Abstract
Background and purpose:
Recent evidence suggests that 5-HT2C receptor activation may inhibit midbrain 5-HT neurones by activating neighbouring GABA neurones. This hypothesis was tested using the putative selective 5-HT2C receptor agonist, WAY 161503.
Experimental approach:
The effect of WAY 161503 on 5-HT cell firing in the dorsal raphe nucleus (DRN) was investigated in anaesthetised rats using single unit extracellular recordings. The effect of WAY 161503 on DRN GABA neurones was investigated using double label immunohistochemical measurements of Fos, glutamate decarboxylase (GAD) and 5-HT2C receptors. Finally, drug occupancy at 5-HT2A receptors was investigated using rat positron emission tomography and ex vivo binding studies with the 5-HT2A receptor radioligand [11C]MDL 100907.
Key results:
WAY 161503 caused a dose-related inhibition of 5-HT cell firing which was reversed by the 5-HT2 receptor antagonist ritanserin and the 5-HT2C receptor antagonist SB 242084 but not by the 5-HT1A receptor antagonist WAY 100635. SB 242084 pretreatment also prevented the response to WAY 161503. The blocking effects of SB 242084 likely involved 5-HT2C receptors because the drug did not demonstrate 5-HT2A receptor occupancy in vivo or ex vivo. The inhibition of 5-HT cell firing induced by WAY 161503 was partially reversed by the GABAA receptor antagonist picrotoxin. Also, WAY 161503 increased Fos expression in GAD positive DRN neurones and DRN GAD positive neurones expressed 5-HT2C receptor immunoreactivity.
Conclusions and implications:
These findings indicate that WAY 161503 inhibits 5-HT cell firing in the DRN in vivo, and support a mechanism involving 5-HT2C receptor-mediated activation of DRN GABA neurones.
Keywords: 5-hydroxytryptamine, 5-HT2C receptor, dorsal raphe nucleus, WAY 161503, SB 242084, [11C]MDL 100907
Introduction
Feedback regulation is an essential aspect of the physiology of central 5-hydroxytryptamine (5-HT, serotonin) neurones (Aghajanian, 1978), and the roles of presynaptic 5-HT autoreceptors are well established. Thus, somatodendritic 5-HT1A autoreceptors inhibit the firing of 5-HT neurones in the dorsal raphe nucleus (DRN), while terminal 5-HT1B autoreceptors inhibit 5-HT release (Barnes and Sharp, 1999).
In addition to 5-HT autoreceptors, recent evidence suggests that postsynaptic 5-HT receptors located on afferent inputs to 5-HT neurones are also involved in 5-HT feedback control. For example, in rats cortical lesions attenuate the inhibitory effect of 5-HT1A receptor agonists on the firing of DRN 5-HT neurones, suggesting the involvement of post-synaptic 5-HT1A receptors (Ceci et al., 1994; Hajós et al., 1999). Also, the inhibition of 5-HT cell firing by 5-HT1A receptor agonists persists following the local inactivation of somatodendritic 5-HT1A autoreceptors (Martin-Ruiz and Ugedo, 2001b).
A role for postsynaptic 5-HT2 receptors in 5-HT feedback control is evident in in vivo electrophysiological findings that 5-HT2 receptor agonists, including (±)-2,5 dimethoxy-4-iodoamphetamine (DOI), inhibit the firing of DRN 5-HT neurones (Aghajanian et al., 1970; Garratt et al., 1991). A recent pharmacological analysis of this effect of DOI suggested a prominent role for the 5-HT2A receptor subtype, but an involvement of 5-HT2B/C receptors was also indicated (Boothman et al., 2003). Accordingly, in vitro electrophysiological findings also emphasize the importance of the 5-HT2A over the 5-HT2C receptor subtype in the induction of inhibitory post-synaptic potentials (IPSPs) in DRN 5-HT neurones by 5-HT and DOI (Liu et al., 2000). However, these studies may under-estimate the importance of the 5-HT2C receptor subtype due to the limited selectivity of the drugs used, and in particular the lack of a selective 5-HT2C receptor agonist.
The neuroanatomical substrates which mediate 5-HT2 receptor agonist-induced inhibition of 5-HT cell firing are unknown but 5-HT neuronal afferents are implicated because 5-HT2 receptors are not expressed by 5-HT neurones (Clemett et al., 2000; Cornea-Hebert et al., 1999). One candidate neuronal substrate is DRN gamma amino-butyric acid (GABA) neurones which synapse onto 5-HT neurones and exert a powerful inhibitory effect (Wang et al., 1992; Varga et al., 2001). Moreover, DRN GABA neurones are the target of several important DRN afferents from the forebrain (Varga et al., 2001; Jankowski and Sesack, 2004). Interestingly, recent immunohistochemical data indicate that DOI administration increases expression of the immediate early gene c-fos in DRN GABA neurones (Boothman and Sharp, 2005a), and the presence of 5-HT2C receptor mRNA in DRN GABA neurones was recently reported (Serrats et al., 2005).
This study investigated the role of 5-HT2C receptors in the regulation of 5-HT cell firing, using the putative selective 5-HT2C receptor agonist, WAY 161503 (Cryan and Lucki, 2000; Rosenzweig-Lipson et al., 2006) and the 5-HT2C receptor antagonist SB 242084 (Kennett et al., 1997). The possible confounding factor of SB 242084 occupancy at 5-HT2A receptors in vivo was studied using positron emission tomography (PET) scanning and ex vivo binding (Hirani et al., 2003). Drug effects on DRN GABA neurones were examined using a combined electrophysiological and Fos immunohistochemical approach. Results indicate that WAY 161503 inhibits 5-HT cell firing in the DRN in vivo, and support a mechanism involving 5-HT2C receptor-mediated activation of DRN GABA neurones. A preliminary account of these experiments was presented to the British Pharmacological Society (Boothman et al., 2005b; Raley et al., 2005).
