Abstract
Background:
Recent studies have implicated the mitogen activated protein kinase (MAPK) in cellular permeability changes following P2X7 receptor activation in native tissues. In this study we have further studied the effect of MAPK inhibitors on recombinant and native P2X7 receptors.
Experimental Approach:
The MAPK inhibitors SB-203580, SB-202190 and SB-242235 were examined in HEK293 cells expressing recombinant P2X7 receptors and in THP-1 cells expressing native human P2X7 receptors using a range of experimental approaches.
Key results:
At human recombinant P2X7 receptors, SB-203580 and SB-202190 were weak, non-competitive inhibitors (pIC50= 4.8 - 6.4) of ethidium accumulation stimulated by 2'- & 3'-O-(4benzoylbenzoyl)-ATP (BzATP) but SB-242235 (0.1-10μM) had no effect. SB-203580 and SB-202190 had no effect on rat or mouse recombinant P2X7 receptors and studies with chimeric P2X7 receptors suggested that SB-203580 was only effective in chimeras containing the N-terminal 255aa of the human P2X7 receptor. SB-203580 did not consistently affect BzATP-mediated increases in cell calcium levels and, in electrophysiological studies, it slightly decreased responses to 30μM BzATP but potentiated responses to 100μM BzATP. In THP1 cells, SB-203580 modestly inhibited BzATP-stimulated ethidium accumulation (pIC50 5.7 – <5) but SB-202190 had no effect. Finally, SB-203580 did not block BzATP-stimulated interleukin-1β release in THP-1 cells.
Conclusions:
This study confirms that high concentrations of SB-203580 and SB-202190 can block human P2X7 receptor-mediated increases in cellular ethidium accumulation but suggest this is not related to MAPK inhibition. Overall, the data cast doubt on a general role of MAPK in mediating P2X7 receptor mediated changes in cellular permeability.
Keywords: P2X7 receptor, MAP Kinase, SB203580, SB202190, ethidium bromide
Introduction
The P2X7 receptor for extracellular ATP, formerly known as the P2Z receptor, exhibits a considerable degree of plasticity in function and affects a wide range of cellular functions. Like other members of the P2X receptor family it functions as an ATP-activated ligand-gated cation channel permeable to Na+, K+ and Ca2+ ions following brief (ms-second) exposures to ATP (Surprenant et al., 1996). However, with prolonged activation (seconds–minutes) the channel properties change dramatically and the channel appears to dilate (Surprenant et al., 1996) or lead to activation of other non-selective channels that are permeable to larger molecules with a MW of up to 800 Da. For simplicity this property will be hereafter referred to as a change in cellular permeability. In addition to these complex effects on cellular permeability the receptor is able to activate a wide range of cellular events. These include stimulation of enzymes such as phospholipase A2, protein kinases and caspases, activation of transcription factors such as NFAT and NF-κB, cytokine release, changes in the cell cytoskeleton, including membrane blebbing and phosphatidylserine translocation, and ultimately to cell lysis and cell death (see North, 2002 and Auger et al., 2005 for references).
There is still debate about the mechanisms underlying the cellular permeability changes that occur following prolonged activation of the channel. Thus, electrophysiological studies on the recombinant receptors expressed in HEK293 cells appeared to have clearly shown that the P2X7, as well as the P2X2 and P2X4, channels can dilate following prolonged activation and that this presumably underlies the change in cellular permeability to molecules with a MW of up to 800 Da (Surprenant et al., 1996, Virginio et al., 1999, Khakh et al., 1999). However, other studies suggest that the permeability of the P2X7 receptor does not change following activation (Petrou et al., 1997, Klapperstuck et al., 2000) or that the changes in cellular permeability occur as a result of the activation of membrane channels or transporters distinct from the P2X7 receptor (Faria et al., 2005, Jiang et al., 2005).
Recent studies have suggested that activation of mitogen-activated protein kinase (MAPK) may be important for the changes in cellular permeability that occur following P2X7 receptor activation. Thus, MAPK inhibitors such as SB-2020190 or SB-203580 have been shown to block P2X7 receptor-mediated changes in cellular permeability in human THP-1 cells (Donnelly-Roberts et al., 2004) and in mouse macrophages (Faria et al., 2005). In the present study, we have investigated the effects of MAPK inhibitors on recombinant P2X7 receptors and partially confirmed their ability to block human P2X7 receptor-mediated permeability changes but also demonstrate that these effects are not observed with rat and mouse P2X7 receptors, not observed with all MAPK inhibitors and may be unrelated to effects on MAPK.
Methods
Test systems used
Assay buffers
For the ethidium accumulation studies, the assay buffer comprised (in mM): HEPES 10, N-methyl-D-glucamine 5, KCl 5. 6, D-glucose 10, CaCl2 0.5 (pH 7.4) and were supplemented with either 280 mM sucrose (sucrose buffer) or 140 mM NaCl (NaCl buffer). In studies on THP-1 cells, the 0.5 mM CaCl2 was omitted and 0.05 mM EDTA included (sucrose-EDTA buffer).
