Abstract
Background and purpose:
The natriuretic peptides, ANP and BNP, modulate vascular smooth muscle tone in human conduit arteries. Surprisingly, the natriuretic peptide receptor-A (NPR-A) has not been visualized using radioligand binding in these vessels. A new member of this peptide family, Dendroaspis natriuretic peptide (DNP) identified from snake venom, has been proposed to be present in human plasma and endothelial cells. Also, recently a novel radioligand, [125I]-DNP, has been characterized as selective for NPR-A in human heart.
Experimental approach:
Our aims were to investigate expression and function of NPR-A receptors in human mammary artery using [125I]-DNP to quantify receptor density, immunocytochemistry to delineate the cellular distribution of the receptor and in vitro pharmacology to compare DNP induced vasodilatation to that of ANP.
Key results:
Saturable, sub-nanomolar affinity [125I]-DNP binding was detected to smooth muscle of mammary artery, with receptor density of ∼2 fmol mg–1 protein, comparable to that of other vasoactive peptides. NPR-A immunoreactivity was localised to vascular smooth muscle cells and this was confirmed with fluorescence dual labelling. NPR-A expression was not detected in the endothelium. Like ANP, DNP fully reversed the constrictor response to ET-1 in endothelium intact or denuded mammary artery, with comparable nanomolar potencies.
Conclusions and Implications:
This is the first characterization of NPR-A in human mammary artery using [125I]-DNP and we provide evidence for the presence of receptor protein on vascular smooth muscle cells, but not endothelial cells. This implies that the observed vasodilatation is predominantly mediated via direct activation of smooth muscle NPR-A.
Keywords: dendroaspis natriuretic peptide, human mammary artery, immunocytochemistry, radioligand binding, natriuretic peptide receptor-A, vascular smooth muscle, vasodilatation
Introduction
The family of natriuretic peptides are important regulators of cardiovascular homeostasis. Two members of this family, atrial natriuretic peptide (ANP) and brain type natriuretic peptide (BNP), circulate in human plasma after their release from cardiac tissue producing vasodilatation as well as natriuresis and diuresis (Inagami, 1994). These effects are predominantly initiated via activation of the cell surface guanylyl-cyclase linked receptor, natriuretic peptide receptor-A (NPR-A; also referred to as GC-A) (Chinkers et al., 1989) that has high affinity for both ANP and BNP (Suga et al., 1992).
Functionally, the natriuretic peptides have been shown to antagonize the vascular actions of endothelin-1 (ET-1) (Wiley and Davenport, 2001, 2002), which is a potent and efficacious vasoconstrictor of human vessels with a prolonged duration of action (Franco-Cereceda, 1989). Levels of ET-1 are increased in cardiovascular disease (Miyauchi et al., 1989; McMurray et al., 1992), which may result in heightened vascular smooth muscle tone. This is of particular importance in pathological states of endothelium dysfunction in which production of the endothelium derived vasodilator nitric oxide (NO), a regulator of vascular smooth muscle tone in vivo, is impaired (Widlansky et al., 2003). Interestingly, circulating levels of natriuretic peptides, especially BNP, are significantly elevated, for example in heart failure patients (Burnett et al., 1986; Mukoyama et al., 1990). Therefore, these peptides may compensate for a lack of NO in disease and oppose the vasoconstriction induced by ET-1. Importantly, plasma BNP levels have been demonstrated to correlate with the degree of left ventricular dysfunction (Abassi et al., 2004; Doust et al., 2005) and are being used as surrogate biomarkers for heart failure. Furthermore, synthetic BNP, nesiritide (Natrecor), is used as a therapeutic agent for this disorder (Keating and Goa, 2003). Therefore, manipulating the natriuretic peptide system is of considerable interest to drug discovery for cardiovascular disease (Abassi et al., 2004).
