Abstract
Background and purpose.
Inhibition of Na+-K+-ATPase is known to attenuate endothelium-dependent relaxation in many arteries. The purpose of this study was to evaluate the role of Na+-K+-ATPase in the regulation of endothelial membrane potential at rest and during stimulation by ACh.
Experimental approach.
Membrane potential was recorded from the endothelium of rat aorta using the perforated patch-clamp technique.
Key results.
Superfusion with K+-free solution produced a depolarization of about 11 mV from the resting value of -42.9±0.9 mV. Reintroduction of 4.7 mM K+ transiently hyperpolarized endothelial cells to −52.4±1.8 mV and the membrane potential recovered within 10 min. Ouabain 500 μM depolarized endothelium by about 11 mV and inhibited the hyperpolarization induced by K+ reintroduction into the K+-free solution. However, 500 nM ouabain did not affect the resting membrane potential or the hyperpolarization induced by K+ reintroduction. Pre-exposure to ouabain 500 μM, but not 500 nM, attenuated the sustained component of hyperpolarization to ACh without affecting the amplitude of the transient peak hyperpolarization. In K+-free solution, the amplitude of peak hyperpolarization to ACh was increased, while the sustained component of hyperpolarization was attenuated.
Conclusions and Implications.
These results indicate that electrogenic Na+-K+-ATPase partially contributes to the sustained hyperpolarization of endothelial cells from rat aorta in response to ACh. They also suggest that the α1, but not α2 or α3 isoforms, is involved in ACh-mediated hyperpolarization.
Keywords: rat aorta, endothelium, membrane potential, Na pump
Introduction
Na+-K+-ATPase is known to have an essential role in the regulation of vascular tone (Marin and Redondo, 1999). Inhibition of Na+-K+-ATPase by cardiac glycosides or K+-free solution has been shown to attenuate or abolish endothelium-dependent relaxation in a number of arteries, including aorta (Rapoport et al., 1985; Hirano et al., 1992; Lee et al., 1992; Edwards et al., 1998; Ferrer et al., 1999; Van de Voorde and Vanheel, 2000; Jiang and Dusting, 2001). By stimulating the release of nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarizing factor (EDHF), ACh induces endothelium-dependent smooth muscle hyperpolarization that then causes endothelium-dependent relaxation. Suppression of endothelium-dependent hyperpolarization by ouabain in a number of vascular preparations, such as canine coronary artery (Feletou and Vanhoutte, 1988), porcine coronary artery (Olanrewaju et al., 1997) and rabbit cerebral artery (Brayden, 1990), has been attributed to inhibition of the smooth muscle Na+-K+-ATPase. Thus, it has been proposed that during stimulation by endothelium-dependent vasodilators, the smooth muscle Na+-K+-ATPase is stimulated by endothelium-derived NO (Rapoport et al., 1985; Gupta et al., 1994) or by K+ released from endothelial cells (Edwards et al., 1998).
Although the triggering role of endothelial hyperpolarization in endothelium-dependent smooth muscle hyperpolarization is widely accepted, the role of Na+-K+-ATPase in endothelial electrical responses to endothelium-dependent vasodilators has only been evaluated in a few studies (Chen and Cheung, 1992; Edwards et al., 1998; White and Hiley, 2000; Jiang et al., 2005). In these studies, only the peak hyperpolarization, which reflects Ca2+ release from internal stores, has been assessed and found not to be sensitive to ouabain. However, the sustained endothelial hyperpolarization, which facilitates Ca2+ influx into endothelial cells required for Ca2+-dependent NO synthesis and EDHF-type responses (Fukao et al., 1997; Tomioka et al., 2001; Ungvari et al., 2002) has not been fully characterized. Moreover, direct transmission of endothelial hyperpolarization to adjacent smooth muscle cells via gap junctions, a phenomenon which has been shown to underpin the EDHF-type response (Coleman et al., 2001), indicates the need to investigate further the mechanisms involved in the attenuation of endothelium-dependent relaxation by ouabain.
Recently, it has been shown that inhibition of the sodium–calcium exchanger (NCX) in rat aortic endothelial cells drastically inhibits the sustained endothelial hyperpolarization to ACh (Bondarenko, 2004), suggesting that activation of reverse NCX and possibly other Na+ extrusion mechanisms are induced by the increased Na+ influx. The ensuing rise in internal Na+ may also stimulate the electrogenic Na+-K+-ATPase, thereby modulating the membrane hyperpolarization and, hence, the driving force for Ca 2+ entry.