Methods
Animals
Experiments were carried out in accordance with the Animals (Scientific Procedures) Act (1986) and a local ethical review process. Male Sprague–Dawley rats (220–320 g; Harlan Olac, Bicester, UK) were group housed (5–6) under conditions of constant temperature (21±1°C) and humidity under a 24 h light–dark cycle (lights on 0800–2000) with food and water freely available. Before immunocytochemical experiments, rats were handled daily for 3–5 days and familiarized with the testing room to minimize stress.
Electrophysiological recording of 5-HT neuronal activity
Rats were anaesthetized with chloral hydrate (460 mg kg−1 i.p. with additional doses as required), supplemented during surgery with saffan (1.2 mg kg−1 i.v.), and maintained at 36°C using a thermoregulated blanket. Extracellular single-unit recordings were made as described previously (Boothman et al., 2003). Single barrel glass electrodes (2 M NaCl, 2% pontamine sky blue; 6–20 MΩ) were stereotactically implanted into the DRN (coordinates relative to Bregma and the dural surface of A/P −7.5 mm, L/M 0.0 mm D/V −4.5 to −5.5 mm, Paxinos and Watson, 1986). Single-unit potentials were amplified and filtered (Gain 1 k; 500 Hz–1.5 kHz band pass; Neurolog system, Digitimer Ltd), captured using a 1401plus interface, and analysed offline using Spike2 software.
The firing properties of DRN neurones fulfilled three or more of the following criteria which are characteristic of 5-HT neurones (Hajós et al., 1995; Allers and Sharp, 2003): slow firing rate (0.2–2 Hz), regular firing pattern (typical coefficient of variation <0.5), triphasic extracellular waveform with a wide spike width (>1.5 ms) and an inhibitory response to the 5-HT1A receptor agonist 8-OH-DPAT (10 μg kg−1 i.v.). Most 5-HT neurones discharged single spikes, but a small number that discharged both single spikes and spikes in short bursts (Hajós et al., 1995) were included.
After 5 min baseline recording, drugs were injected via a lateral tail vein. Rats (n=6–8/group) received WAY 161503 (0.125, 0.25, 0.5 and 1.0 mg kg−1 at 2 min intervals) either alone, or following pre-treatment with SB 242084 (1 mg kg−1). In separate experiments, the following antagonists were administered after WAY 161503: SB 242084 (5-HT2C; 0.5 mg kg−1), ritanserin (5-HT2; 1 mg kg−1), WAY 100635 (5-HT1A; 0.1 mg kg−1) or picrotoxin (GABAA; 0.5–2.0 mg kg−1). At the end of some experiments, 8-OH-DPAT (10 μg kg−1 i.v.) was administered followed by the 5-HT1A receptor antagonist WAY 100635 (0.1 mg kg−1). Finally, dye was expelled by iontophoresis (−3.6 mA pulses, 200 ms duration, 21 ms interpulse interval, 30 min) to allow histological identification of the recording site.
Firing rates were quantified in the final min of each baseline and post-drug period. Regularity of firing was measured by coefficient of variation analysis (COV) of the inter-spike interval (s.d. inter-spike interval/inter-spike interval mean). Neurones discharging spikes in short bursts were analysed using the first spike of each burst.
In vivo and ex vivo binding of [11C]MDL 100907
Rat PET scanning
Rat PET scanning was carried using the high-resolution quad-HIDAC (high-density avalanche chamber) system (Jeavons et al., 1999) as described previously (Hume et al., 2001; Hirani et al., 2003). In brief, rats were maintained under isoflurane anaesthesia with N2O/O2 and positioned in the centre of the field of view of the scanner using a perspex stereotaxic frame. The 5-HT2A receptor radioligand [11C]MDL 100907 (∼10 MBq) was then injected via a tail vein catheter in either drug naïve rats (n=5) or rats pre-treated with SB 242084 (1 mg kg−1 i.v., n=4), or MDL 100907 (0.2 or 0.4 mg kg−1 i.v., n=4) 5 min before radioligand injection. Each rat was scanned for 60 min with data acquired in list-mode.
For data acquisition, quad-HIDAC sinograms were reconstructed into 0.5 mm cubic voxels with the Hamming filter at a cut-off of 0.6. As the current quad-HIDAC scanner technology does not enable quantitative determination of the full dynamics of delivery and development of specific signal, data acquired during a 40 min time frame (20–60 min after radioligand injection) were chosen to represent a compromise between time to reach secular equilibrium and increasing noise in data. Data were transferred into ANALYZE-AVW imaging software and a standard volume of interest (VOI) template was projected onto each volume. Eight VOIs were sampled, including frontal with cingulate cortex (452 voxels), striatum (404 voxels), hippocampus (264 voxels) and cerebellum (576 voxels). As the number of 5-HT2A receptors in the cerebellum are negligible (Lopez-Gimenez et al., 1997), data were expressed relative to cerebellar VOI counts, to give a measure of total:non-specific binding.
Ex vivo measurement of tissue [11C]radioactivity
After PET scanning, rats were administered Euthatal and post-mortem brains were rapidly dissected into regions corresponding to those sampled by the quad-HIDAC (frontal cortex, striatum, hippocampus, cerebellum, olfactory bulbs, hypothalamus/thalamus, superior colliculi and medulla). Extracerebral tissues surrounding the head and within the scanner field of view were also sampled (muscle, skin, submaxillary and lachrymal glands). Carbon-11 radioactivity was measured in whole tissue samples as described previously, using a Wallac gamma-counter, with automatic correction for radioactive decay. Results were normalized to account for radioactivity injected and body weight (‘uptake units'=(c.p.m./g tissue)/(injected c.p.m./g body weight)). For brain regions, tissue:cerebellum ratios were calculated to give a measure of specific binding (total:non-specific).
