Abstract
Background and purpose:
The 2-propyl-1,4 benzoxazine (AM10) shows a peculiar behaviour in skeletal muscle, inhibiting or opening the ATP-sensitive K+ (KATP) channel in the absence and presence of ATP, respectively. We focused on tissue selectivity and mechanism of action of AM10 by testing its effects on pancreatic KATP channels by means of both in vitro and in vivo investigations.
Experimental approach:
In vitro, patch-clamp recordings were performed in native pancreatic beta cells and in tsA201 cells expressing the Kir6.2ΔC36 channel. In vivo, an intraperitoneal glucose tolerance test was performed in normal mice.
Key results:
In contrast with what observed in the skeletal muscle, AM10, in whole cell perforated mode, did not augment KATP current (IKATP) of native beta cells but it inhibited it in a concentration-dependent manner (IC50: 11.5 nM; maximal block: 60%). Accordingly, in current clamp recordings, a concentration-dependent membrane depolarization was observed. On excised patches, AM10 reduced the open-time probability of KATP channels without altering their single channel conductance; the same effect was observed in the presence of trypsin in the bath solution. Moreover, AM10 inhibited, in an ATP-independent manner, the K+ current resulting from expressed Kir6.2ΔC36 (maximal block: 60% at 100 μM; IC50: 12.7 nM) corroborating an interaction with Kir. In vivo, AM10 attenuated the glycemia increase following a glucose bolus in a dose-dependent manner, without, at the dose tested, inducing fasting hypoglycaemia.
Conclusion and implications:
Altogether, these results help to gain insight into a new class of tissue specific KATP channel modulators.
Keywords: native pancreatic beta cells, KATP channel, Kir6.2ΔC36, tsA201 cells, benzoxazine derivative, patch-clamp, intraperitoneal glucose tolerance test
Introduction
The ATP-sensitive K+ (KATP) channel is a macromolecular complex made of four pore-forming inward rectifier K+ channel (Kir) subunits and four regulating sulphonylurea receptor (SUR) subunits that contain two nucleotide-binding folds sensitive to the cytoplasmic ATP/ADP ratio; channel closure occurs when this ratio rises (Aguilar-Bryan et al., 1998; Higgins, 2001). Since its discovery in the cardiac cells (Noma, 1983), the KATP channel has been found in various other tissues where it regulates important physiological functions such as insulin secretion, excitability of muscle fibres and neurons and vascular tone (Rodrigo and Standen, 2005); thus, the search for pharmacological agents able to modulate its activity is an important task, often complicated by the poor tissue specificity (Seino and Miki, 2003; Mannhold, 2004). The characterization of channel molecular structure (Aguilar-Bryan et al., 1995; Inagaki et al., 1996) has allowed us to see how modulators physically interact with KATP channels to exert their effect according to the different subunit composition: SUR1/Kir6.2 in pancreas; SUR2a/Kir6.2 in cardiac and striated muscle fibres; SUR2b/Kir6.2 in smooth muscle cells; SUR2b/Kir6.1 in vascular cells (Seino, 1999). Although recent studies in the heart and in skeletal muscle suggest that the above compositions are not as fixed as proposed previously (Morrissey et al., 2005; Tricarico et al., 2006), they still are the basis for finding tissue-specific modulators, with less side effects with respect to the available drugs.
2-propyl-1,4-benzoxazine (AM10) is a recently synthesized compound that has been shown to exert a dualistic action on the native skeletal muscle KATP channel, showing, in the presence of ATP a potassium channel opener (KCO) activity (nanomolar range), that reverses at higher concentrations, whereas an antagonistic action predominates in the absence of ATP. A dual hypothesis has been proposed to explain this peculiar behaviour: the ability of the compound to bind two distinct sites of skeletal muscle KATP channel complex with opposite action or, rather, a single site whose drug affinity and/or effect are modulated by ATP or generally by tissue metabolism (Tricarico et al., 2003). The use of native pancreatic β cells, which have KATP channels differing from those of skeletal muscle in the SUR subunit while retaining the same Kir subunits, may help to understand if the effects of AM10 depend upon the subunit composition and the tissue metabolism in its native environment. In the pancreatic β cells, the KATP channels control the membrane potential, its glucose-induced fluctuations and are critical in the regulation of insulin secretion (Cook and Hales, 1984; Ashcroft and Rorsman, 1989; Rolland et al., 2002a; Juhl and Hutton, 2004), and are thus the target of clinically relevant drugs. Indeed, SUR1 contains binding sites for two classes of therapeutic drugs, the sulphonylureas and the KCO that inhibit and activate KATP channels, respectively. The sulphonylureas, by blocking the KATP channels, enhance insulin secretion, and are among the most powerful oral hypoglycaemic compounds used to treat type II diabetes (Malik and Trence, 2003). The KCOs such as diazoxide block insulin secretion by opening the KATP channels, and are used for instance to treat children affected by persistent hyperinsulinemic hypoglycaemia of infancy (Hansen et al., 2004).
The present study was performed to examine the effect of the AM10 on KATP channels of native pancreatic β cells. The use of a native system was intended to ensure a proper comparison of the effects of AM10 between the two tissues (present study and Tricarico et al., 2003), as it appears increasingly obvious that both the cellular environment (Krauter et al., 2001) and the plasma membrane itself modulate the properties and the selectivity of the channels (Garavaglia et al., 2004). The results obtained prompted us to look next at the effects of AM10 on the K+ current resulting from the expression of Kir6.2ΔC36 in tsA201 cells, allowing us to assess how much the effect of AM10 was related to an action on the Kir subunit, in the absence of the SUR subunits. Finally, the peculiar action of this compound induced us to an in vivo evaluation of the effects of AM10 on blood glucose.
Methods
All experiments were conducted in accordance with the Italian Guidelines for the use of laboratory animals, which conform with the European Community Directive published in 1986 (86-609-EEC).
Cells
Preparation of cells
The method of preparation of pancreatic β cells and the criteria used to identify them has been described in a previous study (Rolland et al., 2002b). Briefly, the pancreases were taken from non-diabetic Naval Medical Research Institute mice killed by cervical dislocation. Pancreatic islets were isolated, dissociated into single cells. The medium used for the preparation of islet cells contained (in mM): 120 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 24 NaHCO3, 5 HEPES and 10 glucose (pH 7.4). The Ca2+-free medium used to disrupt the islets into single cells contained (in mM): 138 NaCl, 5.6 KCl, 1.2 MgCl2, 5 HEPES and 1 EGTA (pH 7.4). All solutions used for tissue preparation were gassed with O2:CO2 (94:6%) and supplemented with 1 mg ml−1 BSA (fraction V; Roche Molecular Biochemicals; Mannheim, Germany). The β cells were plated on 22 × 40 mm glass coverslips and maintained up to 4 days in Rosewell Park Memorial Institute medium 1640 tissue culture medium containing 10 mM glucose, 10% heat-inactivated foetal calf serum, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin.