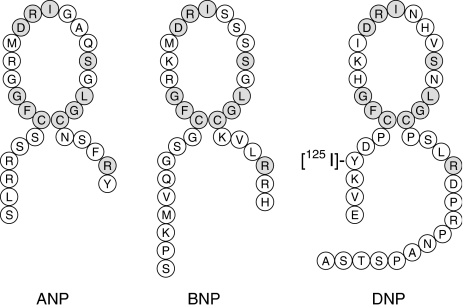
Considering the interest and research into the vascular physiology of the natriuretic peptides, it is surprising that the NPR-A protein had not been pharmacologically characterized in native human tissues using radiolabelled natriuretic peptides. Evolutionary studies have suggested the presence of a natriuretic peptide-like sequence in the venom of numerous snakes (Fry et al., 2006) and, recently, an additional member of the natriuretic peptide family, Dendroaspis natriuretic peptide (DNP), was identified from the venom of the Green Mamba snake (Schweitz et al., 1992). This peptide is more resistant to peptidase degradation than other natriuretic peptides (Chen et al., 2002) and therefore may be more suitable for radiolabelling. Interestingly, evidence for the presence of a DNP-like peptide in mammals (Schirger et al., 1999; Kim et al., 2004; Piao et al., 2004) has been reported. However, the sequence has not yet been identified in the human genome. Pharmacologically DNP resembles ANP (Lisy et al., 1999; Best et al., 2002) and recently we have synthesized a novel radiolabelled analogue of DNP, [125I]-DNP (Figure 1), and demonstrated that it has high affinity and selectivity for NPR-A in adult human heart (Singh et al., 2006).
Figure 1.
Structure of [125I]-DNP and comparison with human ANP and BNP. Identical amino acids in the peptide sequences are shaded.
Our aim was to use radioligand binding, immunocytochemistry and in vitro pharmacology experiments to characterize human vascular NPR-A receptors and further investigate their cellular expression at the protein level and function. The mammary artery was chosen as it has previously been shown to be responsive to ANP and BNP and to express mRNA encoding NPR-A (Ikeda et al., 1996). Using [125I]-DNP we characterized the vascular NPR-A receptor based on the pharmacological criteria of saturable, specific and high-affinity binding and identified the cellular localization of NPR-A protein to vascular smooth muscle cells, but not endothelium, using immunocytochemistry and fluorescence dual labelling. Finally, we demonstrated that DNP-mediated vasodilatation of human mammary artery is predominantly due to activation of smooth muscle NPR-A receptors in this tissue and is comparable, in terms of both potency and efficacy, to that which we have previously reported for ANP and other important directly acting vasodilators (Wiley and Davenport, 2002).
Methods
Tissue collection
Human mammary artery was obtained, with informed consent and local ethical approval, from patients undergoing coronary artery by-pass graft surgery. Drug treatment prior to surgery included ACE inhibitors, β-blockers, diuretics, non-steroidal anti-inflammatory drugs, nitrates and statins. Patients were not treated with synthetic BNP, nesiritide.
For radioligand binding and immunocytochemistry, arteries were snap frozen in liquid nitrogen, stored at −70°C and when required cut into 30 μm sections onto gelatine- and poly-L-lysine-coated slides, respectively. For in vitro pharmacology, tissue was transported to the laboratory in Krebs' solution (4°C).
Preparation of [125I]-DNP
[125I]-DNP (2000 Ci mmol−1) (Amersham Biosciences, GE Healthcare, Bucks, UK) was prepared from unlabelled DNP by direct iodination with sodium [125]-iodide, using the chloramine-T method.
