NS | |||
| Creatinine | 7±0.3 | 8±0.5 | 7±0.8 | 8±0.6 | 7±0.7 | F=0.06 |
| (μg ml−1) | NS | |||||
| Potassium | 5.9±0.3 | 6.8±0.7 | 5.5±0.6 | 5.3±0.6 | 6.6±0.4 | F=1.45 |
| (mEq l−1) | NS | |||||
| Azotemia | 0.48±0.02 | 0.55±0.04 | 0.45±0.03 | 0.62±0.05 | 0.54±0.01 | F=3.8 |
| (mg ml−1) | *P<0.005 | §P<0.02 | ||||
| N (samples) | 10 | 6 | 7 | 7 | 5 | |
Abbreviations: ANOVA, analysis of variance; CK, creatine kinase; LDH, lactate dehydrogenase.
Each row shows the mean±s.e.m. of the plasma parameters measured from the number of samples as indicated.
Significantly different by ANOVA test
Significantly different with respect to control (by Bonferroni's t-test).
Table 3.
Effects of chronic treatment with atorvastatin, fluvastatin and fenofibrate on biochemical parameters in rat urine
| Treatment | |||||
|---|---|---|---|---|---|
| Urinary parameter | Control | Atorvastatin 10 mg kg−1 | Fluvastatin 5 mg kg−1 | Fenofibrate 60 mg kg−1 | ANOVA |
| Myoglobin | 1207±207 | 1701±131 | 1225±105 | 1380±100 | F=1.57 |
| (ng 24 h−1) | *P<0.05 | NS | |||
| Creatinine | 19.0±1.5 | 20.0±3.9 | 15.0±4.3 | 17.6±1.1 | F=0.55 |
| (mg 24 h−1) | NS | ||||
| Na+ | 3.5±0.2 | 2.6±0.2 | 2.1±0.4 | 3.3±0.3 | F=5.95 |
| (mM 24 h−1) | *P<0.01 | *P<0.001 | §P<0.005 | ||
| K+ | 5.0±0.4 | 4.5±0.4 | 3.5±0.4 | 4.3±0.6 | F=1.90 |
| (mM 24 h−1) | *P<0.02 | NS | |||
| Cl− | 3.9±0.3 | 3.2±0.1 | 2.1±0.4 | 4.0±0.6 | F=5.07 |
| (mM 24 h−1) | *P<0.001 | §P<0.01 | |||
| Ca++ | 3.8±1.2 | 2.2±0.4 | 1.8±0.5 | 1.4±0.8 | F=1.24 |
| (mg 24 h−1) | NS | ||||
| Mg++ | 0.9±0.3 | 1.3±0.01 | 1.1±0.2 | 2.5±1.4 | F=1.36 |
| (mg 24 h−1) | *P<0.05 | NS | |||
| Phosphate | 9.4±2.3 | 1.5±0.5 | 2.1±1.2 | 3.0±1.2 | F=4.46 |
| (mg 24 h−1) | *P<0.005 | *P<0.01 | *P<0.02 | §P<0.02 | |
| Protein | 32.6±3.3 | 39.0±8.2 | 19.0±3.0 | 56.6±0.02 | F=6.50 |
| (mg 24 h−1) | *P<0.05 | *P<0.005 | §P<0.005 | ||
| Diuresis | 31.0±4.5 | 37.0±4.2 | 20.0±1.9 | 36.0±5.1 | F=2.35 |
| (ml kg−1 24 h−1) | *P<0.05 | NS | |||
| N samples | 10 | 6 | 5 | 5 | |
Abbreviation: ANOVA, analysis of variance.
Each row shows the mean±s.e.m. of the urinary parameters measured from the number of samples as indicated. The values shown are the amounts excreted in urine in 24 h.
Significantly different by ANOVA test
Significantly different with respect to control (by Bonferroni's t-test).
The urinary parameters tested in treated and control rats were myoglobin, creatinine, protein and electrolytes. Atorvastatin-treated rats showed a significant increase (40%) of myoglobinuria (Table 3). In all the other treated rats this parameter was unchanged. No modification of creatininuria was found in all treatment groups. In addition atorvastatin significantly decreased some urinary parameters such as Na-uria and phosphaturia (Table 3). Surprisingly, in rats treated with the low dose of fluvastatin a significant reduction of many urinary electrolytes, such as Na+-, K+-, Cl− and phosphate, was found. In these animals, the proteinuria was also significantly reduced by 40%, together with a similar decrease in the volume of 24 h urine (Table 3). Although these parameters have not been measured in the 20-mg-fluvastatin-treated rats, a preliminary screening of the urine of these animals revealed a high grade of haematuria (data not shown). Fenofibrate significantly reduced Mg++ and phosphate in urine. In addition it also significantly increased proteinuria (Table 3).