Studies on the Kir6.2ΔC36 channel in tsA201 cells used the Kir6.2ΔC36 (mouse) inserted in the mammalian expression vector pCDNA3 and was generously provided by Professor Frances M Ashcroft. The tsA201 cells (a SV40-transformed variant of the HEK293 human embryonic kidney cell line) were cotransfected with 10 μg of plasmid DNA encoding the channels and lower amount of plasmid DNA encoding CD8 receptors, using the calcium phosphate coprecipitation method (Desaphy et al., 2001). For patch-clamp recordings (36–72 h after transfection), successfully transfected cells were identified using Dynal microbeads coated with anti-CD8 antibody (Dynal AS, Oslo, Norway).
Solutions
For perforated whole-cell patch-clamp recordings, the extracellular solution contained (in mM): 140 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 10 HEPES (pH 7.4), and various concentrations of glucose. The pipette solution contained (in mM): 70 K2SO4, 10 NaCl, 10 KCl, 3.7 MgCl2 and 5 HEPES (pH 7.1).
The electrical contact was established by adding amphotericin B to the pipette solution (stock: 60 mg ml−1 in dimethylsulphoxide (DMSO); final concentration: 300 μg ml−1). The tip of the pipette was filled with amphotericin-free solution, and the pipette was then back-filled with the amphotericin-containing solution. The voltage-clamp was considered satisfactory when the series conductance was >35–40 nS.
For inside-out patch-clamp recordings, the bath solution contained (in mM): 130 KCl, 4.6 CaCl2, 10 EDTA and 20 HEPES (pH 7.2). When indicated, trypsin (100 μg ml−1) was added in the bath solution just after establishment of the inside-out configuration and the compound tested was added 10 min later. The intrapipette solution contained (in mM): 130 KCl, 2 CaCl2, 1 MgCl2, 10 EGTA and 20 HEPES (pH 7.4).
For conventional whole-cell patch-clamp recordings, performed on the tsA201 cells, extracellular solution contained (in mM): KCl 140, HEPES 10, MgCl2 1.4, EGTA 1 and glucose 10 (pH 7.4). The pipette solution had the same composition, but 10 μM ATP was added.
In vitro experiments
Electrophysiological recordings
Patch-clamp measurements were carried out using the voltage-clamp mode (perforated and conventional whole-cell and inside-out configurations) and the current-clamp mode. All experiments were performed at room temperature (20–22°C), using an Axopatch200B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA) and the software pClamp8. Patch pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Hertfordshire, UK) to give a resistance of 4–5 MΩ.
During conventional whole-cell recordings, once a GΩ seal was formed, the patch was ruptured and cells were allowed to dialyse with the pipette solution. All whole-cell recordings were made under symmetrical K+ conditions (140 mM) using hyperpolarizing 200 ms long voltage steps of −100 mV from a holding potential of 0 mV, applied every 10 s. Data were acquired and analysed using the same hardware–software mentioned above. After waiting several minutes for the stabilization of whole-cell current, the amplitude of potassium current was estimated as the current blocked by 2 mM barium chloride. Experiments were started after this initial period.
During perforated whole-cell recordings, KATP channel current (IKATP) was monitored by 100 ms duration pulses of ±20 mV from a holding potential of −70 mV.
During the single-channel current recordings with the inside-out configuration, tolbutamide (0.5 mM) was added in the bath before any recording to assess that IKATP was recorded. The open-time probability (Po) of the single channel was calculated according to the equation Po=(To:Ttot):N; To is the total opened time during the interval considered (Ttot) and N is the number of channels under the patch of membrane. The density of current flowing through the patch membrane after trypsin treatment was so high that it was not possible to determine the Po. In this case, we used a technique described elsewhere by us to measure IKATP and its inhibition by AM10 (Tricarico et al., 2003). Briefly, the current values were calculated by digital average of the sample points over 30 s; IKATP was determined by subtracting the baseline level of the currents (measured in the presence of 10 μM ATP) from the open-channel level.
In vivo experiments
Intraperitoneal glucose tolerance test
These tests were carried out on four groups of 8 weeks-old non-diabetic mice following an overnight fast. AM10 (2 or 10 mg kg−1), Glibenclamide (2 mg kg−1), both dissolved in a solution containing polyoxyethylenesorbitan-monooleate (10%) and NaCl 0.9% (90%) or the latter vehicle solution, were injected intraperitoneally (i.p.) at a constant volume of 0.15 ml, 30 min before glucose loading. Mice were given 2 g kg−1 glucose via i.p. injection just after the first glycaemia determination (time 0). Blood was drawn from a tail vein at 0, 15, 30, 60 and 120 min and the blood glucose level was measured by the glucose oxidase method (Glucometer Elite, Bayer-Schweiz-AG, Zurich, Switzerland). The ‘normalized' area under curves (AUCs) were determined using as reference the fasting glucose concentration (t0-value) to minimize the importance of the ‘starting value'.
Each group was composed of five mice. No macroscopic signs of toxicity were observed in either glibenclamide- or AM10-treated mice during the test and thereafter.
Presentation and analysis of the results
Experiments are illustrated by traces, means or representative results obtained with the indicated number of cells from at least three different cultures. The statistical significance of differences between means was assessed by unpaired Student's t-test. Differences were considered significant (*) at P<0.05. Comparisons between more than two means were based on variance analysis (ANOVA) followed by Bonferroni's t-test.
The concentration of AM10 that produces a 50% block of IKATP (IC50) was determined by using a nonlinear least-squares fit of the concentration–response curve to the following logistic equation: where ‘Effect' is the percentage of change of IKATP, ‘Maximal effect' is the maximal percentage block of IKATP, K is the IC50, n is the logistic slope factor and [AM10] is the molar concentration of the AM10.
Materials
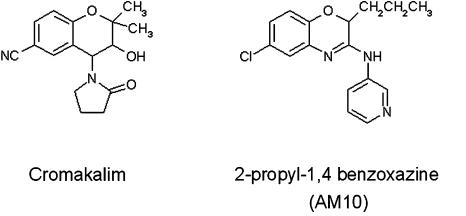
The synthesis of AM10 (Figure 1) has been described previously (Tricarico et al., 2003). As this compound is poorly soluble in the bath solutions used here, AM10 was prepared in a stock solution of 10 mM in DMSO. This allowed the preparation of diluted solutions never exceeding 0.01% DMSO as final percentage, up to 1 μM. This was important as DMSO may have potential effect on the function of β cells (Kemp and Habener, 2002). To test the highest concentration of 100 μM AM10, we had to increase the amount of vehicle, with a final concentration of 1% DMSO; when this concentration was tested, the effect of the vehicle alone was also monitored to verify the lack of any significant effect.
