Abstract
Mutations in the human melanocortin (MC)4 receptor have been associated with obesity, which underscores the relevance of this receptor as a drug target to treat obesity. Infusion of MC4R agonists decreases food intake, whereas inhibition of MC receptor activity by infusion of an MC receptor antagonist or with the inverse agonist AgRP results in increased food intake. This review addresses the role of the MC system in different aspects of feeding behaviour. MC4R activity affects meal size and meal choice, but not meal frequency, and the type of diet affects the efficacy of MC4R agonists to reduce food intake. The central sites involved in the different aspects of feeding behaviour that are affected by MC4R signalling are being unravelled. The paraventricular nucleus plays an important role in food intake per se, whereas MC signalling in the lateral hypothalamus is associated with the response to a high fat diet. MC4R signalling in the brainstem has been shown to affect meal size. Further genetic, behavioural and brain-region specific studies need to clarify how the MC4R agonists affect feeding behaviour in order to determine which obese individuals would benefit most from treatment with these drugs. Application of MCR agonists in humans has already revealed side effects, such as penile erections, which may complicate introduction of these drugs in the treatment of obesity.
Keywords: melanocortins, AgRP, POMC, obesity, feeding behaviour, MC4 receptor
Introduction
In order to maintain stable body weight, energy intake (by ingestion) and energy expenditure (by exercise, basal metabolism and thermogenesis) need to be balanced. The constant availability of highly palatable foods and the lack of exercise strongly contribute to the development of obesity (Woods et al., 2004). Drugs that effectively reduce body weight are urgently needed to fight the obesity epidemic (Boss and Bergenhem, 2006). The unravelling of the genetic defect underlying obesity in the ob/ob mouse, namely the absence of the leptin gene, was key towards identification of neural pathways and neuropeptides that control body weight (Zhang et al., 1994; Friedman and Halaas, 1998). Leptin is an adipose tissue-derived hormone that is released into the circulation proportional to increased energy stores in fat. Leptin stimulates neural circuits that decrease food intake and increase energy expenditure. Both humans and rodents with mutations in the leptin gene or leptin receptor gene are obese (Zhang et al., 1994; Chen et al., 1996; Lee et al., 1996; Montague et al., 1997; Clement et al., 1998; Barsh et al., 2000).
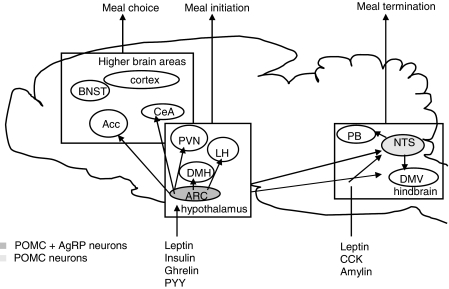
The arcuate nucleus of the hypothalamus is an important relay centre for leptin's effects. The hypothalamic arcuate nucleus integrates and distributes peripheral information from hormonal and neural signals that reflect metabolic status further into the brain (Figure 1). Within the arcuate nucleus, neurons containing melanocortins (MCs), products of the pro-opiomelanocortin (POMC) gene, are activated by leptin (Schwartz et al., 1997). These neurons provide one of the systems via which the leptin signal is propagated further into the brain. Fasting results in loss of adipose tissue and low leptin levels, which causes diminished activation of POMC neurons, whereas overfeeding (high leptin levels) results in a stimulation of POMC neurons (Mizuno et al., 1998; Hagan et al., 1999). Thus, POMC neurons are stimulated during a positive energy balance, and increased plasma leptin levels contribute to this stimulation.
Figure 1.
Simplified scheme of the role of melanocortins in food intake. BNST, bed nucleus of the stria terminalis; Acc, accumbens; CeA, Amygdala; PVN, paraventricular nucleus of the hypothalamus; LH, lateral hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; ARC, arcuate nucleus; PB, parabrachial nucleus; NTS, nucleus of the tractus solitarius; DMV, dorsomotor nucleus of the vagus.
The activity of the MC system is not only regulated by the endogenous MC receptor agonists, α-melanocyte-stimulating hormone (α-MSH), β-MSH and γ-MSH, which are all derived from the POMC precursor, but also by agouti-related protein (AgRP; Table 1). AgRP is also expressed in the arcuate nucleus but in a different subset of neurons than those expressing POMC. In contrast to POMC neurons, AgRP neurons are inhibited by leptin and activated during negative energy balance. Although often described as a competitive antagonist, AgRP acts in fact as an inverse agonist on constitutively active MC3 and MC4 receptors (Haskell-Luevano and Monck, 2001; Nijenhuis et al., 2001), the main brain MC receptors. The unique presence of an endogenous agonist and an inverse agonist acting at the same receptor system implicates a tight regulation of MC function in the brain. Thus, during a negative energy balance, AgRP neurons are activated and AgRP acts to suppress MC receptor activity.
Table 1.
Overview of MCR endogenous ligands, locations and functions
| MC receptor subtype | Endogenous ligand (decreasing affinity) | Endogenous antagonist | Central location | Peripheral location | Central function | Peripheral function |
|---|---|---|---|---|---|---|
| MC1 | αMSH=βMSH=ACTH>γMSH | Agouti | Periaquaductal grey | Melanocyte, pituitary, placenta, testis, corpus luteum, macrophages, monocytes, neutrophils, endothelium, glioma cells, astrocytes, fibroblasts, keratinocytes, Th cells, natural killer (NK) cells | Pigmentation, anti-inflammatory | |
| MC2 | ACTH | Agouti | Adrenals, murine adipocytes, skin, Th cells, NK cells, monocytes, granulocytes | Glucocorticoid production, stress-induced lipolysis | ||
| MC3 | γMSH=αMSH=βMSH=ACTH | AgRP | Brainstem, hypothalamus, thalamus, septum | Placenta, gut, heart, thymus, murine macrophages, Th cells, NK cells, monocytes, granulocytes | Energy homeostasis, anti-inflammatory | Pro-inflammatory cytokine release |
| MC4 | αMSH=βMSH=ACTH>γMSH | AgRP, agouti | Brainstem, hypothalamus, thalamus, striatum, septum, cortex, hippocampus, limbic system, spinal cord | Body weight regulation, grooming, pain processing, sexual behaviour | Penile erections | |
| MC5 |
αMSH>βMSH=ACTH> γMSH |
Agouti | Cortex, cerebellum, striatum, midbrain, pons, medulla, olfactory bulb | Pituitary, skin, adrenals, fat cells, smooth and skeletal muscle, bone marrow, spleen, lymph nodes, thymus, gonadals, uterus, lung, liver, stomach, oesophagus, kidney, mammary glands, exocrine glands, Th cells, NK cells, monocytes, granulocytes | Natriuresis, sebum secretion, preputial lipogenesis | |
| Ref | (Schioth et al., 1996; Adan and Gispen, 1997) | (Ollmann et al., 1997; Yang et al., 1997) | (Roselli-Rehfuss et al., 1993; Griffon et al., 1994; Mountjoy et al., 1994; Fathi et al., 1995; Xia et al., 1995) | (Getting et al., 1999; Wikberg, 1999; Wikberg et al., 2000; Andersen et al., 2005) | (Gispen et al., 1975; Hirsch et al., 1984; Huszar et al., 1997; Chen et al., 2000a; Muceniece et al., 2006) | (Thody et al., 1976; Leiba et al., 1990; Robbins et al., 1993; Bhardwaj et al., 1997; Lipton and Catania, 1998; Boston, 1999; Gantz and Fong, 2003) |
Abbreviations: ACTH, adrenocoticotropic hormone; AgRP, agouti-related protein; MC, melanocortin; MCR, melanocortin receptor; MSH, melanocyte-stimulating hormone.