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on muscle histology, fast-MHC and AQP-4
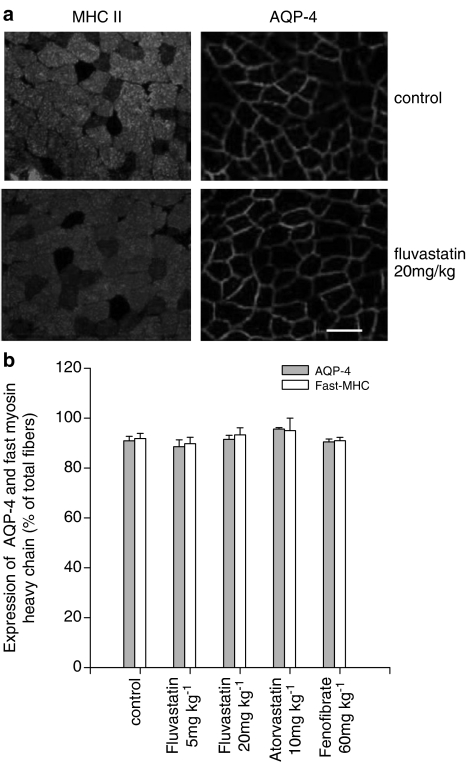
Histological examination of sections from fast-twitch (TA) muscles were used to evaluate their integrity. Muscles were removed from drug-treated and control rats and analysed for the potential presence of histopathological signs. In both transverse and longitudinal sections of all the groups of treated rats, no sign of rhabdomyolysis and/or damage was observed. In particular, muscle sections did not show degenerating areas, hypercellularity, vacuolization, swelling or homogeneous cytoplasm (data not shown). To further investigate possible modifications of the normal structure of skeletal muscle, we looked for changes in AQP-4, a water channel protein typically expressed in fast-twitch muscle, which might contribute to muscle damage. Analysis was performed on five control muscles and five muscles of each treatment group using immunohistochemical techniques. The TA sections analysed included both the deep ‘red' region (with a high proportion of slow-twitch oxidative and fast-twitch oxidative glycolytic fibres) and the superficial ‘white' region (predominantly fast-twitch glycolytic fibres) (Armstrong and Phelps, 1984). No modification in the number of fibres expressing AQP-4 was observed in all TA muscle sections from treated rats as compared to controls (Figure 1). Also, the number of fibres expressing the fast isoform of MHC was not modified in TA muscle sections (Figure 1). These results were in accordance with the absence of drug-induced muscle swelling.
Figure 1.
Effects of atorvastatin, fluvastatin and fenofibrate on AQP-4 and fast MHC (type II) isoform in rat fast-twitch skeletal muscle fibres. (a) Immunofluorescence staining of rat TA muscle from control and fluvastatin-treated rats was performed by using specific antibodies. Top: cryostat transversal section of the same muscle preparation showing fast (MHC type II) fibres (left panel) and AQP-4 (right panel) in control rat muscle. Bottom: cryostat transverse section of the same muscle preparation showing fast (MHC type II) fibres (left panel) and AQP-4 (right panel) in muscle from rats given 20 mg kg−1 day−1 fluvastatin. Bar, 50 μm. (b) quantification of the number of TA muscle fibres expressing AQP-4 and fast-MHC over the total number of fibres. Statistical analysis (by Student's t-test) does not show any difference between treated and control rats.
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on fiber diameter, sarcolemma component ionic conductances and excitability parameters of rat skeletal muscle fibres
The effects of the various drug treatments on skeletal muscle function were evaluated by measuring the resting membrane gCl and sarcolemma excitability.