Figure 1.
Chemical structures of cromakalim and of AM10.
All other chemicals were purchased from Sigma (Milano, Italy).
Results
AM10 does not increase KATP channel activity of native-isolated pancreatic β cells
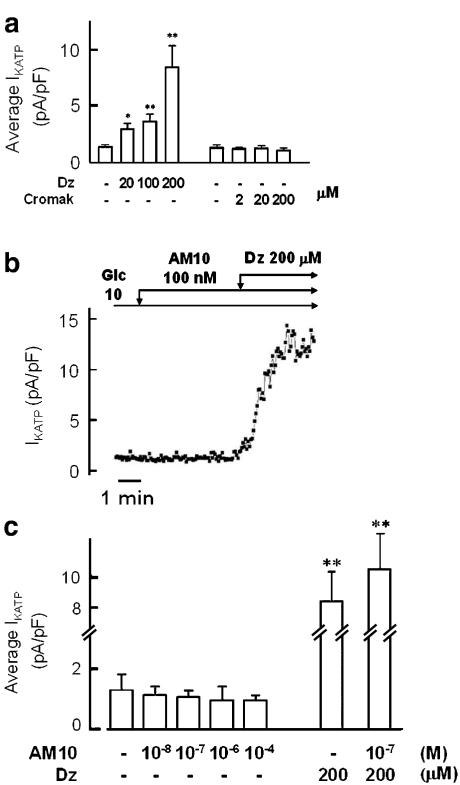
Patch-clamp experiments were performed using the perforated whole-cell configuration. In the presence of 10 mM of glucose in the bath, more than 90% of the KATP channel activity is blocked (Sakura et al., 1998; Garcia-Barrado et al., 2001), the ATP/ADP ratio is high (Detimary et al., 1998), so that it is possible to observe the effect of agonistic compounds. We first verified that the KATP channels were present and functional, by the application of diazoxide, a well-known KCO that interacts mainly with SUR1 and weakly with SUR2b, which in fact augmented IKATP in a dose-dependent manner. On the contrary, cromakalim at 200 μM, which allows it to exert an agonist action on the SUR2 subunits, did not elicit any significant effect (Figure 2a). Using the same protocol, we could not detect any increase of KATP channel activity after AM10 application in the concentration range of 0.1–100 μM (Figure 2b and c). On the contrary, a tendency of AM10 to decrease this activity was observed (Figure 2c). Moreover, AM10 did not seem to interfere with the diazoxide-binding site on SUR1 as the application of 200 μM of this KCO in the absence or in the presence of the benzoxazine derivative induced a similar augmentation of IKATP (Figure 2c).
Figure 2.
Effects of diazoxide, cromakalim and AM10 on IKATP in single native mouse pancreatic β cell. (a) Average amplitude of IKATP in the presence of 10 mM glucose and diazoxide (Dz) or cromakalim (Cromak), at the indicated concentrations. (b) Time course of IKATP amplitude. The glucose concentration was 10 mM throughout the experiment (Glc 10). AM10 (100 nM) and diazoxide (Dz, 200 μM) were added when indicated. Each square represents the amplitude of IKATP elicited by a 100-ms duration pulse of ±20 mV from the holding potential of −70 mV. (c) Average amplitude of IKATP in the presence of 10 mM glucose and AM10 alone or with diazoxide, at the indicated concentrations. Panel (a) and (c) represent the mean of 3–8 experiments and panel (b) is a trace representative of three experiments. *P<0.05; **P<0.01.
AM10 reduces KATP channel activity of native-isolated pancreatic β cells
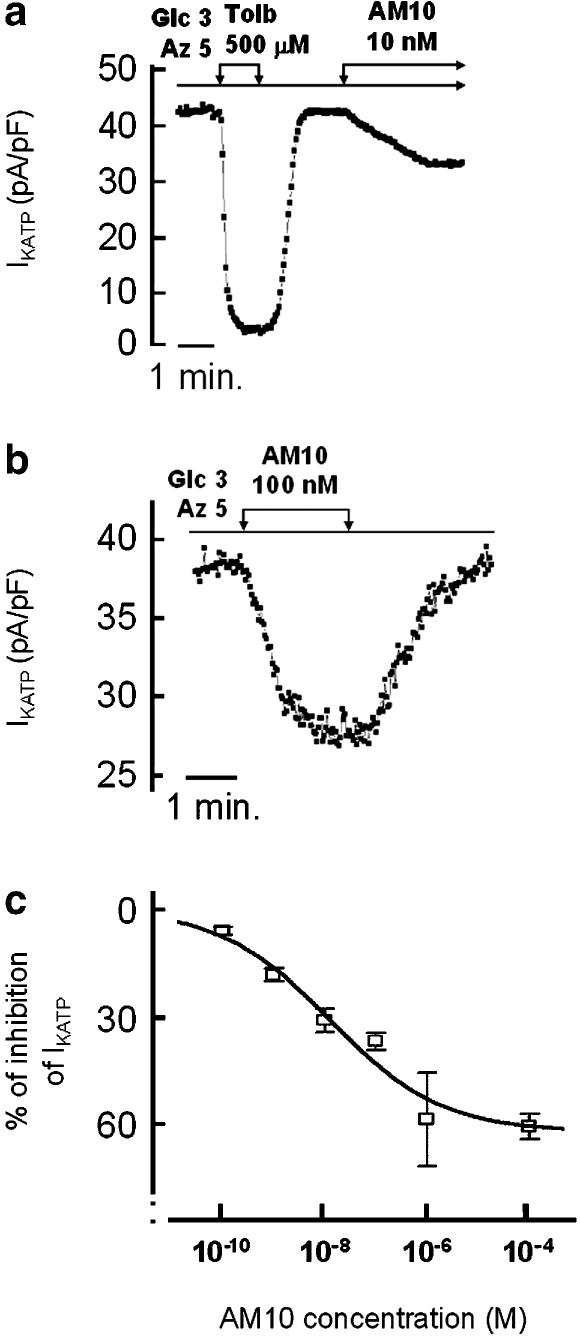
As the above results suggested that AM10 exerts an antagonist effect on pancreatic β cell KATP channels, we set out to evaluate this possibility. In order to open the KATP channels maximally, we used NaN3 a mitochondrial poison that reduces ATP production and ATP/ADP ratio and leads to a maximal KATP channel activity (Atwater et al., 1979). Figure 3a shows the time course of IKATP amplitude of a cell continuously perfused with NaN3 (5 mM) and successively exposed to 0.5 mM tolbutamide or 10 nM AM10 (Figure 3a). Addition of AM10 induced a sustained and reversible inhibition of IKATP (Figure 3b), which was concentration-dependent and reached a maximum of 60% at 1 μM. This degree of block was the maximum, as the application of 100 μM AM10 did not further reduce IKATP. The half-maximal effective concentration (IC50) of AM10 estimated after fitting the data to a sigmoid function, fixing the maximal effect at 60% of inhibition, was 11.5 nM with a Hill coefficient of 0.42 (Figure 3c). Interestingly, the maximal block of IKATP exerted by this class of compounds in the skeletal muscle in the absence of ATP ranged between 40 and 60% (Tricarico et al., 2003).