Although the MC system modulates energy expenditure and insulin sensitivity (Cone, 2005), this review focuses on the role of the MC system in regulation of energy intake. After summarizing the evidence that the MC4 receptor is a promising drug target for the treatment of obesity, it will be discussed how and via which neural circuits the MC system affects energy intake.
Mutations in the MC system: lessons from the MC4 receptor knockout mouse
Spontaneous as well as genetically introduced variations in genes constituting the MC system demonstrate the importance of this system in body weight homeostasis. The first gene belonging to the MC system that was deleted in mice was the MC4 receptor. Overall, disruption of the MC4 receptor in mice results in an obese phenotype, underscoring the potential of the MC4 receptor as drug target for obesity (Huszar et al., 1997; Chen et al., 2000b).
MC4 receptor−/− display maturity onset obesity characterized by hyperphagia, increased adiposity, increased longitudinal growth, normal lean body mass, hyperinsulinaemia and hyperleptinaemia. MC4 receptor+/− show an intermediate phenotype with respect to body weight and food intake, suggesting a gene dosage effect (Huszar et al., 1997; Chen et al., 2000b). When female MC4 receptor−/− mice are pair-fed to controls, their body weight are intermediate between wild-type and non-pair-fed mice, suggesting that inhibition of MC4 receptor activity also affects energy balance independent from food intake (Ste Marie et al., 2000). Furthermore, non-pair-fed MC4 knockout mice consume less oxygen per gram body weight than wild types (Ste Marie et al., 2000). Interestingly, MC4 knockout mice are already heavier than wild types before hyperphagia is significantly increased (Ste Marie et al., 2000). Although body temperature of MC4 receptor−/− is normal, locomotor activity of male MC4 receptor−/− is reduced in the dark phase (Marsh et al., 1999a; Ste Marie et al., 2000). Thus, obesity of MC4 receptor−/− mice is due to both hyperphagia and reduced energy expenditure.
Peripheral as well as central leptin administration does not reduce food intake in obese MC4 receptor−/−, whereas young non-obese MC4 receptor−/− have an attenuated response to leptin (Marsh et al., 1999a). The MC4 receptor does not seem to be involved in the orexigenic or anorexigenic effects of neuropeptide Y (NPY), peptide YY (PYY), orexins and urocortin, as the response to these neuropeptides on food intake is normal in MC4 receptor−/− (Marsh et al., 1999a; Halatchev et al., 2004). On the other hand, cholecystokinin (CCK), a gut-derived satiety factor has an attenuated response (inhibiting food intake) in MC4−/− (Fan et al., 2004), which suggests that intact MC4 receptor signalling is implicated in CCK's effect on satiety (Table 2).
Table 2.
Overview of the effects of administration of neuropeptides on food intake and body weight in wild-type and MC4 receptor−/− animals
↑, increased compared to saline administration; ↓, decreased compared to saline administration; =, normal compared to saline administration.
Effect after MTII administration, empty fields, no data available.
Abbreviations: AgRP, agouti-related protein; CART, cocaine- and amphetamine-regulated transcript; CCK, cholecystokinin; CRH, corticotropin-releasing hormone; MCH, melanin concentrating hormone; MSH, melanocyte-stimulating hormone; NPY, neuropeptide Y; PYY, peptide YY; TRH, thyrotropin-releasing hormone.
Mutations in other genes of the MC system
Genetic deletion of the MC3 receptor, the other main MC receptor in the brain (Table 1), also resulted in an energy balance phenotype. MC3 receptor−/− display adiposity, while not being hyperphagic, and are hypoactive (Butler et al., 2000; Chen et al., 2000a). Besides being expressed in multiple other brain sites, the MC3 receptor is expressed in the arcuate nucleus on POMC neurons (Jegou et al., 2000). This suggests that the MC3 receptor plays a role as autoreceptor, normally suppressing the activity of POMC neurons. If the MC3 receptor normally limits POMC neuronal activity, then lack of the MC3 receptor on POMC neurons would increase its activity, as a negative feedback signal would be disrupted. Subsequent release of MCs would increase MC4 receptor activity and this may contribute to the relative hypophagia observed in MC3 knockout mice. The increased leptin levels owing to adiposity would further contribute to this hypophagia. This might explain why MC3 receptor−/− are not hyperphagic, but hypophagic, despite being fat and hyperleptinemic. Recently, it was demonstrated that peripheral injection of an MC3 receptor selective agonist increased food intake (Marks et al., 2006). This is consistent with an auto-inhibitory role of the MC3 receptor expressed on POMC neurons in the arcuate nucleus.
AgRP knockout mice do not have an obvious phenotype (Qian et al., 2002). As AgRP is co-expressed with NPY in the arcuate nucleus, AgRP knockouts were crossed with NPY knockouts. Surprisingly, double knockouts were not lean, suggesting that NPY and AgRP do not play an essential role in feeding behaviour and body weight homeostasis (Qian et al., 2002). However, upon closer examinations (by comparing younger and older mice), it appeared that older AgRP knockouts display increased locomotor activity, increased thyroid hormone levels and increased energy expenditure, which results in a late-onset lean phenotype (Wortley et al., 2005). This is in agreement with results obtained by suppression of AgRP expression in the adult stage by RNA interference (RNAi), resulting in increased metabolic rate contributing to decreased body weight (Makimura et al., 2002). AgRP expression can also be reduced by damaging AgRP neurons. By expressing the human diphtheria toxin receptor specifically in AgRP neurons, AgRP neurons can be destroyed selectively once diphtheria toxin is administered at chosen moments during development. When the toxin is administered at early stages of development, animals have a normal phenotype. However, administration of diphteria toxin in the adult stage resulted in a lean phenotype (Luquet et al., 2005). Using another strategy, Xu et al. (2005) demonstrated that ablation of AgRP neurons results in decreased fat mass. These data indicate that interpretation of results obtained from knockout mice is complicated by compensations for lack of components of the MC system during development.