The experiments described here were designed to evaluate the contribution of the Na+-K+-ATPase to electrogenesis of endothelial cells in rat aorta, in resting conditions and during stimulation by ACh, particularly with regard to the time course of the response.
Methods
This investigation conforms to the guidelines of the Institutional Committee for Biomedical Research Ethics, the experimental procedures were carried out in accordance with the regulations of the UK Animals (Scientific Procedures) Act 1986.
Experiments were performed on 2–4 months old Wistar–Kyoto rats of either sex. Rats were killed by stunning followed by cervical dislocation. Thoracic aortae were excised and placed in bubbled (95% O2 and 5% CO2) Krebs solution of the following composition (mM): NaCl, 118.3; NaHCO3, 25; KCl, 4.7; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.4; glucose, 11.1 (pH 7.4) and cut into ring segments of 2–3 mm in width. Before the experiments commenced, the segments were cut open, and a strip was pinned to the rubber bottom of a 100 μl chamber and superfused with physiological saline solution at a rate of 0.5 ml min−1. When K+ -free solution was used, KCl was replaced by an equimolar amount of NaCl.
Membrane potential was recorded from intact aortic endothelial cells using the whole cell configuration of the patch-clamp technique in current clamp mode. Electrical contact with the cytosol was established using nystatin. Nystatin was dissolved in dimethyl sulphoxide, and the final nystatin concentration in the pipette solution was 200 μM. Patch pipettes, after being filled with a solution containing (mM) KCl 140, NaCl 10, ethyleneglycol tetraacetate (EGTA) 0.5, 4-(2-hydroxyethyl)-1-piperazineethyl-sulphonic acid (HEPES) 10, had a resistance of 5–7 MΩ. The pH of the pipette solution was adjusted to 7.2 by addition of KOH. Experiments were conducted at room temperature. Pharmacological agents were applied to the preparation by bath perfusion. Comparisons of endothelial hyperpolarization in response to ACh before and after Na+-K+-ATPase inhibition by ouabain or K+-free solution were performed after continuous recordings had been successfully established. Quantitative data are expressed as means±s.e. Statistical significance was evaluated by Student's t-test. P-values <0.05 were considered statistically significant.
Results
Effect of Na+-K+-ATPase inhibition on endothelial membrane potential
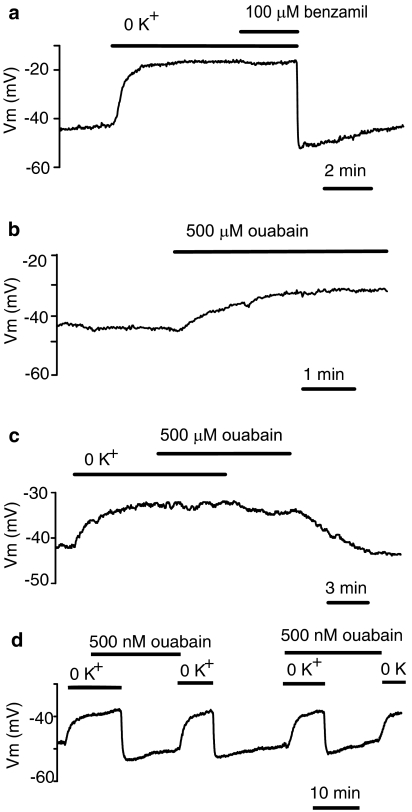
Superfusion of the vessel strip with K+-free solution produced an endothelial depolarization of 11.7±0.6 mV from a resting value of −42.9±0.9 mV (n=50). Subsequent exposure to 100 μM benzamil, a NCX blocker, did not alter the membrane potential (from −28.1±1.0 mV to −28.6±1.4 mV, n=7) (Figure 1a). Addition of 4.7 mM K+ to the bath solution transiently hyperpolarized endothelial cells from a mean value of −31.3±1.9 mV in K+-free solution to a peak level of −52.4±1.8 mV (n=14). Then the membrane potential slowly returned towards a resting value. Five minutes after the peak, the membrane potential (−49.3±1.8 mV, n=14) was still significantly (P<0.05) hyperpolarized compared to the resting level (−43.2±1.6 mV, n=14) and 10 min after the peak, the membrane potential was still more negative (−46.4±1.9 mV, n=14), but the difference was not statistically significant.
Figure 1.