Figure 3.
Effects of AM10 on IKATP in single native mouse pancreatic β cell. The cells were perfused with a solution containing 3 mM glucose and 5 mM of NaN3. (a) and (b) Time course of IKATP amplitude. Tolbutamide (0.5 mM) and AM10 (10 nM), or solely AM10 (100 nM) were added when indicated, respectively in (a) and (b) that are traces representative of three and four experiments, respectively. (c) The concentration dependence of the inhibitory effect of AM10 on IKATP. The values are the mean±s.e.mean of the amplitude of the relative IKATP recorded in 3–5 cells for each AM10 concentration.
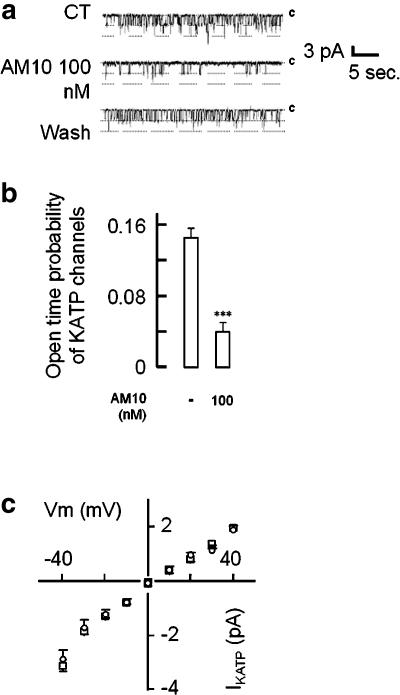
We then performed a series of experiments using the inside-out configuration of the patch-clamp technique and monitoring the spontaneous single-channel currents recorded at a pipette potential of −40 mV (Figure 4). Application of tolbutamide (0.5 mM) or ATP (100 μM) in the bath completely inhibited channel activity (data not shown), indicating that the recorded single-channel current corresponds to IKATP. Application of AM10 (100 nM) to the bath solution lead to a more than threefold reduction of the channel open probability (Figure 4b). The mean open time in our preparations was comparable to that observed by others in native cells (Larsson et al., 1993; Branstrom et al., 1998) (control: 36.4±2.5 ms, n=8 vs AM10 100 nM: 32.9±3.4 ms, n=6) and the average single-channel conductance (control: 54.5±1.7 pS, n=12 vs AM10 100 nM: 55±2.4 pS, n=6) was similar with or without the benzoxazine derivative, whereas the mean interburst time was clearly augmented in the presence of this latter (control: 545±59 ms, n=12 vs AM10 100 nM: 1841±299 ms, n=6, P<0.001). The effect observed was readily reversible, ruling out a possible change in the number of channels as a possible cause of the observed effect. To compare our results with those obtained on the skeletal muscle (Tricarico et al., 2003), we also performed experiments in excised patches where AM10 (0.1 and 100 μM) was applied in the presence of 100 μM ATP in the bath. In these conditions, IKATP was again blocked and no agonistic activity could be detected (n=6 and 3, respectively; data not shown).
Figure 4.
Effects of AM10 on single KATP channel activity. Single-channel current recordings was monitored using the inside-out configuration of the patch-clamp technique. (a) The transmembrane potential was clamped at −40 mV (inside negative). Each trace shows 30 s of recording before (upper trace), during (middle trace) and after the washout (lower trace) of AM10 (100 nM). (b) Average amplitude of the Po of KATP channels in the absence (left bar) or in the presence of 100 nM (right bar) of AM10. (c) I–V relationship of IKATP in the absence (open circles) and in the presence (open square) of AM10 (100 nM). The values are the mean±s.e.mean of the amplitude of IKATP recorded in 12 and six cells, respectively. (a) Traces representative of nine experiments. (c) Mean±s.e.mean of six experiments.
AM10 reduces KATP channel activity via an interaction with Kir6.2
To determine whether AM10 exerts its effect on the SUR or on the Kir subunit, we performed three other series of experiments.
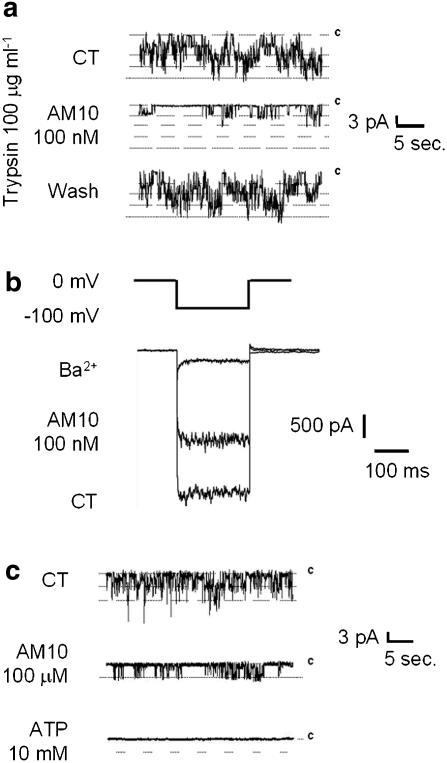
First, again in inside-out patches (from native pancreatic β cells), 10 μg ml−1 of trypsin was added in the bath solution (internal side of the membrane) just after the establishment of the inside-out configuration. This enzymic treatment is known to dissociate the Kir subunit from the SUR subunits. Under these experimental conditions, in agreement with what observed by others (Nichols and Lopatin, 1993), tolbutamide (500 μM) was without any effect on the KATP channel activity, whereas ATP (10 μM) still maintained its blocking activity (data not shown), reflecting their binding to the SUR and the Kir subunit, respectively. Under the same experimental conditions, application of AM10 (100 nM) still inhibited the KATP channel activity (Figure 5a), reducing IKATP by 64±6.1% (n=9), indicating that the benzoxazine derivative may bind to Kir.