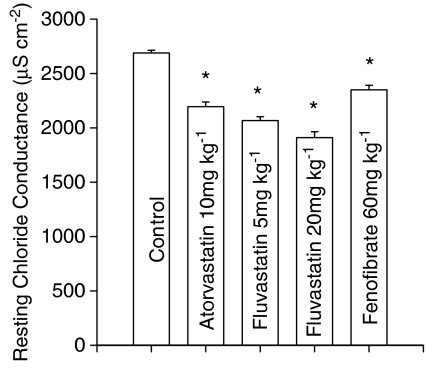
The recording of cable parameters allowed measurement of membrane resistance, from which the ionic conductances were calculated, and fibre diameter. In particular, EDL muscle fibres from rats treated with the high dose of fluvastatin (20 mg kg−1 day−1) showed a significant decrease in fibre diameter (treated, 56.4±3.0 μm (26 fibres); control, 64.9±1.4 μm (101 fibres) P<0.01) in accordance with the atrophy of the TA, another fast-twitch muscle, as described above (Table 1). In this treatment group, all animals showed a significant reduction in gCl with an average reduction of 29% (Figure 2). In these animals the mean resting gK was significantly increased from 339±8 μS cm−2 (10 rats/94 fibres) to 407±34 μS cm−2 (7 rats/41 fibres, P<0.001 by Bonferroni's t-test).
Figure 2.
Resting gCl measured in EDL muscle fibres from rats chronically (2-months) treated with atorvastatin (10 mg kg−1 day−1), fluvastatin (5 and 20 mg kg−1 day−1) and fenofibrate (60 mg kg−1 day−1) and from untreated rats. Each bar represents the mean±s.e.m. of gCl from 49–102 fibres measured in 7–10 animals. Statistical analysis by ANOVA showed significant differences in gCl (F=53, n fibres – k groups=427, P<0.001). *Bonferroni's t-test showed significant differences between all treated groups and the control group (P<0.001).
The fibre diameter in rats treated with atorvastatin and with the low dose of fluvastatin was higher than that of control (atorvastatin, 72±1.6 μm (85 fibres, P<0.005); fluvastatin 77±1.9 μm (102 fibres, P<0.001 vs control as above), respectively. These two treatments also decreased mean resting gCl by 24 and 20%, respectively (Figure 2). Such decreases were observed in 90% of rats given fluvastatin (5 mg kg−1 day−1) and in 70% of those treated with atorvastatin.
The treatment with fenofibrate did not alter EDL fibre diameter, (67±1.3 μm; 86 fibres) but produced a 13% decrease in the mean gCl (Figure 2) with an incidence of 70%. In rats treated with fluvastatin (5 mg kg−1 day−1), atorvastatin and fenofibrate, the mean gK was unchanged, (350±11 μS cm−2 (10 rats/99 fibres), 350±9 μS cm−2 (10 rats/100 fibres) and 351±9 μS cm−2 (10 rats/105 fibres), respectively).
The excitability parameters of the muscles were modified as expected from the observed reduction in gCl (Table 4). In particular, treated fibres showed a tendency to be more excitable with respect to control rats. In fibres from rats treated with the high dose of fluvastatin (20 mg kg−1 day−1), the current needed to elicit one action potential (Ith) was decreased, the latency (Lat) of the action potential was prolonged and the repetitive activity (N spikes) of the fibre was increased (Table 4). The low dose of fluvastatin was also able to prolong the Lat and to increase the N spikes. Moreover, fluvastatin at both doses slightly increased the AP with respect to that of control animals (Table 4). The treatments with atorvastatin and fenofibrate induced an augmentation of Lat and N spikes (Table 4).
Table 4.
Effects of atorvastatin, fluvastatin and fenofibrate treatments on membrane excitability of rat extensor digitorum longus muscle fibres
| Treatment | N rats/n fibres | AP (mV) | Ith (nA) | Lat (ms) | N spikes |
|---|---|---|---|---|---|
| Control | 10/51 | 101±1.3 | 188±7.1 | 5.6±0.2 | 4.0±0.3 |
| Atorvastatin (10 mg kg−1) | 9/34 | 99±2.5 | 205±9.0 | 6.7±0.5 | 5.4±0.5 |
| *P<0.02 | *P<0.02 | ||||
| Fluvastatin (5 mg kg−1) | 8/23 | 106±2.1 | 175±9.1 | 8.3±0.4 | 6.3±0.5 |
| *P<0.05 | *P<0.001 | *P<0.001 | |||
| Fluvastatin (20 mg kg−1) | 7/21 | 107±2.9 | 130±7 | 10.3±0.5 | 7.0±0.7 |
| *P<0.025 | *P<0.001 | *P<0.001 | *P<0.001 | ||
| Fenofibrate (60 mg kg−1) | 9/38 | 100±1.7 | 193±10 | 6.5±0.4 | 5.5±0.6 |
| *P<0.05 | *P<0.01 | ||||
| F=2.57 | F=7.86 | F=19.4 | F=5.16 | ||
| ANOVA | §P<0.05 | §P<0.001 | §P<0.001 | §P<0.001 |
Abbreviation: ANOVA, analysis of variance.