POMC knockout mice are also obese, which is explained by the lack of endogenous MCs. Interestingly, POMC−/− have adrenal insufficiency and, subsequently, very low corticosterone levels and are hypersensitive to the metabolic effects of glucocorticoids (Yaswen et al., 1999). Recently, Low and co-workers demonstrated that when POMC was re-expressed in pituitary gland of POMC knockouts using a transgenic approach, an obesity phenotype was obtained, which was more pronounced than in POMC knockouts (Smart et al., 2006). This may be explained by the presence of restored hypothalamo–pituitary–adrenal (HPA) function in these POMC knockout mice with transgenic expression of POMC in pituitary gland, as compared to overall POMC knockout mice.
Feeding behaviour in humans with mutations in the MC system
Mutations in the MC4 receptor are a relatively common cause of severe childhood obesity (Farooqi et al., 2000, 2003; Vaisse et al., 2000, Mackenzie, 2006). The carrier prevalence for MC4 mutations in a juvenile-onset obese population has been noted to be around 2.5% with a highest prevalence of 6% among severe obese children (Vaisse et al., 2000; Farooqi et al., 2003; Larsen et al., 2005; Hinney et al., 2006). Humans with MC4 mutations show a more or less similar phenotype as has been described for mice with mutations in the MC4 receptor gene. Those people show clear hyperphagia, hyperinsulinaemia, increased fat mass, lean body mass, bone mineral density and linear growth rate, with no changes in cortisol levels, gonadotropin, thyroid and sexsteroid levels (Farooqi et al., 2000, 2003). In contrast to MC4 receptor−/−, hyperphagia and hyperinsulinaemia tends to subside with age in human subjects. Similar to the MC4 knockout mice, the phenotype in heterozygote carriers is intermediate in comparison to homozygote carriers (Farooqi et al., 2003). The exhibited hyperphagia observed upon a test meal is less severe than that observed in people with a leptin deficiency (Farooqi and O'Rahilly, 2005). The severity of MC4 receptor dysfunction seen in assays in vitro can predict the amount of food ingested at a test meal by the subject harbouring that particular mutation and correlates with the onset and severity of the obese phenotype (Farooqi et al., 2003; Lubrano-Berthelier et al., 2006). At least 90 different MC4 receptor mutations have been associated with obesity. Many of these mutations cause retention of the mutated receptors intracellularly, resulting in loss of agonist response (Lubrano-Berthelier et al., 2003; Nijenhuis et al., 2003). In other mutations, a selective loss of agonist affinity and response were reported (Yeo et al., 2003). Even mutations in the human MC4 receptor that only decrease its constitutive activity, without a loss in agonist responsiveness, have been associated with obesity (Srinivasan et al., 2004). This underscores the in vivo relevance of constitutive MC4 receptor activity for normal weight maintenance, and provides a means for AgRP to act beyond α-MSH as inverse agonist. Indeed, even subtle changes in the activity of the MC system, such as in human heterozygous carriers of mutations in the MC4 receptor gene, result in an increased susceptibility to develop obesity (Santini et al., 2004).
Besides mutations in the MC4 receptor, mutations in the POMC gene have been described which lead to severe obesity in humans and to the same phenotype as observed in obese people with an MC4 receptor mutation (Krude et al., 1998, 2003; Challis et al., 2002). Until recently, the effect of mutations in the POMC gene have been attributed to a malfunctioning of α-MSH on the MC4 receptor, as α-MSH is the peptide important for signalling via the MC4 receptor in rodents. However, two recent papers indicate that also mutations in POMC leading to a disruption of β-MSH lead to an obese phenotype similar to that of MC4 receptor mutations, supporting a role for β-MSH in the control of energy balance in humans (Biebermann et al., 2006; Lee et al., 2006).
In contrast to the MC4 receptor and POMC genes, mutations in the human AgRP gene have not been identified so far. However, there are some reports on polymorphisms in the AgRP gene that need to be replicated. Two of such polymorphisms in the human AgRP gene appear to influence energy intake. The 67Thr-allele of AgRP is associated with reduced energy intake from fat and enhanced energy intake from carbohydrates in white subjects (Loos et al., 2005). Another allele, characterized by a −38 C/T in the AgRP promoter region is associated with protein intake in black subjects. Homozygotes of the T allele show reduced protein intake as compared to carriers of the C allele. The TT genotype is associated with a low promoter activity and low affinity for transcription factors and was previously found to be protective of obesity (Mayfield et al., 2001; Argyropoulos et al., 2003). These data are consistent with data described above regarding the phenotype of mice carrying deletions of the AgRP gene and ablation of AgRP neurons: a lean phenotype was induced.
Pharmacological studies using melanocortin receptor ligands
The discovery and application of specific agonists and antagonists for MC receptors contributed strongly to unravel the role of the MC system in energy balance. Central administration of non-specific MC receptor agonists such as α-MSH and melanotan-II (MTII) results in decreased food intake and body weight (Fan et al., 1997). MC receptor knockout mice provide an interesting model to determine which (an)orexigenic signals are dependent on which MC receptor in their effect on appetite. While MTII administration in wild-type control mice inhibits fasting-induced refeeding, this is not observed in MC4 receptor−/− (Marsh et al., 1999a). Furthermore, MTII also does not inhibit nocturnal feeding or 24 h feeding in freely feeding MC4 receptor−/− (Marsh et al., 1999a; Chen et al., 2000b) as it does in wild-type mice. As MTII still inhibits food intake in MC3 knockout mice (Chen et al., 2000a), the MC3 receptor is probably not involved in reduction of food intake induced by MC receptor agonists. This indicates that the central MC pathways that inhibit appetite act through the MC4 receptor.
Centrally administered AgRP, however, does seem to induce hyperphagia in MC4 receptor−/−. Although the increase in food intake is smaller than in wild-type controls, and not always significant, this suggests that unlike the anorectic effects of MTII, the orexigenic effects of AgRP are not exclusively mediated via the MC4 receptor, but may also involve the MC3 receptor (Marsh et al., 1999b; Fekete et al., 2004).
MTII activates similar neuronal circuits as leptin and is able to reduce adiposity in leptin-deficient ob/ob mice (Bluher et al., 2004), which further underscores that MCs act downstream of leptin. MTII also counteracts starvation-induced hyperphagia, suggesting that reduction of MC receptor activity is part of the physiological response to starvation (Fan et al., 1997).