Figure 5.
(a) Effects of AM10 on single KATP channel activity. The transmembrane potential was clamped at −40 mV (inside negative). Each trace shows 30 s of recording before (upper trace), during (middle trace) and after the washout (lower trace) of AM10 (100 nM) in the presence of trypsin (100 μg ml−1) in the bath solution. (b) Effects of AM10 on the K+ current resulting from the expression of Kir6.2ΔC36 in tsA201 cells. Representative traces of the current elicited by a 200 ms hyperpolarization of 100 mV from the holding potential of 0 mV in control condition (K+ equimolar; lower trace), in the presence of Ba2+ (2 mM; upper trace) or in the presence of AM10 (100 nM; middle trace). (c) Effects of AM10 on single Kir6.2ΔC36 channel activity. The transmembrane potential was clamped at −40 mV (inside negative). Each trace shows 30 s of recording in control condition (upper trace) and after application of AM10 (100 μM) (middle trace) and ATP (10 mM) (lower trace) in the bath solution. (a, b and c) Traces representative of six, 10 and three experiments, respectively.
Then, to confirm the latter hypothesis, we tested the effects of AM10 on the K+ current resulting from the expression of Kir6.2ΔC36 in tsA201 cells (see Methods section). This truncated mutant reaches the plasma membrane and leads to a functional channel in the absence of the SUR subunit (Tucker et al., 1997). Figure 5b shows that AM10 exerts a clear block of the current, whereas in the same experimental conditions tolbutamide (100 μM) did not exert any effect (data not shown). The effect of AM10 on the Kir6.2ΔC36 current was concentration-dependent and the block observed at the highest concentration tested (100 μM) was 60±2.1% (n=3). The half-maximal effective concentration (IC50) of AM10 estimated after fitting the data to a sigmoid function, fixing the maximal effect at 60% of inhibition, was 12.7 nM with a Hill coefficient of 0.44.
Finally, we showed that the channel activity that remained after AM10 application was totally blocked by a further addition of 10 mM ATP in the bath solution (inside-out configuration of the patch-clamp technique; heterologous expression of Kir6.2ΔC36 in tsA201 cells) (Figure 5c).
AM10 depolarizes the plasma membrane of isolated native pancreatic β cells
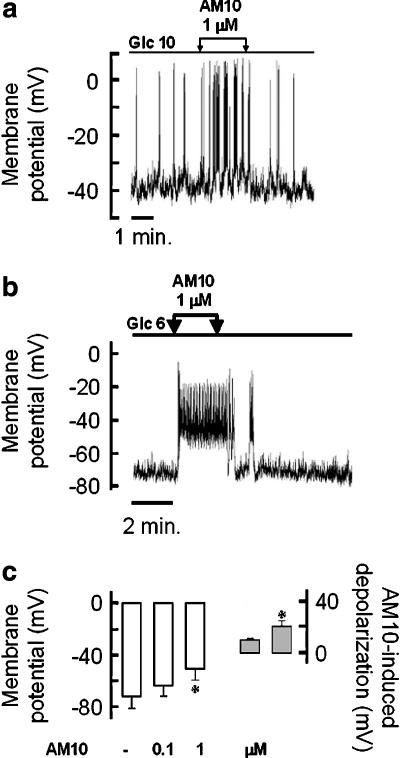
In the light of the importance of the KATP channels in the control of the membrane potential in pancreatic β cells, another series of experiments were undertaken to evaluate the effect of AM10 on this parameter using the current-clamp mode of the patch-clamp technique (perforated whole-cell configuration). All the following experiments were performed on native cells, electrically active in the presence of 10 mM of glucose in the bath solution as the presence of electrical activity in these experimental conditions is a hallmark of the insulin-secreting cell. Addition of diazoxide (0.2 mM) or tolbutamide (0.5 mM) to the extracellular medium hyperpolarized or depolarized the plasma membrane, respectively, whereas application of cromakalim (0.2 mM) was without effect (data not shown). The application of AM10 1 μM in the presence of 10 mM glucose increased the electrical activity (Figure 6a). Thereafter, AM10 was tested when the cells were perfused with 6 mM of glucose (i.e. under the threshold for electrical activity of a pancreatic β cell within mouse islets (Henquin and Meissner, 1984) to see if the benzoxazine derivative was able to induce electrical activity. Under these conditions, AM10 always induced a depolarization of the plasma membrane. This latter leads to the appearance of an electrical activity in 2/4 and 4/4 cells when 0.1 and 1 μM were applied, respectively (Figure 6b). Importantly, AM10 never did hyperpolarize the plasma membrane as a KCO should have done; on the contrary, it exerted a dose-dependent depolarizing effect (Figure 6c).
Figure 6.
Effects of AM10 on the native pancreatic β cell membrane potential. (a and b) The membrane potential of a single mouse β cell was monitored in current-clamp mode of the patch-clamp technique. The glucose concentration of the perfusion medium was maintained stable (10 and 6 mM, in (a) and (b), respectively) and AM10 (1 μM) was added and withdrawn as indicated by the arrows. (c, left part) Average of the membrane potential of single β cells perfused with a solution containing 6 mM glucose, in the presence or not of AM10 at the indicated concentration. (c, right part) Average of the AM10 induced depolarization (0.1 and 1 μM left and right bar, respectively), *P<0.05 vs CT. (a) and (b) Traces representative of three and five experiments, respectively. (c) Mean±s.e.mean of nine experiments (four and five for 0.1 and 1 μM of AM10, respectively).