The columns from left to right indicate as follows: N rats/n fibres, number of rats examined on number of fibres sampled; AP, amplitude of the first action potential (peak); Ith, threshold current (minimum current intensity that would elicit a single action potential); Lat, latency of the first action potential (maximal delay from the beginning of the current pulse to the onset of the spike); N spikes, maximum number of action potentials elicited by raising the intensity of a 100 ms-duration pulse in the same fibre. As detailed in the Methods section AP and Lat were calculated from traces obtained using Ith current. The values are mean±s.e.m.
Significantly different by ANOVA test
Significantly different with respect to control (by Bonferroni's t-test).
Effects of chronic treatment with fluvastatin, atorvastatin and fenofibrate on the MT for contraction of rat skeletal muscle fibres
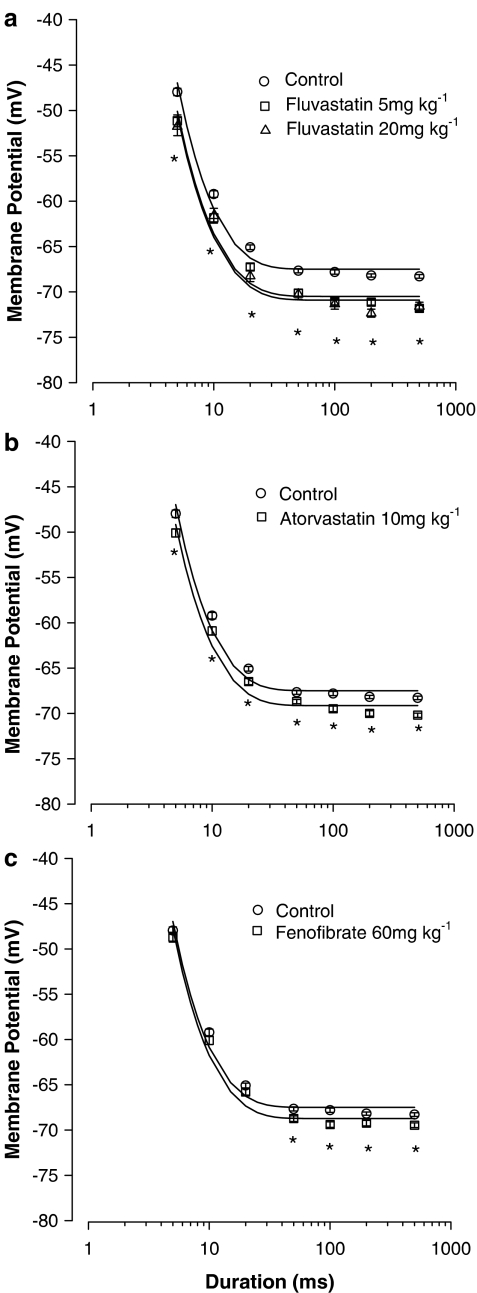
To find out if the modifications induced by hypolipidemic drug treatments were accompanied by changes in calcium handling mechanisms and contraction, we measured the voltage threshold for mechanical activation (MT) in single fibres from EDL muscles of control and treated animals. The threshold potential needed to elicit contraction was determined by applying current pulse of different durations (5–500 ms) and showed the typical dependence on command pulse duration, being the more negative the longer the duration of the pulse (Figure 3). The rheobase voltage (R) calculated from the experimental points showed differences between treated and control animals. Muscle fibres (EDL) from fluvastatin-treated rats needed less depolarization to contract at each pulse duration and consequently the threshold potential–duration relationship was shifted toward more negative potentials as compared to controls. The R-values calculated from the data shown in Figure 3 were for fluvastatin, at low or high doses, respectively, −70.5±0.7 mV (308 fibres/10 rats) and −71±0.7 mV (124 fibres/5 rats) and less than R-values in controls, −67.5±0.6 mV (364 fibres/10 rats; P<0.001). Atorvastatin treatment also shifted the strength–duration curves toward more negative potentials, R being −69±0.5 mV (335 fibres/10 rats, P<0.025). However, in muscles from fenofibrate-treated rats, R was not modified (−68.7±0.4 mV; 374 fibres/10 rats).
