Abstract
Background and purpose:
The anticancer drugs doxorubicin and bleomycin are well-known for their oxidative stress-mediated side effects in heart and lung, respectively. It is frequently suggested that iron is involved in doxorubicin and bleomycin toxicity. We set out to elucidate whether iron chelation prevents the oxidative stress-mediated toxicity of doxorubicin and bleomycin and whether it affects their antiproliferative/proapoptotic effects.
Experimental approach:
Cell culture experiments were performed in A549 cells. Formation of hydroxyl radicals was measured in vitro by electron paramagnetic resonance (EPR). We investigated interactions between five iron chelators and the oxidative stress-inducing agents (doxorubicin, bleomycin and H2O2) by quantifying oxidative stress and cellular damage as TBARS formation, glutathione (GSH) consumption and lactic dehydrogenase (LDH) leakage. The antitumour/proapoptotic effects of doxorubicin and bleomycin were assessed by cell proliferation and caspase-3 activity assay.
Key results:
All the tested chelators, except for monohydroxyethylrutoside (monoHER), prevented hydroxyl radical formation induced by H2O2/Fe2+ in EPR studies. However, only salicylaldehyde isonicotinoyl hydrazone and deferoxamine protected intact A549 cells against H2O2/Fe2+. Conversely, the chelators that decreased doxorubicin and bleomycin-induced oxidative stress and cellular damage (dexrazoxane, monoHER) were not able to protect against H2O2/Fe2+.
Conclusions and implications:
We have shown that the ability to chelate iron as such is not the sole determinant of a compound protecting against doxorubicin or bleomycin-induced cytotoxicity. Our data challenge the putative role of iron and hydroxyl radicals in the oxidative stress-mediated cytotoxicity of doxorubicin and bleomycin and have implications for the development of new compounds to protects against this toxicity.
Keywords: iron chelation, drug toxicity, lipid peroxidation, cell proliferation, apoptosis, EPR
Introduction
Modern chemotherapy employs a wide range of efficient cytostatic agents. However, dangerous side effects sometimes hamper the therapy and may lead to serious or even fatal organ dysfunctions. Among the anticancer drugs, doxorubicin is well-known for its cardiotoxicity (Keizer et al., 1990; Minotti et al., 2004) while bleomycin (BLM) is known to elicit severe interstitial pulmonary fibrosis (Mir et al., 1996; Azambuja et al., 2005). These two drugs belong to different classes: doxorubicin is an anthracycline antibiotic whereas bleomycin is a glycosylated peptide antibiotic. They, however, share some properties. Thus, chronic organ toxicity frequently develops upon administration of cumulative doses of both drugs (Singal and Iliskovic, 1998; Azambuja et al., 2005). Also, reactive oxygen species (ROS) were shown to be involved in the toxicity of both doxorubicin and bleomycin (Zhou et al., 2001; Manoury et al., 2005). Finally, interactions of both drugs with iron are considered to be of importance in exerting their deleterious effects on healthy tissues as well as in their antineoplastic activity. The exact mechanisms leading to doxorubicin-induced cardiotoxicity and bleomycin-induced lung toxicity, however, remain unclear and it is generally accepted that several mechanisms are involved.
The interactions of doxorubicin with iron are complex (for review, see Xu et al. (2005)). Some of these include involvement of ROS whereas others are oxidative stress-independent. Iron can either promote hydroxyl radical (HO•) generation via the Fenton or Haber–Weiss reaction or form doxorubicin-Fe(III) redox active complexes. ROS-independent effects include, for example, disturbance of either iron regulatory proteins (Minotti et al., 1999) or iron mobilization from ferritin (Kwok and Richardson, 2003). The role of iron in doxorubicin cardiotoxicity became particularly evident after successful application of the iron chelator dexrazoxane (ICRF-187) as a cardioprotector (Hasinoff et al., 2003; Cvetkovic and Scott, 2005). Dexrazoxane, a prodrug of an EDTA analogue, likely acts via displacing iron from doxorubicin-Fe(III) complexes and removing free iron from the vicinity of biomolecules. We have previously shown that another iron chelating compound, monohydroxyethylrutoside (monoHER), was protective against doxorubicin-induced cardiotoxicity (Van Acker et al., 2000; Abou El Hassan et al., 2003a). Besides iron chelation, the antioxidant or anti-inflammatory effects might contribute to the cardioprotective effect of the flavonoid monoHER (Van Acker et al., 1998; Abou El Hassan et al., 2003b).
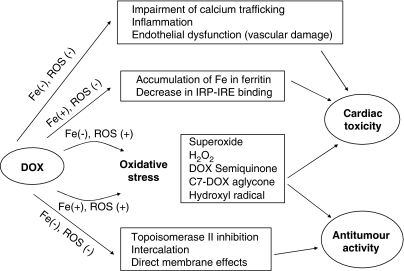
We also found that monoHER did not affect the antitumour effects of doxorubicin in MCF-7, OVCAR-3 and A2780 cell lines and in graft hosted nude mice (Van Acker et al., 1997). The antiproliferative action of doxorubicin is complex. Interaction with the DNA–topoisomerase II complex is considered a primary trigger for cell growth arrest (Gewirtz, 1999). Formation of free radicals, directly affecting the cell membrane or the effects via DNA cross-linking, DNA intercalation or alkylation have also been reported. The precise contribution of free radicals to death of cancer cells is still under debate. It has been shown that doxorubicin retains toxicity under hypoxic conditions when ROS cannot be formed (Tannock and Guttman, 1981). Co-treatment of doxorubicin with the iron chelator dexrazoxane also did not affect the antitumour effects of doxorubicin (Wu and Hasinoff, 2005). On the other hand, some other studies demonstrated that dexrazoxane compromised the antitumour properties of doxorubicin (Zhang et al., 1996). The mechanisms by which doxorubicin can interact with cardiac and cancer cells are summarized in Figure 1.
Figure 1.
A simplified scheme of the proposed interactions of doxorubicin with tumour and cardiac cells illustrates their complexity. With regard to iron, the effects of doxorubicin can be divided to Fe-dependent and Fe-independent. The most prominent characteristic of doxorubicin is its ability to induce oxidative stress. ROS can be formed in the absence of Fe (semiquinone radical, C7-aglycone radical, superoxide, H2O2) but their generation can also be Fe-catalyzed (Fenton reaction, Haber–Weiss reaction), creating highly damaging HO• radicals. Doxorubicin (DOX) can also form DOX–Fe(III) complexes that also lead to ROS. ROS may induce damage to both cardiac and neoplastic cells. The higher susceptibility of heart tissue to oxidative stress is often explained by its poor antioxidant defences and/or abundance of mitochondria, which are both important source, and target of ROS. The major mechanisms that lead to cancerous cell death are, most probably, inhibition of topoisomerase II and DNA intercalation effects independent of oxidative stress and iron. Other effects that do not depend on ROS are thought to contribute predominantly to their cardiotoxicity: dysregulation of iron homeostasis via interaction with iron regulatory protein and inhibition of Fe mobilization from ferritin are examples of Fe-mediated effects whereas inflammation, endothelial dysfunction and calcium homeostasis impairment are both iron and ROS independent.
In the case of bleomycin, there is convincing evidence that its antitumour action is linked with free radical formation. Bleomycin binds iron and oxygen thus forming an activated complex capable of releasing damaging oxidants in close proximity to DNA (El-Medany et al., 2005). However, some investigators observed that bleomycin was equally effective in normal and iron-deprived mice (Lyman et al., 1989). In another study, O-phenanthroline, a metal ion chelator, fully inhibited bleomycin-induced DNA cleavage (Larramendy et al., 1989). Bleomycin does not only form ROS but it can also act as an intercalating agent thanks to its bithiazole structural moiety. ROS are clearly involved in the development of lung fibrosis. Studies with antioxidants such as N-acetylcysteine or bilirubin showed effective protection of rats against bleomycin-induced lung fibrosis. There is also growing evidence that imbalances in various metalloproteinases and their inhibitors are crucial elements in the fibrogenic process associated with bleomycin (Manoury et al., 2005).