Modulation of endothelial membrane potential by Na+-K+-ATPase inhibition. (a) Effects of external K+ withdrawal followed by 100 μM benzamil administration and K+ reintroduction on the membrane potential. (b) Effect of 500 μM ouabain on endothelial membrane potential. (c) Lack of depolarizing effect of 500 μM ouabain in the absence of external K+ and inhibitory effect of 500 μM ouabain on the hyperpolarization induced by K+ reintroduction. (d) Lack of inhibitory effect of 500 nM ouabain on the hyperpolarization induced by K+ reintroduction.
Superfusion of the vessel strip with a solution containing ouabain (500 μM; an inhibitor of Na+-K+-ATPase) at a concentration sufficient to block both α1 and α2 ion-transporting subunits, depolarized endothelial cells by 11.4±0.9 mV from a resting value of −45.1±1.5 mV (n=24) (Figure 1b). However, the membrane potential was not modified when 500 μM ouabain was administered in the absence of external K+ (n=3). Addition of 4.7 mM K+ in the presence of 500 μM ouabain produced only minor changes in the membrane potential (n=3) (Figure 1c), indicating effective inhibition of Na+-K+-ATPase by 500 μM ouabain. Ouabain withdrawal caused a gradual return of the membrane potential towards baseline.
In contrast, a low concentration of ouabain (500 nM), which has been reported to block α2 and α3 isoforms of the Na+-K+-ATPase selectively (Blanco and Mercer, 1998), did not affect the resting membrane potential (from −45.9±2.9 to −45.7±2.8 mV, n=6) (Figure 1d). Essentially, ouabain at 500 nM was not effective at inhibiting the hyperpolarization induced by K+ reintroduction into K+-free solution (n=3) (Figure 1d).
Effect of Na+-K+-ATPase inhibition on ACh-induced endothelial hyperpolarization
Vasoactive agonists cause Na+ entry into endothelial cells via a variety of pathways (Nilius and Droogmans, 2001). Conceivably, following ACh administration, the Na+-K+-ATPase of endothelial cells may be further stimulated, thus contributing to membrane hyperpolarization. To test this assumption, we compared endothelial electrical responses to ACh before and after Na+-K+-ATPase inhibition. ACh was applied at intervals not less than 10 min. During this period, a control bath solution was switched to either K+-free or ouabain-containing solution.
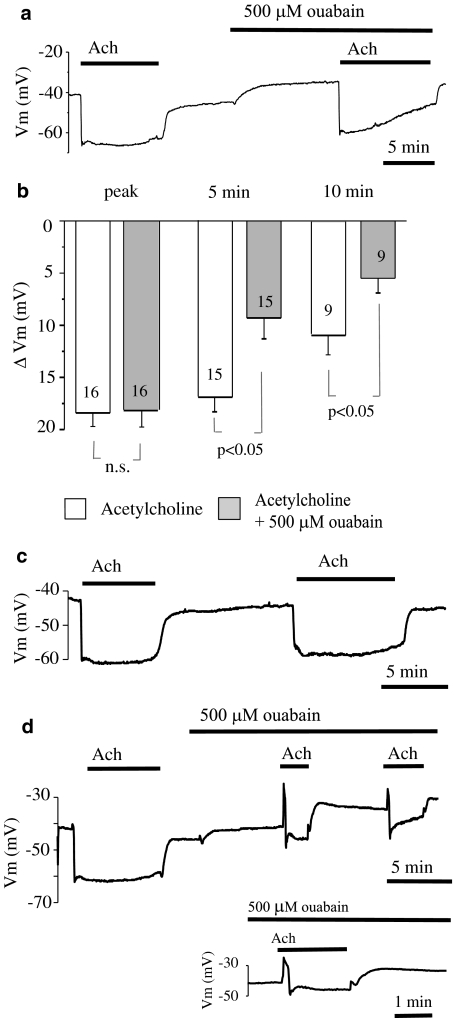
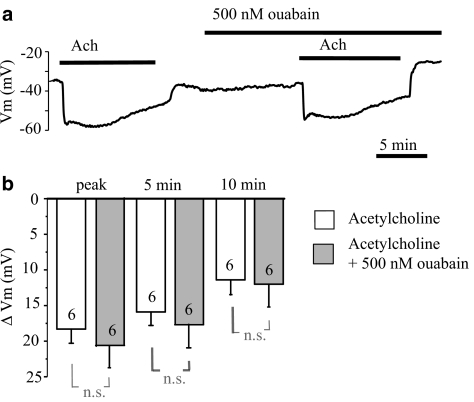
ACh (2 μM) produced a transient peak hyperpolarization of 18.4±1.3 mV (n=16). This transient hyperpolarization was followed by a sustained hyperpolarization with a partial reduction in amplitude. In the presence of 500 μM ouabain, the membrane potential was significantly depolarized. When ACh was again applied in the presence of 500 μM ouabain, the initial peak hyperpolarization (18.1±1.6 mV, n=16) was not altered and the membrane potential (−57.3±1.6 mV) attained during the peak hyperpolarization