Saturation binding assay and receptor autoradiography
Binding experiments were carried out as previously described (Davenport and Kuc, 2005a). Briefly, sections of mammary artery were preincubated for 15 min in binding buffer (50 mM Tris-HCl buffer, containing 10 mM MgCl2 and 5 mM EDTA; pH 7.2). For saturation analysis, sections were incubated with increasing concentrations of [125I]-DNP (8 pM–1 nM), for 1 h, at 22°C, with nonspecific binding defined in adjacent sections at each concentration of radiolabel by inclusion of 1 μM DNP. For receptor autoradiography, sections were incubated with 0.2 nM [125I]-DNP, for 1 h, at 22°C with nonspecific binding determined as before. All sections were washed in 50 mM Tris-HCl buffer, dipped in de-ionized water for 1 s, air-dried and apposed to radiation sensitive film for 5 days, together with [125I] standards. The resulting autoradiograms were analysed by means of computer assisted image analysis (Quantimet 970, Cambridge Instruments) to give values of receptor density in a mol mm−2. Saturation data were analysed using the iterative, nonlinear curve fitting programs EBDA and LIGAND in the KELL package (Biosoft, Cambridge, UK). The presence of one or two sites was determined using the F-ratio test in LIGAND. Pooled KD, Bmax and Hill slope (nH) were expressed as mean±s.e.m; n-values are the number of patients from whom arteries were obtained.
Immunocytochemistry
Detection of NPR-A immunoreactivity (IR) was carried out as previously described (Davenport and Kuc, 2005b). Briefly, sections of mammary artery were fixed in acetone, blocked for nonspecific protein interactions with 5% swine serum and incubated with rabbit anti-NPR-A serum (raised against human sequence of NPR-A294-308 LKQLKHLAYEQFNFT), for 72 h at 4°C, at a 1:300 dilution in phosphate-buffered saline solution/Tween (PBS/T) containing 2% swine serum. After washing in PBS/T, sections were incubated for 1 h, at 22°C, with swine anti-rabbit serum (1:200) containing 1% swine serum. Sections were washed and incubated with rabbit peroxidase/antiperoxidase complex (1:400) for 1 h, at 22°C, containing 1% swine serum. Following a final wash, NPR-A like IR was visualized using 3,3′-diaminobenzidine in 0.05 M Tris-HCl buffer containing 0.3% H2O2.
For controls, adjacent sections were incubated without the primary antiserum. BLAST analysis of this immunizing peptide revealed minimal sequence identity to other human proteins.
Fluorescence dual staining and confocal microscopy image analysis
Sections of mammary artery, fixed in acetone and blocked with 5% goat serum, were incubated with rabbit anti-NPR-A serum (1:300) and either mouse anti-von Willebrand Factor (vWF) monoclonal antibody (1:50) or mouse anti-smooth muscle α-actin (SMαA) antiserum (1:100) in PBS/T containing 1% goat serum, for 48 h, at 4°C, as previously described (Kleinz et al., 2005). After being washed in PBS/T, sections were incubated with the secondary antibody solution containing both AlexaFluor 488 conjugated goat anti-rabbit serum (1:200) and AlexaFluor 568 conjugated goat anti-mouse serum (1:200) with 1% goat serum, for 1 h, at 22°C. Following a final wash, sections were mounted using Vectashield mounting medium and imaged using a confocal laser-scanning microscope (Leica Microsystems, Heidelberg, Germany).
In vitro pharmacology
Mammary artery was freed of connective tissue, cut into 4 mm rings, either left intact or denuded of endothelium and mounted in 5 ml baths containing oxygenated (95% O2/5% CO2) Krebs' solution (mM: NaCl, 90; NaHCO3, 45; KCl, 5; MgSO4·7H2O, 0.5; Na2HPO4·2H2O, 1; CaCl2, 2.25; fumaric acid, 5; glutamic acid, 5; glucose, 10, sodium pyruvate, 5; pH 7.4 at 37°C) for the measurement of isometric tension. Optimal basal tension was determined by repeated application of 100 mM KCl at increasing levels of basal tension until no further increase in isometric tension developed was obtained. Vessels were constricted with 1 μM phenylephrine (PE) and the presence or absence of a functional endothelium was confirmed by addition of the 1 μM ACh. Artery rings were then washed, allowed to re-equilibrate for 60 min and contracted with 10 nM ET-1. Once a stable response was established, cumulative concentration–response curves were constructed to DNP (1 pM–300 nM). Adjacent rings, preconstricted with ET-1 but to which no DNP was added, served as time-matched controls. Experiments were terminated by addition of 1 μM S-nitroso-N-acetylpenicillamine (SNAP) to determine maximal possible direct vasodilatation of the smooth muscle. Responses to DNP and SNAP were expressed as the percentage reversal of the ET-1 mediated constriction. For DNP, values of pD2 and maximum response (Emax) were determined using the iterative curve fitting software Fig P (Biosoft, Cambs, UK). All data are expressed as mean±s.e.m., and n-values are the number of patients from whom arteries were obtained. Values of pD2 and Emax for DNP were compared in endothelium intact and endothelium-denuded vessels and to other dilators using Student's two-tailed t-test, with statistical significance taken as P<0.05.