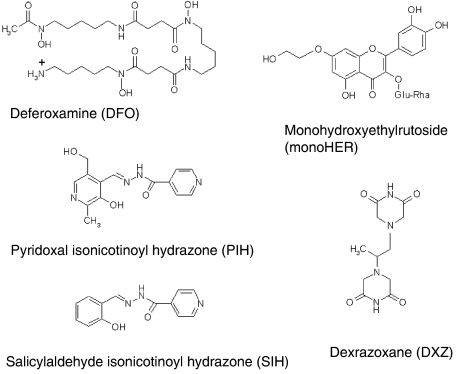
In the present study, we have employed five iron chelators with clinical applicability: dexrazoxane, monoHER, deferoxamine (DFO) and two aroylhydrazones (pyridoxal isonicotinoyl hydrazone, PIH and salicylaldehyde isonicotinoyl hydrazone, SIH; Figure 2). These compounds differ in their physico-chemical properties but their iron-chelating capacity has been well documented in a variety of in vitro and in vivo models (Table 1). We investigated the interactions between these iron chelators and the oxidative stress-inducing agents (doxorubicin, bleomycin and for comparison H2O2) in order to find out whether the iron chelators were able to prevent doxorubicin and bleomycin oxidative stress-mediated cytotoxicity and whether the antiproliferative effects of doxorubicin and bleomycin in A549 human lung adenocarcinoma cells were affected by iron chelation.
Figure 2.
Chemical structures of the iron chelators used in this study: DFO, PIH, SIH, monoHER and DXZ (ICRF-187).
Table 1.
Iron-chelating capacity of the chelators under investigation
| Chelator | Data on iron chelation | References |
|---|---|---|
| DFO | DFO decreased the intracellular calcein-chelatable iron pool and protected Jurkat cells against H2O2-induced DNA damage | (Tenopoulou et al., 2005) |
| DFO displaced iron from iron–calcein complexes in solution as quickly as PIH or SIH but it was much less efficient in K562 cells | (Cabantchik et al., 1996) | |
| DFO reduced hepatic iron concentration and serum ferritin and increased urinary iron excretion in iron overloaded thalassemic patients | (Taher et al., 2001; Yarali et al., 2006) | |
| DXZ | DXZ and its hydrolysis products displaced iron(III) from its complex with anthracyclines | (Buss and Hasinoff, 1993) |
| Hydrolysis products of DXZ displaced Fe(II) from its complex with calcein both in solution and in isolated neonatal rat cardiac myocytes | (Hasinoff et al., 2003) | |
| monoHER | MonoHER released iron (II) from iron(II)–EDTA complex | (Van Acker et al., 1996) |
| MonoHER treatment decreased plasma iron in β-thalassemic mice | (De Franceschi et al., 2004) | |
| PIH | PIH was about as efficient as SIH in displacing iron from iron–calcein complexes in K562 cells | (Cabantchik et al., 1996) |
| PIH reduced uptake of rat transferrin-59Fe and its incorporation into ferritin by hepatocytes; the effect was comparable to that of DFO | (Baker et al., 1985) | |
| SIH | SIH rapidly and completely displaced iron from iron–calcein complexes in solution, resealed ghosts and cultured K562 cells | (Cabantchik et al., 1996) |
| SIH quickly chelated calcein-bound iron in HUVEC cells while deferoxamine acted more slowly | (Kartikasari et al., 2004) |
Abbreviations: DFO, Deferoxamine; DXZ, dexrazoxane; monoHER, monohydroxyethylrutoside; PIH, pyridoxal isonicotinoyl hydrazone; SIH, salicylaldehyde isonicotinoyl hydrazone.
Methods
Cell culture
Human lung adenocarcinoma cell line (A549) was maintained in DMEM supplemented with 10% FBS, 100 U ml−1 of penicillin, 100 μg ml−1 of streptomycin and 2 mM of L-glutamine under humidified atmosphere containing 5% CO2 at 37°C. Cell passages between 25 and 40 were used for experiments described in this study.
For lipid peroxidation assay, GSH determination and LDH leakage, subconfluent cultures were trypsinized and seeded in 6 cm Petri dishes at a density of 1.9 × 105 cells ml−1 (in a total volume of 4.5 ml). Cells were left to attach for 24 h after which the culture medium was renewed for the medium containing test compounds and cells were cultivated for further 48 h. Cells were 40–50% confluent when treated and confluent after 48 h incubation. The concentration of dimethyl sulphoxide (DMSO) used to dissolve PIH, SIH and monoHER was adjusted to 0.2% in all the solutions including controls. After the end of incubation period 200 μl of medium was aspirated from each dish, mixed with 50 μl of 15% BSA and stored at −80°C for later determination of LDH activity. The rest of the medium was removed and the dishes were kept on ice. Cell monolayers were washed with ice cold PBS and scraped from the dishes with 600 μl of fresh PBS per dish. Cells were sonicated on ice briefly (15 s) and 300 μl of the cell lysate were removed to another microtube containing 33 μl of 15% sulphosalicylic acid and stored at −20°C for glutathione (GSH) determination. The rest of the lysate was also stored at −20°C and used for determination of thiobarbituric acid reactive substances (TBARS).
Cell proliferation assay (Trypan blue exclusion)
For cell proliferation assay, subconfluent cultures were trypsinized and seeded in six-well plates at a density of 1.9 × 105 cell ml−1 (in a total volume of 2 ml). They were left to adapt for 24 h after which the medium was changed for the medium containing test compounds using the same solutions as for the assays described above. Cells were exposed to substances for 48 h and then they were trypsinized with 300 μl of trypsin per well, 700 μl of serum containing medium was added, and cell suspension was mixed thoroughly in the well and transferred to a microtube. Samples were then vortexed and an aliquot was mixed with a Trypan blue solution (5 mg ml−1) 1:1. Living cells were counted using a Bürker's chamber and cell viability was expressed as a percentage of control.
Caspase-3 activity determination
Cells were seeded on 10-cm Petri dishes at the density of 1.9 × 105 cells ml−1 (in 9 ml total volume). After the 24 h adaptation period, cells were exposed to the compounds. The chelators were added 30 min before doxorubicin or bleomycin. At 12, 24 and 48 h cells were harvested by centrifugation (600 g, 5 min) and lysed on ice for 20 min in a lysis buffer containing 50 mM HEPES, 5 mM CHAPS and 5 mM DTT. The lysates were centrifuged at 14 000 g, 10 min, 4°C, the supernatants were collected and stored at −80°C. The enzyme activity was measured in a 96-well microplate using a kinetic fluorometric method based on the hydrolysis of the peptide substrate Ac-DEVD-AMC by caspase-3, resulting in the release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety. Ac-DEVD-CHO, a specific inhibitor of caspase-3, was used to confirm the specificity of the cleavage for caspase-3. Fluorescence was recorded at λex 360 nm and λem 465 nm. The concentration of the AMC released was calculated from a standard curve constructed with known concentrations of AMC. Caspase-3 activity was expressed as nmol AMC min−1 ml−1 (mg protein)−1.