Immunohistochemistry
Pilot experiments (n=3 rats/group) tested the effect of 1, 3 and 10 mg kg−1 WAY 161503 compared to saline vehicle. In the main experiments (n=6 rats/group), rats received two i.p. injections 30 min apart as follows: (i) vehicle–vehicle, (ii) vehicle–WAY 161503 (3 mg kg−1), (iii) SB 242084 (1 mg kg−1)–vehicle or (iv) SB 242084 (1 mg kg−1)–WAY 161503 (3 mg kg−1). At 2 h after the last injection rats were anaesthetized with pentobarbital (300 mg kg−1 i.p.), perfused with 200 ml 0.9 % saline followed by 200 ml fixative (4% paraformaldehyde in 0.1 M sodium phosphate buffer with 0.4% picric acid), and brains were post-fixed overnight (4°C). Free-floating sections (40 μm) were cut on a vibratome and stored at 4°C before further use.
Fos/GAD double labelling
Sections were incubated in hydrogen peroxide (0.3%, 10 min), washed in phosphate-buffered saline (PBS: 140 mM NaCl, 30 mM KCl, 80 mM Na2HPO4, 15 mM KH2PO4, in distilled water), and then treated (30 min) with standard blocking serum (10% normal goat serum, in PBS with 0.3% Triton), prior to overnight incubation (4°C) in rabbit anti-glutamate decarboxylase (GAD)65/67 antibody (Chemicon, Hampshire, UK, AB 5992, 1:2000 dilution). Sections were then washed (PBS) and incubated in biotinylated secondary antibody (1:500 dilution, 2 h). GAD65/67 immunoreactivity was visualized using a chromagen reaction to give a brown product (Vectastain ABC elite and DAB kit, Vector, Burlinghame, CA, USA). Sections were again washed (PBS), before incubation (72 h at 4°C) with rabbit anti-Fos antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, sc-253, 1:2000 dilution) followed by biotinylated secondary antibody (goat anti-rabbit, Vector BA-1000 1:500 dilution, 2 h). Fos immunoreactivity was visualized using a chromagen reaction giving a dark-blue product (Vectastain ABC elite and SG kit, Vector).
5-HT2C receptor and GAD65/67 or Fos double labelling
Sections were treated with blocking serum before incubation at 4°C in mouse anti-5-HT2C receptor antibody (Pharmigen 556335, 1:200 dilution, overnight, see Bubar et al., 2005) together with rabbit anti-GAD65/67 antibody (1:2000 dilution, overnight), or in rabbit anti-Fos antibody (1:2000 dilution, 60 h) with the subsequent addition of mouse anti-5-HT2C receptor antibody (1:200 dilution, overnight). Sections were washed (PBS) and then incubated with a green fluorescent goat anti-rabbit antibody (Alexa Fluor-488, InVitrogen, Carlsbad, CA, USA, A-11034, 1:500 dilution, 1 h) before further incubation in a red fluorescent goat anti-mouse antibody (Alexa Fluo--568, InVitrogen A-11004, 1:250 dilution, 2 h).
Cell counting and image collection
Counts of GAD65/67/Fos double-labelled cells were made in a defined area of the DRN (250 × 170 μm grid in eyepiece of × 40 objective: Lietz Diaplan light microscope) by an operator blind to treatment. Counts were made bilaterally on six sections per animal. Bright-field images were captured using a colour video camera (Sony) and image software (Scion Image Software, version 1.62c).
5-HT2C/Fos and 5-HT2C/GAD65/67 double-labelled cells were visualized using fluorescence microscopy. Fluorescent images captured using a digital camera (Xillix microimager, Richmond BC, Canada) with the application of false colour (Openlab software, version 3.0.2).
Drugs and materials
The drugs used (with supplier) were as follows: WAY 161503 (8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5(6 H)-one; Tocris, UK), SB 242084 (6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy) pyridin-3-yl carbamoyl] indoline; Eli Lilly & Co, UK), ritanserin (Janssen Phamaceuticals, Belgium), MDL 100907 (R-(+)-(2,3-dimethoxyphenil)-1-[2-(4-flurophenylethyl)]-4-piperidone – methanol; ABX advanced biochemical compounds, Germany), WAY 100635 (n-[2-[4-(2-methoxyphenyl)-1-piperazinylethyl]-n-(2-pyridinyl) cyclohexane carboxamide trihydrochloride; Wyeth Pharmaceuticals, UK), 8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)-tetralin; Sigma-Aldrich, UK), picrotoxin (Sigma-Aldrich, UK). Drugs were dissolved in deionized water except SB 242084 (10% cyclodextrin in 25 mM citric acid) and ritanserin and MDL 100907 (few drops of glacial acetic acid and 5% glucose).
[11C]MDL 100907 was prepared by Hammersmith Imanet radiochemistry according to published methods (Lundkvist et al., 1996). The radioligand had a purity of ∼99%, specific activity (at time of injection) of ∼45 GBq μmol−1 and the dose of MDL 100907 associated with ∼10 MBq injection was estimated to be 1.3 nmol kg−1.
Statistical analysis
Electrophysiological data were analysed using one-way ANOVA with Dunnett's post hoc tests (for the effect of agonists alone), two-way ANOVA with Bonferroni post hoc tests (for the effect of antagonist pretreatment) and Student's two-tailed paired t-test (effect of antagonists alone). [11C]MDL 100907 binding data were analysed on a region-by-region basis using Student's unpaired t-test (two-tailed). Cell count data were analysed using one-way ANOVA followed post hoc with either Dunnett's t-test (multiple group comparison) or Bonferroni's test (between group comparison). P-values of ⩽0.05 were considered statistically significant.
