Abstract
Background and purpose:
According to the two-domain model for the corticotropin-releasing factor receptor type 1 (CRF1), peptide antagonists bind to the N-terminal domain (N-domain), non-peptide antagonists to the transmembrane region (J-domain), whereas peptide agonists attach to both the N- and J-domain of the receptor to express activity. The aim of this study was to search for possible differences in the antagonism of the Gs- and Gi-protein coupling of CRF1 by a peptide (α-helical CRF(9–41)) and non-peptide antagonist (antalarmin), to determine whether the conformational requirements of the activated CRF1 states for Gs and Gi coupling are similar or different.
Experimental approach:
We studied the inhibitory effect of α-helical CRF(9–41) and antalarmin on the coupling of CRF1 to Gs- and Gi-protein in human embryonic kidney cells, using the [35S]-GTPγS binding stimulation assay.
Key results:
The non-peptide antagonized the receptor coupling to Gs competitively but that to Gi noncompetitively, and its antagonistic potency was different for urocortin- and sauvagine-evoked G-protein activation. In contrast, the peptide antagonist exhibited uniformly competitive antagonism.
Conclusions and Implications:
The results allow us to extend the two-domain model of CRF1 activation by assuming that CRF1 agonists activate the receptor by binding to at least two ensembles of J-domain configurations which couple to Gs or Gi, that are in turn antagonized by a non-peptide antagonist competitively and allosterically, respectively. It is further concluded that the allosteric mechanism of non-peptide antagonism is not valid for the Gs-mediated physiological activities of CRF1.
Keywords: CRF receptor 1, two-domain model, G-protein coupling, non-peptide antagonist
Introduction
Corticotropin-releasing factor (CRF) receptor type 1 (CRF1) is a class B G-protein-coupled receptor (GPCR) belonging to the secretin receptor family. It is activated by several peptide ligands and is thought to be the principal physiological mediator of stress responses (for a review, see Dautzenberg and Hauger, 2002; Hillhouse and Grammatopoulos, 2006). For the activation of the receptor by peptide ligands, a two-domain model has been established, in which the C-terminal part of the ligand binds to the extracellular N-terminal domain of the receptor (N-domain) and the N-terminal ligand part to the juxtamembrane region consisting of transmembrane regions and intervening loops (J-domain) (Nielsen et al., 2000; Assil et al., 2001; Hoare et al., 2003, 2004). Also, there is evidence that the C- and N-terminal portions of the peptides are functionally independent (Beyermann et al., 2000). Within this model, the N-domain of the receptor is required for ligand binding, whereas the J-domain is involved in the activation of the receptor evoking the intracellular signalling processes. Furthermore, whereas peptide antagonists bind only to the N-domain with high affinities, non-peptide antagonists bind only to the J-domain (Liaw et al., 1997; Hoare et al., 2003, 2004; Zhang et al., 2003), suggesting that the antagonism shown by the latter is at least partly allosteric rather than simply competitive. This has been confirmed in receptor binding studies (Hoare et al., 2003; Zhang et al., 2003) but not in functional assays (Zhang et al., 2003).
By measuring [35S]-GTPγS stimulation and the activity of the adenylyl cyclase, we recently found that the CRF1 expressed in human embryonic kidney (HEK) cells couples to Gs- and Gi-proteins that are differently desensitized and regulated by ligand and GTPγS concentrations. Correspondingly to a high-affinity ligand binding site, sauvagine and other peptide CRF1 ligands evoked high-potency activation of the Gs-protein and stimulation of adenylyl cyclase activity, whereas a low-affinity site corresponded to low-potency activation of Gi-proteins and low-potency inhibition of the cyclase (Wietfeld et al., 2004).
The two-domain model describes the general mechanism of ligand binding to CRF1, whereas our studies have been concerned with the regulation of the coupling of the receptor to different G-proteins. Therefore, it seemed to be important to determine whether our results on the G-protein coupling could be fitted into the two-domain model. For this reason, we studied the effects of peptide and non-peptide antagonists on the Gs and Gi coupling of the receptor and found that their different antagonizing activities can be described within the framework of the two-domain model.