Thiobarbituric acid reactive substances assay for lipid peroxidation
The TBARS generated were measured by means of HPLC according to Lepage et al. (1991). Briefly, 100 μl of cell lysate or malondialdehyde (MDA) standard were mixed with 900 μl reagent composed of 10 parts of reagent A (0.012 M 2-thiobarbituric acid (TBA), 0.32 M H3PO4 and 0.01% EDTA) and one part of reagent B (butylated hydroxytoluene (BHT) in ethanol 1.5 mg ml−1). Standards were prepared using 0–10 μM MDA solutions in PBS and derivatized during the same analytical measurement as the samples. The tubes were covered with marbles and heated for 1 h at 99°C. After cooling, the product was extracted in 500 μl of butanol by vigorous shaking and the tubes were centrifuged for 5 min at 5000 g 30 μl of the extract were injected on to a Nucleosil C18 column, 150 mm × 3.2 mm (Supelco Inc., Bellefonte, PA, USA) and eluted with 65% water and 35% methanol+0.05% trifluoroacetic acid (TFA). Fluorescence was recorded at λex 532 nm and λem 553 nm. The peak of TBA-MDA product was integrated and the concentration of MDA was calculated by means of linear regression. The amount of MDA was corrected for protein content of the lysates and the results were expressed as a percentage of TBARS formation, taking the control sample as 100%.
GSH determination
Samples preserved in sulphosalicylic acid (see above) were thawed, sonicated for 5 min on ice, centrifuged for 5 min at 5000 g and the supernatants were used for the assay. Total GSH was determined according to Tietze (1969) using the enzymatic recycling method with DTNB and GSH reductase in a microplate format. The rate of TNB formation was recorded at 405 nm for 2 min and the slope was compared to that of the standard curve of GSSG. The concentration of total GSH was calculated using the method of linear regression, the results were corrected for protein content and expressed as a percentage of the total GSH in control sample (100%).
Lactic dehydrogenase leakage assay
For lactic dehydrogenase (LDH) activity determination, 50 μl of sample was mixed with 950 μl of reagent consisting of 1.2 mM of sodium pyruvate and 0.1 mM NADH in 50 mM Na+/K+ phosphate buffer pH 7.4. The rate of NADH oxidation was followed at 340 nm for 1 min. Enzyme activity was calculated using the extinction coefficient of NADH as 6.32 l mmol−1 cm−1. LDH leakage was expressed as percentage of control taking LDH leakage in control sample (medium from non treated cells) as 100% release.
Protein determination
Protein was determined spectrophotometrically using bicinchoninic acid (Brown et al., 1989), with bovine serum albumin as a standard.
Electron paramagnetic resonance
H2O2/Fe2+ induced HO• formation and its modulation by iron chelators was measured by electron paramagnetic resonance (EPR) spectroscopy. Measurements were performed under the following conditions: microwave power, 2 mW; modulation amplitude, 1 G; scan width, 50 G; modulation frequency, 100 kHz; and temperature, 30°C using spectrometer Bruker EMX (Bruker GmbH, Freiburg, Germany). A typical reaction mixture contained 66 μl of 750 mM 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), 300 μl of 6.6 mM H2O2, 2 μl of 50 mM PIH or SIH in DMSO (or DMSO alone in case of control sample) and 582 μl of de-aerated MilliQ water. The reaction was started with 50 μl of 200 μM ferrous sulphate and the spectrum was recorded, after 2–3 min DFO and dexrazoxane were dissolved in MilliQ instead of DMSO. DMSO was adjusted to 0.2% (v/v) in all the measurements. The final concentrations of H2O2, Fe2+, chelators and DMSO for the EPR experiments were the same as used for cell culture experiments.
Data analysis
Results are given as mean±s.d. Statistical analysis was performed by SigmaStat for Windows 3.0.1 (SPSS). Comparisons between groups were made by one-way ANOVA with Tukey's post hoc test. The differences were considered significant when P<0.05.
Drugs and chemicals
PIH and salicylaldehyde hydrazone (SIH) were a kind gift from P Ponka (Mc Gill University, Montréal, Canada). MonoHER was kindly provided by Novartis Consumer Health (Nyon, Switzerland). Dexrazoxane (Cardioxane) was from Chiron BV (Amsterdam, The Netherlands), and DFO (Desferal) was from Novartis (Switzerland). Doxorubicin (Adriblastina R.T.U.) was from Pfizer (USA) and bleomycin (Bleomycine 15U PCH) was purchased from Pharmachemie B.V. (Haarlem, the Netherlands). Note that 1 μg ml−1 of bleomycin the concentration used in these experiments, approximates to 0.7 μM. Bleomycin is a mixture of several molecules (Bleomycin A, Bleomycin B, etc.) in variable ratios and thus the molecular weight is not specified in most commercially available products.
TBA, butylated hydroxytoluene (BHT), oxidized glutathione (GSSG) and reduced glutathione (GSH), 5-5′-dithiobis(2-nitrobenzoic acid) (DTNB), GSH reductase, β-NADPH, Trypan blue, pyruvate, hydrogen peroxide, ferrous sulphate, Dulbecco's modified Eagle's medium (DMEM), bovine serum albumin (BSA), sulphosalicylic acid (SSA), dimethylsulphoxide (DMSO), 7-amino methyl coumarin (AMC), HEPES, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and malondialdehyde (MDA) were purchased from Sigma (St Louis, MO, USA). Hanks' balanced salt solution (HBSS), trypsin, foetal bovine serum (FBS), penicillin and streptomycin (P/S) were from Life Technologies (Breda, The Netherlands). NADH was obtained from ICN Biochemicals (OH, USA) and bicinchoninic acid (BCA) was obtained from Pierce. N-acetyl Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) and N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde) (Ac-DEVD-CHO) were from Alexis Biochemicals. CHAPS was from Fluka.
Results
The effects of iron chelation on H2O2/Fe2+-induced oxidative stress
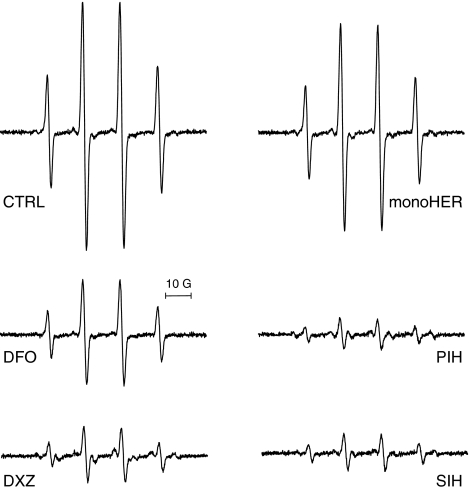
The iron chelators DFO, DXZ, PIH and SIH markedly decreased the H2O2/Fe2+-induced HO• formation, measured as DMPO-OH adduct, to 43, 23, 13 and 15% respectively, compared to control (Figure 3). MonoHER was a weak HO• scavenger, decreasing the formation of this radical to only 84% of control. Besides the characteristic spectra of DMPO-OH adducts, additional peaks were observed by EPR after adding dexrazoxane and to a smaller extent in spectra after PIH. This suggests that besides HO•, other radicals were formed. In contrast, simple spectra, similar to that of H2O2/Fe2+ alone, resulted when DFO, SIH or monoHER were added.
Figure 3.
Representative EPR spectra of DMPO-OH radicals produced by Fenton reagents (2 mM H2O2, 10 μM Fe2+) (control) and its modulation by 100 μM of DFO, DXZ, monoHER, PIH and SIH. The concentration of the reagents used in this experiment correspond to those used in cell culture. For details, see Materials and methods and Results.
Incubation of intact A549 cells with the chelators and subsequent exposure to H2O2/Fe2+ showed that SIH effectively reduced H2O2/Fe2+-induced oxidative damage as demonstrated by diminished TBARS formation and conserved GSH levels (Tables 3 and 4, the last column). Cell viability was increased and LDH leakage was prevented by SIH (Table 2, the last column) suggesting its high efficacy in prevention of Fenton-derived toxicity. On the other hand, DFO offered only partial protection (marked preservation of GSH levels while the other parameters did not change significantly). The other chelators PIH, dexrazoxane and monoHER, were not effective. To exclude a confounding effect of DMSO, which by itself can act as a HO• scavenger, the DMSO concentration was adjusted to 0.2% in both EPR experiments and the cell culture experiments.