Drugs, chemical reagents and other materials
ET-1 was (10−4 M stock solution dissolved in 0.01% acetic acid, stored at −20°C) was from the Peptide Institute (Osaka, Japan) and DNP (10−4 M stock solution dissolved in water, stored at −20°C) was from Phoenix Pharmaceuticals (Belmont, CA, USA). Rabbit anti-NPR-A serum was from Abcam (Cambridge, UK). The secondary antibodies rabbit-PAP and swine anti-rabbit serum were from DAKO (Ely, UK). AlexaFluor 488-conjugated goat anti-rabbit serum and AlexaFluor 568-conjugated goat anti-mouse serum were obtained from Molecular Probes (Leiden, The Netherlands). All other reagents were from Sigma-Aldrich (Poole, UK), DAKO (Ely, UK) or BDH Ltd (Dorset, UK).
Results
[125I]-DNP binding characteristics in mammary artery
Over the concentration range tested (8 pM–1 nM), [125I]-DNP bound to sections of mammary artery with subnanomolar affinity (KD=0.07±0.01 nM) and with receptor density (Bmax) of 2.22±0.30 fmol mg−1 protein (n=3). A one-site fit was preferred over a two-site model and Hill slope was close to unity (nH=0.92±0.11).
Autoradiographical visualization of [125I]-DNP binding in mammary artery
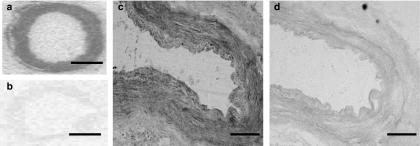
Specific binding of [125I]-DNP accounted for ∼70% of total binding and localized to the vascular smooth muscle layer of mammary artery (n=3; Figure 2a and b).
Figure 2.
Localization of NPR-A protein in human mammary artery by receptor autoradiography and immunocytochemistry. Total [125I]-DNP binding was detected to the vascular smooth muscle of (a) mammary artery (n=3) with nonspecific binding shown in (b). Scale bar=2 mm. Representative photomicrographs showing NPR-A IR (c) present in the vascular smooth muscle layer (n=4) of mammary artery. Staining was attenuated when the primary antibody was omitted (d). Scale bar=200 μm.
Immunocytochemistry
NPR-A IR was visualized in vascular smooth muscle cells of mammary artery (n=4; Figure 2c), but was absent or below the level for detection in the endothelium of these arteries. Specific staining was absent when the primary antibody for NPR-A was omitted (Figure 2d).
Fluorescence dual staining and confocal microscopy image analysis
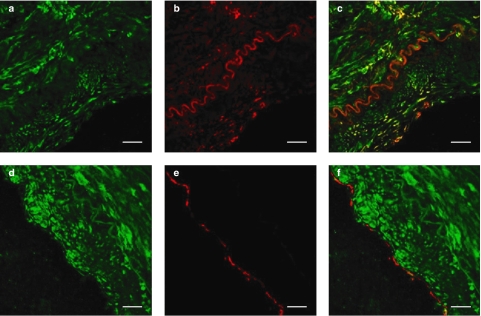
In agreement with the immunocytochemical distribution, confocal laser scanning microscopy revealed that NPR-A IR co-localized with SMαA IR to the smooth muscle layer of mammary artery (n=3; Figure 3a–c), but co-localization of NPR-A IR was not detected with vWF IR, a specific marker of endothelial cells (n=3; Figure 3d–f).