Results
Electrophysiological characteristics of DRN neurones
Presumed 5-HT neurones in the DRN (n=27) fired broad triphasic spikes (waveform length 2.01±0.06 ms, range 1.5–2.7 ms), in a slow and regular firing pattern (baseline firing rate 0.91±0.07 Hz, range 0.32–1.74 Hz; baseline COV 0.26±0.03, range 0.18–0.4). There was no significant difference in baseline firing rate or COV between treatment groups. The baseline firing rate of these neurones is constant over 20–30 min following an initial 2–3 min stabilization period, and administration of the vehicles used in the current study (deionized water, 10% cyclodextrin in 25 mM citric acid, dilute glacial acetic acid in 5% glucose) have no significant effect firing rate or regularity.
Effect of WAY 161503 on 5-HT cell firing
Systemic administration of the putative 5-HT2C receptor agonist WAY 161503 (0.125–0.5 mg kg−1 i.v.) caused a dose-related inhibition of 5-HT cell firing compared to pre-drug values. This effect was apparent at 0.125 mg kg−1, and the highest dose tested reduced firing to 21% of pre-drug values (Figures 1a and 2). WAY 161503 (0.125–0.5 mg kg−1 i.v.) had no significant effect on the regularity of 5-HT cell firing (data not shown).
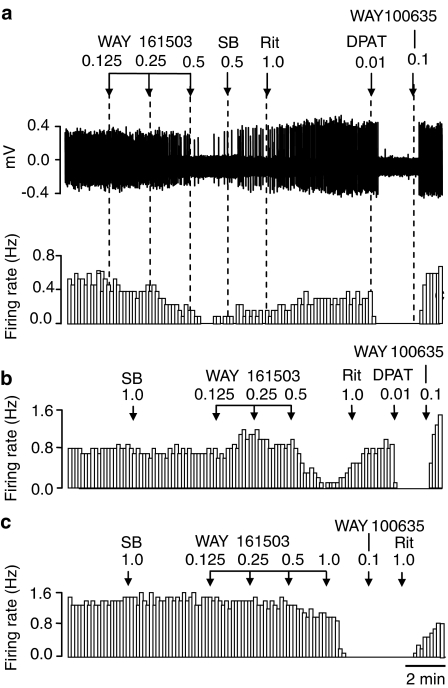
Figure 1.
Spike train (upper trace) and rate-meter recordings showing the inhibitory effect of the putative 5-HT2C receptor agonist WAY 161503 on the firing rate of individual DRN 5-HT neurones in the anaesthetized rat. WAY 161503 produced a dose-related inhibition of 5-HT cell firing (a), which was attenuated by pretreatment with the 5-HT2C receptor antagonist SB 242084 (b, c). The effect of WAY 161503 was reversed by both SB 242084 (a) and the 5-HT2 receptor antagonist ritanserin (a, b), but not by the 5-HT1A receptor antagonist WAY 100635 (c). Note also the characteristic inhibitory response of 5-HT neurones to the 5-HT1A receptor agonist 8-OH-DPAT, which was reversed by the 5-HT1A receptor antagonist WAY 100635 (a, b). Drug administration (mg kg−1 i.v.) as indicated by arrows. Abbreviations: 8-OH-DPAT (DPAT), SB 242084 (SB), ritanserin (Rit).
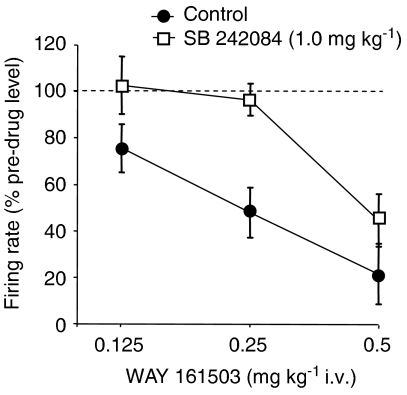
Figure 2.
Inhibition of 5-HT cell firing by WAY 161503 and its blockade by the 5-HT2C receptor antagonist SB 242084 (1.0 mg kg−1). Control animals received WAY 161503 alone and showed a dose-related inhibition of cell firing. Note that pre-treatment with SB 242084 caused a significant shift to the right of the dose response to WAY 161503. Data are mean±s.e.m. of n observations at agonist doses of 0.125, 0.25, 0.5 mg kg−1, respectively: control n=8,8,7; SB 242084 n=6,6,5. Effect of WAY 161503 alone, P<0.0001, one-way ANOVA; P<0.01 at 0.25 and 0.5 mg kg−1, Dunnett's post hoc test. Effect of SB 242084 pre-treatment, P<0.01, two-way ANOVA; P<0.05 at 0.25 mg kg−1 WAY 161503, Bonferroni post hoc test.
Effect of WAY 161503 on 5-HT cell firing in the presence of 5-HT receptor antagonists
As illustrated in Figure 1, the inhibitory effect of WAY 161503 (0.125–0.5 mg kg−1 i.v.) was reversed by administration of the 5-HT2A/C receptor antagonist ritanserin (1.0 mg kg−1 i.v.; 15/18 neurones). The effect of WAY 161503 was also reversed by the 5-HT2C receptor antagonist SB 242084 (1.0 mg kg−1 i.v.; 3/4 neurones), but not the 5-HT1A receptor antagonist WAY 100635 (0.1 mg kg−1 i.v.; 2/2 neurones) (Figure 1). The reversal of the effects of WAY 161503 is not due to rapid clearance of the drug because the reversal was always coincident with administration of the 5-HT2C receptor antagonist and under the same time-frame (2 min post-agonist), there was no reversal of the effect of WAY 161503 by a 5-HT1A receptor antagonist (Figure 1c). Also recent experiments (Queree et al., unpublished observation) demonstrate that the inhibition of 5-HT cell firing by WAY 161503 has a duration of at least 5–10 min.