Table 3.
Effects of various iron chelators on doxorubicin, bleomycin and H2O2/Fe2+-induced TBARS formation in A549 cells
| Chelator | Controla (%) | DOX (%) | BLM (%) | H2O2/Fe2+ (%) |
|---|---|---|---|---|
| Controlb | 100 | 389±132* | 176±51 | 8998±940* |
| DXZ | 435±16* | 513±55* | 435±91*† | 11936±1744* |
| DFO | 386±17* | 469±72* | 441±83*† | 9457±2158* |
| monoHER | 100±9 | 458±88* | 207±58 | 11110±2258* |
| PIH | 133±34 | 409±82* | 144±43 | 8856±2702* |
| SIH | 282±149* | 659±263*† | 470±262*† | 3224±76† |
A549 cells were pretreated with 100 μM of the chelator for 30 min at 37°C before doxorubicin (1 μM), bleomycin (1 μg mg−1) or H2O2/Fe2+ (2 mM /10 μM) were added. The exposure period was 48 h. Values represent the mean±s.d. (n⩾3). Data are expressed as a percentage of TBARS formation relative to untreated controls. Control values were 0.29±0.06 nmol MDA/mg.
Cells not treated with any oxidative stress-inducing agent.
Cells not pretreated with any chelator.
P< 0.05 vs untreated control
P<0.05 vs DOX, bleomycin and H2O2/Fe2+, respectively.
Table 4.
Effects of various iron chelators on doxorubicin, bleomycin and H2O2/Fe2+-induced GSH decrease in A549 cells
| Chelator | Controla (%) | DOX (%) | BLM (%) | H2O2/Fe2+ (%) |
|---|---|---|---|---|
| Controlb | 100 | 50±9* | 82±5* | 8±1* |
| DXZ | 99±7 | 81±13*† | 82±4* | 11±5* |
| DFO | 56±5* | 26±13*† | 51±6*† | 39±5*† |
| monoHER | 103±10 | 83±15† | 98±5 | 10±4* |
| PIH | 102±16 | 50±10* | 76±7* | 14±6* |
| SIH | 54±4* | 44±6* | 81±18* | 109±11† |
The A549 cells were pretreated with 100 μM of the chelator for 30 min at 37°C before doxorubicin (1 μM), bleomycin (1 μg mg−1) or H2O2/Fe2+ (2 mM /10 μM) were added. The exposure period was 48 h. Values represent the mean±s.d. (n⩾3). Data are expressed as a percentage of GSH content relative to untreated controls. Control values were 51±5 nmol GSH/mg.
Cells not treated with any oxidative stress-inducing agent.
Cells not pretreated with any chelator.
P< 0.05 vs untreated control
P< 0.05 vs DOX, bleomycin and H2O2/Fe2+, respectively.
Table 2.
Effects of various iron chelators on doxorubicin, bleomycin and H2O2/Fe2+-induced LDH leakage in A549 cells
| Chelator | Controla (%) | DOX (%) | BLM (%) | H2O2/Fe2+ (%) |
|---|---|---|---|---|
| Controlb | 100 | 232±19* | 86±5 | 259±11* |
| DXZ | 137±11 | 121±14† | 135±7† | 279±26* |
| DFO | 260±37* | 260±37* | 221±49*† | 234±19* |
| MonoHER | 86±3 | 150±20*† | 93±8 | 305±22*† |
| PIH | 91±11 | 241±24* | 97±4 | 262±9* |
| SIH | 214±32* | 262±37* | 223±26*† | 131±6*† |
A549 cells were pretreated with 100 μM of the chelator for 30 min at 37°C before doxorubicin (1 μM), bleomycin (1 μg mg−1) or H2O2/Fe2+ (2 mM /10 μM) were added. The exposure period was 48 h. Values represent the mean±s.d. (n⩾3). Data are expressed as a percentage of LDH leakage relative to untreated controls. Control values were 18.3±0.7 U l−1 min−1.
Cells not treated with any oxidative stress-inducing agent.
Cells not pretreated with any chelator.
P< 0.05 vs untreated control
P<0.05 vs DOX, bleomycin and H2O2/Fe2+, respectively.
The effects of iron chelation on doxorubicin and bleomycin-induced cytotoxicity and oxidative stress
Leakage of the cytosolic enzyme LDH from A549 cells increased upon 48 h exposure to 1 μM doxorubicin while a comparable concentration of bleomycin did not induce LDH leakage (Table 2). No LDH release was observed with PIH- or monoHER-treated cells. Dexrazoxane caused a slight increase in LDH leakage while the effect of SIH and DFO was a significant increase. Combining the chelators with either doxorubicin or bleomycin resulted in a significant cellular protection against doxorubicin induced LDH leakage by dexrazoxane and monoHER, whereas the other chelators were not protective.
A marker of lipid peroxidation, TBARS, increased in the cells exposed to doxorubicin and to a lesser extent also upon exposure to bleomycin. Except for monoHER and PIH, all the chelators were able to induce TBARS formation directly. None of the chelators showed any protection against doxorubicin or bleomycin-induced lipid peroxidation (Table 3).
The intracellular antioxidant GSH decreased after doxorubicin and to a lesser extent also after the bleomycin treatment (Table 4). Notably, the chelators that were effective in reducing the H2O2/Fe2+-induced decrease in GSH (i.e. SIH and DFO) failed to protect the A549 cells against the fall in GSH induced by doxorubicin and bleomycin. On the contrary, the chelators dexrazoxane and monoHER, which did not prevent the loss in GSH induced by H2O2/Fe2+ significantly protected against the doxorubicin induced GSH decrease. Only monoHER prevented bleomycin-induced GSH depletion.
The effects of iron chelation on the antiproliferative and proapoptotic activity of doxorubicin and bleomycin
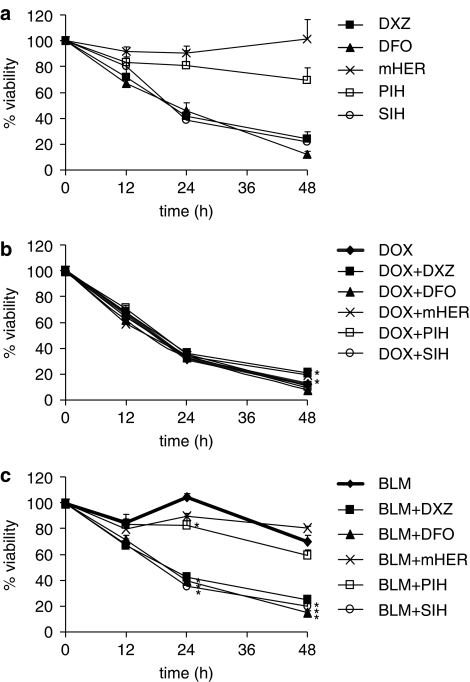
The influence of the two anticancer drugs and the iron chelators on cell proliferation was followed in time (12, 24 and 48 h; Figure 4). In comparable concentrations (1 μM of doxorubicin; 1 μg ml−1 of bleomycin), doxorubicin was a much more efficient antiproliferative agent than bleomycin with IC50 values of 0.03 μM and 5 μg ml−1, respectively, after 48 h exposure (data not shown). Among the chelators, DFO, dexrazoxane and SIH (all at 100 μM) exerted their own remarkable antiproliferative effect. In contrast, PIH was only moderately effective and monoHER did not have any direct influence on cell proliferation. When combined with doxorubicin, the chelators DFO, PIH, SIH did not affect its cytotoxic properties. A minor attenuation of the antiproliferative effect of doxorubicin by dexrazoxane and monoHER after 48 h incubation was observed. The antiproliferative effect of bleomycin, which could not be observed earlier than 48 h of treatment, was not diminished by any of the chelators.