did not reach (P<0.05) the level attained in the absence of ouabain (−63.1±0.9 mV). However, as shown in Figure 2a, in the presence of ACh, the membrane potential recovered faster after inhibition of the Na+-K+-ATPase by 500 μM ouabain. Close inspection revealed that at 5 min after the peak, the amplitude of sustained hyperpolarization was significantly (P<0.05) attenuated by 500 μM ouabain (from 16.9±1.4 mV, n=15 to 9.3±2.0 mV, n=15). At 10 min, the hyperpolarization was further attenuated (P<0.05) by ouabain (from 10.9±1.9 mV, n=9 to 5.5±1.4 mV, n=9). This effect was not seen when repeated ACh applications were administered in the control Krebs solution (n=5) (Figure 2c). In one preparation, ouabain (500 μM) administration resulted in attenuation of all phases of the ACh-evoked hyperpolarization and the hyperpolarization was preceded by a transient depolarization of up to 15 mV (Figure 2d). In contrast, 500 nM ouabain failed to affect either the transient (from 18.3±2.1 mV, n=6 to 20.7±3.0 mV, n=6) or the sustained component of the hyperpolarization (at 5 min, from 15.9±1.9 mV, n=6 to 17.9±3.3 mV, n=6, and at 10 min, from 11.4±2.1 mV n=6 to 12.0±3.1 mV, n=6) (Figure 3).
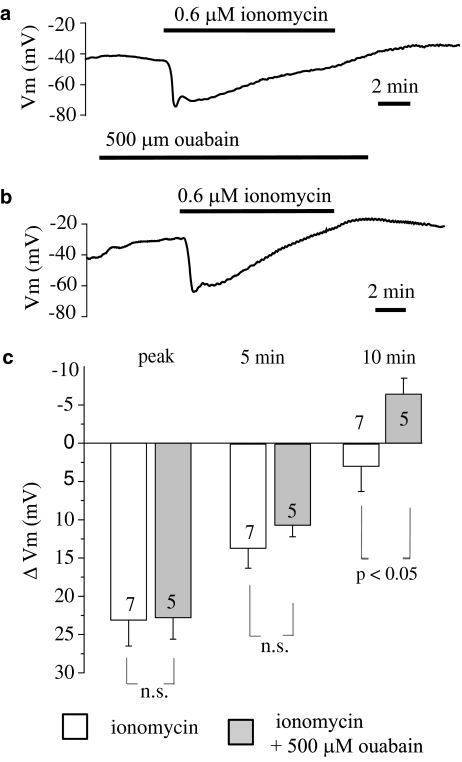
Figure 2.
Effect of 500 μM ouabain on the hyperpolarization of endothelial cells from rat aorta produced by acetylcholine. (a) Original tracing showing the influence of 500 μM ouabain on the hyperpolarization of endothelial cells produced by 2 μM acetylcholine. (b) Graphical representation of the time dependency of the effect of 500 μM ouabain on the mean changes in membrane potential evoked by 2 μM acetylcholine. The changes in membrane potential were calculated as the difference between the membrane potential values before and after acetylcholine administration. Numbers associated with bar plot indicate the number of aorta preparations. (c) Representative record of repeated applications of 2 μM acetylcholine. (d) Original tracing showing attenuation of all phases of acetylcholine-evoked hyperpolarization by 500 μM ouabain that unmasked a transient depolarization phase in response to acetylcholine. For clarity, a portion of the trace has been expanded.
Figure 3.
Influence of a low concentration of ouabain (500 nM) on the hyperpolarization of endothelial cells produced by acetylcholine. (a) Original tracing showing a lack of effect of 500 nM ouabain on the hyperpolarization of endothelial cells produced by 2 μM acetylcholine. (b) Graphical representation of the time dependency of the effect of 500 nM ouabain on the mean changes in membrane potential evoked by 2 μM acetylcholine. The changes in membrane potential were calculated as the difference between the membrane potential values before and after acetylcholine administration. Numbers associated with bar plot indicate the number of aorta preparations.