For studies on interleukin-1β (IL1β) release, NaCl buffer containing 0.1 mM calcium was used. For electrophysiological studies the extracellular solution contained (in mM): NaCl 145, KCl 2, CaCl2 0.5, HEPES 10, D-glucose 10 (pH 7.3, osmolarity 300 mOsm).
Construction of chimeric P2X7 receptors
Rat (Surprenant et al., 1996), human (Rassendren et al., 1997) and mouse (Chessell et al., 1998) P2X7 receptor cDNAs in pcDNA3.1 were bisected at a unique BglII restriction site (5′-A↓GATC↑-3′) at the base position equivalent with amino acid (aa) 255 within the coding region of the receptor and the corresponding N and C termini ligated to form the various chimeric receptors. The resulting cDNA fragments were directionally subcloned into pcDNA3.1 (+ or −) vectors, the cDNA were transfected into HEK293 cells and stable clonal cell lines expressing the chimeric receptors were selected using standard procedures. In total, four chimeric receptors were used in this study. These are referred to as human–rat, human–mouse, mouse–human and rat–human, P2X7 receptors. These chimeric receptors are described in terms of the origins of the N and C termini, respectively. For example, in the human–rat chimeric receptor the first 255 aa sequence originated from the human receptor while the C-terminal region (post aa 255) was derived from the rat receptor. Note that an additional HindIII restriction site was engineered into the non-coding region of the rat P2X7 receptor by site-directed mutagenesis to enable an additional BglII site, originally present in the 3′ untranslated region of this receptor, to be removed.
Measurements made
Ethidium accumulation measurements in HEK293 cells
Studies were performed as described previously (Hibell et al., 2001) with minor modifications. HEK293 cells, expressing rat, mouse, human or chimeric recombinant P2X7 receptors, were grown in poly-L-lysine pre-treated 96-well plates (Costar, High Wycombe, UK) for 18–24 h. Thereafter, cells were washed twice with 350 μl of assay buffer and incubated for 20 min at room temperature (19–21°C) in the presence or absence of MAPK inhibitor before addition of ATP or 2′- & 3′-O-(4benzoylbenzoyl) ATP (BzATP) and ethidium (100 μM final assay concentration). Incubations were continued until approximately 10–30% of maximal dye accumulation occurred (see below). Reactions were rapidly terminated by addition of 25 μl of 1.3 M sucrose assay buffer containing 5 mM of the P2X7 receptor antagonist, reactive black 5. Cellular accumulation of ethidium in the cell monolayer was determined by measuring fluorescence (excitation wavelength of 530 nm and emission wavelength of 620 nm) from below the plate with a Canberra Packard Fluorocount (Pangbourne, UK).
Agonist exposure times in these studies were adjusted to ensure that measurements of agonist potency were made when approximately 10–30% of the maximal agonist-stimulated dye accumulation had occurred. Thus, in sucrose buffer, incubation times with agonist were 1.5, 2 and 10 min when studying rat, human and mouse receptors, respectively. In NaCl buffer, incubation times with agonist were 4 and 8 min when studying rat and human receptors, respectively. For the human–rat, rat–human, human–mouse and mouse–human chimeric receptors agonist exposure times were 2, 2, 8 and 16 min, respectively.
Ethidium accumulation measurements in THP-1 cells
The human THP-1 promonocytic cell line was grown as a suspension culture in RPMI 1640 media containing 10% foetal bovine serum at 37°C in a humidified atmosphere (95% air, 5% CO2). Cells (50 000 well−1) were added to poly-lysine 96-well plates (Costar, High Wycombe, UK) and differentiated by addition of 100 nM phorbol 12-myristate 13-acetate (PMA) for 3 h and adherent cells were cultured for 18–24 h before use. Ethidium accumulation studies were performed as described for HEK293 cells above with the following modifications. Calcium was omitted from the assay buffer and 0.1 mM EDTA included in its place (sucrose-EDTA buffer) and agonist exposure time was 30 min.