Application of the MC 3/4 receptor antagonist SHU9119 results in voluntary overfeeding, obesity and increased plasma leptin levels (Fan et al., 1997; Adage et al., 2001). Consistent with data obtained in MC4−/− mice, SHU9119 increases body adiposity and plasma leptin levels when rats are pair-fed to controls, suggesting that metabolic effects of reduced brain MC activity are also independent of food intake. A lower body temperature and reduced locomotor activity contribute to these effects (Adage et al., 2001). Moreover, MTII is not able to increase metabolic rate in MC4 receptor−/−, whereas it does in wild-type littermates, suggesting that the MC4 receptor is indeed necessary for the regulation of metabolism (Chen et al., 2000b).
A low dose of SHU9119 prevents compensatory reduction in food intake after involuntary overfeeding (Hagan et al., 1999). This illustrates the physiological relevance of activation of the MC system during a positive energy balance. The inverse agonist AgRP is a potent stimulant of food intake and is implicated in the maintenance of feeding rather than the initiation of feeding (Wirth and Giraudo, 2000). Therefore, AgRP is most effective when injected in the natural feeding period or following food deprivation. A single central administration of AgRP increases food intake for at least 24 h (up to 7 days) and blocks α-MSH/MTII-induced hypophagia (Rossi et al., 1998; Hagan et al., 2000; Wirth and Giraudo, 2000). Chronic treatment of rats with central AgRP increases body weight gain by enhancing food intake and reducing energy expenditure (Small et al., 2001, 2003). Taken together, pharmacological evidence suggests that the central MC system affects energy intake as well as energy expenditure and underscores the physiological involvement of the MC system in energy balance.
Effects of melanocortinergic signalling on feeding behaviour
As described above, the central MC system is clearly involved in regulation of feeding behaviour. The question remains, however, which specific biological processes related to appetite control are regulated by MC signalling?
In order to survive, it is essential for an animal to search for food, remember food sources, prepare for consumption, ingest sufficient amounts of food and digest foods efficiently. The decision to eat is controlled by endogenous drives (hunger and satiety), and also by environmental cues such as availability of palatable or novel foods and predator exposure when searching for food (Blundell and Gillett, 2001). Furthermore, different factors involved in food intake influence each other. For example, meal termination is controlled by internal and external cues (Kral, 2006). Meal size is influenced by extent of negative energy balance preceding the meal as well as meal composition (Strubbe and Woods, 2004). Even when a high degree of satiety is achieved, the availability of a highly palatable food may overrule this and increase meal size (Erlanson-Albertsson, 2005). Both, anatomical and pharmacological evidence suggests that satiety, hunger and rewarding aspects associated with feeding are regulated in different anatomical sites, many of which contain MC receptors (Berthoud, 2004). To answer the question of how the MC system affects feeding behaviour, pharmacological and genetic interference studies have been combined with behavioural paradigms that address these different processes.
For example, it was found that similar to leptin, MTII reduces food intake by affecting meal size and duration as shown by meal pattern analysis studies, whereas meal frequency and inter-meal interval were unaffected (Williams et al., 2002; Zheng et al., 2005). Oppositely, SHU9119 increases food intake by selectively increasing meal size (Zheng et al., 2005). These data suggest that the MC system is involved in meal termination rather than meal initiation. This is consistent with the demonstration that MTII is less effective in suppressing food intake when rats expect a meal (Benoit et al., 2003) which underscores the lack of MC effects on affecting meal initiation. Other studies by Benoit et al. (2003) showed that the efficacy of MC receptor agonists appears attenuated during scheduled feeding, when rats have learned to consume large amounts of food in a short period of time, suggesting that the MC system is not involved in anticipation to food.
Data from analysis of MC4−/− mice do not always fit with these pharmacological data. In a paradigm where mice have to press a lever to get a meal, MC4 receptor−/− are not hyperphagic and lose body weight, while control animals show normal food intake and body weight gain. Meal size as well as frequency is normal in MC4 receptor−/− in this paradigm, suggesting that a functional MC4 receptor is not required for the normal regulation of meal patterns (Vaughan et al., 2005). However, data about meal patterns during hyperphagia and in a normal environment are necessary to clarify this. Furthermore, in MC4−/− mice, compensatory adaptation may mask a physiological role of the MC system in determining meal size.
Ligands that suppress food intake may do so because they induce a state of illness. This can be tested by pairing ligand infusion with a flavour, and subsequent measurement of the intake of the flavoured food in the absence of ligand infusion, as in conditioned taste aversion (CTA) tests. Although it has been reported that MTII, the mixed MC3/4 receptor agonist, induced CTA, more selective MC4 receptor agonists do not induce CTA, suggesting that the MC4 receptor is the candidate MC receptor for development of agonists to suppress energy intake (Butler, 2006). Thus, the MC system affects meal size, but not meal initiation, meal frequency or anticipation and a reduction of food intake by activation of the MC4 receptor is not caused by inducing nausea.
Effects of melanocortins on different diets
Relevant to the evaluation of MC receptor agonists as potential drugs to reduce food intake in obesity, is whether the efficacy of MC receptor agonists depends on the type of diet and whether MC receptor agonists are still effective in obese subjects.
When MC receptor agonists are infused over days to weeks in rats, the efficacy to suppress food intake is high in the first days but fades away over time (McMinn et al., 2000; Jonsson et al., 2002). Rats that are fed a high-fat diet for several weeks also show an attenuated response to MTII (Benoit et al., 2003; Clegg et al., 2003). It was recently shown that chronic MTII treatment still reduces food intake in rats fed a high-fat diet as well as in food-deprived rats. MTII was, however, less effective in reducing food intake and body weight in rats with a lower body fat mass (Seeley et al., 2005). This indicates that activity of the MC system is related to the defended level of body adiposity, suggesting that the main function of the MC system is regulating body adiposity rather than food intake.
Interestingly, when the high-fat diet is low in carbohydrates (mimicking the ‘Atkins' diet), MTII sensitivity is maintained, although the level of adiposity on this diet is increased as compared to controls (Kinzig et al., 2005). It is important to note that not all laboratories that investigated the effects of MCs have used the same diet, which complicates direct comparisons. Further studies need to clarify whether the type of (high fat) diet affects the responsiveness of the MC system.
Another aspect of different diets is that these not only differ in caloric density but also in palatability. MC receptors are expressed in brain centres that relay information on taste and palatability, such as the amygdala, nucleus of the tractus solitarius and parabrachial nucleus. This provides an anatomical basis for an effect of MCs on food choice. As MCs may affect taste processing, this might contribute to an overall effect on food intake. There are several reports on the role of the MC system in preference for certain foods. MTII specifically reduces intake of fat (but not of protein or carbohydrates) on a three-choice macronutrient diet (Samama et al., 2003) and the inverse agonist AgRP enhances the intake of specifically high-fat diets in rats (Hagan et al., 2001). In addition, obese mice with ectopic overexpression of Agouti (which mimics the action of AgRP) have enhanced preference for fat meals (Koegler et al., 1999).