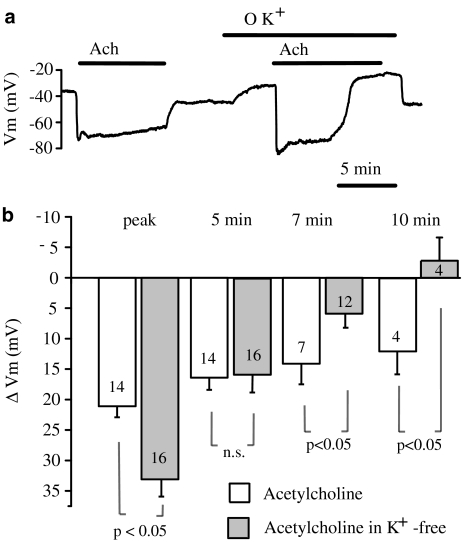
When K+-free solution was used to inhibit the Na+-K+-ATPase, the membrane potential was depolarised and the amplitude of peak hyperpolarization (33.1±2.8 mV, n=16) produced by 2 μM ACh was significantly (P<0.05) larger then that observed before omission of external K+ (21.1±1.8 mV, n=14). At 5 min after the peak, the amplitude of sustained hyperpolarization to ACh in K+-free solution (15.8±3.1 mV, n=16) was not significantly different from that obtained before omission of external K+ (16.4±2.0 mV, n=14) (Figure 4). However, at 7 min after the peak, the amplitude of hyperpolarization in K+-free solution (5.9±2.3 mV, n=12) decreased significantly (P<0.05) compared with the control response (14.1±3.4 mV, n=7). This difference was even more significant at 10 min after the peak. At this point, ACh-induced hyperpolarization in K+-free solution completely decayed (the membrane potential values were −31.3±2.5 and −28.5±2.4 mV (n=4) before and after ACh administration, respectively), whereas in the control solution, the amplitude of hyperpolarization still amounted to 12.0±3.7 mV (n=4). These data demonstrate that Na+-K+-ATPase blockade with either 500 μM ouabain or omission of external K+ attenuates the sustained hyperpolarization of endothelial cells induced by ACh.
Figure 4.
Effect of K+-free solution on the hyperpolarization of endothelial cells from rat aorta produced by acetylcholine. (a) Original tracing showing the influence of external K+ substitution on the resting membrane potential and acetylcholine-induced hyperpolarization of endothelial cells. (b) Graphical representation of the time dependency of the effect of K+-free solution on the mean changes in membrane potential evoked by 2 μM acetylcholine. The changes in membrane potential were calculated as the difference between the membrane potential values before and after acetylcholine administration. Numbers associated with bar plot indicate the number of aorta preparations.
We further sought to investigate whether Na+-K+-ATPase contributes to the hyperpolarization of endothelial cells produced independently of receptor stimulation. Ca2+ ionophores at low concentrations are thought to act selectively on Ca2+ storage sites without a significant direct increase in the Ca2+ permeability of the plasma membrane (Itoh et al., 1985). This leads to a depletion of stored Ca2+ with subsequent activation of store-operated channels. Hence, to evoke this we used a low concentration of ionomycin. Superfusion of the vessel strip with ionomycin (0.6 μM) produced endothelial cell hyperpolarization followed by a recovery of the membrane potential that turned to a depolarization of several mV beyond the resting level (Figure 5). The initial hyperpolarization to ionomycin developed substantially slower than that produced by ACh. However, the membrane potential recovered faster. Ouabain 500 μM did not modify the amplitude of ionomycin-induced peak hyperpolarization (from 23.1±3.4 mV, n=7 to 22.8±2.8 mV, n=5). Although 500 μM ouabain slightly decreased the amplitude of ionomycin-evoked hyperpolarization at 5 min after the peak (from 13.6±2.6 mV, n=7 to 10.6±1.5 mV, n=5), the difference did not reach statistical significance. At 10 min, the hyperpolarization to ionomycin amounted to 2.9±3.3 mV (n=7), whereas in the presence of ouabain the membrane response turned to a depolarization of 6.4+2.0 mV (n=5, P<0.05) (Figure 5).
Figure 5.