Figure 3.
Photomicrographs showing cellular localization of NPR-A in human mammary artery using confocal microscopy and fluorescent dual-labelling immunocytochemistry. NPR-A IR, (a) shown in green, co-localized with SMαA IR, (b) shown in red, to (c) the vascular smooth muscle (n=3). NPR-A IR (d) did not co-localize with vWF IR (e), shown in red, to (f) the endothelial cell layer (n=3). Scale bar=25 μm.
In vitro pharmacology
Vasodilator responses to ACh were present in endothelium-intact arteries (% reversal of 1 μM PE constriction= 71.5±5.6%, n=6) but absent in endothelium-denuded vessels (% reversal of 1 μM PE=2.4±1.6%, n=8). Responses to ET-1 and KCl are expressed as force developed (in mN mm−1) above optimized basal resting tension. Basal resting tension (15.7±1.9 vs 15.1±0.9 mN mm−1), isometric force developed to 10 nM ET-1 (11.1±2.9 vs 8.8± 1.8 mN mm−1), isometric force developed to 100 mM KCl (12.2±2.8 vs 6.9±1.1 mN mm−1) and percentage reversal of ET-1-induced constriction using 10 μM SNAP (117±7 vs 113±3%) was not different between the endothelium intact and endothelium denuded groups, respectively (P>0.05).
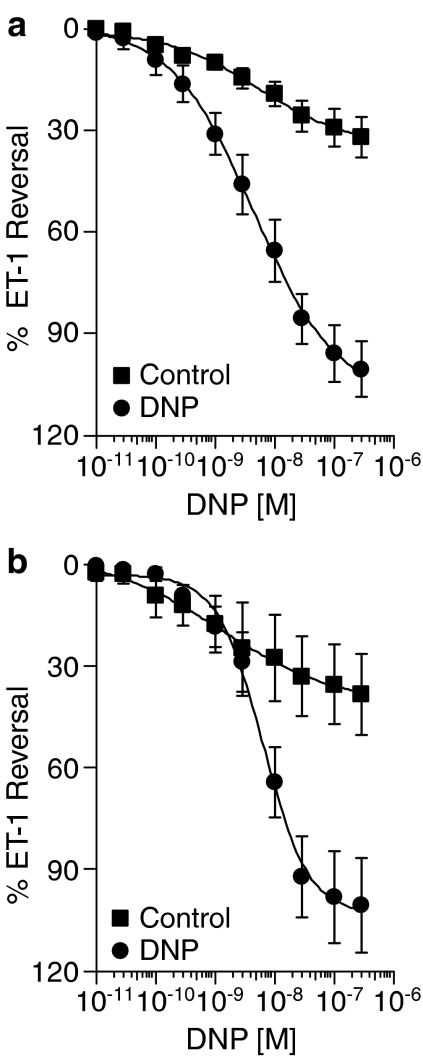
In endothelium intact mammary artery, DNP completely reversed ET-1-mediated vasoconstriction (Emax=102.9±8.4%, n=6) with nanomolar potency (pD2=8.31±0.19). Removal of the endothelium did not significantly (P>0.05) affect either the efficacy (Emax=101.3±14.0%) or potency (pD2=8.22±0.11, n=8) of DNP (Figure 4). Compared to the vasodilator response to ANP in endothelium-denuded mammary artery that we have previously reported (Wiley and Davenport, 2002), DNP produced a comparable maximum response but was significantly more potent (P<0.05) (Table 1). DNP was comparable to CGRP (calcitonin gene related peptide), but more effective as a vasodilator than adrenomedullin in this artery.
Figure 4.