Pre-treatment with SB 242084 (1.0 mg kg−1 i.v.) caused a rightward shift in the dose response to WAY 161503 (Figures 1 and 2). When administered alone, SB 242084 (0.5 mg kg−1 i.v.) had no significant effect on the rate or regularity of 5-HT cell firing (Figures 1b and c).
In some animals, the 5-HT1A agonist 8-OH-DPAT (10 μg kg−1 i.v.) was administered following the reversal of the effect of WAY 161503 by a 5-HT2 receptor antagonist. In all cases (15/15 neurones) 8-OH-DPAT inhibited cell firing and this effect was typically reversed by subsequent administration of WAY 100635 (11/15 neurones) (Figure 1).
Occupancy of SB 242084 at 5-HT2A receptors
As 5-HT2A receptors modulate 5-HT cell firing, the occupancy of SB 242084 at these receptors was investigated. In rat PET studies, [11C]MDL 100907 demonstrated high levels of binding (VOI:cerebellum ratios) in the frontal cortex and striatum with lower levels in the olfactory bulbs, hypothalamus, hippocampus, superior colliculi and medulla (Figure 3a). Pre-treatment with SB 242084 (1 mg kg−1 i.v.) had no statistically significant on [11C]MDL 100907 binding in any region tested. In comparison, pre-treatment with unlabelled MDL 100907 (0.2 or 0.4 mg/kg i.v.) reduced [11C]MDL 100907 specific binding to unity in all regions sampled.
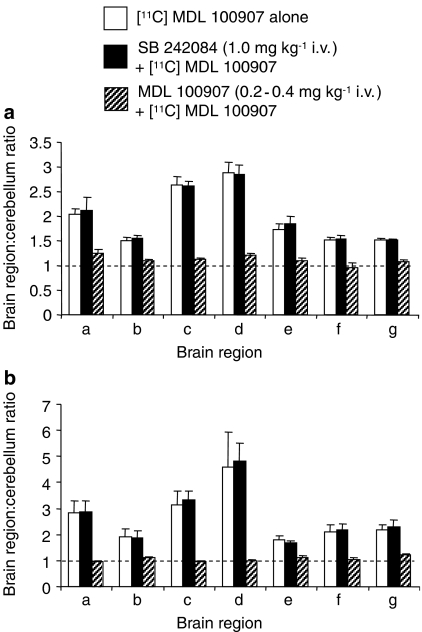
Figure 3.
Binding of the radioligand [11C]MDL 100907 in rat brain determined by (a) PET and (b) ex vivo methods. Rats received [11C]MDL 100907 alone (open columns, n=5), 5 min after SB 242084 1 mg kg−1 i.v. (filled columns, n=4), or 5 min after non-radioactive MDL 100907 (0.2–0.4 mg kg−1 i.v.) (striped columns, n=4). Brain regions: a, olfactory bulbs; b, hypothamus; c, striatum; d, frontal with cingulate cortex; e, hippocampus, f, superior colliculi; g, medulla. Data are mean±s.d. values. Note that SB 242084 did not affect [11C]MDL 100907 binding in any region whereas unlabelled MDL 100907 reduced [11C]MDL 100907 binding to unity (P<0.05: Student's t-test).
Ex vivo binding of [11C]MDL 100907 (tissue:cerebellum [11C]radioactivity ratios) confirmed the PET data (Figure 3b). As with the PET data, pre-treatment with SB 242084 (1 mg kg−1 i.v.) had no statistically significant effect on [11C]MDL 100907 binding whereas unlabelled MDL 100907 reduced specific binding to unity.
Effect of WAY 161503 in presence of GABAA receptor antagonist
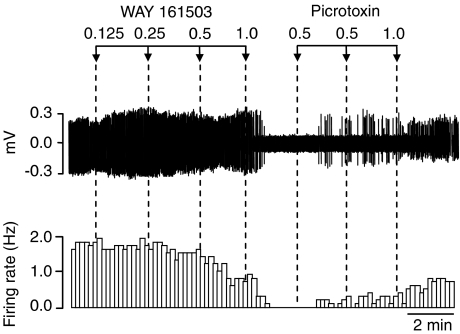
Additional experiments tested whether the inhibitory effect of WAY 161503 on 5-HT cell firing involved GABA. WAY 161503 (0.125–1.0 mg kg−1 i.v.) inhibited 5-HT cell firing, and this effect was partially restored (4/7 neurones) by administration of the GABAA receptor antagonist picrotoxin (⩽2 mg kg−1) (Figure 4). Picrotoxin (0.5–1.0 mg kg−1) alone caused a slight (+25%) increase in the firing rate of 5-HT cells (3/3 neurones).
Figure 4.
Spike train (upper trace) and rate-meter recording illustrating a partial reversal of the inhibitory effect of WAY 161503 on 5-HT cell firing by administration of the GABAA receptor antagonist picrotoxin. Drug administration (mg kg−1 i.v.) as indicated by arrows.
Effect of WAY 161503 on Fos expression in GAD positive DRN neurones
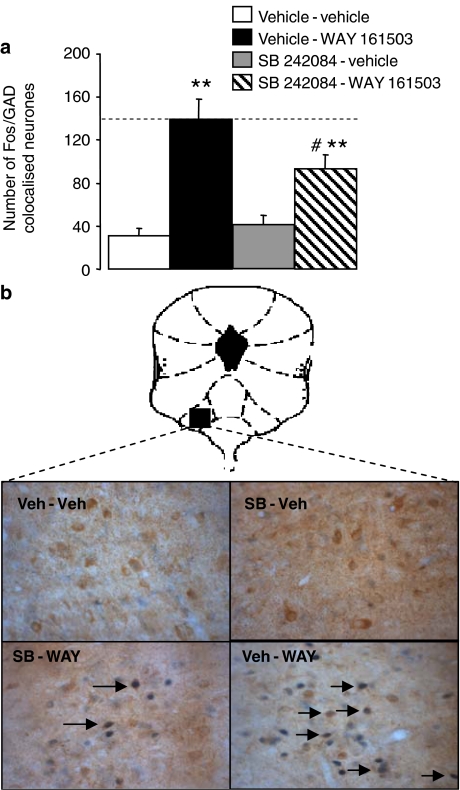
In sections from vehicle-treated animals, GAD immunoreactivity was abundant in the lateral wings of the DRN with few GAD positive cells present in the midline. Moreover, in vehicle-treated animals the number of Fos positive cells was low, and there were few cells in which Fos and GAD immunoreactivity was colocalized (Figure 5).