Electrophysiological studies
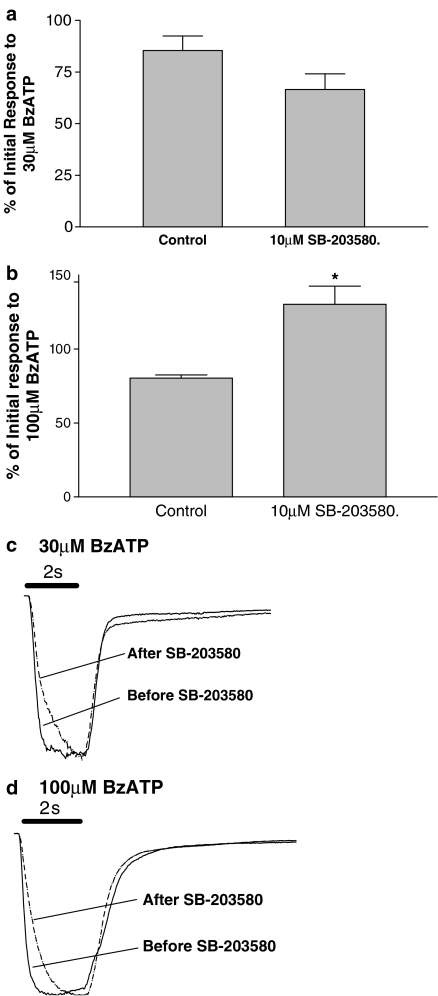
Studies were conducted essentially as described previously (Hibell et al., 2000). Briefly, HEK293 cells expressing recombinant P2X7 receptors were plated onto coverslips and, after overnight culture (18 h), were transferred to a perfused recording chamber (volume approximately 400 μl, flow rate 2 ml min−1). Agonist-evoked inward currents were recorded in NaCl extracellular solution (see above) using the whole cell configuration of the patch clamp technique (Hamill et al., 1981). Patch electrodes, with resistances of 3–8 MΩ, were pulled from 1.2 mm borosilicate glass (GC120F-10, Clarke Electromedical Supplies, Pangbourne, UK). Electrodes were filled with internal solution (in mM): Cs aspartate 145, EGTA 11, HEPES 5, NaCl 2 (pH 7.3, osmolarity 290 mOsm). In all experiments, cells were voltage clamped at –90 mV (junction potential was uncorrected). Responses to a 2 s application of BzATP (30 and 100 μM, P2X7) were recorded before and after a 20 min pre-incubation with 10 μM SB-203580 or its vehicle that was applied in the extracellular solution bathing the cells. Responses in the control and SB-203580 treated cells were expressed as a percentage of the initial response in each case to account for any time-dependent changes in BzATP response. In the studies illustrated in Figure 7c and d, the inward currents in cells incubated with SB-203580 or its vehicle were normalized to the maximum inward current produced in each cell and then these normalized data were averaged to provide mean data to illustrate the kinetics of responses.
Figure 7.
The effect of SB-203580 on BzATP-induced inward currents in cells expressing human P2X7 receptors. Inward currents evoked by 30 or 100 μM BzATP were obtained before (control) and after pre-incubation with 10 μM SB-203580 for 20 min. Subsequently, 30 μM (a) or 100 μM (b) BzATP was added in the continued presence of 10 μM SB-203580. (a, b) The effects of SB-203580 on peak responses to BzATP while (c, d) show normalized and averaged currents over time to illustrate changes in kinetics of the response. In (c, d) Horizontal bars represent the 2 s drug application. For the control cells, the control mean peak currents at 30 and 100 μM BzATP were 835 and 3060 pA, respectively. For the SB-203580-treated cells the control mean peak currents at 30 and 100 μM BzATP were 758 and 2097 pA, respectively. The data are the mean±s.e.m. from three separate experiments. *Significantly different from control group (Student's t-test).
Measurement of [Ca2+]i using a FLIPR
HEK293 cells stably expressing the human P2X7 receptor were plated at 30 000 cells well−1 in black-walled clear-bottomed 96-well plates (Costar, UK) 24 h before use, and incubated under 5% CO2 at 37°C. Cells were loaded with the calcium sensitive fluorescent dye Fluo-4AM (2 μM) for 2 h at room temperature and then washed four times in Tyrodes buffer (mM: NaCl 145, KCl 2.5, HEPES 10, glucose 10, CaCl2 0.5, pH7.4). Thereafter, cells were incubated at room temperature for 25 min in the presence or the absence of SB-203580 before addition of BzATP. Changes in cell calcium were monitored on a FLIPR (Molecular Devices, UK) by measuring fluorescence (excitation wavelength of 488 nm and emission wavelength of 540). Data were expressed as a percentage of the maximal BzATP response in the absence of SB-203580.
BzATP-stimulated IL1β release from LPS-treated THP-1 cells
Studies were performed as described previously (Buell et al., 1998). Briefly, THP-1 cells were pre-treated for 18 h with 10 μg ml−1 of lipopolysaccharide (LPS). Cells were harvested and resuspended in NaCl buffer containing 0.1 mM calcium and pre-incubated with SB-203580 for 40 min at 37°C before addition of BzATP. Reactions were stopped after 30 min at 37°C by addition of ice-cold DMEM media containing 10% FBS. The cells were centrifuged at 150 g for 5 min and 10 μl of the supernatant removed for determination of mature IL1β content using an A549 cell bioassay that only detects released mature IL1β (Buell et al., 1998). Data were expressed as a percentage of the maximal BzATP-stimulated IL1β release measured in the absence of SB-203580.
Experimental design
Irreversible blockade of human P2X7 receptors with OxATP and receptor protection studies
In some ethidium accumulation experiments, human P2X7 receptor expression levels were reduced by pre-incubating HEK293 cells with 100 μM of the irreversible antagonist, periodate oxidized ATP (OxATP), for 60 min in NaCl buffer at 37°C. The cells were then washed three times with NaCl assay buffer before use in experiments with SB-203580 and SB-202190 (see Figure 2).
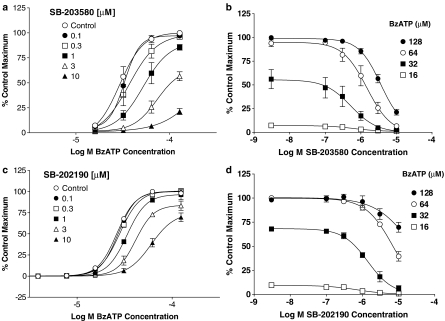
Figure 2.
