Abstract
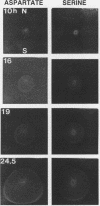
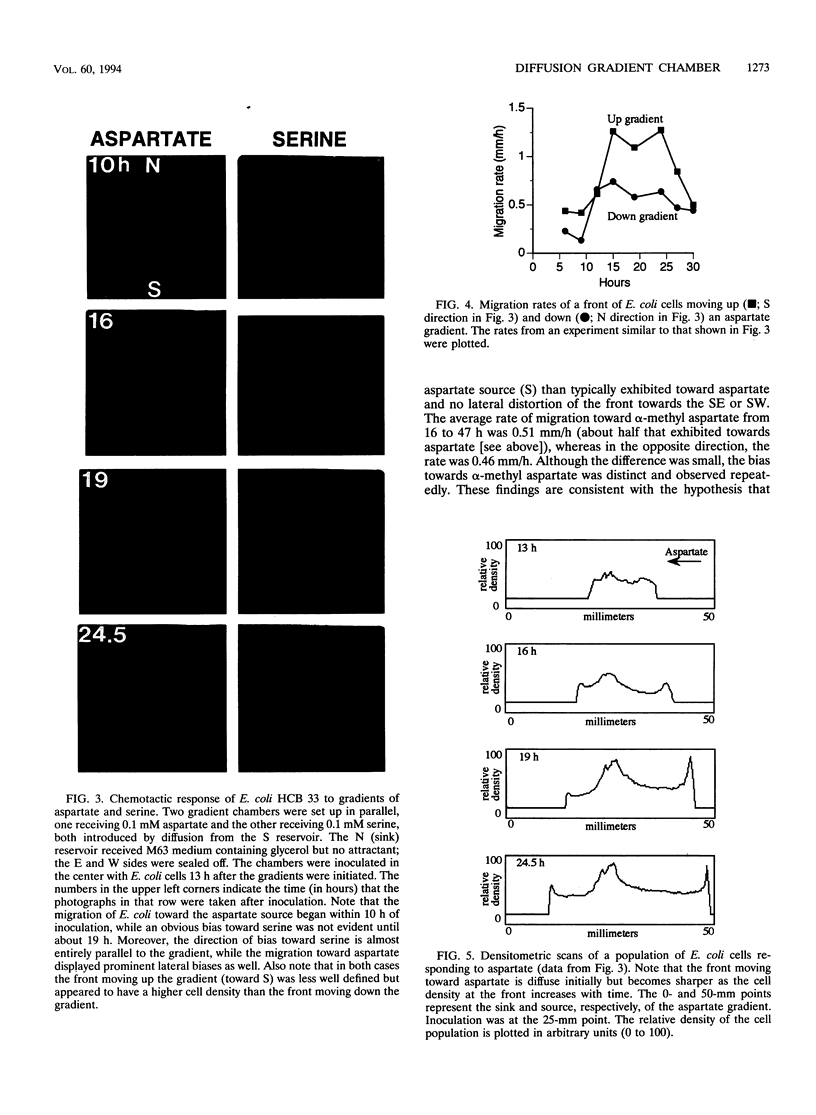
The natural habitats of most microbes are dynamic and include spatial gradients of growth substrates, electron acceptors, pH, salts, and inhibitory compounds. To mimic this diffusive aspect of nature, we developed an analytical diffusion gradient chamber (DGC) that can be used to separate, enrich for, isolate, and study the behavior of microorganisms. The chamber is a polycarbonate box containing an arena (5 by 5 by 2 cm) into which is cast a semisolid growth medium. Continuously replenished solute reservoirs positioned on each side of the arena but separated from it by a porous membrane enable the formation throughout the gel of multiple, intersecting gradients of solutes in two dimensions. With glucose as the solute, a gradient which spanned a 100-fold range in concentration was established across the arena in about 4 days. The shape of the glucose gradient was accurately predicted by a mathematical model based on Fickian diffusion. The growth and migratory behavior of Escherichia coli in response to imposed gradients of attractants (aspartate, α-methyl aspartate, and serine) and a repellent (valine) were examined. Cells responded in predictable ways to such gradients by forming distinctive growth and migration patterns in the DGC. This was true for wild-type E. coli as well as specific chemotaxis and motility mutants. The patterns yielded information about the threshold concentration of chemoeffectors needed to elicit a response as well as their saturating concentration. It was also evident that the metabolism of attractants significantly affected the gradients and, hence, the movement of cells. Finally, it was possible to separate E. coli and Pseudomonas fluorescens in the DGC on the basis of their differential responses to gradients of various chemoeffectors.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Amsler C. D., Cho M., Matsumura P. Multiple factors underlying the maximum motility of Escherichia coli as cultures enter post-exponential growth. J Bacteriol. 1993 Oct;175(19):6238–6244. doi: 10.1128/jb.175.19.6238-6244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. A physicist looks at bacterial chemotaxis. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):1–9. doi: 10.1101/sqb.1988.053.01.003. [DOI] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Caldwell D. E., Hirsch P. Growth of microorganisms in two-dimensional steady-state diffusion gradients. Can J Microbiol. 1973 Jan;19(1):53–58. doi: 10.1139/m73-008. [DOI] [PubMed] [Google Scholar]
- Langer R., Fefferman M., Gryska P., Bergman K. A simple method for studying chemotaxis using sustained release of attractants from inert polymers. Can J Microbiol. 1980 Feb;26(2):274–278. doi: 10.1139/m80-045. [DOI] [PubMed] [Google Scholar]
- Manson M. D. Bacterial motility and chemotaxis. Adv Microb Physiol. 1992;33:277–346. doi: 10.1016/s0065-2911(08)60219-2. [DOI] [PubMed] [Google Scholar]
- Møller M. M., Nielsen L. P., Jørgensen B. B. Oxygen Responses and Mat Formation by Beggiatoa spp. Appl Environ Microbiol. 1985 Aug;50(2):373–382. doi: 10.1128/aem.50.2.373-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Revsbech N. P., Jørgensen B. B. Microoxic-Anoxic Niche of Beggiatoa spp.: Microelectrode Survey of Marine and Freshwater Strains. Appl Environ Microbiol. 1986 Jul;52(1):161–168. doi: 10.1128/aem.52.1.161-168.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel L. A., Jackson J. L. Theoretical analysis of chemotactic movement in bacteria. J Mechanochem Cell Motil. 1973 May;2(1):25–34. [PubMed] [Google Scholar]
- Wimpenny J. W., Waters P. Growth of micro-organisms in gel-stabilized two-dimensional diffusion gradient systems. J Gen Microbiol. 1984 Nov;130(11):2921–2926. doi: 10.1099/00221287-130-11-2921. [DOI] [PubMed] [Google Scholar]
- Wolfaardt G. M., Lawrence J. R., Hendry M. J., Robarts R. D., Caldwell D. E. Development of steady-state diffusion gradients for the cultivation of degradative microbial consortia. Appl Environ Microbiol. 1993 Aug;59(8):2388–2396. doi: 10.1128/aem.59.8.2388-2396.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Berg H. C. Migration of bacteria in semisolid agar. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6973–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]