Figure 4.
Inhibition of tumour cell proliferation by various iron chelators (a) and the effects of these chelators on the antiproliferative activity of doxorubicin (b) and bleomycin (c). The A549 cells were pretreated with the chelators (100 μM) for 30 min at 37°C before 1 μM of doxorubicin or 1 μg mg−1 of bleomycin were added. Cells were incubated for further 12, 24 or 48 h, harvested by trypsinization and viable cells were counted using Trypan blue staining. Cell viability (mean±s.d.) is expressed as percentage of viable control (untreated) cells at the three respective time points. *P<0.05 vs doxorubicin and BLM, respectively. For sake of clarity, significance vs control is not indicated in the graphs.
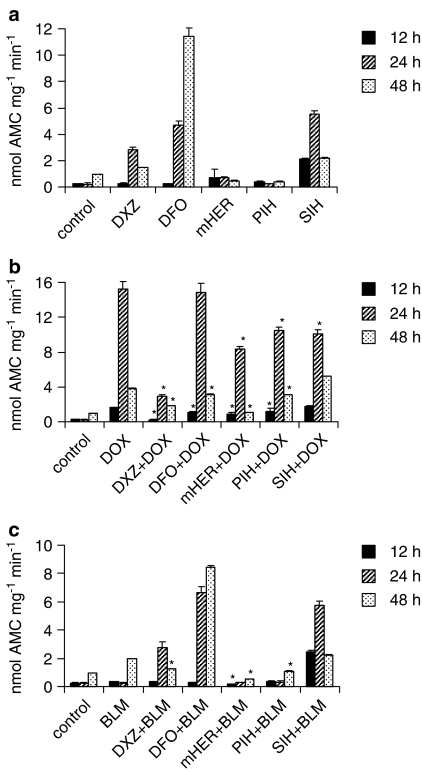
To investigate the nature of cell death induced by doxorubicin, bleomycin and the chelators, we employed the caspase-3 activity assay to quantify apoptosis at different times (Figure 5). The onset of apoptosis in A549 cells was much more rapid with doxorubicin than with bleomycin. With doxorubicin, an increase in caspase-3 activity was detected as early as after 12 h of incubation with maximum activity after 24 h, whereas bleomycin only induced apoptosis after 48 h of incubation. Moreover, bleomycin-induced caspase-3 activation was relatively weak compared to the effect of doxorubicin. Among the chelators, PIH and monoHER did not induce apoptosis whereas dexrazoxane, SIH and DFO acted as proapoptotic agents, SIH being able to induce the earliest cellular response (at 12 h). All chelators, except for DFO, reduced the doxorubicin-induced apoptosis after 24 h, where the differences between the groups were the most obvious. Dexrazoxane was the most efficient chelator in this respect. At 12 and 48 h, SIH was the only chelator, which did not cause a significant decrease in apoptosis induced by doxorubicin. Among the chelators that prevented doxorubicin-induced apoptosis, dexrazoxane and monoHER were the most effective compounds at all time points.
Figure 5.
Induction of apoptosis in A549 cells treated by iron chelators (a) and the effects of these chelators on the doxorubicin (b) and bleomycin induced (c) apoptosis at 12, 24 and 48 h. Caspase-3 activity was measured as described in Materials and methods. The results are expressed as nmol of the fluorescent product (AMC) formed by a caspase-3 cleavage of its specific substrate. Data are given as means±s.d. *P<0.05 vs doxorubicin and bleomycin, respectively. For sake of clarity, significance vs control is not indicated in the graphs.
Interestingly, we observed that the treatment of the cells with DFO+doxorubicin caused less caspase-3 activation than DFO alone. Evaluation of the effects of the chelators on bleomycin-induced apoptosis was only meaningful after 48 h incubation. MonoHER, PIH and dexrazoxane effectively reduced bleomycin-induced caspase-3 activation, but not SIH and DFO.
Discussion and conclusions
We investigated the role of iron in the oxidative stress-mediated cytotoxicity of doxorubicin and bleomycin and the consequences of iron chelation on the anti-proliferative effects of these two drugs. We would like to emphasize that our intention was not to aim our study at the question of protection against doxorubicin cardiotoxicity or bleomycin lung toxicity as such, but rather at the iron-dependent and oxidative stress-related component of the cytotoxicity of these compounds. To this end, we used the A549 human lung adenocarcinoma cells and five different chelators of iron. Dexrazoxane (ICRF-187) is a cell permeable prodrug of an EDTA-like chelator (known as ADR-925) and is approved for clinical use in the prevention of doxorubicin cardiotoxicity. Dexrazoxane was also shown to act against hypoxia-reoxygenation damage (Hasinoff, 2002) and more recently, it has successfully been used to prevent the extravasation tissue injury by anthracyclines (Langer et al., 2000; Hasinoff, 2006). DFO is a strongly hydrophilic iron chelator that is currently used for the treatment of iron-overload diseases. MonoHER is a flavonoid, which was shown to protect against doxorubicin cardiotoxicity (Van Acker et al., 2000; Abou El Hassan et al., 2003a) and recently entered phase II clinical trials. It has both antioxidant properties as well as iron-chelating capacity (Haenen et al., 1993; Van Acker et al., 1998). PIH and SIH are two low molecular weight, lipophilic, iron chelators of the aroylhydrazone class (Ponka et al., 1979; Hoy et al., 1979; Baker et al., 1992).
We have shown that the chelators presented here induced different cellular responses in many respects. The most striking difference in the activity of the compounds is the one between PIH and SIH, the most structurally similar compounds in this study. A mere replacement of the pyridoxal moiety for the salicylaldehyde moiety completely changed the behaviour of the molecule in the cell. SIH acted as a pro-oxidant and apoptosis inducer whereas PIH was devoid of these effects. On the other hand, SIH but not PIH, was as an excellent protector against H2O2/Fe2+-induced injury. In EPR experiments, both chelators were equally effective in prevention of HO• formation induced by H2O2/Fe2+. Their effect was comparable to that of dexrazoxane and greater than that of DFO and monoHER. However, of all the chelators tested only SIH and, partially, DFO protected the A549 cells against H2O2/Fe2+-induced oxidative damage. The different efficacy of these compounds is probably due to different lipophilicity. Sufficient lipophilicity is important to allow the compounds to reach the cellular compartments where ROS are produced. This would certainly explain the effect of SIH, which was previously also found to be protective against H2O2-induced injury in isolated cardiomyocytes (Horackova et al., 2000) and in the H9c2 cardiomyoblast cells (Simunek et al., 2005a). However, the effects of DFO, a relatively large, water-soluble molecule with a low partition coefficient are not as easily explained. Although the cellular protection by DFO was weaker than that of SIH, DFO was still more effective than other more lipophilic chelators, such as PIH. Membrane permeability is therefore not the only prerequisite for an intracellular action of an iron chelator. Although DFO is not freely diffusible through the biological barriers, it is taken up by the cells by endocytosis (Persson et al., 2003). The effective antioxidant behaviour of DFO is also because it is a hexadentate chelator, which binds to all six coordination sites of iron making it unreactive. On the other hand, we have found that in the A549 cells, DFO and SIH were able to induce considerable oxidative stress on their own. However, when the cells were preincubated with DFO or SIH and subsequently exposed to H2O2, these chelators efficiently prevented oxidative damage. Dexrazoxane was shown to have relatively weak pro-oxidant properties while PIH and monoHER had no such effects at all. In conclusion, SIH and partially also DFO protected against H2O2/Fe2+-induced oxidative stress, whereas dexrazoxane, monoHER and PIH did not.