Potency (pIC50) of SB-203580 and SB-202190 as a function of BzATP concentration in ethidium accumulation studies. (a) pIC50 values for SB-203580 and SB-202190, obtained from Figure 1b and d, respectively, are plotted at each of the BzATP concentrations. In b) data obtained from similar studies in which cells were pre-incubated with buffer or 100 μM OxATP for 40 min before studying the effects of SB-202190 or SB-203580, as in Figure 1, are presented. The data are the mean±s.e.m. of three separate experiments.
In other studies (see Figure 4), the ability of SB-203580 to prevent the irreversible blockade of the human P2X7 receptor produced by OxATP was examined by pre-incubating cells with either SB-203580 (10 μM) or pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) in sucrose buffer for 5 min at 37°C before addition of various concentrations of OxATP in the absence or continued presence of SB-203580 or PPADS. After 40 min incubation at 37°C, the cells were washed three times, by repeated aspiration of solutions and addition of 350 μl of NaCl assay buffer. Cells were incubated for 90 min at 37°C, to enable complete dissociation of SB-203580 or PPADS, before addition of a mixture of 100 μM ethidium and 2 mM ATP. After 8 min, reactions were terminated and cellular accumulation of ethidium was measured as described above.
Figure 4.
Antagonism of OxATP receptor inactivation by PPADS, but not SB-203580. Cells expressing human P2X7 receptors were pre-incubated with PPADS (1 μM) or SB-203580 (10 μM) for 5 min in sucrose buffer at 37°C before addition of OxATP. After a further 40 min incubation, cells were washed three times with NaCl buffer and incubated at 37°C for 90 min before measuring ATP-stimulated ethidium accumulation. The data are expressed as a percentage of the maximal ATP-induced ethidium accumulation measured in control cells. The data are the mean±s.e.m. of three separate experiments.
Data analysis
In all studies the data are the mean±s.e.m. of three to four experiments. All curve fitting and statistical analysis was performed using GraphPad Prism 3 (GraphPad Software Inc., San Diego, CA, USA).
Drugs, chemical reagents and other materials
ATP was obtained from Promega (Southampton, UK) or Sigma (Poole, UK). BzATP, ethidium, LPS, OxATP, PMA, reactive black 5 and SB-202190, were obtained from Sigma (Poole, UK). PPADS was from Tocris (Bristol, UK). SB-203580 and SB-242235 was obtained from Sigma or obtained from GSK (Harlow, UK). Fluo-4AM was from Teflabs, Austin, USA. All culture media were obtained from Invitrogen (Paisley, Scotland) while other reagents were obtained from Fisons (Loughborough, UK).
Results
Effects of MAPK inhibitors at human recombinant P2X7 receptors
The MAPK inhibitor, SB-203580, produced a concentration-dependent antagonism of BzATP-induced ethidium accumulation in HEK293 cells expressing the human P2X7 receptor (Figure 1a and b). In NaCl buffer, the pIC50 values for SB-203580 varied from 6.4 to 5.4 depending upon agonist concentration (Figure 2a).
Figure 1.
Antagonism by SB-203580 or SB-202190 of BzATP-induced ethidium accumulation in HEK293 cells expressing human recombinant P2X7 receptors. Studies were performed in NaCl buffer at 19–21°C. (a, c) Concentration–effect curves to BzATP in the presence of SB-203580 and SB-202190. (b, d) Inhibition curves at each of the BzATP concentrations used in (a, c), respectively, are presented. The data are expressed as a percentage of the maximal BzATP-induced ethidium accumulation measured in the control group. Basal accumulation, which represented 4% of the maximum, has been subtracted. The data are the mean±s.e.m. of three separate experiments.
The MAPK inhibitor, SB-202190, also antagonized human P2X7 receptor-mediated responses (Figure 1c and d) but was generally less potent than SB-203580 (Figures 1 and 2a) with pIC50 values varying from 6.2 to 4.8, depending on BzATP concentration. In marked contrast, another MAPK inhibitor, SB-242235, which has some structural differences to SB-202190 and SB-203580, had no effect at concentrations up to 10 μM (e.g. responses to 64 μM BzATP in the presence of 0, 0.1, 0.3, 1, 3 and 10 μM SB-242235 were 82±11, 84±10, 85±9, 84±7, 85±4 and 87±2 of the control maximal response, respectively).
Effect of receptor density on potency of MAPK inhibitors
To assess if receptor density affected the potency of the MAPK inhibitors, cells expressing human P2X7 receptors were treated with OxATP for 1 h and then potencies of MAPK inhibitors were assessed. OxATP decreased the maximal response to BzATP but there was no marked difference in MAPK inhibitor potency in control and OxATP treated cells (Figure 2b).