Methods
Membranes from HEK293 cells stably expressing rat CRF1 and exhibiting Gs or Gi activity
HEK293 cells stably transfected with rat CRF1 (rCRF1) were used as described previously (Wietfeld et al., 2004) and designated as HEK-rCRF1 cells. Their membranes contained a high- and low-affinity [125I]-Tyr0-sauvagine binding site with Kd(h) 3.85 × 10−11 M and Kd(l) 1.47 × 10−8 M. Before preparation of membranes, the cells were pretreated with 100 ng ml−1 pertussis toxin (PTX), which abolished the activation of Gi-proteins through the low-affinity site, or with 0.1 μM sauvagine, which selectively desensitized the activation of Gs-protein through the high-affinity site. This allowed us to differentiate between the coupling of CRF1 to the two G-protein subclasses (Wietfeld et al., 2004).
Receptor/G-protein coupling estimated by binding of [35S]-GTPγS to HEK-rCRF1 cell membranes
The activation of G-proteins by CRF1 was measured by ligand-evoked stimulation of [35S]-GTPγS binding in HEK-rCRF1 cell membranes as described previously (Wietfeld et al., 2004). Briefly, about 5 μg of membranes exhibiting Gs or Gi activity were incubated in triplicate at 25°C with 125 pM [35S]-GTPγS in a medium consisting of Tris/HCl (50 mM, pH 7.4), 100 mM NaCl, 0.1 μM guanosine diphosphate, 10 mM MgCl2, 0.2 mM ethyleneglycol tetraacetate, 1 mg ml−1 bovine serum albumine and 0.15 mM bacitracin for 120 min. The reaction was terminated by filtration through Whatman GF/B filters using a Brandel harvester (Gaithersburg, MD, USA). Concentration–response curves for the stimulation of [35S]-GTPγS binding, induced by activation of the CRF receptor by the peptide ligands sauvagine and urocortin in the absence and presence of the peptide antagonist α-helical CRF(9–41) and the non-peptide antagonist antalarmin, were fitted by nonlinear regression using the programme PRISM 4 (GraphPad Software, San Diego, CA, USA). From the parameters obtained, the antagonistic constants Kb were calculated by constructing Schild plots for competitive antagonism, or by using the method of Gaddum for non-competitive antagonism. The specificity of the Gi and Gs responses to sauvagine in the absence and presence of antalarmin was further tested by immunoprecipitation of [35S]-GTPγS-bound Gαs and Gαi subunits, using the specific anti-G-protein antibodies mentioned under materials as described previously (Wietfeld et al., 2004). Briefly, after sauvagine had evoked stimulation of the [35S]-GTPγS binding in the absence and presence of antalarmin, the membranes were dissolved, incubated with the subunit-specific anti-G-protein antibodies and the [35S]-GTPγS-bound subunit antibody complexes were pelleted with protein A-Sepharose CL-4B and directly counted for 35S. The amount of radioactivity was corrected for the basal activity as observed in parallel incubations without sauvagine. This method was also used for determining the activation of Gαq.
Materials
The CRF1 peptide ligands, sauvagine, urocortin and α-helical CRF(9–41), were synthesized in our laboratory. The non-peptide antagonist antalarmin, PTX and protein A-Sepharose CL-4B were from Sigma (Taufkirchen, Germany). [35S]-GTPγS (1250 Ci mmol−1) was purchased from Perkin-Elmer Life Sciences (Boston, MA, USA). Three affinity-purified rabbit polyclonal anti-G-protein antibodies from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) were used: Gαs/olf (C-18), raised against a peptide mapping at the carboxy terminus of Gαs of rat origin; Gαi3 (C-10), raised against a peptide mapping at the carboxyl terminus of Gαi3 of rat origin, which reacts with Gαi3, Gαi1, and to a lesser extent with Gαi2 of mouse, rat, human and bovine origin; and Gαq/11 (C-19), raised against a peptide mapping within a domain common to Gαq and Gα11 of mouse origin, which reacts with Gαq and Gα11 of mammalian origin.