Vasodilator responses to DNP: lack of effect of endothelium removal. Data are the mean concentration–response curves for DNP in (a) endothelium intact (n=6) and (b) endothelium denuded (n=8) rings of mammary artery. DNP fully reversed ET-1 preconstricted vessels independently of the presence of endothelium. Controls are time matched and results are expressed as a percentage reversal of the constrictor response to 10 nM ET-1. Data are expressed as mean±s.e.m.
Table 1.
Vasodilator responses to DNP compared to other peptides in human mammary artery
| Peptide | Potency (pD2) | Efficacy (Emax, % ET-1 reversal) | n |
|---|---|---|---|
| DNP | 8.26±0.10 | 102.0±8.9 | 14 |
| ANPa | 7.75±0.14 | 106.3±2.0 | 5 |
| Adrenomedullina | 7.63±0.28 | 58.0±7.3 | 9 |
| CGRPa | 8.08±0.17 | 76.0±15 | 5 |
Data for DNP are combined from endothelium-intact and denuded arteries.
Comparative data for ANP, adrenomedullin and CGRP are taken from previously published results (Wiley and Davenport, 2002) in endothelium-denuded mammary artery. Values are mean±s.e.m.Abbreviations: ANP, atrial natriuretic peptide; CGRP; DNP, dendroaspis natriuretic peptide.
Discussion
We have recently described binding of [125I]-DNP in human heart and demonstrated selectivity of this radiolabel for the NPR-A receptor compared to NPR-B and NPR-C, the putative clearance receptor (Singh et al., 2006). We now provide the first evidence of [125I]-DNP binding to NPR-A in human mammary artery that was saturable and occurred with a single high affinity. Using in vitro receptor autoradiography, specific [125I]-DNP binding sites were detected to the arterial smooth muscle layer and this was confirmed using immunocytochemistry and confocal microscopy. We observed unambiguous co-localization of NPR-A IR with the smooth muscle marker SMαA IR, consistent with the reported expression of NPR-A mRNA (Ikeda et al., 1996). In contrast, we did not detect co-localization of NPR-A IR with the endothelial cell marker von Willebrand Factor.
The vascular physiology of natriuretic peptides is well established. Both in vivo and in vitro studies have shown than these peptides induce potent concentration-dependent vasodilatation of large and small diameter human blood vessels (Wiley and Davenport, 2001, 2002; Schmitt et al., 2003, 2004). In the present study, we confirmed that the newly described natriuretic peptide, DNP, is a vasodilator and completely reversed the potent and efficacious vasoconstriction induced by ET-1 in human mammary artery, as we have previously reported for ANP (Wiley and Davenport, 2002). This action is consistent with data from both human (Best et al., 2002) and animal studies (Schweitz et al., 1992; Collins et al., 2000; Pan et al., 2004) where vasodilatation was suggested to result predominantly from a direct action on the vascular smooth muscle. The density of NPR-A that we measured in the smooth muscle of mammary artery was comparable to that for other vasoactive peptides such as ET-1 (∼2 fmol mg−1 protein) in this tissue (Davenport et al., 1995).
Although the presence of the sequence has not yet been identified in the human genome; interestingly, DNP IR has been detected in human plasma (Schirger et al., 1999), with circulating levels increasing in heart failure patients (Schirger et al., 1999). The source of this circulating peptide is currently unclear. However, the presence of DNP IR has been reported in the endothelium of large conduit blood vessels (Best et al., 2002). Therefore, endogenous DNP if released from the endothelium may act in a paracrine fashion on the underlying vascular smooth muscle to activate NPR-A, thus stimulating cGMP production and resulting in vasodilatation. Dysfunction of the endothelium that is associated with hypertension (Bolad and Delafontaine, 2005) and cardiovascular disease (Elliott, 1998) results in the loss of endothelial derived vasodilators, such as NO, and increases in vasoconstrictors such as ET-1 (Lerman et al., 1991). It is therefore possible that the increased levels of circulating ANP, BNP and, most recently, DNP reported in patients with cardiovascular disease may represent a compensatory response to the loss of physiologically important, locally acting vasodilators, and therefore help to counteract the increased contribution to vascular tone by vasoconstrictor substances such as ET-1 observed in these conditions.