In vivo effects of AM10
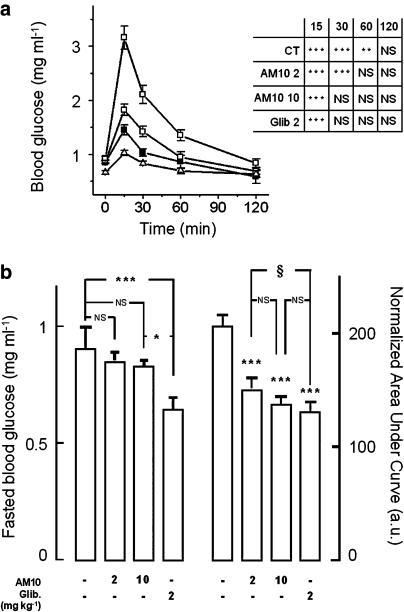
The pharmacological profile of AM10 is unusual in that it inhibits the KATP channels in the pancreatic β cell while it activates them in the skeletal muscle, in the same concentration range. This peculiarity prompted us to evaluate its effects in vivo. Because of the differences observed between AM10 and the sulphonylurea, we compared their respective effect by an i.p. glucose tolerance test, as described in the Methods section. Administration of 2 g kg−1 of glucose to control mice injected with the vehicle, 30 min before the first measurement of blood glucose, resulted in a typical increase in blood glucose levels from a basal value of 0.92±0.04 to a peak of 3.16±0.21 mg ml−1. The animals treated with AM10 at either 2 or 10 mg kg−1 had a fasting blood glucose level not significantly different from that of the control mice (Figure 7b, left panel). However, treatment with the benzoxazine derivative lead to a dose-dependent reduction of the blood glucose response to the i.p. glucose load (peak value: 1.82±0.11 and 1.45±0.09 mg ml−1 for 2 and 10 mg kg−1, respectively, P<0.05 vs control values; Figure 7a). AM10 also limited the duration of the rise in blood glucose. In control animals, the blood glucose was still raised above the fasting level 60 min after the bolus (1.35±0.10 and 0.97±0.10 mg ml−1, respectively, P<0.05), but in animals pre-treated with AM10, at the two concentrations tested, blood glucose was back to normal at this time. The dose-dependent effects of AM10 are also underlined by the fact that 30 min after pre-treatment with the higher dose (10 mg kg−1), blood glucose levels were normal, whereas in the animals pre-treated with 2 mg kg−1 AM10, normalization of the blood glucose needed 60 min. Glibenclamide pre-treated animals (2 mg kg−1) showed the most significantly improved glucose tolerance curve, with a peak glucose of 1.024±0.05 mg ml−1 and also a marked decrease in fasting blood glucose concentration (0.66±0.032 mg ml−1; P<0.05 vs comtrol; Figure 7b, left panel). Finally, it has to be underlined that the peaks of blood glucose following the glucose loading for the three groups of treated animals are different between each group (Figure 7a). Accordingly, mice treated with AM10 and glibenclamide showed a significant decrease in the normalized AUC (nAUC) of blood glucose during the i.p. glucose tolerance test with respect to control animals (Figure 7b, right panel). Interestingly, no difference in the nAUC of blood glucose was observed between the group of mice treated with AM10 (10 mg kg−1) and the one treated with glibenclamide (2 mg kg−1) (Figure 7b, right panel).
Figure 7.
i.p. glucose tolerance test. (a) Each curve represents the blood glucose levels of mice, fasted for 14 h and then treated 30 min before the first blood glucose measurement and the glucose loading (2 g kg−1), with the vehicle, AM10 (2 or 10 mg kg−1) or glibenclamide (2 mg kg−1) (open circle, open square, closed square and open triangle, respectively). Each point shows the mean±s.e.mean of nine measurements. Inset panel: statistical significance of differences between the fasting blood glucose (t=0) and the blood glucose determined after 15, 30, 60 and 120 min, in all the experimental conditions. ***P<0.001. Although not shown, the values of blood glucose at 15 min were significantly different between treatment groups (ANOVA test (F=64; P<0.0001). The Bonferroni's t-test correction for individual comparison showed significant differences between each couples of values with P<0.02. (b, left hand graph) Mean values of initial (t=0) blood glucose in mice given vehicle-, AM10- or glibenclamide-treated animals. Note that these samples were taken 30 min after treatment. (b, right hand graph) nAUC (arbitrary units) of blood glucose during the i.p. glucose tolerance test in vehicle-, AM10- or glibenclamide-treated animals. Each column represents the mean±s.e.mean of five experiments. ***P<0.001 vs control; §P<0.5.
Discussion
This work was aimed at evaluating the effects of a newly synthesized AM10 in native mouse pancreatic β cells in order to better define its pharmacological profile and tissue specificity. Surprisingly, we observed that in our experimental conditions, AM10 did not show a KCO activity in the pancreatic β cell, while it provoked a concentration-dependent closure of KATP channels via a direct inhibition on the Kir subunit, which lead to depolarization of the plasma membrane. As already demonstrated (Tricarico et al., 2003), within the same range of concentrations, AM10 is clearly a KCO in skeletal muscle. In vivo experiments show that AM10 was able to accelerate the restoration of blood glucose to normal after an i.p. glucose bolus.
AM10 exerts a different action on KATP channels of native pancreatic β cells and skeletal muscle
The present results clearly show than AM10 mainly blocks pancreatic β cell KATP channels, whereas in the same concentration range it presents a dualistic action in the skeletal muscle. Two hypotheses, based on a mathematical model (Rovati and Nicosia, 1994), were previously proposed to explain the dualistic effect of the benzoxazine derivatives on the KATP channels in skeletal muscle fibres (Tricarico et al., 2003). First, these molecules may exert two opposite effects by binding to two distinct sites of the channel: a stimulatory site available for drug binding in the presence of ATP, and an inhibitory one which is unmasked in the absence of the nucleotide. Alternatively, both effects may result from a unique binding site changing its affinity according to the ATP concentration. The results obtained in the present study with the pancreatic β cell reinforce the first hypothesis. Indeed, we show here, with patch-clamp experiments using the inside-out configuration, that AM10, in the concentration range used, does not enhance IKATP either in the absence or in the presence of ATP. These results are in line with those obtained in perforated patch (voltage- and current-clamp). Moreover, apart from the different absolute potency, AM10 maintained a very similar cromakalim-like profile in skeletal muscle. In this regard, it is important to remember that, in general, KCOs may recognize the different SUR subunits based on their relative affinity and the metabolic state of the cell. Recent molecular biology experiments underlined that the basis for the high affinity of SUR2 for cromakalim-like openers mostly resides in the presence of a hydrophobic amino acid (leucine) on the last transmembrane segment of SUR2 which is replaced in SUR1 by a polar amino acid (threonine) (Moreau et al., 2000). This difference may account for the weak ability of SUR1 to establish a strong hydrophobic interaction with the aromatic moiety of cromakalim and other KCOs, including the benzoxazine derivative under investigation. With this in mind, it is reasonable to propose that, in our experimental conditions, the lack of agonistic effect of AM10 in the concentration range used in pancreatic β cells is due to the reported structural differences in the KCO binding site on SUR subunits between tissues.
In the skeletal muscle, the dualistic action may result from the fact that at low concentrations the inhibitory effect of AM10 is masked by the action on the stimulatory site that the benzoxazine derivatives activate with higher potency (Tricarico et al., 2003). Thus, the compound first saturates the stimulatory site and then the inhibitory one, reflecting the dualistic action. In contrast, on the pancreatic β cell KATP channel, the stimulatory binding site for AM10, if present, has such a low affinity that, in our experimental conditions, we only observe an antagonistic effect.