Figure 3.
MT for contraction measured in EDL muscle fibres of fluvastatin (a), atorvastatin (b) and fenofibrate (c) chronically treated rats with respect to controls. Each point, expressed as mean value±s.e.m. from 124 to 374 fibres of 5–10 rats, show the voltage potential for fibre contraction at each pulse duration measured in different experimental conditions (control and treated animals). *Significantly more negative with respect to control (by Student's t-test, P<0.001). A single asterisk indicates the significant differences of the mean voltage potential measured in rats treated with fluvastatin (5 or 20 mg kg−1 day−1) with respect to that of control rats. The values have been fitted to the equation shown in the Methods section to obtain the strength–duration curves and the calculated values of rheobase (R) are given in the Results section.
Discussion
The occurrence of rhabdomyolysis with statin and fibrate monotherapy is generally very low, while the incidence of less-serious events, such as myalgia and cramps is higher (Thompson et al., 2003). Here, we tested the effects of chronic treatment of rats with the widely used atorvastatin and fluvastatin. These lipophilic statins are more likely able to modify muscle function, because we previously showed that the lipophilic simvastatin can alter the electrical and contractile properties of rat skeletal muscle fibres, whereas the hydrophilic pravastatin had no effect (Pierno et al., 1995). The statins used in the present work are more potent than simvastatin in modifying muscle function. Either fluvastatin or atorvastatin, at doses lower than simvastatin, induced a marked reduction of the resting gCl with a concomitant increase of sarcolemma excitability and shifted the MT for contraction towards more negative potentials. Animals treated with the higher dose of fluvastatin also showed an increased gK, suggesting that this drug can affect the KATP channel activity, as already described for other statins (Pierno et al., 1995). In these animals, the plasma levels of myoglobin and CK were increased, although not as much as during rhabdomyolysis (Thompson et al., 2003). As there were no relevant histological signs of pathology except a reduced muscle weight and a reduced fibre diameter, we propose that the plasma parameters may represent early markers of myopathy occurring before rhabdomyolysis. Accordingly much higher statin doses were required to induce histological modifications in rat skeletal muscle (Smith et al., 1991; Westwood et al., 2005). Another important finding is that the two statins do not affect the number of fibres expressing AQP-4, a protein typical of fast-twitch muscles and important for the regulation of myofiber volume after contraction (Frigeri et al., 2004). This suggests that the mechanisms of water transport that are likely to contribute to swelling and possibly oedema in muscle cells were not altered at this stage. Surprisingly, atorvastatin-treated rats showed an increased TA muscle weight and an increase of fibre diameter in samples of EDL muscle. If water balance is not involved, this effect might be attributable to an increase of protein expression due to transcriptional control by statins (Morikawa et al., 2005).
The lower dose of fluvastatin did not induce muscle atrophy or biochemical and histological changes, suggesting that the functional parameters such as gCl, excitability and contractility may represent the most basic targets of fluvastatin action. Atorvastatin was more potent than fluvastatin as a dose of 10 mg kg−1 day−1 already produced skeletal muscle cell injury, revealed by the increased myoglobin, LDH enzyme and CK in the plasma. This greater ability to induce side effects may be related to the longer elimination half-life of atorvastatin with respect to fluvastatin (Corsini et al., 1999), suggesting that drug accumulation may contribute to increased risk of myopathy. Nevertheless, for atorvastatin also, there was neither alteration of muscle histology nor decrease of weight gain. The increased myoglobinuria indicated early renal involvement, consequent on muscle injury.
The modification of MT suggests that alteration of muscle calcium homeostasis may in turn be responsible for the onset of muscle damage. Indeed, we can hypothesize that a calcium increase may contribute to the modification of gCl by altering the function of calcium-dependent regulatory proteins and of biochemical parameters (such as myoglobinemia) by activating proteolytic enzymes. At this regard we have previously demonstrated that a cytosolic Ca2+ increase activates protein kinase C, that in turn can close muscle chloride channels and consequently reduce gCl (Bryant and Conte Camerino, 1991; De Luca et al., 1994). Moreover, the role of Ca2+ in the proteolytic activity has been described during simvastatin-induced damage of human muscle cells (Sacher et al., 2005).