Data analysis
The quantitative data are expressed as mean±s.e., obtained from at least three independent experiments each performed in triplicate. Statistical analyses were performed by Student's t-test.
Results
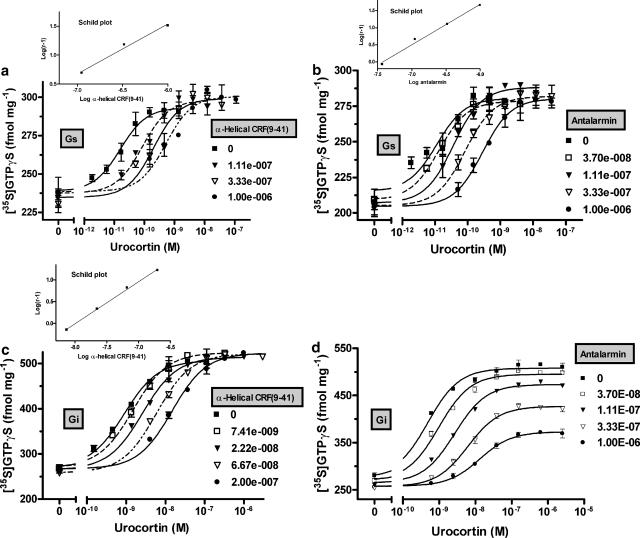
Concentration–response curves for Gs and Gi activation by CRF1, as measured by sauvagine-evoked stimulation of [35S]-GTPγS binding in HEK-rCRF1 cell membranes obtained from cells pretreated with PTX and sauvagine, respectively, resulted in EC50 values of 1.68 × 10−11 and 3.85 × 10−9 M (Figure 1a–d). These values confirmed the large difference between the potencies of ligand-stimulated Gs and Gi activation, as found previously (Wietfeld et al., 2004). The peptide antagonist α-helical CRF(9–41) shifted the Gs as well as Gi response curves induced by sauvagine to the right, in a competitive manner, with Kb values of 4.41 × 10−9 and 2.86 × 10−9 M, respectively, obtained by use of Schild analysis for competitive antagonism (Figure 1a and c). Similarly, the non-peptide antagonist antalarmin antagonized the activation of Gs competitively with a Kb of 9.05 × 10−9 M (Figure 1b). However, the Gi-induced response was non-competitively antagonized by antalarmin, which shifted the sauvagine concentration–response curves to the right (Figure 1d) and, at the same time, suppressed the maximum stimulation by sauvagine down to 28% (at 1 μM antalarmin; Figure 3). Gaddum analysis of this non-competitive antagonism produced a Kb of 7.95 × 10−9 M, a value close to that (9.05 × 10−9) obtained for antalarmin antagonizing the Gs response by competitive antagonism.
Figure 1.
Effect of the peptide antagonist α-helical CRF(9–41) and the non-peptide antagonist antalarmin on the concentration–response curves for sauvagine-stimulated binding of [35S]-GTPγS to HEK-rCRF1 cell membranes. To observe Gs- (a, b) and Gi-coupled activity selectively (c, d), cell membranes were obtained from HEK-rCRF1 cells pretreated with 100 ng ml−1 PTX for 24 h and with 0.1 μM sauvagine for 4 h, respectively. About 120 pM [35S]-GTPγS and 5–7 μg of membrane protein were incubated in binding medium (see Methods) with increasing concentrations of sauvagine in the absence and presence of fixed concentrations of α-helical CRF(9–41) (a, c) or antalarmin (b, d) at 25°C for 2 h. The data shown represent a single experiment and are given as means±s.d. from triplicate incubations. They were fitted by nonlinear regression and from the EC50 values obtained, Schild plots were drawn, when competitive antagonism was observed (insets in a, b, and c).
Figure 3.
Maximum stimulation of sauvagine- and urocortin-stimulated [35S]-GTPγS binding to HEK-rCRF1 cell membranes in the presence of the non-peptide antagonist antalarmin. Data for Gs and Gi activity were estimated from concentration–response curves, as shown in Figures 1 and 2. The data points were normalized with the maximum response in the absence of antagonist taken as 100% (means±s.d.).