Mechanism of action studies with SB-203580
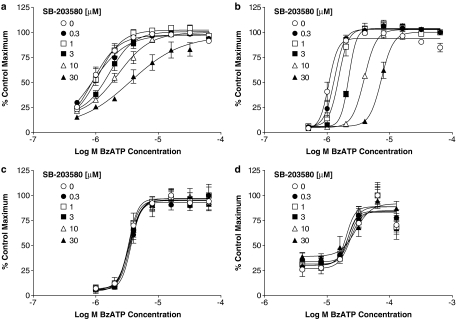
In order to evaluate if SB-203580 was a competitive antagonist, additional studies were performed in sucrose buffer to enable a greater range of agonist concentrations to be used. In sucrose buffer, SB-203580, produced a decrease in agonist pEC50 with no effect on the maximal response at human P2X7 receptors (Figure 3a). However, the Schild plot possessed a slope of 0.5 (data not shown) suggesting that SB-203580 was not behaving as a competitive antagonist of the human P2X7 receptor.
Figure 3.
Antagonism by SB-203580 of BzATP-induced ethidium accumulation in HEK293 cells expressing human, rat or mouse recombinant P2X7 receptors. Studies were performed in sucrose buffer at 19–21°C. The figure shows the effects of SB-203580 on human (a), rat (b) or mouse (c) P2X7 receptors. (d) Inhibition curves for SB-203580 against 1 μM (rat), 4 μM (human) or 64 μM (mouse) BzATP concentrations are presented. In all figures the data are expressed as a percentage of the maximal BzATP-induced ethidium accumulation measured in the control group. Basal accumulation, which represented 10–20% of the maximum, has been subtracted. The data are the mean±s.e.m. of four separate experiments. A Schild plot of the data from (a) is inset into (a).
The effects of SB-203580 were rapid in onset and offset in both sucrose and NaCl assay buffers (data not shown). Thus, similar effects of SB-203580 were observed with or without pre-incubation, suggesting that equilibrium of SB-203580 binding is achieved within at least 2–8 min, depending on the buffer used. The effects of SB-203580 were also rapidly reversible with complete washout of effects within 10 min (data not shown).
We have previously shown that PPADS can protect P2X7 receptors from irreversible blockade by OxATP (Michel et al., 2000). PPADS (1 μM) clearly prevented the ability of OxATP to irreversibly block the human P2X7 receptor whereas SB-203580 (10 μM) had little effect (Figure 4).
Effects of MAPK inhibitors on rat, mouse and chimeric P2X7 receptors
In contrast to their effects at human recombinant P2X7 receptors, neither SB-20350 nor SB-202190 had any effect on BzATP or ATP-stimulated ethidium accumulation in cells expressing rat or mouse recombinant P2X7 receptors when examined at concentrations up to 10 μM (see Figure 3b–d for data with SB-203580, data for SB-202190 not shown).
To further examine the species selectivity of MAPK inhibitors, additional studies were performed in cells expressing chimeric P2X7 receptors (Figure 5). SB-203580 was effective in cells expressing chimeric P2X7 receptors with an N-terminal sequence (255 aa) of human origin (human–mouse and human–rat receptors, Figure 5a and b) but had no effect in cells expressing chimeric receptors with the N-terminal sequence (255 aa) from either the rat (rat–human, Figure 5c) or mouse (mouse–human receptors, Figure 5d) P2X7 receptor.
Figure 5.
Antagonism by SB-203580 of BzATP-induced ethidium accumulation in HEK293 cells heterologously expressing chimeric human, rat or mouse P2X7 receptors. Studies were performed in sucrose buffer at 19–21°C. Cells expressing human–rat (a), human–mouse (b), rat–human (c) or mouse–human (d) chimeric P2X7 receptors were pre-incubated with the indicated concentrations of SB-203580 for 40 min and then BzATP-induced ethidium accumulation was measured over a 2 min (human–rat, rat–human), 8 min (human–mouse) or 16 min (mouse–human) period in the continued presence of SB-203580. In all figures, the data are expressed as a percentage of the maximal BzATP-induced ethidium accumulation measured in the control group. The data are the mean±s.e.m. of four separate experiments.
Effect of MAPK inhibitors in THP-1 cells
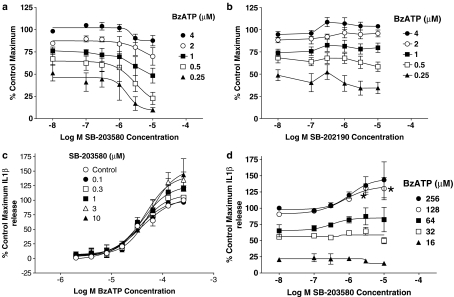
THP-1 cells express endogenous P2X7 receptors and were used in the studies of Donnelly-Roberts et al. (2004). Our studies with THP-1 cells could only be conducted on PMA-treated cells as interferon-γ and LPS-treated cells did not adhere to the culture plates. In PMA-treated THP-1 cells, BzATP-stimulated ethidium accumulation could be measured in sucrose-EDTA and in this buffer SB-203580 produced a modest inhibition of responses while SB-202190 produced very little effect (Figure 6a and b). Note that KN62 produced a marked inhibition of responses to BzATP in the THP-1 cells (pIC50=7.7±0.05 vs 1 μM BzATP).
Figure 6.