It is remarkable that the lower dose of fluvastatin already produced modification of many of the urinary electrolyte, together with a decrease of diuresis and proteinuria. In this regard, the partial elimination of fluvastatin (being slightly more hydrophilic than atorvastatin) (Corsini et al., 1999) through the kidney may contribute to the adverse side effects observed at this level. It may be that the deaths occurring with the higher fluvastatin dose could be associated with renal failure. Indeed, the rats treated with 20 mg kg−1 day−1 fluvastatin also showed urinary haematuria, which can be a sign of serious renal toxicity. In addition the increased azotemia and kidney hypertrophy indicated a progressive renal damage. An appealing hypothesis is the possible interaction of the drug with chloride channels present in the kidney, which belong to the same ClC family as the muscle ClC-1 channel (Liantonio et al., 2004; Jentsch, 2005). Indeed, modification in the activation of these channels can produce serious consequences for renal function, as seen in hereditary diseases characterized by mutations in ClC genes (Jeck et al., 2004). Further studies are required to address this hypothesis with the aim of preventing adverse side effects in patients under therapy.
Interestingly, all the statin effects reported here were independent of their lowering of plasma cholesterol, as the total cholesterol level in these rats was only slightly modified. This finding excludes the possibility that the modification of the electrical and contractile properties could be related to a drug-induced reduction of membrane cholesterol. At this point other explanations are more likely, such as a direct interaction with specific proteins affecting muscle function.
Regarding fenofibrate, the muscle damage may occur through quite different mechanisms. Indeed, although the reduction of gCl was observed, the MT for contraction was not modified, suggesting no change in calcium handling. Effects on gCl may result from fenofibrate metabolites, since we previously showed that the clofibrate metabolite, 2-p-chloro-phenoxy-propionic acid, is a potent inhibitor of gCl (Conte Camerino et al., 1984). Moreover, the increase of plasma myoglobin and LDH activity after fenofibrate treatment may involve mechanisms other than those related to alterations in calcium homeostasis. A renal involvement was also suggested by the increase of proteinuria after fenofibrate.
Interestingly, the gCl appears as a early target common to all the drugs tested and its long-term modification might play a role in determining toxicity. As gCl is responsible for membrane electrical stabilization following the action potential (Bryant and Conte Camerino, 1991; Jentsch et al., 2002), the functional modification induced by the drugs may contribute to the milder side effects, such as the muscle cramps, frequently reported by the patients under hypolipidemic drug therapy.
In conclusion, either statin or fenofibrate can affect skeletal muscle function, although only doses much higher than the therapeutic doses were highly toxic. This study underlines the importance of reducing the clinical use of these drugs in individuals showing a basic alteration of gCl. For instance, side effects of hypolipidemic drugs are more frequent in the elderly, a condition already characterized by a reduced gCl (Pierno et al., 1999b). Moreover, since renal involvement was found with fluvastatin, the presence of renal dysfunction should be considered before initiating therapy. On the other hand, individuals with unaltered muscle and kidney function may be exposed to higher doses of drugs due to genetic variability in drug metabolism or excretion and thus at higher risk of muscle and renal side effects (Kajinami et al., 2004). Finally, the higher occurrence of side effects in patients receiving a combination statin-fibrate therapy because they have a mixed dyslipidaemia, is well established. The cause of such a correlation is still unclear and may involve pharmacokinetic and/or pharmacodynamic mechanisms. Our results suggest that the concomitant action of both drugs on the gCl and sarcolemma excitability may contribute to increase the risk of muscle side effects and rhabdomyolysis.
Acknowledgments
This study was supported by Italian ‘Ministero dell'Istruzione, dell'Università e della Ricerca' (FIRB RBAU015E9T) to D Conte Camerino. We are grateful to Dr Jean-François Desaphy for helpful discussion and to Dr Gianpatrizio Bianco for technical computer assistance.
Abbreviations
- AQP-4
aquaporin-4
- CK
creatine kinase
- EDL
extensor digitorum longus
- gCl
chloride conductance
- gK
potassium conductance
- HMGCoA reductase
3-hydroxy-methyl-glutaryl Coenzyme A reductase
- LDH
lactate dehydrogenase
- MHC
myosin heavy chain
- MT
mechanical threshold
- TA
tibialis anterior
Conflict of interest
The authors state no conflict of interest.