Next, we investigated the effect of iron chelation on doxorubicin and bleomycin-induced cytotoxicity. Based on preliminary experiments, the concentrations of 1 μM and 1 μg ml−1 were found to be optimal for doxorubicin and bleomycin-induced oxidative toxicity, respectively. At these concentrations both doxorubicin and bleomycin were capable of inducing cellular oxidative damage after a 48 h incubation. A shorter incubation period did not result in significant differences in oxidative stress markers between the groups (data not shown). However, for studies on cell proliferation, shorter exposures of 12 and 24 h were used.
Although DFO and SIH were effective in prevention of oxidative stress induced by H2O2/Fe2+, these chelators failed to protect against doxorubicin and bleomycin-induced oxidative injury. On the contrary, dexrazoxane and monoHER reduced doxorubicin cellular toxicity and partially also the toxicity of bleomycin but had no effect on H2O2/Fe2+-induced oxidative stress. These data imply that the Fenton-type reaction is not involved in the toxicity of doxorubicin. We suggest that the lack of protection against doxorubicin and bleomycin-induced oxidative stress can be explained by the involvement of free radicals, other than the HO•, in doxorubicin and bleomycin toxicity, while the iron chelators protect via prevention of HO• formation. In mice doxorubicin cardiotoxicity in vivo was relieved by administration of lecithinized copper-zinc superoxide dismutase (Den Hartog et al., 2004) showing that it is rather the superoxide radical, which is crucial in pathophysiology of doxorubicin cardiotoxicity. As for bleomycin, protection against lung fibrosis in mice overexpressing extracellular SOD has also been described (Bowler et al., 2002).
However, there is evidence that iron chelators can protect against doxorubicin cardiotoxicity and dexrazoxane (ICRF-187) has become a clinically approved drug with this indication. The same compound was successfully used to prevent bleomycin lung toxicity in mice in vivo (Herman et al., 1995). PIH attenuated daunorubicin-induced histological and biochemical changes in the rabbit heart in vivo and increased the survival of the animals (Simunek et al., 2005b). In isolated hepatocytes, SIH also prevented daunorubicin-induced loss in CYP450 activities (Schroterova et al., 2004). It seems safe to conclude that iron is somehow involved in the cellular toxicity of doxorubicin but not via the previously accepted Fenton-derived production of HO• radicals. For example, Minotti et al. (1999) suggested that the anthracyclines (or possibly their C13-alcohol metabolites) disrupt a delicate iron homeostasis through their interaction with iron regulatory proteins, which regulate the expression of transferin receptor and ferritin according to the cellular needs. Also, doxorubicin as well as other redox-cycling agents induce an accumulation of iron in ferritin in both myocardial and neoplastic cells and this effect can be prevented by some iron chelators (DFO but not dexrazoxane) (Kwok and Richardson, 2003). It is, however, not clear, whether and in which way this effect contributes to the anthracycline toxicity.
The cardioprotective potential of another compound, the flavonoid monoHER, although originally selected because of its iron chelating and antioxidant properties, has recently been linked with other features like anti-inflammatory effects, which could be of importance (Abou El Hassan et al., 2003a). PIH analogues also seem to possess antioxidant properties in addition to their iron chelating capacity (Hermes-Lima et al., 2000). Interestingly, it was shown that systolic heart failure induced by the anthracyclines is accompanied by chronic calcium overload and dexrazoxane was able to restore normal myocardial calcium content (Simunek et al., 2005c). Interference of daunorubicin with calcium-handling proteins such as the ryanodine receptor and its normalization by dexrazoxane was also demonstrated (Burke et al., 2000). It is therefore possible that the protective effects by many chelators – including dexrazoxane – are not exclusively due to chelation of iron.
Before introducing new iron chelators in chemotherapy protocols, it is essential to establish whether the compounds interfere with the antitumour effect of the chemotherapeutic agents. Our results have shown that pretreatment of the A549 cells with 100 μM PIH, SIH and DFO did not affect the antiproliferative effects of either doxorubicin or bleomycin. On the other hand, we have observed a weak reduction of doxorubicin-induced apoptosis and cell death by dexrazoxane and monoHER. Although clinical trials showed that dexrazoxane did not decrease the effectiveness of doxorubicin chemotherapy in patients (Marty et al., 2006), studies in hypertensive rats and in CHO cells, however, confirmed an antagonism (Hasinoff et al., 1996; Zhang et al., 1996). It was suggested that the mechanism by which dexrazoxane diminishes the antiproliferative effects of doxorubicin is that the active chelating form of dexrazoxane (ADR-925)-Fe(III) complexes oxidatively degrade the α-ketol side chain of doxorubicin, possibly changing its antitumour efficiency (Malisza and Hasinoff, 1995). This interaction promotes formation of HO•, which would also explain certain pro-oxidant activities of dexrazoxane. On the other hand, addition of dexrazoxane to the doxorubicin-containing chemotherapy protocols allows the cumulative dose of the anthracycline to be increased without concurrent increased cardiac risk. Moreover, in vitro, dexrazoxane significantly delayed the development of multidrug resistance, a frequent complication of doxorubicin chemotherapy (Sargent et al., 2001). All the chelators, except for monoHER, were shown to possess antiproliferative properties by themselves (DFO≈SIH≈dexrazoxane>PIH). We had expected to see an additive effect when these chelators were combined with doxorubicin or bleomycin, because of the apparently different mechanisms by which these compounds inhibit cell growth. Nevertheless, this was not the case. It might be that a possible interference of the chelators with the antitumour activity of the cytostatic drugs was sufficiently counteracted by the antiproliferative action of the chelators. On the other hand, Wu et al. (2004) previously showed that the antiproliferative effect of bleomycin was not compromised by dexrazoxane in a specially developed dexrazoxane-insensitive CHO cell line in which the confounding effect of the intrinsic antiproliferative effect of the chelator was eliminated.
Three of the five chelators tested (DFO, SIH, dexrazoxane) induced apoptosis in the A549 cells. SIH derivatives (aroylhydrazones) were previously shown to induce apoptosis in Jurkat T lymphocytes and K562 cells (Buss et al., 2003) and other chelators with enhanced antiproliferative and proapoptotic properties are continuously being developed for further evaluation as new anticancer drugs (Lovejoy and Richardson, 2003). It may be that the apoptosis caused by these compounds is triggered by redox-cycling of their iron complexes (Buss et al., 2004). Other studies demonstrated that dexrazoxane also induced apoptosis in tumour cells, which is likely to be due to its inhibitory action on topoisomerase II (Hasinoff et al., 2001). Interestingly, DFO was recently shown to act as a cytostatic agent in the MCF-7 breast cancer cells but it caused neither apoptosis nor cell cycle arrest (Hoke et al., 2005). The concentration of DFO used by Hoke et al. was, however, 4–30 μM, compared to 100 μM used in this study. We therefore propose that the mechanism of the antitumour effect of DFO is concentration-dependent. It is well known that iron is involved in some crucial metabolic pathways in the cell such as DNA synthesis or oxygen transport (Mladenka et al., 2006). We suggest that at high concentrations of the chelator, not only free iron, but also the iron bound to macromolecules might be depleted and cellular apoptosis initiated. Possible differences between various cell types also have to be considered when extrapolating the data collected from the A549 cells to non-cancerous cells, for example, cardiomyocytes. Nevertheless, we suggest that the mechanisms by which the compounds (doxorubicin and bleomycin vs H2O2) induce oxidative stress, and the way in which this can be prevented, may not be cell-type dependent. Moreover, our data offer a reasonable argument to support this statement, as the two iron chelators that had been previously shown to protect cardiac cells against doxorubicin toxicity (dexrazoxane, monoHER) also prevented the doxorubicin-induced oxidative damage and increased the cell survival of the A549 cells in the present study.