Effects of MAPK inhibitors in THP-1 cells. (a, b) The effect of SB-203580 (a) or SB-202190 (b) on BzATP-induced ethidium accumulation in PMA-differentiated THP-1 cells expressing human, native P2X7 receptors. Studies were performed in sucrose-EDTA buffer at 19–21°C. The data are the mean±s.e.m. of five separate experiments and are expressed as a percentage of the maximal BzATP-induced ethidium accumulation measured in the control group. Basal accumulation, which represented 40% of the maximum, has been removed. (c, d) The effect of SB-203580 on BzATP-induced IL1β release from LPS-treated THP-1 cells. (c) The effect of the indicated concentrations of SB-203580 on responses to BzATP while (d) shows inhibition curves for SB-203580 at selected concentrations of BzATP used. The data are expressed as a percentage of the maximal BzATP-induced IL1β release (1.7±0.8 ng IL1β) measured in the control group. Basal release, which represented 13% of the maximum, has been removed. The data are the mean±s.e.m. of three separate experiments.
Although SB-203580 produced a slight inhibition of ethidium accumulation in THP-1 cells it did not block BzATP-stimulated IL1-β release from THP-1 cells (Figure 6c). Indeed, it even modestly increased responses to the higher concentrations of BzATP, although this was only significant (P<0.05, one-way ANOVA with Dunnetts test) for 128 μM BzATP (Figure 6d).
Effect of SB-203580 on P2X7 receptor mediated electrophysiological responses and changes in intracellular calcium
In electrophysiological studies, SB-203580 (10 μM) produced complex effects on responses to BzATP (Figure 7). The rise times of currents elicited by 30 and 100 μM BzATP were increased two- to threefold (30 μM BzATP t1/2 control=177±9 ms, t1/2 SB-203580=437±32 ms; 100 μM BzATP t1/2 control=120±3 ms, t1/2 SB-203580=316±92 ms). The plateau response to 30 μM BzATP was not significantly decreased whereas that to 100 μM BzATP was significantly increased. SB-203580 (10–10 000 nM) produced no consistent effects on P2X7 receptor-mediated increases in intracellular calcium when studied in the FLIPR assay (Figure 8).
Figure 8.
Effect of SB-203580 on BzATP-induced increases in cell calcium. Studies were performed in HEK293 cells heterologously expressing the human P2X7 receptor. (a) The effect of the indicated concentrations of SB-203580 on responses to BzATP while (b) shows inhibition curves for SB-203580 at selected concentrations of BzATP used in (a). In both figures the data are expressed as a percentage of the maximal BzATP-induced calcium increase measured in the control group. The data are the mean±s.e.m. of four separate experiments.
Discussion
The main aim of this study was to evaluate the effect of MAPK inhibitors on recombinant P2X7 receptor function. The results confirm the ability of high concentrations of SB-203580 and SB-202190 to affect human P2X7 receptor-mediated changes in cellular permeability but also show that these effects are modest, not observed on rat or mouse P2X7 receptors and that another MAPK inhibitor, SB-242235, has no effect. Furthermore, although SB-203580 affected P2X7 receptor-mediated cellular permeability changes it had little or no effect on other measures of human P2X7 receptor function.
In agreement with previous studies, we found that SB-203580 was a relatively weak antagonist of human P2X7 receptor-mediated ethidium accumulation at native and recombinant human P2X7 receptors. Antagonist potency was highly dependent upon agonist concentration with pIC50 values varying from 5.4 to 6.4. The lower potency estimate is close to the IC50 of 9 μM reported in THP-1 cells (Donnelly-Roberts et al., 2004). In contrast, SB-202190 was inactive in THP-1 cells and was shown to have a pIC50 of 6.2–4.8 at recombinant human P2X7 receptors. The low potency of SB-202190 in THP-1 cells contrasts with the relatively potent inhibition previously reported in these cells (IC50 75 nM; Donnelly-Roberts et al., 2004) but the potency in our recombinant assays is similar to the IC50 of 1.5 μM previously reported at human recombinant P2X7 receptors in 1321N1 astrocytoma cells (Donnelly-Roberts et al., 2004). The difference in potency of SB-202190 between native and recombinant P2X7 receptors was anecdotally attributed to differences in receptor expression (Donnelly-Roberts et al., 2004). However, when we reduced P2X7 receptor levels in HEK293 cells by using receptor inactivation with OxATP, the potency of SB-203580 and SB-202190 was not greatly affected suggesting that receptor density differences do not readily explain the differences in the potency of SB-202190 between cell types.
It is difficult to provide a definitive explanation for the differences between the various studies but several explanations can be suggested. First, there may be multiple mechanisms for ethidium accumulation in cells and it is possible that our HEK293 and THP-1 cells lack one of these key mechanisms regulated by MAPK. Certainly there is evidence for a maitotoxin-sensitive change in cellular permeability in THP-1 and other cells (Verhoef et al., 2004, Schilling et al., 1999, Lundy et al., 2004) that is separate from the P2X7 receptor and more recent studies have also suggested that ethidium may accumulate in cells through a mechanism distinct from the P2X7 ion channel (Jiang et al., 2005). Secondly, we found SB-203580 potency exhibited a very strong dependence on agonist concentration with IC50 values varying 10-fold when agonist concentration was increased only fourfold. In the previous studies, MAPK inhibitors were only examined against a single agonist concentration and it is possible this was optimal for demonstrating high potency of SB-202190.