Figure 5.
Effect of WAY 161503 on the expression of Fos in GAD-positive DRN neurones. (a) Drug treatments were vehicle-vehicle, vehicle-WAY 161503 (3 mg kg−1), SB 242084 (1.0 mg kg−1)-vehicle and SB 242084 (1.0 mg kg−1)-WAY 161503 (3 mg kg−1). Data (n=6) are mean±s.e.m. Note that WAY 161503 increased the number of Fos/GAD double-labelled cells and that this effect was reduced by pre-treatment with SB 242084. **P<0.001 versus vehicle–vehicle (one way ANOVA with Dunnett's post hoc test). #P<0.05 versus vehicle–WAY 161503 (one-way ANOVA with Bonferroni's post hoc test). (b) Photomicrographs showing cells double labelled (arrows) with Fos and GAD immunoreactivity in the DRN of rats administered one the following drug treatments: vehicle–vehicle, SB 242084 (1.0 mg kg−1)–vehicle, SB 242084 (1.0 mg kg−1)–WAY 161503 (3 mg kg−1), vehicle–WAY 161503 (3 mg kg−1). Images at × 40 magnification.
Pilot experiments established that WAY 161503 (1, 3 and 10 mg kg−1 i.p.) caused a dose-related increase in Fos positive cells in the rat DRN (data not shown). A subsequent more detailed analysis confirmed this effect and revealed that, in comparison to vehicle controls, WAY 161503 (3 mg kg−1 i.p.) increased the number of Fos/GAD double-labelled cells in the lateral wings around three-fold and this effect was significantly reduced by pre-treatment with SB 242084 (1 mg kg−1 i.p.) (Figure 5). SB 242084 alone did not alter the number of Fos/GAD double-labelled cells (Figure 5).
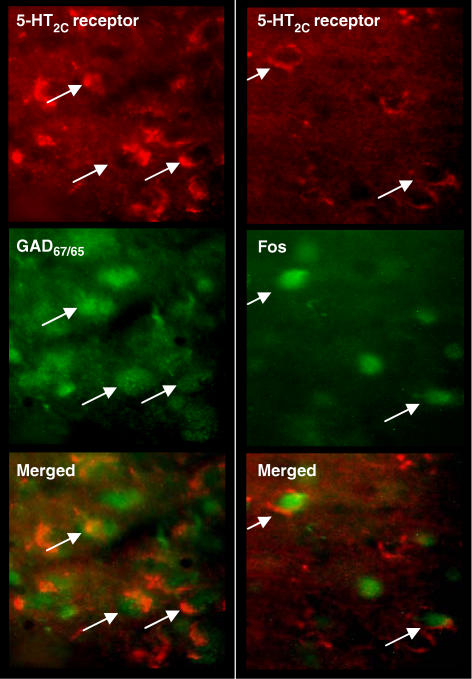
In sections from drug-naive rats, cells with 5-HT2C receptor immunoreactivity were sparsely distributed in the ventrolateral DRN with low levels in other DRN regions. In the ventrolateral DRN many GAD positive cells were double-labelled with 5-HT2C receptor immunoreactivity (Figure 6). In DRN sections from rats treated with WAY 161503 (3 mg kg−1 i.p.), numerous Fos positive cells were double-labelled with 5-HT2C receptor immunoreactivity (Figure 6).
Figure 6.
Photomicrographs of the rat DRN showing fluorescent images of 5-HT2C receptor immunoreactivity with either GAD65/67 immunoreactivity (left) or Fos immunoreactivity induced by WAY 161503 (3 mg kg−1 i.p.) (right). Arrows indicate co-localized neurones as seen in the merged images. Note the presence of GAD positive cells double-labelled with 5-HT2C receptor immunoreactivity. Also Fos positive cells were double-labelled with 5-HT2C receptor immunoreactivity after WAY 161503 administration.
Discussion and conclusions
Recent evidence suggests that postsynaptic 5-HT2 receptors are involved in the feedback control of 5-HT neurones in the midbrain raphe nuclei, and that neighbouring GABA neurones play a role (see Introduction). In particular, a combination of electrophysiological and Fos immunohistochemical studies show that 5-HT2 receptor agonists both inhibit 5-HT neurones and activate GABA neurones in the DRN (Liu et al., 2000; Boothman et al., 2003). While these studies emphasize the contribution of the 5-HT2A receptor subtype, the importance of the 5-HT2C receptor subtype may be underestimated due to a lack of sufficiently selective drugs. The present study addressed this issue using the putative selective 5-HT2C receptor agonist WAY 161503. In addition, the possible involvement of DRN GABA neurones in 5-HT2C receptor-mediated feedback was investigated.
An important finding was that WAY 161503 caused a dose-dependent inhibition of the firing of DRN 5-HT neurones in anaesthetized rats. WAY 161503 is a recently developed 5-HT2C receptor agonist, with reported ∼6-fold selectivity over 5-HT2A receptors and 20-fold selectivity over 5-HT2B receptors in radioligand binding studies, and weak or negligible affinity for other sites reported to date (5-HT1A,1B,1D,1F receptors, 5-HT transporter) (Rosenzweig-Lipson et al., 2000, 2006). These data are supported by findings in functional in vitro assays that indicate WAY 161503 has 9- and 12-fold higher potency at 5-HT2C than 5-HT2A receptors (calcium mobilization) and 5-HT2B receptors ([3H]-inositol phosphate formation), respectively (Rosenzweig-Lipson et al., 2006). Moreover, at doses comparable to those used in the present study (0.1–3.0 mg kg−1) WAY 161503 is active in behavioural models of 5-HT2C receptor function in rats, and sensitive to 5-HT2C receptor antagonist blockade (Cryan and Lucki, 2000; Rosenzweig-Lipson et al., 2006).