Influence of 500 μM ouabain on the hyperpolarization of endothelial cells from rat aorta produced by ionomycin. (a) Original tracing of an experiment showing the influence of ionomycin (0.6 μM) on the membrane potential of endothelial cells. (b) Original tracing of an experiment showing the influence of 500 μM ouabain on the hyperpolarization of endothelial cells produced 0.6 μM ionomycin. (c) Graphical representation of the time dependency of the effect of 500 μM ouabain on the mean changes in membrane potential evoked by 0.6 μM ionomycin. The changes in membrane potential were calculated as the difference between the membrane potential values before and after ionomycin administration. Numbers associated with bar plot indicate the number of aorta preparations.
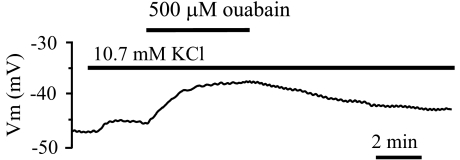
The inhibitory effect of ouabain and K+-free solution on the sustained component of endothelial hyperpolarization may indicate that the Na+-K+-ATPase promotes a sustained endothelial hyperpolarization in response to ACh. Apart from the intracellular Na+ concentration ([Na+]i) rise, an increase in K+ concentration close to the endothelial cell membrane owing to activation of calcium-dependent potassium channels may also stimulate the endothelial Na+-K+-ATPase. To test this possibility, the extracellular K+ concentration was increased from 4.7 to 10.7 mM. This increase consistently depolarized endothelial cells by 2.2±0.2 mV (n=5). Conceivably, if extracellular K+ stimulates the Na+-K+-ATPase, a resulting drop in internal Na+ may cause stimulation of the forward mode of NCX, producing depolarization (Kim et al., 2005). Under these conditions, ouabain is expected to inhibit partially K+-induced depolarization. However, as shown in Figure 6, additional administration of 500 μM ouabain to a K+-enriched solution further depolarized endothelium by 7.2±0.9 mV (n=5), providing no evidence that rise in external K+ stimulates the Na+-K+-ATPase in rat aortic endothelium.
Figure 6.
Original tracing of the membrane potential responses to increased KCl (from 4.7 to 10.7 mM) followed by 500 μM ouabain administration.
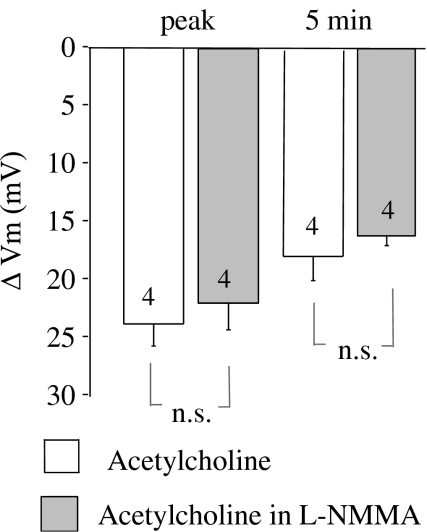
ACh-induced, endothelium-derived NO was demonstrated to promote a sustained hyperpolarization of smooth muscle cells in rat aorta (Vanheel et al., 1994). This effect of NO may be mediated by the opening of K+ channels (Satake et al., 1997) or by the stimulation of the smooth muscle Na+-K+-ATPase (Rapoport et al., 1985). To rule out the possibility that NO-attributed smooth muscle hyperpolarization is transmitted to the endothelium, thus promoting endothelial hyperpolarization to ACh, we examined the effect of the NO synthase inhibitor NG-monomethyl-L-arginine monoacetate (L-NMMA) (200 μM) on ACh-evoked endothelial hyperpolarization. If a sustained smooth muscle cell hyperpolarization stimulated by endothelium-derived NO is transmitted to endothelial cells, inhibition of NO synthesis would partially inhibit the sustained component of endothelial hyperpolarization evoked by ACh. However, the hyperpolarization was unaffected by L-NMMA (n=4) (Figure 7). Further administration of 500 μM ouabain in the continued presence of L-NMMA attenuated the sustained hyperpolarization to ACh (at 5 min, from 16.0±0.7 mV, n=3 to 7.9±1.8, n=3) (data not shown).
Figure 7.
Effect of NO synthase inhibitor L-NMMA (200 μM) on the mean changes in membrane potential of endothelial cells evoked by 2 μM acetylcholine. The changes in membrane potential were calculated as the difference between the membrane potential values before and after acetylcholine administration. Numbers associated with bar plot indicate the number of aorta preparations.