Also, experiments using MC4−/− mice indicate an involvement of the MC system in fat preference. When exposed to a high-fat diet, MC4 receptor−/− display an increased caloric intake, which is, unlike in control animals, not normalized after 48 h. Together with an enlarged feed efficiency, this results in an even more increased body weight gain. Additionally, whereas normal animals increase their oxygen consumption on a high-fat diet, this effect is absent in MC4 receptor−/−. This indicates that the MC4 receptor is required for a normal metabolic and behavioural response to increased dietary fat. Thus, MC4−/− have a deficit in the normal response (reduced intake) to a high-fat diet, which may, besides a reduced metabolic response to high-fat diet, be explained by a deficit in sensing foods with an increased caloric density or by increased liking of fat foods. When given the choice between a high-fat, high-protein and high-carbohydrate diet, wild-type animals treated with MTII reduce specifically the intake of the high-fat diet, whereas the intake of high protein and high carbohydrate derived calories remains unchanged. This effect is absent in MC4 receptor−/−, suggesting that the MC4 receptor is necessary for the MTII-induced reduction of fat intake (Samama et al., 2003).
Taken together, accumulating evidence in rodents, but also in humans carrying mutations in the MC system, indicate that reduction of MC receptor activity is associated with preference for fat meals. However, there are long-term effects of different diets, with the ratio of fat to protein and carbohydrates in diets as important factors affecting the sensitivity for MC receptor agonists. It might be that the level of adiposity (which is increased by high-fat diets) sets the sensitivity of the brain MC system. Future studies need to clarify whether treatment with MC4 receptor agonists are able to shift this adiposity set point.
The sites in the brain where MC receptor signalling affects energy intake
The MC4 receptor is expressed in several sites in the brain, including hypothalamus, forebrain and hindbrain, that have been implicated in energy balance (Mountjoy et al., 1994; Kishi et al., 2003). This suggests that a large variety of processes and anatomical sites involved in energy balance are modified by MC4 receptors. Studies investigating the site of action of (an)orexigenic effects of MC receptor ligands within the hypothalamus and amygdala identified the paraventricular (PVN) and dorsomedial (DMH) hypothalamus and medial preoptic (MPO) area as primary sites (Figure 1) (Kim et al., 2000).
The PVN receives multiple projections from various areas in the brain and its central location makes it an integrator of signals regulating food intake and body weight. The PVN receives input from the ARC where neuropeptides of the MC system are produced. Local injection of the MC4 receptor agonists MTII or α-MSH into the PVN results in a reduction of food intake in mice as well as in rats. Interestingly, the effects of MCs on food intake are only observed when feeding is stimulated, for instance by the onset of the dark period, by fasting or by central NPY injection (Giraudo et al., 1998; Cowley et al., 1999; Wirth et al., 2001), which underscores an effect on meal termination rather than meal initiation. MC4 receptor antagonists such as AgRP, SHU9119 and HS014 stimulate feeding when administered directly into the PVN (Giraudo et al., 1998; Cowley et al., 1999; Kask and Schioth, 2000; Wirth and Giraudo, 2000). Again, these antagonists are merely effective when meal initiation is triggered, suggesting that signalling via the MC4 receptor in the PVN is implicated in meal duration rather than meal initiation.
A subpopulation of corticotropin-releasing hormone (CRH) neurons in the PVN contains MC4 receptors, and MTII induces a rapid induction of CRH gene transcription in the PVN (Lu et al., 2003). The CRH receptor antagonist α-helical-CRH(9–41) partly antagonizes the MTII-food-suppressing effect when injected intracerebroventricularly (i.c.v.), which suggest that CRH acts as a downstream mediator of MC signalling and contributes to the mechanisms by which the central MC system controls feeding (Lu et al., 2003). Thyrotropin-releasing hormone (TRH)-expressing neurons in the PVN also have MC4 receptors, and MC signalling increases TRH expression (Harris et al., 2001). It is not clear to what extent activation of TRH signalling contributes to the effect of MCs to suppress food intake.
As mentioned above, a second hypothalamic site that shows involvement of the MC system in appetite is the DMH. The DMH plays an important role in relaying information to neural pathways mediating neuroendocrine, autonomic and behavioural responses to stress. [norleucine4; D-phenylalanine7]-α-MSH and AgRP administration into the DMH result in hypophagia or hyperphagia, respectively (Kim et al., 2000; Wirth and Giraudo, 2001). Furthermore, AgRP administration in the DMH increases sucrose intake, whereas it does not affect the intake of an isocaloric product as corn starch, suggesting an influence of the palatability of a diet (Wirth and Giraudo, 2001).
Also, brainstem MC4 receptors are involved in feeding behaviour. MC4 receptors are expressed in the dorsal motor nucleus of the vagus nerve and in the nucleus of the tractus solitarius, both these brain centres are important for autonomic control. Grill et al. (1998) found that fourth ventricle administration of the MC4 receptor ligands MTII and SHU9119 has comparable effects on food intake as administration to the lateral ventricle, indicating that the caudal brainstem is contributing to the effects of the MC system on appetite. More specifically, Williams et al. (2000) injected MTII directly into the dorsal vagal complex (DVC) and this reduced food intake and body weight, whereas SHU9119 stimulates food intake, suggesting that brainstem effects on appetite are mediated by the DVC (Williams et al., 2000). Administration of SHU9119 in the third or fourth ventricle revealed that the CCK-mediated inhibition of food intake is affected by MC4 receptor activity in the brainstem but not in the hypothalamus (Fan et al., 2004). CCK-sensitive neurons in the nucleus of the tractus solitarius (NTS) have been implicated in mediating the effect of MCs to reduce meal size (Sutton et al., 2005).
Two other sites expressing MC receptors are the central amygdala (CeA) and the lateral hypothalamus (LH). The CeA is an important relay centre for taste perception. Whereas effects on food intake are absent after administration of the MC4 receptor agonist NDP-MSH into the CeA, the antagonists AgRP or HS014 do increase food intake, although to a lesser extent than injection into the PVN, DMH or DVC (Kask and Schioth, 2000; Kim et al., 2000). The LH connects the hypothalamus with the mesolimbic reward system and electrical stimulation of the LH induces food intake in satiated rats. The LH contains two types of neurons expressing orexigenic neuropeptides, namely melanin concentrating hormone (MCH) and orexin neurons. AgRP-increased food intake is associated with increased c-fos expression in orexin, but not MCH-containing neurons in the LH (Zheng et al., 2002). However, AGRP administered i.c.v. into rats augmented MCH but not orexin gene expression (Hanada et al., 2000). It remains to be determined whether MCH and/or orexin are implicated as downstream effectors of the MC system. MC4 receptor agonists or antagonists injected into the LH, arcuate nucleus, ventromedial hypothalamus and nucleus accumbens have little or no effect on food intake (Kask and Schioth, 2000; Kim et al., 2000). Taken together, multiple MC receptor-expressing sites in the brain have been implicated in regulating food intake with the PVN, DMH and DVC/NTS as the most sensitive sites. Further studies need to clarify which aspect of appetite is affected precisely when MC receptor activity in these brain sites is modulated.