The effect of SB-202190 in THP-1 cells was utilized to suggest a central role of the MAPK pathway in the cellular permeability changes following P2X7 receptor activation in THP-1 cells (Donnelly-Roberts et al., 2004). While it still remains possible that P2X7 receptor activation can lead to cellular permeability changes through activation of a MAPK-dependent pathway, the present results cast doubt on the universal role of MAPK in mediating the permeability changes following native and recombinant P2X7 receptor activation for a number of reasons. First, we could not observe any effect of SB-202190 in our THP-1 cells that may or may not, reflect differences in cell lineage or assay conditions. Second, the concentrations of SB-203580 and SB-202190 that affected cellular permeability changes in our studies (pIC50 6.4–4.8; IC50∼400–16 000 nM) are considerably higher than the IC50 values of these compounds for affecting LPS-stimulated IL1β release (50–80 nM; Gallagher et al., 1995) raising concerns about compound specificity. This is especially relevant as it is known that these two MAPK inhibitors have limited specificity and interact with other kinases (Laping et al., 2002). Third, SB-242235, which is a more specific, but equipotent, MAPK inhibitor to SB-203580 and SB-202190 (Badger et al., 2000), did not affect P2X7 responses. Fourth, SB-203580 and SB-202190 are generally equipotent in studies on MAPK (Gallagher et al., 1995) but exhibited very different potency in the studies of Donnelly-Roberts et al. (2004). Finally, the marked species selectivity described below seems inconsistent with a general role of MAPK activation in mediating permeability changes following P2X7 receptor activation. Note that IC50 values of SB-203580, SB-202190 and SB-224435 were 48 nM, 129 and 95 nM, respectively, for inhibiting MAPK in intact HEK-293 cells (AD Michel, unpublished observation).
While the present findings cast doubts on the universal role of MAPK in mediating permeability changes following P2X7 receptor activation, it is important to acknowledge that there is a growing body of evidence supporting a role of MAPK in cellular events initiated by P2X7 receptor activation. Thus, Pfeiffer and colleagues (2004), demonstrated that MAPK inhibitors could antagonize P2X7 receptor-dependent membrane blebbing in rat RAW264.7 macrophages although they did not examine changes in cellular permeability. Auger et al. (2005) found that ATP stimulation of P2X7 receptors led to extracellular signal-related protein kinase 1/2 activation and thymocyte cell death and that MAP kinase kinase 1/2 (MEK1/2) inhibitors prevented cell death. Interestingly, MEK1/2 inhibitors had no effect on P2X7 receptor-mediated changes in cellular permeability in that study. The elegant studies of Faria et al. (2005) provided very convincing evidence for a role of MAPK in mediating the cellular permeability changes that occur after P2X7 receptor activation in mouse peritoneal macrophages and 2BH4 thymic cells. However, the cellular permeability changes in that study were highly calcium dependent. This contrasts markedly with the inhibitory effect of calcium ions on P2X7 receptor-mediated cellular permeability changes (Michel et al., 1999) suggesting that the permeation pathway activated in 2BH4 thymic cells is distinct from the P2X7 receptor. Overall it seems plausible that P2X7 receptor activation leads to changes in cellular permeability through at least two pathways. One pathway is sensitive to MAPK inhibitors and leads to activation of distinct channels or transporters that are found in some cell types. The second, MAPK independent, pathway studied here may be more directly associated with or could still be, the P2X7 receptor ion channel itself as originally suggested (Surprenant et al., 1996).
A further observation of the present study was that SB-203580 and SB-202190 did not antagonize responses mediated by rat or mouse recombinant P2X7 receptors. This was also evident in studies conducted using chimeric P2X7 receptors in which the N-terminal parts of the rat, human and mouse P2X7 receptor proximal to aa 255 were ligated to the corresponding species C-terminal part of the receptor distal to aa 255. In these chimeric receptors, SB-203580 was only effective in the human–rat and human–mouse chimeras but inactive in the rat–human or mouse–human chimeras. Overall, these studies suggest that the effects of SB-203580 on human P2X7 receptor function observed in this study require the N-terminal 255 aa of the human P2X7 receptor.
This raises the question of how the effects of SB-203580 and SB-202190 that we observed are mediated. It is possible that activation of human P2X7 receptors selectively activates a kinase or protein sensitive to relatively high concentrations of SB-203580 and SB-202190 that affects changes in cellular permeability. However, the effects of SB-203580 were very rapid in onset and offset that seems to make this unlikely. Alternatively, these compounds could be relatively weak blockers of the human P2X7 receptor. Certainly, SB-203580 inhibits ATP binding to MAPK (Frantz et al., 1998) so it is not inconceivable that SB-203580 could bind to the ATP-binding site of the P2X7 receptor. However, if SB-203580 is a P2X7 receptor antagonist it is either a non-competitive or allosteric antagonist since the Schild slope for SB-203580 was only 0.5. Furthermore, SB-203580 did not interact with compounds known to affect the ATP-binding site of the P2X7 receptor. Thus, we have previously shown that PPADS interacts at the same site on the P2X7 receptor as OxATP and will prevent its irreversible blockade of the human P2X7 receptor (Michel et al., 2000). SB-203580 did not affect the irreversible blockade produced by OxATP suggesting that it does not interact with the ATP-binding site. Finally, SB-203580 produced effects in various assays on the human P2X7 receptor that were clearly not consistent with it functioning as a competitive P2X7 receptor antagonist. Thus, in the electrophysiology studies and IL1β release studies, the compound increased responses to the highest doses of BzATP and had no consistent effect on BzATP-stimulated rise in intracellular calcium.