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Assmann G, Carmena R, Cullen P, Fruchart JC, Jossa F, Lewis B, et al. Coronary heart disease: reducing the risk: a worldwide view. International Task Force for the Prevention of Coronary Heart Disease. Circulation. 1999;100:1930–1938. doi: 10.1161/01.cir.100.18.1930. [DOI] [PubMed] [Google Scholar]
- Bernini F, Poli A, Paoletti R. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc Drugs Ther. 2001;15:211–218. doi: 10.1023/a:1011908004965. [DOI] [PubMed] [Google Scholar]
- Bryant SH, Conte Camerino D. Chloride channel regulation in the skeletal muscle of normal and myotonic goats. Pflügers Arch. 1991;417:605–610. doi: 10.1007/BF00372958. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- Chapman AF. Fibrates in 2003: therapeutic action in atherogenic dyslipidaemia and future perspectives. Atherosclerosis. 2003;171:1–13. doi: 10.1016/s0021-9150(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Chiesa G, Parolini C, Canadesi M, Colombo N, Sirtori CR, Fumagalli R, et al. Human apolipoproteins A-I and A-II in cell cholesterol efflux: studies with transgenic mice. Arterioscler Thromb Vasc Biol. 1998;18:1417–1423. doi: 10.1161/01.atv.18.9.1417. [DOI] [PubMed] [Google Scholar]
- Conte Camerino D, Tortorella V, Ferrannini E, Bryant SH. The toxic effects of clofibrate and its metabolite on mammalian skeletal muscle: an electrophysiological study. Arch Toxicol. 1984;7:482–484. doi: 10.1007/978-3-642-69132-4_101. [DOI] [PubMed] [Google Scholar]
- Corsini A, Bellosta S, Baetta R, Fumagalli R, Paletti R, Bernini F. New insight into pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- De Luca A, Conte Camerino D. Effects of aging on the mechanical threshold of rat skeletal muscle fibers. Pflugers Arch. 1992;420:407–409. doi: 10.1007/BF00374477. [DOI] [PubMed] [Google Scholar]
- De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, et al. A multidisciplinary evaluation of the effectiveness of cyclosporine A in dystrophic mdx mice. Am J Pathol. 2005;1696:477–489. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Conte Camerino D. Effect of taurine depletion on excitation-contraction coupling and Cl− conductance of rat skeletal muscle. Eur J Pharmacol. 1996;296:215–222. doi: 10.1016/0014-2999(95)00702-4. [DOI] [PubMed] [Google Scholar]
- De Luca A, Tricarico D, Pierno S, Conte Camerino D. Aging and chloride channel regulation in rat fast-twitch muscle fibers. Pflugers Arch. 1994;427:80–85. doi: 10.1007/BF00585945. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. Effects of membrane potential on mechanical activation in skeletal muscle. J Gen Physiol. 1982;79:233–251. doi: 10.1085/jgp.79.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraysse B, Desaphy J-F, Pierno S, De Luca A, Liantonio A, Mitolo CI, et al. Decrease in resting calcium and calcium entry associated with slow-to-fast transition in unloaded rat soleus muscle. FASEB J. 2003;17:1916–1918. doi: 10.1096/fj.02-1012fje. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Balena R, Nico B, Svelto M. Aquaporins in skeletal muscle: reassessment of the functional role of aquaporin-4. FASEB J. 2004;18:1417–1423. doi: 10.1096/fj.03-0987fje. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Desaphy J-F, Pierno S, De Luca A, Conte Camerino D, et al. Muscle loading modulates aquaporin-4 expression in skeletal muscle. FASEB J. 2001;15:1282–1284. doi: 10.1096/fj.00-0525fje. [DOI] [PubMed] [Google Scholar]
- Green JR, Margerison D. Statistical Treatment of Experimental Data. Elsevier: New York; 1978. pp. 86–88. [Google Scholar]
- Heiny JA, Jong D, Bryant SH, Conte Camerino D, Tortorella V. Enantiomeric effects on excitation-contraction coupling in frog skeletal muscle by a chiral phenoxy carboxylic acid. Biophys J. 1990;57:147–152. doi: 10.1016/S0006-3495(90)82515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Tanabe M, Kono K, Maruyama K, Ikemoto T, Endo M. Ca2+-releasing effect of cerivastatin on the sarcoplasmic reticulum of mouse and rat skeletal muscle fibers. J Pharmacol Sci. 2003;93:279–288. doi: 10.1254/jphs.93.279. [DOI] [PubMed] [Google Scholar]