The present data show that the inhibitory effect of WAY 161503 on 5-HT cell firing was reversed by both the 5-HT2 receptor antagonist ritanserin (Leysen et al., 1985) and the 5-HT2C receptor antagonist, SB 242084 but not the 5-HT1A antagonist WAY 100635. In addition, pretreatment with SB 242084 shifted the WAY 161503 dose response curve to the right. The blocking effects of SB 242084 in particular, suggest a role for 5-HT2C receptors in the inhibition of 5-HT cell firing by WAY 161503. However, since activation of 5-HT2A receptors causes inhibition of 5-HT cell firing (Boothman et al., 2003) experiments were carried out to exclude the possible in vivo occupancy of SB 242084 at 5-HT2A receptors.
SB 242084, at a dose (1 mg kg−1 i.v.) that blocked the effect of WAY 161503 on 5-HT cell firing, showed no displacement of the 5-HT2A receptor radioligand [11C]MDL 100907 in either rat PET or ex vivo binding studies. In comparison unlabelled MDL 100907 (0.2 or 0.4 mg kg−1 i.v.) fully displaced the [11C]MDL 100907 signal in both models. Radioactivity levels in extracerebral tissues were unaffected by SB 242084 pre-treatment, indicating that the antagonist did not affect [11C]MDL 100907 delivery. Thus, these data indicate that SB 242084 (1 mg kg−1 i.v.) has no significant occupancy of rat brain 5-HT2A receptors in vivo. These results are consistent with radioligand binding studies showing that SB 242084 has over 100-fold selectivity for 5-HT2C versus 5-HT2A and 5-HT2B receptors (Kennett et al., 1997) and exerts a 5-HT2C receptor antagonist action in vivo (Kennett et al., 1997).
Taken together, the above data indicate that 5-HT2C receptor activation contributes to the inhibition of 5-HT cell firing by WAY 161503. This evidence of a role for 5-HT2C receptors in 5-HT neurone control accords with observations that the inhibition of 5-HT cell firing by the 5-HT2 receptor agonist DOI was attenuated by pre-treatment with the 5-HT2B/C receptor antagonist SB 206553 in vivo (Boothman et al., 2003). Also, in a raphe slice preparation the increase of IPSPs in 5-HT neurones induced by 5-HT and DOI was attenuated by SB 242084 (Liu et al., 2000).
It is noteworthy that the inhibition of 5-HT cell firing by WAY 161503 was not completely reversed by SB 242084, and that full reversal was only achieved by subsequent administration of ritanserin. As activation of 5-HT2A receptors inhibits 5-HT cell firing (Boothman et al., 2003), these results suggest that the inhibitory effect of WAY 161503 may be partially 5-HT2A receptor-mediated. This would be consistent with radioligand and functional assay data showing that even though WAY 161503 demonstrates preference for 5-HT2C versus 5-HT2A receptors, the selectivity is only of the order of 10-fold (see above).
In the present study, administration of the GABAA receptor antagonist picrotoxin restored the inhibition of 5-HT cell firing induced by WAY 161503. In agreement with previous studies (Gallager and Aghajanian, 1976), picrotoxin by itself had little effect on the firing of 5-HT neurones (+25% increase). These data are consistent with an earlier report in that the inhibition of 5-HT cell firing induced by DOI was restored by picrotoxin (Martin-Ruiz et al., 2001a). Moreover, the data suggest that an activation of GABA neurones may be involved in the inhibitory action of WAY 161503.
A possible action of WAY 161503 on GABA neurones within the DRN was investigated using Fos immunohistochemistry. It was found that WAY 161503 caused a marked increase in Fos expression in DRN cells that were immunoreactive for the GABA neurone marker, GAD. As this effect of WAY 161503 was attenuated by pre-treatment with SB 242084 the involvement of 5-HT2C receptors is implicated. Recently, we reported that DOI administration increased the number of Fos/GAD double-labelled cells in the DRN and it is possible that 5-HT2C (as well as 5-HT2A) receptors contribute to this effect (Raley et al., 2004).
The 5-HT2C receptor-mediated action of WAY161503 on DRN GABA cells may be a direct effect as these cells were found to express 5-HT2C immunoreactivity, a finding which agrees with a recent report describing the presence of 5-HT2C receptor mRNA in DRN GABA neurones (Serrats et al., 2005). Also, WAY161503 increased Fos expression in DRN cells immunoreactive for 5-HT2C receptors.
Taking together the above findings, it is plausible that WAY 161503 inhibits 5-HT cell-firing in the DRN by activating local GABA neurons. This idea is consistent with earlier data demonstrating extensive synaptic interactions between GABA and 5-HT neurons in the DRN (Wang et al., 1992), and that local application of GABA agonists into this nucleus inhibits 5-HT cell firing (Gallager and Aghajanian, 1976). Thus, current data support a model of 5-HT feedback control in which 5-HT2C receptors activate DRN GABA neurones to inhibit 5-HT neuronal activity. However, 5-HT2C receptors are abundant in other brain regions and the present data do not exclude the additional possibility that 5-HT2C receptors also play a role in the modulation of DRN afferents from more distant regions such as the lateral habenula or prefrontal cortex.