Analogous to activation by sauvagine, activation of Gs and Gi by urocortin was competitively antagonized by α-helical CRF(9–41) with similar Kb values of 1.31 × 10−8 and 1.04 × 10−8 M, respectively (Figure 2a and c). Furthermore, the competitive and non-competitive nature of the antagonism of, respectively, Gs and Gi activation by antalarmin was also observed when urocortin was used as a stimulus (Figure 2b and d). However, compared to activation by sauvagine, antalarmin and, to a lesser extent, α-helical CRF(9–41) were weaker at antagonizing the urocortin-stimulated responses. The Schild constant Kb of antalarmin for antagonizing Gs activity was, at 8.33 × 10−8 M, nearly 10-fold higher than that for the sauvagine-evoked response, and higher concentrations of antalarmin were needed to suppress Gi activation by urocortin compared to that by sauvagine (Figure 3). A summary of the constants characterizing the potencies of the stimulating ligands and the antagonists is given in Table 1.
Figure 2.
Effect of the peptide antagonist α-helical CRF(9–41) and the non-peptide antagonist antalarmin on the concentration–response curves for urocortin-stimulated binding of [35S]-GTPγS to HEK-rCRF1 cell membranes selectively expressing Gs (a, b) and Gi-coupled activity (c, d). Experimental conditions were exactly the same as given for sauvagine-stimulated binding in Figure 1.
Table 1.
Inhibition of [35S]-GTPγS binding stimulation in HEK-rCRF1 cell membranes by the peptide antagonist α-helical CRF(9-41) and the non-peptide antagonist antalarmin
| Stimulating ligand | Activity | Stimulation | Antagonism by α-helical CRF(9-41) | Antagonism by antalarmin |
|---|---|---|---|---|
| EC50 (M) (pEC50) | Kb (M) (pKb) | Kb (M) (pKb) | ||
| Sauvagine | Gs | 1.68 × 10−11 (10.78±0.05) | 4.41 × 10−9 a (8.36±0.09) | 9.05 × 10−9 a (8.04±0.04) |
| Gi | 3.85 × 10−9 (8.41±0.05) | 2.86 × 10−9 a (8.54±0.03) | 7.95 × 10−9 b ±7.85 × 10−10 | |
| Urocortin | Gs | 1.11 × 10−11 (10.96±0.06) | 1.31 × 10−8 a (7.88±0.10) | 8.33 × 10−8 a (7.08±0.09) |
| Gi | 4.90 × 10−10 (9.31±0.06) | 1.04 × 10−8 a (7.98±0.01) | 2.85 × 10−8 b ±1.91 × 10−9 |
The results are from concentration–response curves of sauvagine- and urocortin-evoked binding of [35S]-GTPγS to HEK-rCRF1 cell membranes at conditions selectively representing activation of Gs- or Gi-proteins in the absence and presence of the antagonists. As shown in Figures 1 and 2, EC50 values for the stimulation by sauvagine and urocortin and Kb values for the antagonists were calculated. The constants and their pK values are presented as means±s.e.
Between sauvagine and urocortin, the Schild pKb and Gaddum Kb values of antalarmin were significantly different (P<0.001), as were the Schild pKb values of α-helical CRF(9-41) (P<0.05).
Schild analysis for competitive antagonism.
Gaddum analysis for non-competitive antagonism.
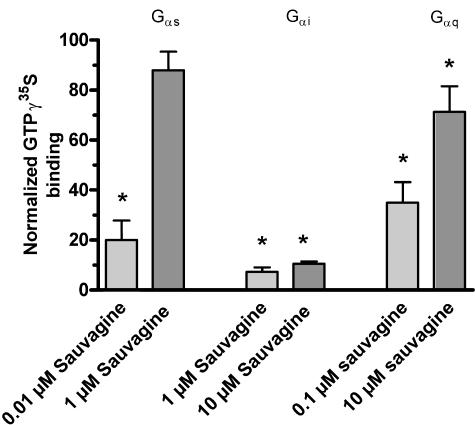
Specificity and differences in the antagonism by antalarmin of the Gs and Gi responses to sauvagine were further confirmed by immunoprecipitation of the [35S]-GTPγS-bound Gαs and Gαi subunits. Figure 4 shows that 1 μM antalarmin decreased the amount of Gαs subunits activated by 0.01 μM sauvagine by 80% and that this decrease was competitively overcome by 1 μM sauvagine. In contrast, a 10-fold increase in sauvagine concentration did not change the nearly complete inhibition (93%), induced by antalarmin, of the response mediated by Gαi activation by 1 μM sauvagine, confirming the non-competitive nature of the antagonism of Gi activation by antalarmin.