- Jeck N, Waldegger P, Doroszewicz J, Seyberth H, Waldegger S. A common sequence variation of the CLCNKB gene strongly activates ClC-Kb chloride channel activity. Kidney Int. 2004;65:190–197. doi: 10.1111/j.1523-1755.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Chloride transport in the kidney: lesson from human disease and knockout mice. J Am Soc Nephrol. 2005;16:1549–1561. doi: 10.1681/ASN.2005020207. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin and fluvastatin in patients with hypercholesterolemia (the CURVES study) Am J Cardiol. 1998;81:582–587. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate+statin versus gemfibrozil+any statin. Am J Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- Kajinami K, Brousseau ME, Ordovas JM, Schaefer EJ. Polymorphisms in the multidrug resistance-1 (MDR1) gene influence the response to atorvastatin treatment in a gender-specific manner. Am J Cardiol. 2004;93:1046–1050. doi: 10.1016/j.amjcard.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Liantonio A, Pusch M, Picollo A, Guida P, De Luca A, Pierno S, et al. Investigations of pharmacologic properties of the renal CLC-K1 chloride channel co-expressed with barttin by the use of 2-(p-chlorophenoxy)propionic acid derivatives and other structurally unrelated chloride channels blockers. J Am Soc Nephrol. 2004;15:13–20. doi: 10.1097/01.asn.0000103226.28798.ea. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Murakami T, Yamazaki H, Izumi A, Saito Y, Hamakubo T, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J Atheroscler Thromb. 2005;12:121–131. doi: 10.5551/jat.12.121. [DOI] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Beck CL, George AL, Jr, Conte Camerino D. Aging-associated down-regulation of ClC-1 expression in skeletal muscle: phenotypic-independent relation to the decrease of chloride conductance. FEBS Lett. 1999b;449:12–16. doi: 10.1016/s0014-5793(99)00202-1. [DOI] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Desaphy J-F, Fraysse B, Liantonio A, Didonna MP, et al. Growth hormone secretagogues modulate the electrical and contractile properties of rat skeletal muscle through a ghrelin-specific receptor. Br J Pharmacol. 2003;139:575–584. doi: 10.1038/sj.bjp.0705284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Liantonio A, Camerino C, Conte Camerino D. Effects of HMG-CoA reductase inhibitors on exitation-contraction coupling of rat skeletal muscle. Eur J Pharmacol. 1999a;364:43–48. doi: 10.1016/s0014-2999(98)00817-6. [DOI] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Tricarico D, Ferrannini E, Conte T, D'Alò G, et al. Experimental evaluation of the effects of pravastatin on electrophysiological parameters of rat skeletal muscle. Pharmacol Toxicol. 1992;71:325–329. doi: 10.1111/j.1600-0773.1992.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Tricarico D, Roselli A, Natuzzi F, Ferrannini E, et al. Potential risk of myopathy by HMG-CoA reductase inhibitors: a comparison of pravastatin and simvastatin effects on membrane electrical properties of rat skeletal muscle fibres. J Pharmacol Exp Ther. 1995;275:1490–1496. [PubMed] [Google Scholar]
- Pierno S, Desaphy J-F, Liantonio A, De Bellis M, Bianco G, De Luca A, et al. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 2002;125:1510–1521. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Furberg CD, Ray WA, Weiss NS. Potential for conflict of interest in the evaluation of suspected adverse drug reactions: use of cerivastatin and risk of rhabdomyolysis. JAMA. 2004;292:2622–2631. doi: 10.1001/jama.292.21.2622. [DOI] [PubMed] [Google Scholar]
- Sacher J, Weigl L, Werner M, Szegedi C, Hohenegger M. Delineation of myotoxicity induced by 3-hydroxy-3-methylglutaryl CoA reductase inhibitors in human skeletal muscle cells. J Pharmacol Exp Ther. 2005;314:1032–1041. doi: 10.1124/jpet.105.086462. [DOI] [PubMed] [Google Scholar]
- Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca2+ signalling alteration in skeletal muscle. Biochem Biophys Res Commun. 2005;329:1067–1075. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- Smith PF, Eydelloth RS, Grossman SJ, Stubbs RJ, Schwartz MS, Germershausen JI, et al. HMG-CoA reductase inhibitor-induced myopathy in the rat: cyclosporine A interaction and mechanism studies. J Pharmacol Exp Ther. 1991;257:1226–1235. [PubMed] [Google Scholar]
- Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- Stein EA. The power of statins: aggressive lipid lowering. Clin Cardiol. 2003;26:25–31. doi: 10.1002/clc.4960261506. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development and fibre selectivity. Toxicol Pathol. 2005;33:246–257. doi: 10.1080/01926230590908213. [DOI] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]