AM10 and the sulphonylureas block the KATP channels of native pancreatic β cells in a different manner
Our results strongly implied that the block of IKATP exerted by AM10 is different from the one exerted by the sulphonylureas. First, we found that the Hill coefficient for the concentration–response curve of AM10 on both native channels and expressed Kir6.2ΔC36 is lower than 1. This finding strongly suggests that the inhibition of the KATP channel requires the binding of more than one molecule of AM10 on the channel complex. The question of how many molecules of sulphonylurea must bind to the IKATP channel to induce its inhibition is still debated (Dorschner et al., 1999; Russ et al., 1999). Anyway, these two studies showed that the curves resulting from KATP inhibition by the sulphonylureas always gave a Hill coefficient close to 1. Then, it should be underlined that the chemical structure of the sulphonylureas and the benzoxazine derivatives are very different. In particular, the latter have a positive charge owing to the protonation of the pyridine nitrogen at physiological pH (Figure 1), and also do not present the anionic group that has been defined as an essential requisite for the sulphonylureas to bind to their specific site on the SUR subunit (Meyer et al., 1999). We have also shown here that the benzoxazine derivative was still able to block the KATP channel activity after exposure of the internal face of the membrane patches to trypsin. This enzymic treatment has been reported to prevent the modulation of the KATP channels by agents that interact with the SUR subunit (MgADP, or sulphonylureas), whereas inhibition by ATP that binds to Kir remains unchanged (Nichols and Lopatin, 1993; Proks and Ashcroft, 1993; Branstrom et al., 1998). Finally and most importantly, Kir6.2ΔC36 current is diminished by AM10 in the same range of concentrations that inhibit IKATP in native pancreatic β cells and the residual current is inhibited by ATP. Thus, the present study demonstrated that AM10 binds to the Kir6.2 subunit and probably at a site different from that binding ATP. Further studies using specific mutants for the ATP-binding domain of the Kir6.2ΔC36 (Reimann et al., 1999) will be helpful to verify our finding. It is however of interest to point out that the presence of an inhibitory binding site on Kir6.2 has been already hypothesized (Proks and Ashcroft, 1997; Gribble et al., 1998; Henquin, 2004).
In vivo implications
A preliminary in vivo assessment of the effect of AM10 allowed us to observe a dose-dependent attenuation of the induced elevation of blood glucose. Surprisingly, AM10, at the dose of 10 mg kg−1 reduces the nAUC of the blood glucose to the same extent as 2 mg kg−1 glibenclamide, but this effect is not accompanied by a reduction of the fasting blood glucose level. It has to be pointed out that AM10 was used only at a fivefold higher dose that glibenclamide, which has an affinity for pancreatic β cell KATP channels about 200-fold higher (Gromada et al., 1995). Thus, we cannot rule out that the lack of effect on fasting glucose of our compound results from the different potency of the two drugs on the target tissue. An alternative interpretation for the difference between the two classes of compounds on fasting glucose could be that the decrease of blood glucose owing to an insulinotropic effect of AM10 through the closure of pancreatic β cell KATP channels is somehow counterbalanced by its KCO action at the skeletal muscle (Tricarico et al., 2003). On the other hand, the inhibitory effect exerted by glibenclamide on both tissues (Pulido et al., 1996; Minami et al., 2004) accounts for the damaging reduction of the fasting blood glucose level (Rendell, 2004). In fact, it is now well documented that the closure of KATP channels in skeletal muscle is a mechanism through which a drug may increase glucose uptake, whereas the opposite is observed with KCOs (Wasada et al., 2001). The detailed pharmacokinetics of AM10 are not known and thus a proper comparison of the in vivo effects of these compounds is not yet possible and no therapeutic outcome can be predicted from this preliminary in vivo study. Nevertheless, it must be emphasized that the observed hypoglycaemic effect is supported by the in vitro results in the two tissues.
Deeper insights in the effects of AM10 on the other KATP channels and in the structure–activity relationship of these benzoxazine derivatives, both in vitro and in vivo, may lead to improvements in their physico-chemical properties as well as to the design of new drugs. For instance, it has been proposed that compounds able, within the same range of concentration, to inhibit IKATP in the pancreatic β cell and to increase it in vascular muscle that contains SUR2b, the preferential target of KCO (Gribble and Reimann, 2002) may be of relevance to the treatment of type 2 diabetic patients with hypertension (Henquin, 2004).
Acknowledgments
JF Rolland was supported by Telethon-Italy postdoctoral grant (Project No. 1208). We thank Professor Frances M Ashcroft for providing them pCDNA3 containing Kir6.2ΔC36 and Dr Ernesto Carbonara for the technical support.
Abbreviations
- AM10
2-propyl-1,4-benzoxazine
- AUC
area under curve
- Glc
glucose
- IKATP
KATP channel current
- KATP channel
ATP-sensitive potassium channel
- KCO
potassium channel opener
- Kir
inward rectifier potassium channel
- SUR
sulphonylurea receptor
Conflict of interest
The authors state no conflict of interest.