Long-term disturbance of melanocortinergic signalling in specific brain sites
Most pharmacological studies show short-term effects on MC signalling and do not study the effects on body weight regulation. Moreover, pharmacological interventions are limited by the relatively short time of action of these ligands, which does not allow measurement of obesity development over weeks or months. By using mice that carry a lox-stop-lox MC4 receptor allele, which can be reactivated by Cre recombinase expression, Balthasar et al. (2005) recently demonstrated that in mice carrying a loss of function allele of the MC4 receptor, local re-expression of MC4 receptors in the PVN and a subpopulation of amygdala neurons normalizes excessive food intake and reduce body weight, but does not reduce energy expenditure (Balthasar et al., 2005). By means of a different, but complementary approach, Kas et al. (2004) demonstrated that inhibition of MC receptor signalling in the rat PVN by injecting an adeno-associated virus (AAV) expressing Agouti resulted in increased food intake and body weight (Kas et al., 2004). Thus, by locally rescuing the obese phenotype in a knockout mouse (Balthasar et al., 2005) and by a brain region-specific induction of an obese phenotype in a normally developed rat (Kas et al., 2004), these studies provide matching evidence for a role of MC signalling in the PVN for regulating food intake. These observations are consistent with studies showing that acute injections of MC ligands in the PVN affect food intake (Kim et al., 2000; Wirth et al., 2001).
Chronic inhibition of MC signalling through Agouti expression in the LH in rats (Kas et al., 2004) does not induce obesity on normal chow. However, it was discovered before that in MC4 receptor−/− mice, obesity is strongly increased by ongoing exposure to a high-fat diet (Butler et al., 2001). When exposed to a high-fat diet, LH AAV-agouti-injected rats develop an obesity phenotype, suggesting that MC signalling in the LH is involved in a normal response to high-fat diets (Kas et al., 2004). Thus, different aspects of MC regulation of energy balance, such as food intake, energy expenditure and coping with high-fat diets, are regulated by distinct nuclei in the brain expressing MC receptors.
A promising new development is to use viral vectors for delivery of short hairpin (sh) RNAs that knock down gene expression. A lentiviral system has been build now, which allows inducible micro-RNA-based shRNA expression under control of PolII promoters (Stegmeier et al., 2005). Certainly, this will contribute to further increase timing and cell-type specificity of gene knockdown. We have successfully generated transgenic mice, expressing an shRNA targeting the MC4 receptor. The transgenic MC4 receptor knockdown animals showed a similar phenotype as described for the MC4 receptor−/−. They had a 1.3-fold increase in body weight in comparison to their non-transgenic littermates and control lentiviral transgenic mice. This phenotype could be enhanced on a high-fat diet, as has been described for MC4 receptor−/− before (De Krom et al., unpublished observations).
The melanocortin 4 receptor as drug target
The MC4 receptor is an attractive candidate drug target to treat obesity, as it not only affects several aspects of feeding behaviour as discussed in this review, but activation of the MC system also increases insulin sensitivity and energy expenditure, part of which effect is independent of food intake (Banno et al., 2004; Heijboer et al., 2005). Several pharmaceutical industries are active in the development of MC4 receptor-specific drugs (Tian et al., 2005; Bakshi et al., 2006; Nicholson et al., 2006). It has proven difficult to design selective MC4 receptor agonists, in particular those that completely lack affinity for the MC3 receptor (Holder and Haskell-Luevano, 2004; Todorovic and Haskell-Luevano, 2005). However, mixed MC3/4 agonism might provide a therapeutic advantage, as reduced MC3 receptor activity (as in MC3−/− mice) has been associated with increased adipogenesis. Early rodent and human studies revealed some side effects of MC receptor agonists. Administration of MC4 receptor agonists is associated with penile erections as well as flushing, which has resulted in a new application area for these drugs: erectile dysfunction (Van der Ploeg et al., 2002; Diamond et al., 2004; Wessells et al., 2005). Preclinical studies have identified roles of the MC system in blood pressure regulation (MC receptor agonists have a depressor effect most probably via the NTS in brainstem) (Versteeg et al., 1998), in the inflammatory responses (where agonists limit thermogenic responses to pyrogens) (Catania et al., 2004) and in pain processing (where antagonists suppress pain sensation) (Vrinten et al., 2001). With the development of MC4 receptor agonists in the treatment of obesity, it should be carefully monitored whether these other MC effects provide unwanted side effects.
Concluding remarks
Interference within the central MC system (e.g. at the level of AgRP, POMC, MC3 or of MC4 receptors) revealed a wide variety of energy balance phenotypes. Hyperphagia and obesity are observed in both mice and humans with mutations in MC system. Reduction of MC receptor activity is associated with pushing the energy balance towards positive. The MC system affects multiple factors affecting energy balance, such as meal size, food choice and energy expenditure, which are controlled in different brain sites expressing MC receptors. The PVN plays a major role in MC4 receptor-mediated hyperphagia, whereas interactions between hypothalamic (e.g., LH) and mesolimbic signalling may play a role in the normal response to high-fat diets. The role of the MC system in feeding behaviour needs to be unravelled further, in order to select those groups of obese individuals that may benefit from treatment with MC4 receptor agonists. Careful analysis of feeding behaviour in humans treated with MC4 receptor agonists (when they become available for clinical studies) provides an interesting approach to achieve this.
Acknowledgments
This research is supported by the Technology Foundation STW, applied science division of NWO and the technology programme of the Ministry of Economic Affairs.
Abbreviations
- AgRP
agouti-related protein
- CCK
cholecystokinin
- CRH
corticotropin-releasing hormone
- CTA
conditioned taste aversion
- DMH
dorsomedial hypothalamus
- DVC
dorsovagal complex
- i.c.v.
intracerebroventricular
- LH
lateral hypothalamus
- LHSS
lateral hypothalamic self stimulation
- MC
melanocortin
- MCH
melanin concentrating hormone
- MSH
melanocyte-stimulating hormone
- MTII
melanotan-II
- NTS
nucleus of the tractus solitarius
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- TRH
thyrotropin-releasing hormone
- VMH
ventromedial hypothalamus
Conflict of interest
The authors state no conflict of interest.