In conclusion, using A549 cells we established that the mechanism by which doxorubicin and bleomycin induce oxidative stress to the cells is not iron-mediated (i.e. Fenton reaction-dependent). We have clearly shown that the ability to chelate iron, as such, is not the sole determinant of an effective protective compound. Further, the effect of iron chelation on the antiproliferative activity of doxorubicin and bleomycin is only minor. These findings will have influence the direction in which new protective agents should be developed.
Acknowledgments
This work was supported by Grant Agency of Charles University in Prague (Grant No.97/2005) and Ministry of Education of Czech Republic (Grant MSM 0021620820). We thank Marc AJG Fischer for his excellent technical assistance.
Abbreviations
- EPR
electron paramagnetic resonance
- GSH
glutathione
- HO•
hydroxyl radical
- LDH
lactate dehydrogenase
- monoHER
monohydroxyethylrutoside
- PIH
pyridoxal isonicotinoyl hydrazone
- SIH
salicylaldehyde isonicotinoyl hydrazone
- TBARS
thiobarbituric acid reactive substances
Conflict of interest
The authors state no conflict of interest.
References
- Abou El Hassan MA, Rabelink MJ, Van der Vijgh WJF, Bast A, Hoeben RC. A comparative study between catalase gene therapy and the cardioprotector monohydroxyethylrutoside (MonoHER) in protecting against doxorubicin-induced cardiotoxicity in vitro. Br J Cancer. 2003a;89:2140–2146. doi: 10.1038/sj.bjc.6601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou El Hassan MA, Verheul HM, Jorna AS, Schalkwijk C, Van Bezu J, Van der Vijgh WJF, et al. The new cardioprotector monohydroxyethylrutoside protects against doxorubicin-induced inflammatory effects in vitro. Br J Cancer. 2003b;89:357–362. doi: 10.1038/sj.bjc.6601022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk. Pulm Pharmacol Ther. 2005;18:363–366. doi: 10.1016/j.pupt.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Baker E, Richardson D, Gross S, Ponka P. Evaluation of the iron chelation potential of hydrazones of pyridoxal, salicylaldehyde and 2-hydroxy-1-naphthylaldehyde using the hepatocyte in culture. Hepatology. 1992;15:492–501. doi: 10.1002/hep.1840150323. [DOI] [PubMed] [Google Scholar]
- Baker E, Vitolo ML, Webb J. Iron chelation by pyridoxal isonicotinoyl hydrazone and analogues in hepatocytes in culture. Biochem Pharmacol. 1985;34:3011–3017. doi: 10.1016/0006-2952(85)90142-x. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol-Lung C. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989;180:136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Burke BE, Gambliel H, Olson RD, Bauer FK, Cusack BJ. Prevention by dexrazoxane of down-regulation of ryanodine receptor gene expression in anthracycline cardiomyopathy in the rat. Br J Pharmacol. 2000;131:1–4. doi: 10.1038/sj.bjp.0703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss JL, Hasinoff BB. The one-ring open hydrolysis product intermediates of the cardioprotective agent ICRF-187 (dexrazoxane) displace iron from iron-anthracycline complexes. Agents Actions. 1993;40:86–95. doi: 10.1007/BF01976756. [DOI] [PubMed] [Google Scholar]
- Buss JL, Neuzil J, Gellert N, Weber C, Ponka P. Pyridoxal isonicotinoyl hydrazone analogs induce apoptosis in hematopoietic cells due to their iron-chelating properties. Biochem Pharmacol. 2003;65:161–172. doi: 10.1016/s0006-2952(02)01512-5. [DOI] [PubMed] [Google Scholar]
- Buss JL, Neuzil J, Ponka P. Oxidative stress mediates toxicity of pyridoxal isonicotinoyl hydrazone analogs. Arch Biochem Biophys. 2004;421:1–9. doi: 10.1016/j.abb.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Glickstein H, Milgram P, Breuer W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal Biochem. 1996;233:221–227. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- Cvetkovic RS, Scott LJ. Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65:1005–1024. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- De Franceschi L, Turrini F, Honczarenko M, Ayi K, Rivera A, Fleming MD, et al. In vivo reduction of erythrocyte oxidant stress in a murine model of beta-thalassemia. Haematologica. 2004;89:1287–1298. [PubMed] [Google Scholar]
- Den Hartog GJM, Haenen GRMM, Boven E, Van der Vijgh WJF, Bast A. Lecithinized copper, zinc-superoxide dismutase as a protector against doxorubicin-induced cardiotoxicity in mice. Toxicol Appl Pharm. 2004;194:180–188. doi: 10.1016/j.taap.2003.09.008. [DOI] [PubMed] [Google Scholar]
- El-Medany A, Hagar HH, Moursi M, At Muhammed R, El-Rakhawy FI, El-Medany G. Attenuation of bleomycin-induced lung fibrosis in rats by mesna. Eur J Pharmacol. 2005;509:61–70. doi: 10.1016/j.ejphar.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Haenen GRMM, Jansen FP, Bast A. The antioxidant properties of five O-(β-hydroxyethyl)-rutosides of the flavonoid mixture Venoruton. Phlebology Suppl. 1993;1:10–17. [Google Scholar]
- Hasinoff BB. Dexrazoxane (ICRF-187) protects cardiac myocytes against hypoxia-reoxygenation damage. Cardiovasc Toxicol. 2002;2:111–118. doi: 10.1385/ct:2:2:111. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB. Dexrazoxane use in the prevention of anthracycline extravasation injury. Future Oncol. 2006;2:15–20. doi: 10.2217/14796694.2.1.15. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Abram ME, Barnabe N, Khelifa T, Allan WP, Yalowich JC. The catalytic DNA topoisomerase II inhibitor dexrazoxane (ICRF-187) induces differentiation and apoptosis in human leukemia K562 cells. Mol Pharmacol. 2001;59:453–461. doi: 10.1124/mol.59.3.453. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Schnabl KL, Marusak RA, Patel D, Huebner E. Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc Toxicol. 2003;3:89–99. doi: 10.1385/ct:3:2:89. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Yalowich JC, Ling Y, Buss JL. The effect of dexrazoxane (ICRF-187) on doxorubicin- and daunorubicin-mediated growth inhibition of Chinese hamster ovary cells. Anticancer Drugs. 1996;7:558–567. doi: 10.1097/00001813-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Herman EH, Hasinoff BB, Zhang J, Raley LG, Zhang TM, Fukuda Y, et al. Morphologic and morphometric evaluation of the effect of ICRF-187 on bleomycin-induced pulmonary toxicity. Toxicology. 1995;98:163–175. doi: 10.1016/0300-483x(94)02987-6. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Ponka P, Schulman HM. The iron chelator pyridoxal isonicotinoyl hydrazone (PIH) and its analogues prevent damage to 2-deoxyribose mediated by ferric iron plus ascorbate. Biochim Biophys Acta. 2000;1523:154–160. doi: 10.1016/s0304-4165(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Hoke EM, Maylock CA, Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med. 2005;39:403–411. doi: 10.1016/j.freeradbiomed.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Horackova M, Ponka P, Byczko Z. The antioxidant effects of a novel iron chelator salicylaldehyde isonicotinoyl hydrazone in the prevention of H(2)O(2) injury in adult cardiomyocytes. Cardiovasc Res. 2000;47:529–536. doi: 10.1016/s0008-6363(00)00088-2. [DOI] [PubMed] [Google Scholar]
- Hoy T, Humphrys J, Jacobs A, Williams A, Ponka P. Effective iron chelation following oral administration of an isoniazid-pyridoxal hydrazone. Br J Haematol. 1979;43:443–449. doi: 10.1111/j.1365-2141.1979.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Kartikasari AE, Georgiou NA, Visseren FL, Van Kats-Renaud H, Van Asbeck BS, Marx JJ. Intracellular labile iron modulates adhesion of human monocytes to human endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:2257–2262. doi: 10.1161/01.ATV.0000147406.00871.b3. [DOI] [PubMed] [Google Scholar]