Figure 4.
Effect of antalarmin on immunoprecipitation of sauvagine-stimulated [35S]-GTPγS-bound Gαi, Gαs and Gαq subunits in membranes obtained from HEK-rCRF1 cells. The membranes (40 μg) were incubated with 0.4 nM [35S]-GTPγS in the absence (basal) and in the presence of sauvagine (stimulated) with and without 1 μM antalarmin. The Gαi-, Gαs- and Gαq-[35S]-GTPγS complexes were immunoprecipitated using antibodies directed against the Gα subunits, and the [35S]-GTPγS activities in the precipitates were directly counted. The data shown are the responses to sauvagine in the presence of antalarmin as percentage of those in absence of antalarmin. Data are given as mean±s.e. of at least four experiments carried out in triplicate. Statistically significant changes in sauvagine-evoked [35S]-GTPγS binding caused by antalarmin are indicated as *P<0.05.
As shown previously (Wietfeld et al., 2004), the Gq activation could only be studied with the immunoprecipitation assay, not by use of the binding assay. Antalarmin inhibited the stimulation of [35S]-GTPγS-bound Gαq by 0.1 μM sauvagine by 65%, and this inhibition was decreased to 29% when sauvagine was increased to 10 μM (Figure 4). This could indicate that the Gq-mediated response cannot be totally antagonized by antalarmin. However, because of the low potency of sauvagine at activating Gq, it was not possible to find out if the residual inhibition of 29% was further abolished by higher ligand concentrations.
Discussion
It has been shown that different receptor domains are responsible for the binding of peptide and non-peptide antagonists (Liaw et al., 1997; Hoare et al., 2004) and that non-peptide antagonists only partially inhibit peptide ligand binding to CRF1, with mutual, weak negative cooperativity occurring between their binding (Hoare et al., 2003, 2004; Zhang et al., 2003). Nevertheless, in functional assays, for example, CRF-mediated adrenocorticoid hormone release from rat pituitary cells and cyclic AMP accumulation in HEK cells expressing CRF1, the non-peptide antagonists, like the peptide antagonists, have been shown to exhibit competitive antagonism (Zhang et al., 2003; Hoare et al., 2004). The present results provide a possible solution to this discrepancy by taking into account different G-protein-coupling states of the receptor.
In contrast to the peptide antagonist α-helical CRF(9–41), the non-peptide antagonist antalarmin exhibited competitive antagonism only towards the activation of Gs, but strongly antagonized Gi activation non-competitively in HEK-rCRF1 cell membranes (Figures 1 and 2). The decrease in the efficacy of Gi activation by antalarmin implicates an allosteric mechanism in the non-peptide antagonism and supports similar conclusions obtained from the above-mentioned studies. However, the present results restrict the putative allosteric mechanism of non-peptide antagonism to the activation of Gi. Because functional responses to the activation of CRF1 are generally the result of activation of adenylate cyclase, that is, Gs activation, they should also be competitively antagonized by non-peptide antagonists, as observed previously (Zhang et al., 2003; Hoare et al., 2004). For this reason, the non-peptide CRF1 antagonists presently available may prove not to possess the therapeutic advantages allosteric GPCR ligands are thought to have over classic orthosteric ligands (Christopoulos and Kenakin, 2002; Soudijn et al., 2002; Jensen and Spalding, 2004). At present, it is not clear if the low expression of the receptor in vivo allows any effective Gi coupling, which would restrict the magnitude of Gs activation. Wietfeld et al. (2004) have shown that the Gi response of the CRF1 in HEK-rCRF1 cell membranes is associated with a low-affinity binding site, as opposed to a high-affinity site responsible for Gs activation. Conclusively the Gi-coupled binding site is not accessible to competition by non-peptide antagonists and this would explain their partly allosteric behaviour in receptor binding studies (Hoare et al., 2003, 2004; Zhang et al., 2003).