Although further research is required to prove the existence of DNP as an endogenous peptide in humans, we have demonstrated that this peptide is able to abolish ET-1 induced vasoconstriction, potently and fully, by binding to NPR-A protein on vascular smooth muscle cells. Synthetic BNP has recently been approved for the treatment of acute decompensated heart failure (Keating and Goa, 2003), and as DNP has been reported to have enhanced resistance to degradative enzymes (Chen et al., 2002) we hypothesize that this peptide may also have therapeutic potential.
In conclusion, we have demonstrated that [125I]-DNP can be used as a pharmacological tool to identify and quantify NPR-A in human tissues. Use of this novel radioligand may therefore provide further insights into the role of NPR-A in cardiovascular physiology and pathophysiology.
Acknowledgments
This work was supported by grants from the British Heart Foundation. GS was supported by a Biotechnology and Biological Sciences Research Council Cooperative Award in Science and Engineering studentship with Pfizer. We thank Jean Chadderton and the Consultant and theatre staff of Papworth Hospital for tissue collection.
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
brain type natriuretic peptide
- DNP
dendroaspis natriuretic peptide
- ET-1
endothelin-1
- IR
immunoreactivity
- NO
nitric oxide
- NPR-A
natriuretic peptide receptor-A
- PBS/T
phosphate buffer saline/Tween
- PE
phenylephrine
- SMαA
smooth muscle alpha actin
- SNAP
S-nitroso-N-acetylpenicillamine
- vWF
von Willebrand Factor.
Conflict of interest
The authors state no conflict of interest.
References
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther. 2004;102:223–241. doi: 10.1016/j.pharmthera.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Best PJ, Burnett JC, Wilson SH, Holmes DR, Jr, Lerman A. Dendroaspis natriuretic peptide relaxes isolated human arteries and veins. Cardiovasc Res. 2002;55:375–384. doi: 10.1016/s0008-6363(02)00402-9. [DOI] [PubMed] [Google Scholar]
- Bolad I, Delafontaine P. Endothelial dysfunction: its role in hypertensive coronary disease. Curr Opin Cardiol. 2005;20:270–274. doi: 10.1097/01.hco.0000167719.37700.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- Chen HH, Lainchbury JG, Burnett JC., Jr Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J Am Coll Cardiol. 2002;40:1186–1191. doi: 10.1016/s0735-1097(02)02127-7. [DOI] [PubMed] [Google Scholar]
- Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, et al. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Collins E, Bracamonte MP, Burnett JC, Jr, Miller VM. Mechanism of relaxations to dendroaspis natriuretic peptide in canine coronary arteries. J Cardiovasc Pharmacol. 2000;35:614–618. doi: 10.1097/00005344-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Radioligand-binding and molecular-imaging techniques for the quantitative analysis of established and emerging orphan receptor systems. Methods Mol Biol. 2005a;306:93–120. doi: 10.1385/1-59259-927-3:093. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Immunocytochemical localization of receptors using light and confocal microscopy with application to the phenotypic characterization of knock-out mice. Methods Mol Biol. 2005b;306:155–172. doi: 10.1385/1-59259-927-3:155. [DOI] [PubMed] [Google Scholar]
- Davenport AP, O'Reilly G, Kuc RE. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br J Pharmacol. 1995;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott HL. Endothelial dysfunction in cardiovascular disease: risk factor, risk marker, or surrogate end point. J Cardiovasc Pharmacol. 1998;32 Suppl 3:S74–S77. [PubMed] [Google Scholar]