References
- Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, III, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Atwater I, Dawson CM, Ribalet B, Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Branstrom R, Efendic S, Berggren PO, Larsson O. Direct inhibition of the pancreatic beta-cell ATP-regulated potassium channel by alpha-ketoisocaproate. J Biol Chem. 1998;273:14113–14118. doi: 10.1074/jbc.273.23.14113. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Desaphy JF, De Luca A, Tortorella P, De Vito D, George AL, Jr, Conte CD. Gating of myotonic Na channel mutants defines the response to mexiletine and a potent derivative. Neurology. 2001;57:1849–1857. doi: 10.1212/wnl.57.10.1849. [DOI] [PubMed] [Google Scholar]
- Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J. 1998;333 Part 2:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner H, Brekardin E, Uhde I, Schwanstecher C, Schwanstecher M. Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol Pharmacol. 1999;55:1060–1066. doi: 10.1124/mol.55.6.1060. [DOI] [PubMed] [Google Scholar]
- Garavaglia M, Dopinto S, Ritter M, Furst J, Saino S, Guizzardi F, et al. Membrane thickness changes ion-selectivity of channel-proteins. Cell Physiol Biochem. 2004;14:231–240. doi: 10.1159/000080332. [DOI] [PubMed] [Google Scholar]
- Garcia-Barrado MJ, Ravier MA, Rolland JF, Gilon P, Nenquin M, Henquin JC. Inhibition of protein synthesis sequentially impairs distinct steps of stimulus–secretion coupling in pancreatic beta cells. Endocrinology. 2001;142:299–307. doi: 10.1210/endo.142.1.7910. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Pharmacological modulation of KATP channels. Biochem Soc Trans. 2002;30:333–339. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998;47:1412–1418. doi: 10.2337/diabetes.47.9.1412. [DOI] [PubMed] [Google Scholar]
- Gromada J, Dissing S, Kofod H, Frokjaer-Jensen J. Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia. 1995;38:1025–1032. doi: 10.1007/BF00402171. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Arkhammar PO, Bodvarsdottir TB, Wahl P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr Med Chem. 2004;11:1595–1615. doi: 10.2174/0929867043365026. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Pathways in beta-cell stimulus–secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53 Suppl 3:S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Meissner HP. Significance of ionic fluxes and changes in membrane potential for stimulus–secretion coupling in pancreatic B-cells. Experientia. 1984;40:1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: physiology, structure and mechanism – an overview. Res Microbiol. 2001;152:205–210. doi: 10.1016/s0923-2508(01)01193-7. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Juhl K, Hutton J. Stimulus–secretion coupling in the pancreatic beta-cell. Adv Exp Med Biol. 2004;552:66–90. [PubMed] [Google Scholar]
- Kemp DM, Habener JF. Synergistic effect of dimethyl sulfoxide on glucagon-like peptide 1 (GLP-1)-stimulated insulin secretion and gene transcription in INS-1 cells: characterization and implications. Biochem Pharmacol. 2002;64:689–697. doi: 10.1016/s0006-2952(02)01212-1. [DOI] [PubMed] [Google Scholar]
- Krauter T, Ruppersberg JP, Baukrowitz T. Phospholipids as modulators of KATP channels: distinct mechanisms for control of sensitivity to sulphonylureas, K+ channel openers, and ATP. Mol Pharmacol. 2001;59:1086–1093. [PubMed] [Google Scholar]
- Larsson O, Ammala C, Bokvist K, Fredholm B, Rorsman P. Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta-cells. J Physiol. 1993;463:349–365. doi: 10.1113/jphysiol.1993.sp019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik T, Trence DL. Treating diabetes using oral agents. Primary Care. 2003;30:527–541. doi: 10.1016/s0095-4543(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Mannhold R. KATP channel openers: structure–activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- Meyer M, Chudziak F, Schwanstecher C, Schwanstecher M, Panten U. Structural requirements of sulphonylureas and analogues for interaction with sulphonylurea receptor subtypes. Br J Pharmacol. 1999;128:27–34. doi: 10.1038/sj.bjp.0702763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Miki T, Kadowaki T, Seino S. Roles of ATP-sensitive K+ channels as metabolic sensors: studies of Kir6.x null mice. Diabetes. 2004;53 Suppl 3:S176–S180. doi: 10.2337/diabetes.53.suppl_3.s176. [DOI] [PubMed] [Google Scholar]
- Moreau C, Jacquet H, Prost AL, D'hahan N, Vivaudou M. The molecular basis of the specificity of action of K(ATP) channel openers. EMBO J. 2000;19:6644–6651. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar CP, Han S, et al. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Trypsin and alpha-chymotrypsin treatment abolishes glibenclamide sensitivity of KATP channels in rat ventricular myocytes. Pflugers Arch. 1993;422:617–619. doi: 10.1007/BF00374011. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Proks P, Ashcroft FM. Modification of K-ATP channels in pancreatic beta-cells by trypsin. Pflugers Arch. 1993;424:63–72. doi: 10.1007/BF00375103. [DOI] [PubMed] [Google Scholar]
- Proks P, Ashcroft FM. Phentolamine block of KATP channels is mediated by Kir6.2. Proc Natl Acad Sci USA. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido N, Casla A, Suarez A, Casanova B, Arrieta FJ, Rovira A. Sulphonylurea stimulates glucose uptake in rats through an ATP-sensitive K+ channel dependent mechanism. Diabetologia. 1996;39:22–27. doi: 10.1007/BF00400409. [DOI] [PubMed] [Google Scholar]
- Reimann F, Ryder TJ, Tucker SJ, Ashcroft FM. The role of lysine 185 in the kir6.2 subunit of the ATP-sensitive channel in channel inhibition by ATP. J Physiol. 1999;520 Part 3:661–669. doi: 10.1111/j.1469-7793.1999.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–1358. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11:1915–1940. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- Rolland JF, Henquin JC, Gilon P. Feedback control of the ATP-sensitive K+ current by cytosolic Ca2+ contributes to oscillations of the membrane potential in pancreatic beta-cells. Diabetes. 2002a;51:376–384. doi: 10.2337/diabetes.51.2.376. [DOI] [PubMed] [Google Scholar]
- Rolland JF, Henquin JC, Gilon P. G protein-independent activation of an inward Na+ current by muscarinic receptors in mouse pancreatic beta-cells. J Biol Chem. 2002b;277:38373–38380. doi: 10.1074/jbc.M203888200. [DOI] [PubMed] [Google Scholar]
- Rovati GE, Nicosia S. Lower efficacy: interaction with an inhibitory receptor or partial agonism. Trends Pharmacol Sci. 1994;15:140–144. doi: 10.1016/0165-6147(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Russ U, Hambrock A, Artunc F, Loffler-Walz C, Horio Y, Kurachi Y, et al. Coexpression with the inward rectifier K(+) channel Kir6.1 increases the affinity of the vascular sulfonylurea receptor SUR2B for glibenclamide. Mol Pharmacol. 1999;56:955–961. [PubMed] [Google Scholar]
- Sakura H, Ashcroft SJ, Terauchi Y, Kadowaki T, Ashcroft FM. Glucose modulation of ATP-sensitive K-currents in wild-type, homozygous and heterozygous glucokinase knock-out mice. Diabetologia. 1998;41:654–659. doi: 10.1007/s001250050964. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Barbieri M, Antonio L, Tortorella P, Loiodice F, Camerino DC. Dualistic actions of cromakalim and new potent 2H-1,4-benzoxazine derivatives on the native skeletal muscle KATP channel. Br J Pharmacol. 2003;139:255–262. doi: 10.1038/sj.bjp.0705233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Jr, Camerino DC. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci USA. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Wasada T, Yano T, Ohta M, Yui N, Iwamoto Y. ATP-Sensitive potassium channels modulate glucose transport in cultured human skeletal muscle cells. Endocr J. 2001;48:369–375. doi: 10.1507/endocrj.48.369. [DOI] [PubMed] [Google Scholar]