Discussion
In the present study, the role of Na+-K+-ATPase in the regulation of endothelial membrane potential at rest, during K+ reintroduction, and following ACh administration was evaluated in rat aorta, in vitro. Inhibition of the Na+-K+-ATPase by either withdrawal of external K+ or with 500 μM ouabain produced a gradual endothelial cell depolarization of ≈11 mV, indicating strong basal activity of the Na+-K+-ATPase in endothelial cells. Activation of the Na+-K+-ATPase by reintroduction of external K+ produced an abrupt endothelial hyperpolarization, which was abolished by 500 μM ouabain, confirming that this concentration of ouabain was effective at inhibiting Na+-K+-ATPase activity in rat aortic endothelium. In contrast, 500 nM ouabain failed to affect either the resting membrane potential or the hyperpolarization produced by K+ reintroduction. Because of the different ouabain affinity among rodent Na+-K+-ATPase isozymes (Blanco and Mercer, 1998), the results obtained indicate that the ouabain-resistant α1 isoform, but not α2 or α3, is involved in setting the resting membrane potential in rat aortic endothelium. This conclusion is compatible with previous observations made in porcine aortic (Gruwel et al., 1995) and human umbilical vein (Oike et al., 1993) endothelial cells, which require high ouabain concentrations to inhibit the Na+-K+-ATPase. These results are also in agreement with the findings that the endothelium of large vessels predominantly expresses the α1 isoform of Na+-K+-ATPase (Zahler et al., 1996).
Inhibition of the Na+-K+-ATPase is expected to result in internal Na+ accumulation and stimulation of the reversed mode of NCX. The latter, being electrogenic, is likely to attenuate the depolarizing effect caused by Na+-K+-ATPase inhibition. A lack of effect of benzamil on endothelial membrane potential in K+-free solution provides no evidence for stimulation of the reversed NCX following Na+-K+-ATPase inhibition in rat aortic endothelial cells under the current experimental conditions. Similar conclusions have been drawn from experiments on cultured endothelial cells derived from rat aorta (Dong et al., 2004), bovine atria (Laskey et al., 1990) and pulmonary artery (Sage et al., 1991), human umbilical vein (Oike et al., 1993) and rat brain (Domotor et al., 1999), where Na+-K+-ATPase inhibition was without effect or decreased resting intracellular Ca2+ concentration ([Ca2+]i). These results are at variance with those obtained in intact rabbit cardiac valve endothelial cells (Li and van Breemen, 1995), where fura 2 fluorescent ratio was moderately increased by ouabain, indicating an increase in [Ca2+]i. The reason for this discrepancy is not easy to explain, but it may reflect the level of expression of different Na+-K+-ATPase α isoforms. Both α2 and α3 isoforms have been shown to be ouabain-sensitive and colocalized with NCX in plasma membrane microdomains that overlie the endoplasmic reticulum. These isoforms regulate [Na+]i in a restricted cytosolic space between the plasma membrane and endoplasmic reticulum, whereas the α1 isoform is uniformly distributed over the cell surface (Juhaszova and Blaustein, 1997) and responsible for the regulation of bulk [Na+]i. Owing to the close proximity of α2/α3 subunits to the NCX, their inhibition may lead to stimulation of reverse NCX, whereas in vascular beds, where α2/α3 isoforms of Na+-K+-ATPase are dormant or absent, this may not be the case.
Another explanation for the aforementioned discrepancy may arise from variations in the resting [Ca2+]i values and the contribution of the Na+-K+-ATPase to the resting membrane potential. The ouabain-induced membrane depolarization to ∼−30 mV observed in the present study decreases the driving force for basal Ca2+ entry. But operation of NCX in reversed mode requires a permissive [Ca2+]i (Blaustein and Lederer, 1999). In native endothelial cells from rat aorta, the resting [Ca2+]i was reported to be 95 nM (Usachev et al., 1995). Therefore, an anticipated increase in [Na+]i caused by Na+-K+-ATPase inhibition that is accompanied by drop in [Ca2+]i below 90 nM may not result in stimulation of the reverse NCX.