References
- Adage T, Scheurink AJ, de Boer SF, de Vries K, Konsman JP, Kuipers F, et al. Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats. J Neurosci. 2001;21:3639–3645. doi: 10.1523/JNEUROSCI.21-10-03639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan RA, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Andersen GN, Hagglund M, Nagaeva O, Frangsmyr L, Petrovska R, Mincheva-Nilsson L, et al. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand J Immunol. 2005;61:279–284. doi: 10.1111/j.1365-3083.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- Argyropoulos G, Rankinen T, Bai F, Rice T, Province MA, Leon AS, et al. The agouti-related protein and body fatness in humans. Int J Obes Relat Metab Disord. 2003;27:276–280. doi: 10.1038/sj.ijo.802201. [DOI] [PubMed] [Google Scholar]
- Bakshi RK, Hong Q, Tang R, Kalyani RN, Macneil T, Weinberg DH, et al. Optimization of a privileged structure leading to potent and selective human melanocortin subtype-4 receptor ligands. Bioorg Med Chem Lett. 2006;16:1130–1133. doi: 10.1016/j.bmcl.2005.11.095. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Banno R, Arima H, Sato I, Hayashi M, Goto M, Sugimura Y, et al. The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats. Peptides. 2004;25:1279–1286. doi: 10.1016/j.peptides.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Barrera JG, Seeley RJ, Woods SC. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52:2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Becher E, Mahnke K, Hartmeyer M, Schwarz T, Scholzen T, et al. Evidence for the differential expression of the functional alpha-melanocyte-stimulating hormone receptor MC-1 on human monocytes. J Immunol. 1997;158:3378–3384. [PubMed] [Google Scholar]
- Biebermann H, Castaneda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, et al. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006;3:141–146. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bluher S, Ziotopoulou M, Bullen JW, Jr, Moschos SJ, Ungsunan L, Kokkotou E, et al. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53:82–90. doi: 10.2337/diabetes.53.1.82. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Gillett A. Control of food intake in the obese. Obes Res. 2001;9 Suppl 4:263S–270S. doi: 10.1038/oby.2001.129. [DOI] [PubMed] [Google Scholar]
- Boss O, Bergenhem N. Adipose targets for obesity drug development. Expert Opin Ther Targets. 2006;10:119–134. doi: 10.1517/14728222.10.1.119. [DOI] [PubMed] [Google Scholar]
- Boston BA. The role of melanocortins in adipocyte function. Ann NY Acad Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull. 1984;12:571–575. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11:1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000a;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000b;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D'Alessio D, et al. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144:2941–2946. doi: 10.1210/en.2002-0218. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res. 2004;16:51–59. doi: 10.1038/sj.ijir.3901139. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol. 2005;97:61–73. doi: 10.1111/j.1742-7843.2005.pto_179.x. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Z, Iben LG, Parker EM. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem Res. 1995;20:107–113. doi: 10.1007/BF00995160. [DOI] [PubMed] [Google Scholar]
- Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, et al. Effect of agouti-related protein (AGRP) in regulation of the hypothalamic–pituitary–thyroid (HPT) axis in the MC3-R KO mouse. Endocrinology. 2004;145:4816–4821. doi: 10.1210/en.2004-0476. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162:7446–7453. [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Wiegant VM, Greven HM, De Wied D. The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure–activity studies. Life Sci. 1975;17:645–652. doi: 10.1016/0024-3205(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am J Physiol Endocrinol Metab. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP-(83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol. 2001;280:R814–R821. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van der Ploeg LH, et al. Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, et al. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19:2362–2367. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–2590. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T. Differential regulation of melanin-concentrating hormone and orexin genes in the agouti-related protein/melanocortin-4 receptor system. Biochem Biophys Res Commun. 2000;268:88–91. doi: 10.1006/bbrc.1999.2081. [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Peptides. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, et al. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48:1621–1626. doi: 10.1007/s00125-005-1838-8. [DOI] [PubMed] [Google Scholar]
- Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, et al. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab. 2006;91:1761–1769. doi: 10.1210/jc.2005-2056. [DOI] [PubMed] [Google Scholar]
- Hirsch MD, O'Donohue TL, Wilson R, Sawyer TK, Hruby VJ, Hadley ME, et al. Structural and conformational modifications of alpha-MSH/ACTH4-10 provide melanotropin analogues with highly potent behavioral activities. Peptides. 1984;5:1197–1201. doi: 10.1016/0196-9781(84)90187-6. [DOI] [PubMed] [Google Scholar]
- Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure–activity relationship (SAR) studies. Med Res Rev. 2004;24:325–356. doi: 10.1002/med.10064. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jegou S, Boutelet I, Vaudry H. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol. 2000;12:501–505. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- Jonsson L, Skarphedinsson JO, Skuladottir GV, Watanobe H, Schioth HB. Food conversion is transiently affected during 4-week chronic administration of melanocortin agonist and antagonist in rats. J Endocrinol. 2002;173:517–523. doi: 10.1677/joe.0.1730517. [DOI] [PubMed] [Google Scholar]
- Kas MJ, Tiesjema B, van Dijk G, Garner KM, Barsh GS, Brake OT, et al. Induction of brain-region-specific forms of obesity by agouti. J Neurosci. 2004;24:10176–10181. doi: 10.1523/JNEUROSCI.3442-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Rago L, Wikberg JE, Schioth HB. Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC(3) receptor in the regulation of food intake. Neurosci Lett. 2000;283:1–4. doi: 10.1016/s0304-3940(00)00837-5. [DOI] [PubMed] [Google Scholar]
- Kask A, Schioth HB. Tonic inhibition of food intake during inactive phase is reversed by the injection of the melanocortin receptor antagonist into the paraventricular nucleus of the hypothalamus and central amygdala of the rat. Brain Res. 2000;887:460–464. doi: 10.1016/s0006-8993(00)03034-1. [DOI] [PubMed] [Google Scholar]
- Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, et al. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49:177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Altered hypothalamic signaling and responses to food deprivation in rats fed a low-carbohydrate diet. Obes Res. 2005;13:1672–1682. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Koegler FH, Schaffhauser RO, Mynatt RL, York DA, Bray GA. Macronutrient diet intake of the lethal yellow agouti (Ay/a) mouse. Physiol Behav. 1999;67:809–812. doi: 10.1016/s0031-9384(99)00104-3. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Kral TV. Effects on hunger and satiety, perceived portion size and pleasantness of taste of varying the portion size of foods: a brief review of selected studies. Appetite. 2006;46:103–105. doi: 10.1016/j.appet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, et al. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88:4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab. 2005;90:219–224. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Leiba H, Garty NB, Schmidt-Sole J, Piterman O, Azrad A, Salomon Y. The melanocortin receptor in the rat lacrimal gland: a model system for the study of MSH (melanocyte stimulating hormone) as a potential neurotransmitter. Eur J Pharmacol. 1990;181:71–82. doi: 10.1016/0014-2999(90)90246-3. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kneip J, Grace M, Morley JE. Effect of centrally administered neurotensin on multiple feeding paradigms. Pharmacol Biochem Behav. 1983;18:19–23. doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Catania A. Mechanisms of antiinflammatory action of the neuroimmunomodulatory peptide alpha-MSH. Ann NY Acad Sci. 1998;840:373–380. doi: 10.1111/j.1749-6632.1998.tb09576.x. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Rankinen T, Rice T, Rao DC, Leon AS, Skinner JS, et al. Two ethnic-specific polymorphisms in the human agouti-related protein gene are associated with macronutrient intake. Am J Clin Nutr. 2005;82:1097–1101. doi: 10.1093/ajcn/82.5.1097. [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo–pituitary–adrenal responses. J Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype–phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab. 2006;91:1811–1818. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12:145–153. doi: 10.1093/hmg/ddg016. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- MacKenzie RG. Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides. 2006;27:395–403. doi: 10.1016/j.peptides.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV. Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci. 2002;3:18. doi: 10.1186/1471-2202-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999a;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999b;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- Mayfield DK, Brown AM, Page GP, Garvey WT, Shriver MD, Argyropoulos G. A role for the agouti-related protein promoter in obesity and type 2 diabetes. Biochem Biophys Res Commun. 2001;287:568–573. doi: 10.1006/bbrc.2001.5600. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and (corrected) in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Muceniece R, Zvejniece L, Liepinsh E, Kirjanova O, Baumane L, Petrovska R, et al. The MC(3) receptor binding affinity of melanocortins correlates with the nitric oxide production inhibition in mice brain inflammation model. Peptides. 2006;27:1443–1450. doi: 10.1016/j.peptides.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317:771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Garner KM, van Rozen RJ, Adan RA. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem. 2003;278:22939–22945. doi: 10.1074/jbc.M211326200. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Rohner-Jeanrenaud F, Craft LS, Bridwell J, Suter TM, Tinsley FC, Smiley DL, et al. Chronic central infusion of cocaine- and amphetamine-regulated transcript (CART 55–102): effects on body weight homeostasis in lean and high-fat-fed obese rats. Int J Obes Relat Metab Disord. 2002;26:143–149. doi: 10.1038/sj.ijo.0801863. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000;141:850–853. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- Samama P, Rumennik L, Grippo JF. The melanocortin receptor MCR4 controls fat consumption. Regul Peptides. 2003;113:85–88. doi: 10.1016/s0167-0115(02)00299-9. [DOI] [PubMed] [Google Scholar]
- Santini F, Maffei M, Ceccarini G, Pelosini C, Scartabelli G, Rosellini V, et al. Genetic screening for melanocortin-4 receptor mutations in a cohort of Italian obese patients: description and functional characterization of a novel mutation. J Clin Endocrinol Metab. 2004;89:904–908. doi: 10.1210/jc.2003-031175. [DOI] [PubMed] [Google Scholar]
- Schick RR, Stevens CW, Yaksh TL, Go VL. Chronic intraventricular administration of cholecystokinin octapeptide (CCK-8) suppresses feeding in rats. Brain Res. 1988;448:294–298. doi: 10.1016/0006-8993(88)91266-8. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Burklow ML, Wilmer KA, Matthews CC, Reizes O, McOsker CC, et al. The effect of the melanocortin agonist, MT-II, on the defended level of body adiposity. Endocrinology. 2005;146:3732–3738. doi: 10.1210/en.2004-1663. [DOI] [PubMed] [Google Scholar]
- Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26:1720–1727. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, et al. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes. 2001;50:248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- Small CJ, Liu YL, Stanley SA, Connoley IP, Kennedy A, Stock MJ, et al. Chronic CNS administration of agouti-related protein (Agrp) reduces energy expenditure. Int J Obes Relat Metab Disord. 2003;27:530–533. doi: 10.1038/sj.ijo.0802253. [DOI] [PubMed] [Google Scholar]
- Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, York DA, Bray GA. Chronic cerebroventricular galanin does not induce sustained hyperphagia or obesity. Peptides. 1994;15:1267–1272. doi: 10.1016/0196-9781(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, et al. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci USA. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- Thody AJ, Cooper MF, Bowden PE, Meddis D, Shuster S. Effect of alpha-melanocyte-stimulating hormone and testosterone on cutaneous and modified sebaceous glands in the rat. J Endocrinol. 1976;71:279–288. doi: 10.1677/joe.0.0710279. [DOI] [PubMed] [Google Scholar]
- Tian X, Field T, Mazur AW, Ebetino FH, Wos JA, Crossdoersen D, et al. Design, synthesis, and evaluation of proline based melanocortin receptor ligands. Bioorg Med Chem Lett. 2005;15:2819–2823. doi: 10.1016/j.bmcl.2005.03.120. [DOI] [PubMed] [Google Scholar]
- Todorovic A, Haskell-Luevano C. A review of melanocortin receptor small molecule ligands. Peptides. 2005;26:2026–2036. doi: 10.1016/j.peptides.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Bray GA. Acetylation alters the feeding response to MSH and beta-endorphin. Brain Res Bull. 1989;23:165–169. doi: 10.1016/0361-9230(89)90142-1. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, et al. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci USA. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiol Behav. 2005;84:129–133. doi: 10.1016/j.physbeh.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Versteeg DH, Van Bergen P, Adan RA, de Wildt DJ. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- Vijayan E, McCann SM. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH) Endocrinology. 1977;100:1727–1730. doi: 10.1210/endo-100-6-1727. [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. Neuropathic pain: a possible role for the melanocortin system. Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005;26:1972–1977. doi: 10.1016/j.peptides.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikberg JE. Melanocortin receptors: perspectives for novel drugs. Eur J Pharmacol. 1999;375:295–310. doi: 10.1016/s0014-2999(99)00298-8. [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, et al. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000;42:393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Weiss SM, Baird JP, Kaplan JM. Behavioral processes underlying the intake suppressive effects of melanocortin 3/4 receptor activation in the rat. Psychopharmacology (Berl) 2002;161:47–53. doi: 10.1007/s00213-002-1022-5. [DOI] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Giraudo SQ. Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides. 2000;21:1369–1375. doi: 10.1016/s0196-9781(00)00280-1. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Giraudo SQ. Effect of agouti-related protein delivered to the dorsomedial nucleus of the hypothalamus on intake of a preferred versus a non-preferred diet. Brain Res. 2001;897:169–174. doi: 10.1016/s0006-8993(01)02087-x. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ. Paraventricular hypothalamic alpha-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides. 2001;22:129–134. doi: 10.1016/s0196-9781(00)00367-3. [DOI] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, et al. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. NeuroReport. 1995;6:2193–2196. doi: 10.1097/00001756-199511000-00022. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, et al. Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern MM, Crousillac SM, Patterson LM, Phifer CB, Berthoud HR. Neurochemical phenotype of hypothalamic neurons showing Fos expression 23 h after intracranial AgRP. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1773–R1781. doi: 10.1152/ajpregu.00019.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]