- Franco-Cereceda A. Endothelin- and neuropeptide Y-induced vasoconstriction of human epicardial coronary arteries in vitro. Br J Pharmacol. 1989;97:968–972. doi: 10.1111/j.1476-5381.1989.tb12038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF, et al. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Itoh H, Komatsu Y, Hanyu M, Yoshimasa T, Matsuda K, et al. Natriuretic peptide receptors in human artery and vein and rabbit vein graft. Hypertension. 1996;27:833–837. doi: 10.1161/01.hyp.27.3.833. [DOI] [PubMed] [Google Scholar]
- Inagami T. Atrial natriuretic factor as a volume regulator. J Clin Pharmacol. 1994;34:424–426. doi: 10.1002/j.1552-4604.1994.tb04982.x. [DOI] [PubMed] [Google Scholar]
- Keating GM, Goa KL. Nesiritide: a review of its use in acute decompensated heart failure. Drugs. 2003;63:47–70. doi: 10.2165/00003495-200363010-00004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yang SH, Yu MY, Lee HK, Kim SY, Kim SH. Dendroaspis natriuretic peptide system and its paracrine function in rat colon. Regul Pept. 2004;120:93–98. doi: 10.1016/j.regpep.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP. Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res. 2005;69:227–235. doi: 10.1016/j.cardiores.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Lisy O, Jougasaki M, Heublein DM, Schirger JA, Chen HH, Wennberg PW, et al. Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 1999;56:502–508. doi: 10.1046/j.1523-1755.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ray SG, Abdullah I, Dargie HJ, Morton JJ. Plasma endothelin in chronic heart failure. Circulation. 1992;85:1374–1379. doi: 10.1161/01.cir.85.4.1374. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Yanagisawa M, Tomizawa T, Sugishita Y, Suzuki N, Fujino M, et al. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989;2:53–54. doi: 10.1016/s0140-6736(89)90303-6. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakao K, Saito Y, Ogawa Y, Hosoda K, Suga S, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;323:757–758. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- Pan S, Gulati R, Mueske CS, Witt TA, Lerman A, Burnett JC, Jr, et al. Gene transfer of a novel vasoactive natriuretic peptide stimulates cGMP and lowers blood pressure in mice. Am J Physiol Heart Circ Physiol. 2004;286:H2213–H2218. doi: 10.1152/ajpheart.00465.2003. [DOI] [PubMed] [Google Scholar]
- Piao FL, Park SH, Han JH, Cao C, Kim SZ, Kim SH. Dendroaspis natriuretic peptide and its functions in pig ovarian granulosa cells. Regul Pept. 2004;118:193–198. doi: 10.1016/j.regpep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Schirger JA, Heublein DM, Chen HH, Lisy O, Jougasaki M, Wennberg PW, et al. Presence of Dendroaspis natriuretic peptide-like immunoreactivity in human plasma and its increase during human heart failure. Mayo Clin Proc. 1999;74:126–130. doi: 10.4065/74.2.126. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Broadley AJ, Nightingale AK, Payne N, Gunaruwan P, Taylor J, et al. Atrial natriuretic peptide regulates regional vascular volume and venous tone in humans. Arterioscler Thromb Vasc Biol. 2003;23:1833–1838. doi: 10.1161/01.ATV.0000084826.86349.1D. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Gunaruwan P, Payne N, Taylor J, Lee L, Broadley AJ, et al. Effects of exogenous and endogenous natriuretic peptides on forearm vascular function in chronic heart failure. Arterioscler Thromb Vasc Biol. 2004;24:911–917. doi: 10.1161/01.ATV.zhq0504.7914. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- Singh G, Kuc RE, Maguire JJ, Fidock M, Davenport AP. The novel snake venom ligand Dendroaspis natriuretic peptide is selective for natriuretic peptide receptor-A in human heart: down-regulation of natriuretic peptide receptor-A in heart failure. Circ Res. 2006;99:183–190. doi: 10.1161/01.RES.0000232322.06633.d3. [DOI] [PubMed] [Google Scholar]
- Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br J Pharmacol. 2001;133:568–574. doi: 10.1038/sj.bjp.0704119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol. 2002;136:1146–1152. doi: 10.1038/sj.bjp.0704815. [DOI] [PMC free article] [PubMed] [Google Scholar]