One possible explanation of these data is that SB-203580 and SB-202190 are allosteric regulators of the human P2X7 receptor and bind to a site that prevents activation-dependent permeability changes in the channel or associated structures but does not affect the flux of small ions through the channel. Certainly, there are precedents for such a differential effect of antagonists, as calmidazolium has been shown to exhibit the converse selectivity to SB-203580 and affect P2X7 receptor-mediated responses in electrophysiological but not dye accumulation studies (Virginio et al., 1997).
Overall, these studies confirm that MAPK inhibitors can affect human recombinant P2X7 receptor-mediated changes in cellular permeability but failed to find any evidence that this effect was due to selective MAPK inhibition. The studies highlight considerable differences between results obtained in different laboratories with respect to responses obtained in native tissues and on recombinant channels and suggest that there is still much to learn about the function of the P2X7 receptor despite the considerable increase in our understanding of its function since its molecular identity was established 10 years ago.
Abbreviations
- BzATP
2′- & 3′-O-(4benzoylbenzoyl) ATP
- IL1β
interleukin-1β
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MEK1/2
MAP kinase kinase 1/2
- OxATP
periodate oxidized ATP
- PMA
phorbol 12-myristate 13-acetate
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
Conflict of interest
AD Michel, E Fonfria and I Boyfield are employed by GlaxoSmithKline, PPA Humphrey by Theravance and K Thompson by Astex.
References
- Auger R, Motta I, Benihoud K, Ojcius DM, Kanellopoulos JM. A role for mitogen-activated protein kinase (Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J Biol Chem. 2005;280:28142–28151. doi: 10.1074/jbc.M501290200. [DOI] [PubMed] [Google Scholar]
- Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthr Rheumat. 2000;43:175–183. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor. Am J Physiol – Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Frantz B, Klatt T, Pang M, Parsons J, Rolando A, Williams H, et al. The activation state of p38 mitogen-activated protein kinase determines the efficiency of ATP competition for pyridinylimidazole inhibitor binding. Biochemistry. 1998;37:13846–13853. doi: 10.1021/bi980832y. [DOI] [PubMed] [Google Scholar]
- Gallagher TF, Fier-Thompson SM, Garigipati RS, Sorenson ME, Smietana JM, Lee D, et al. 2, 4, 5-Triarylimidazole inhibitors of IL-1 biosynthesis. Bioorg Med Chem Lett. 1995;5:1171–1176. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:195–198. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hibell AD, Kidd EJ, Chessell IP, Humphrey PP, Michel AD. Apparent species differences in the kinetic properties of P2X7 receptors. Br J Pharmacol. 2000;130:167–173. doi: 10.1038/sj.bjp.0703302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. Complexities of measuring antagonist potency at P2X7 receptor orthologs. J Pharmacol Exp Ther. 2001;296:947–957. [PubMed] [Google Scholar]
- Jiang L-H, Rassendren F, Mackenzie A, Zhang Y-H, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am J Physiol – Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nature Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- Klapperstuck M, Buttner C, Bohm T, Schmalzing G, Markwardt F. Characteristics of P2X7 receptors from human B lymphocytes expressed in Xenopus oocytes. Biochim Biophys Acta. 2000;1467:444–456. doi: 10.1016/s0005-2736(00)00245-5. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Nelson P, Mi L, Frew R, Minaker S, Vair C, et al. Pharmacological differentiation of the P2X7 receptor and the maitotoxin-activated cationic channel. Eur J Pharmacol. 2004;487:17–28. doi: 10.1016/j.ejphar.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Nauyn Schmied Arch Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- Michel AD, Kaur R, Chessell IP, Humphrey PP. Antagonist effects on human P2X7 receptor-mediated cellular accumulation of YO-PRO-1. Br J Pharmacol. 2000;359:513–520. doi: 10.1038/sj.bjp.0703368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Phys Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Petrou S, Ugur M, Drummond RM, Singer JJ, Walsh JV., Jr P2X7 purinoceptor expression in Xenopus oocytes is not sufficient to produce a pore-forming P2Z-like phenotype. FEBS Letts. 1997;411:339–345. doi: 10.1016/s0014-5793(97)00700-x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leuk Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitotoxin and P2Z/P2X(7) purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol. 1999;277:C766–C776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Verhoef PA, Kertesy SB, Estacion M, Schilling WP, Dubyak GR. Maitotoxin induces biphasic interleukin-1beta secretion and membrane blebbing in murine macrophages. Mol Pharmaocl. 2004;66:909–920. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacol. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nature Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]