The finding that CRF1 couples to Gs-, Gi-, and Gq-proteins (Grammatopoulos et al., 1999, 2000; Wietfeld et al., 2004) suggests that different active receptor states, or conformation ensembles, are responsible for the different couplings, or alternatively that the affinities of the different G-proteins to a common activated receptor conformation ensembles differ (Kenakin, 2002). The present results favour the hypothesis that different conformations of the CRF1 J-domain are responsible for the coupling of the receptor to Gs and Gi. This conclusion can be drawn from the two-domain model for activation of CRF1, which was established on the basis of numerous findings (Nielsen et al., 2000; Assil et al., 2001; Hoare et al., 2003, 2004). According to this model, peptide ligands, agonists as well as antagonists, bind primarily to the N-terminus of the receptor, whereas non-peptide antagonists bind to the J-domain. As α-helical CRF(9–41) invariably inhibited Gs as well as Gi activation competitively, it is concluded that at the level of the N-domain, antagonists are not able to discriminate between Gs and Gi activation. Further contact by the peptide agonists with the J-domain was shown to be necessary for the receptor to become activated, which means to couple to G-proteins. If the same activated receptor J-domain conformations were responsible for the Gs as well as Gi coupling, a non-peptide like antalarmin should antagonize both couplings by the same competitive or non-competitive mechanism; this was not observed. With respect to the Gq coupling, the type of antagonism induced by antalarmin cannot be determined from the present results, as mentioned above. However, if the Gq coupling is also non-competitively antagonized by antalarmin, it will only be partly involved, because most of the activity inhibited by antalarmin was restored by the addition of a higher concentration of sauvagine (Figure 4).
Urocortin-stimulated Gs and Gi activation was only weakly antagonized by antalarmin compared to the sauvagine-stimulated activities (Table 1, Figure 3). These results parallel those on the neurokinin 1 receptor, where a non-peptide antagonist differently blocked the responses to the agonists substance P and septide (Pradier et al., 1994). Furthermore, urocortin and CRF were shown to regulate the G-protein receptor kinase 3 activity differently in human retinoblastoma Y79 cells (Dautzenberg et al., 2002) and the Gq-protein-mediated mitogen-activated protein kinase signal-transduction pathway in human pregnant myometrium and transfected cells (Grammatopoulos et al., 2000). This suggests that the J-domain conformations are not exactly the same for all CRF1 agonists. Furthermore, the α-helical CRF(9–41) antagonist was also less potent at antagonizing the urocortin-stimulated G-protein activation than that evoked by sauvagine (Table 1), which complicates the mechanism even more.
The present results suggesting that Gs and Gi protein activation by the CRF1 is accomplished through different conformations of the receptor extends the two-domain model describing the activation of the CRF1 (Nielsen et al., 2000; Assil et al., 2001; Hoare et al., 2003, 2004). With rare exceptions (Reinhart et al., 2004), it is the transmembrane domain of the receptor that forms the major binding sites for non-peptide ligands of the GPCRs whose binding domains have been characterized so far, whether the natural ligands are small molecules or peptides (for a review, see Strader et al., 1994). In addition, the present findings show that non-peptide ligands may also differentially influence different signalling chains evoked by the receptor, which could have important implications for the development of non-peptide drugs.
Acknowledgments
We thank M Georgi for technical assistance.
Abbreviations
- CRF
corticotropin-releasing factor
- CRF1
CRF receptor type 1
- GPCR
G-protein-coupled receptor
- HEK
human embryonic kidney
- HEK-rCRF1 cells
HEK293 cells stably transfected with rCRF1
- Kd(h) and Kd(l)
high- and low-affinity Kd, respectively
- PTX
pertussis toxin
- rCRF1
rat CRF1
Conflict of interest
The authors state no conflict of interest.