- Keizer HG, Pinedo HM, Schuurhui GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther. 1990;47:219–231. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Richardson DR. Anthracyclines induce accumulation of iron in ferritin in myocardial and neoplastic cells: inhibition of the ferritin iron mobilization pathway. Mol Pharmacol. 2003;63:849–861. doi: 10.1124/mol.63.4.849. [DOI] [PubMed] [Google Scholar]
- Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6:3680–3686. [PubMed] [Google Scholar]
- Larramendy ML, Lopez-Larraza D, Vidal-Rioja L, Bianchi NO. Effect of the metal chelating agent o-phenanthroline on the DNA and chromosome damage induced by bleomycin in Chinese hamster ovary cells. Cancer Res. 1989;49:6583–6586. [PubMed] [Google Scholar]
- Lepage G, Munoz G, Champagne J, Roy CC. Preparative steps necessary for the accurate measurement of malondialdehyde by high-performance liquid chromatography. Anal Biochem. 1991;197:277–283. doi: 10.1016/0003-2697(91)90392-7. [DOI] [PubMed] [Google Scholar]
- Lovejoy DB, Richardson DR. Iron chelators as anti-neoplastic agents: current developments and promise of the PIH class of chelators. Curr Med Chem. 2003;10:1035–1049. doi: 10.2174/0929867033457557. [DOI] [PubMed] [Google Scholar]
- Lyman S, Taylor P, Lornitzo F, Wier A, Stone D, Antholine WE, et al. Activity of bleomycin in iron- and copper-deficient cells. Biochem Pharmacol. 1989;38:4273–4282. doi: 10.1016/0006-2952(89)90526-1. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Hasinoff BB. Doxorubicin reduces the iron(III) complexes of the hydrolysis products of the antioxidant cardioprotective agent dexrazoxane (ICRF-187) and produces hydroxyl radicals. Arch Biochem Biophys. 1995;316:680–688. doi: 10.1006/abbi.1995.1091. [DOI] [PubMed] [Google Scholar]
- Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17:614–622. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- Minotti G, Cairo G, Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song. FASEB J. 1999;13:199–212. [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mir LM, Tounekti O, Orlowski S. Bleomycin: Revival of an old drug. Gen Pharmacol. 1996;27:745–748. doi: 10.1016/0306-3623(95)02101-9. [DOI] [PubMed] [Google Scholar]
- Mladenka P, Simunek T, Hubl M, Hrdina R. The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radic Res. 2006;40:263–272. doi: 10.1080/10715760500511484. [DOI] [PubMed] [Google Scholar]
- Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic Biol Med. 2003;34:1295–1305. doi: 10.1016/s0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- Ponka P, Borova J, Neuwirt J, Fuchs O. Mobilization of iron from reticulocytes. Identification of pyridoxal isonicotinoyl hydrazone as a new iron chelating agent. FEBS Lett. 1979;97:317–321. doi: 10.1016/0014-5793(79)80111-8. [DOI] [PubMed] [Google Scholar]
- Sargent JM, Williamson CJ, Yardley C, Taylor CG, Hellmann K. Dexrazoxane significantly impairs the induction of doxorubicin resistance in the human leukaemia line, K562. Br J Cancer. 2001;84:959–964. doi: 10.1054/bjoc.2001.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroterova L, Kaiserova H, Baliharova V, Velik J, Gersl V, Kvasnickova E. The effect of new lipophilic chelators on the activities of cytosolic reductases and P450 cytochromes involved in the metabolism of anthracycline antibiotics: studies in vitro. Physiol Res. 2004;53:683–691. [PubMed] [Google Scholar]
- Simunek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AM, Sterba M, et al. SIH – a novel lipophilic iron chelator – protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. J Mol Cell Cardiol. 2005a;39:345–354. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Simunek T, Klimtova I, Kaplanova J, Sterba M, Mazurova Y, Adamcova M, et al. Study of daunorubicin cardiotoxicity prevention with pyridoxal isonicotinoyl hydrazone in rabbits. Pharmacol Res. 2005b;51:223–231. doi: 10.1016/j.phrs.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Simunek T, Sterba M, Holeckova M, Kaplanova J, Klimtova I, Adamcova M, et al. Myocardial content of selected elements in experimental anthracycline-induced cardiomyopathy in rabbits. Biometals. 2005c;18:163–169. doi: 10.1007/s10534-004-4491-7. [DOI] [PubMed] [Google Scholar]
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. New Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Taher A, Sheikh-Taha M, Koussa S, Inati A, Neeman R, Mourad F. Comparison between deferoxamine and deferiprone (L1) in iron-loaded thalassemia patients. Eur J Haematol. 2001;67:30–34. doi: 10.1034/j.1600-0609.2001.067001030.x. [DOI] [PubMed] [Google Scholar]
- Tannock I, Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981;43:245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenopoulou M, Doulias PT, Barbouti A, Brunk U, Galaris D. Role of compartmentalized redox-active iron in hydrogen peroxide-induced DNA damage and apoptosis. Biochem J. 2005;387:703–710. doi: 10.1042/BJ20041650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Van Acker FAA, Van Acker SABE, Kramer K, Haenen GRMM, Bast A, Van der Vijgh WJF. 7-monohydroxyethylrutoside protects against chronic doxorubicin-induced cardiotoxicity when administered only once per week. Clin Cancer Res. 2000;6:1337–1341. [PubMed] [Google Scholar]
- Van Acker SABE, Boven E, Kuiper K, Van den Berg DJ, Grimbergen JA, Kramer K, et al. Monohydroxyethylrutoside, a dose-dependent cardioprotective agent, does not affect the antitumor activity of doxorubicin. Clin Cancer Res. 1997;3:1747–1754. [PubMed] [Google Scholar]
- Van Acker SABE, Van Balen GP, Van den Berg DJ, Bast A, Van der Vijgh WJF. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharmacol. 1998;56:935–943. doi: 10.1016/s0006-2952(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Van Acker SABE, Van den Berg DJ, Tromp MN, Griffioen DH, Van Bennekom WP, Van der Vijgh WJF, et al. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Hasinoff BB. The antitumor anthracyclines doxorubicin and daunorubicin do not inhibit cell growth through the formation of iron-mediated reactive oxygen species. Anticancer Drugs. 2005;16:93–99. doi: 10.1097/00001813-200501000-00014. [DOI] [PubMed] [Google Scholar]
- Wu X, Patel D, Hasinoff BB. The iron chelating cardioprotective prodrug dexrazoxane does not affect the cell growth inhibitory effects of bleomycin. J Inorg Biochem. 2004;98:1818–1823. doi: 10.1016/j.jinorgbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 2005;68:261–271. doi: 10.1124/mol.105.013383. [DOI] [PubMed] [Google Scholar]
- Yarali N, Fisgn T, Duru F, Kara A, Ecin N, Fitoz S, et al. Subcutaneous bolus injection of deferoxamine is an alternative method to subcutaneous continuous infusion. J Pediatr Hematol Oncol. 2006;28:11–16. [PubMed] [Google Scholar]
- Zhang J, Clark JR, Jr, Herman EH, Ferrans VJ. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J Moll Cell Cardiol. 1996;28:1931–1943. doi: 10.1006/jmcc.1996.0186. [DOI] [PubMed] [Google Scholar]
- Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121:151–157. doi: 10.1016/s0378-4274(01)00329-0. [DOI] [PubMed] [Google Scholar]