Interestingly, we observed many instances of DRN 5-HT neurones that were inhibited by both WAY 161503 as well as 8-OH-DPAT. This finding is evidence of 5-HT neurones that are sensitive to feedback control by both 5-HT2C and 5-HT1A receptors, the latter probably involving both pre- and post-synaptic mechanisms (Hajós et al., 1999). Our observations suggest that individual 5-HT neurones are subject to 5-HT feedback control at several levels.
In summary, this study demonstrates that WAY 161503 inhibits 5-HT cell firing in the DRN in vivo, and support a mechanism involving 5-HT2C receptor-mediated activation of DRN GABA neurones. This mechanism may be one means by which postsynaptic 5-HT receptors located on afferent inputs to 5-HT neurones contribute to 5-HT feedback control. In this context, evidence that 5-HT2C receptor antagonists augment the neurochemical effects of some antidepressants is of particular interest (Cremers et al., 2004; Boothman et al., 2006). Thus, by analogy to 5-HT autoreceptor-mediated feedback mechanisms, it is possible that 5-HT2C feedback may provide a source of targets for drug therapies addressing neuropsychiatric disorders.
Acknowledgments
We thank Sue Hume for her expert assistance with the PET imaging in this study. This work was supported by the EC 6th Framework Programme (NEWMOOD, LMSH-CT-2004-503474, TS), and by Wellcome Trust studentships (JR, FD).
Abbreviations
- 5-HT
5-hydroxytryptamine
- DRN
dorsal raphe nucleus
- GABA
gamma amino-butyric acid
- GAD
glutamate decarboxylase
- HIDAC
high-density avalanche chamber
- PET
positron emission tomography
Conflict of interest
The authors state no conflict of interest.
References
- Aghajanian G.Feedback regulation of central monoaminergic neurones: Evidence from single cell recording studies Essays in Neurochemistry and Neuropharmacology 1978J Wiley and Sons Ltd: Chichester; 1–32.In: Youdim D, Lovenberg W, Lagnado J (eds) [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970;171:178–187. [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman LJ, Mitchell SN, Sharp T. Investigation of the SSRI augmentation properties of 5-HT2 receptor antagonists using in vivo microdialysis. Neuropharmacology. 2006;50:726–732. doi: 10.1016/j.neuropharm.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Raley J, Sharp T. Electrophysiological evidence of a role for 5-HT2C receptors in the feedback inhibition of 5-HT neuronal activity. Proc Br Pharmacol Soc. 2005b;15 [Google Scholar]
- Boothman LJ, Sharp T. A role for midbrain raphe gamma-aminobutyric acid neurons in 5-hydroxytryptamine feedback control. Neuroreport. 2005a;16:891–896. doi: 10.1097/00001756-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Seitz PK, Thomas MJ, Cunningham KA. Validation of a seclective serotonin 5-HT2C receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063:105–113. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, et al. Inactivation of 5-HT2C receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur J Pharmacol. 1976;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Garratt JC, Kidd EJ, Wright IK, Marsden CA. Inhibition of 5-hydroxytryptamine neuronal activity by the 5-HT agonist, DOI. Eur J Pharmacol. 1991;199:349–355. doi: 10.1016/0014-2999(91)90499-g. [DOI] [PubMed] [Google Scholar]
- Hajós M, Gartside SE, Villa AE, Sharp T. Evidence for a repetitive (burst) firing pattern in a sub-population of 5-hydroxytryptamine neurons in the dorsal and median raphe nuclei of the rat. Neuroscience. 1995;69:189–197. doi: 10.1016/0306-4522(95)00227-a. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hajós-Korcsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani E, Sharp T, Sprakes M, Grasby P, Hume S. Fenfluramine evokes 5-HT2A receptor-mediated responses but does not displace [11C]MDL 100907: small animal PET and gene expression studies. Synapse. 2003;50:251–260. doi: 10.1002/syn.10268. [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, et al. Effect of 5-HT on binding of [(11)C] WAY 100635 to 5-HT1A receptors in rat brain, assessed using in vivo microdialysis nd PET after fenfluramine. Synapse. 2001;41:150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jeavons AP, Chandeler RA, Dettmar CAR. A 3D HIDAC-PET camera with sub-millimetre resolution for imaging small laboratory animals. IEEE Trans Nucl Sci. 1999;46:468–473. [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Guadalupe H, Palacios JM, Vilaro MT. Selective visualisation of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedeberg's Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lundkvist C, Halldin C, Ginovart N, Nyberg S, Swahn CG, Carr AA, et al. [11C]MDL 100907, a radioligland for selective imaging of 5-HT2A receptors with positron emission tomography. Life Sci. 1996;58:187–192. doi: 10.1016/0024-3205(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, et al. Control of serotonergic function in medial prefrontal cortex by serotonin2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001a;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz R, Ugedo L. Electrophysiological evidence for postsynaptic 5-HT1A receptor control of dorsal raphe 5-HT neurones. Neuropharmacology. 2001b;41:72–78. doi: 10.1016/s0028-3908(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. In The Rat Brain in Stereotaxic Coordinates. Academic Press Ltd: London; 1986. [Google Scholar]
- Raley J, Boothman LJ, Sharp T. Immunohistochemical evidence that 5-HT1A and 5-HT2A receptor agonists activate GABA neurones in the DRN. Proc Br Pharmacol Soc. 2004.
- Raley J, Denk F, Boothman LJ, Sharp T. Immunohistochemical evidence that 5-HT2C receptor agonist administration activates GABA neurones in the rat DRN. Proc Br Pharmacol Soc. 2005.
- Rosenzweig-Lipson S, Coupet J, Dunlop J, McGonigle P. Anti-obesity-like efeects of the selective 5-HT2C agonist WAY 161503. FASEB. 2000;14:A1321. [Google Scholar]
- Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, et al. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res. 2006;1073–1074:240–251. doi: 10.1016/j.brainres.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortes R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajós M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]