References
- Assil IQ, Qi L, Arai M, Shomali M, Abou SA. Juxtamembrane region of the amino terminus of the corticotropin releasing factor receptor type 1 is important for ligand interaction. Biochemistry. 2001;40:1187–1195. doi: 10.1021/bi001758y. [DOI] [PubMed] [Google Scholar]
- Beyermann M, Rothemund S, Heinrich N, Fechner K, Furkert J, Dathe M, et al. A role for a helical connector between two receptor binding sites of a long-chain peptide hormone. J Biol Chem. 2000;275:5702–5709. doi: 10.1074/jbc.275.8.5702. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochem Biophys Res Commun. 2002;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Dai YL, Randeva HS, Levine MA, Karteris E, Easton AJ, et al. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–2202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hilhouse EW. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1 alpha and R2 beta CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol. 2000;14:2076–2091. doi: 10.1210/mend.14.12.0574. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Ling N, Crowe PD, Grigoriadis DE. Mechanism of corticotropin-releasing factor type I receptor regulation by nonpeptide antagonists. Mol Pharmacol. 2003;63:751–765. doi: 10.1124/mol.63.3.751. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Schwarz DA, Ling N, Vale WW, Crowe PD, et al. Ligand affinity for amino-terminal and juxtamembrane domains of the corticotropin releasing factor type I receptor: regulation by G protein and nonpeptide antagonists. Biochemistry. 2004;43:3996–4011. doi: 10.1021/bi036110a. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. Allosteric modulation of G protein coupled receptors. Eur J Pharm Sci. 2004;21:407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy at G protein-coupled receptors. Nat Rev Drug Discov. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- Liaw CW, Grigoriadis DE, Lorang MT, de Souza EB, Maki RA. Localization of agonist- and antagonist-binding domains of human corticotropin-releasing factor receptors. Mol Endocrinol. 1997;11:2048–2053. doi: 10.1210/mend.11.13.0034. [DOI] [PubMed] [Google Scholar]
- Nielsen SM, Nielsen LZ, Hjorth SA, Perrin MH, Vale WW. Constitutive activation of tethered-peptide/corticotropin-releasing factor receptor chimeras. Proc Natl Acad Sci USA. 2000;97:10277–10281. doi: 10.1073/pnas.97.18.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradier L, Menager J, Le-Guern J, Bock M-D, Heuillet E, Fardin V, et al. Septide: an agonist for the NK1 receptor acting at a site distinct from substance P. Mol Pharmacol. 1994;45:287–293. [PubMed] [Google Scholar]
- Reinhart GJ, Xie Q, Liu XJ, Zhu YF, Fan J, Chen C, et al. Species selectivity of nonpeptide antagonists of the gonadotropin-releasing hormone receptor is determined by residues in extracellular loops II and III and the amino terminus. J Biol Chem. 2004;279:34115–34122. doi: 10.1074/jbc.M404474200. [DOI] [PubMed] [Google Scholar]
- Soudijn W, van-Wijngaarden I, Ijzerman AP. Allosteric modulation of G protein-coupled receptors. Curr Opin Drug Discov Dev. 2002;5:749–755. [PubMed] [Google Scholar]
- Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- Wietfeld D, Heinrich N, Furkert J, Fechner K, Beyermann M, Bienert M, et al. Regulation of the coupling to different G proteins of rat corticotropin-releasing factor receptor type 1 in human embryonic kidney 293 cells. J Biol Chem. 2004;279:38386–38394. doi: 10.1074/jbc.M405335200. [DOI] [PubMed] [Google Scholar]
- Zhang G, Huang N, Li YW, Qi X, Marshall AP, Yan XX, et al. Pharmacological characterization of a novel nonpeptide antagonist radioligand, (+/−)-N-[2-methyl-4-methoxyphenyl]-1-(1-(methoxymethyl) propyl)-6-methyl-1H-1,2,3-triazolo[4,5-c]pyridin-4-amine ([3H]SN003) for corticotropin-releasing factor1 receptors. J Pharmacol Exp Ther. 2003;305:57–69. doi: 10.1124/jpet.102.046128. [DOI] [PubMed] [Google Scholar]