To quantify the change in ACh-induced hyperpolarization elicited by Na+-K+-ATPase inhibition, the amplitude and the duration of the hyperpolarization were simultaneously considered. A lack of effect of ouabain on the peak hyperpolarization to ACh observed in the present study is in agreement with previous findings where ouabain was tested either alone (Chen and Cheung, 1992) or in combination with low Ba2+ concentrations (Edwards et al., 1998; White and Hiley, 2000; Jiang et al., 2005). A novel finding in the present study is that the sustained hyperpolarization to ACh was attenuated by Na+-K+-ATPase inhibition either by ouabain or K+-free solution. This effect was clearly observed after 5 min of hyperpolarization in the ouabain experiments and after 7 min when K+-free solution was used. In the latter case, the difference in amplitude was not seen at 5 min, because the peak hyperpolarization in K+-free solution was increased due to an enhanced driving force for K+. However, the inhibitory effect of K+-free solution on the sustained hyperpolarization was still observed at 5 min, as the decline in amplitude at this point was larger than that observed in the control solution.
In contrast, a low ouabain concentration that selectively inhibits α2 and α3 isoforms of the Na+-K+-ATPase was not effective in modifying either the resting membrane potential, the hyperpolarization to K+ reintroduction or the hyperpolarization to ACh, suggesting that the α1, but not the α2 or α3 isoforms, mediates prolongation of the hyperpolarization to ACh in rat aortic endothelial cells. The results obtained are thus compatible with a previously reported observation that relatively high concentrations of ouabain (300 μM) are required to inhibit an endothelium-derived increase in 86Rb+ efflux induced by ACh in the rat aorta (Bray and Quast, 1991). Furthermore, wire myograph studies confirmed that ouabain at up to 1 mM is required for maximal inhibition of the relaxation in response to muscarinic stimulation (Rapoport et al., 1985; Hirano et al., 1992; Lee et al., 1992).
These results, however, are not in line with the observation that nanomolar concentrations of ouabain induce a bradykinin-induced [Ca2+]i increase and NO release from rat aortic endothelial cells (Dong et al., 2004). This discrepancy may reflect differences in the mechanisms of action of ACh and bradykinin or the experimental conditions applied (transient application vs superfusion in the present study). Alternatively, the disparity may originate from possible alterations in [Ca2+]i regulation in cultured vs in situ endothelial cells.
Although the precise mechanisms underlying prolongation of endothelial hyperpolarization to ACh by Na+-K+-ATPase remain to be determined, they may be connected either to internal Na+ loading or a rise in extracellular K+. Inhibition of NO synthesis by L-NMMA failed to affect endothelial hyperpolarization to ACh as well as prevent the inhibitory effect of ouabain. Therefore, it is unlikely that prolongation of the hyperpolarization is mediated by the stimulation of Na+-K+-ATPase of the smooth muscle cells by endothelium-derived NO with subsequent transmission of the signal to the endothelial cell layer.
The stimulant effect of K+ on Na+-K+-ATPase has been proposed to underlie the EDHF-type response in rat hepatic and mesenteric arteries (Edwards et al., 1998). However, in a number of vascular beds, support for the involvement of this EDHF pathway has not been obtained (Quignard et al, 1999; Coleman et al., 2001). Based on the differences in ouabain affinity between rodent Na+-K+-ATPase isozymes (Blanco and Mercer, 1998), the present study indicates that the ouabain-resistant α1 isoform is implicated in the membrane potential modulation by ouabain in rat aortic endothelial cells. It is also known that α isoforms in rats differ by their affinities to extracellular K+. A strong argument in support of our assumption that a rise in external K+ does not mediate the increase in Na+-K+-ATPase activity in rat aortic endothelium is the finding that, whereas the α2 or α3 isoforms increase their activity in response to a small elevation in extracellular K+, the α1 isoform is nearly fully activated at a physiological concentration of extracellular K+ (Juhaszova and Blaustein, 1997; Blanco and Mercer, 1998; Weston et al., 2002). Indeed, in support of the view that the α1 isoform is predominantly responsible for the membrane potential modulation in response to ouabain in rat aortic endothelium, we demonstrated that an elevation in external K+ produces endothelial cell depolarization; an effect that is not mediated by the forward mode of NCX secondary to stimulation of the Na+-K+-ATPase. Collectively, these observations do not favour the view that the rise in external K+ stimulates the Na+-K+-ATPase in rat aortic endothelial cells.
Under normal physiological conditions, the Na pump is unsaturated with respect to intracellular Na+ (Therien and Blostein, 2000), and therefore [Na+]i is another potent determinant of pump activity. In endothelial cells from porcine aorta, a 14% increase in [Na+]i produced by nystatin, augmented the Na pump rate approximately two-fold (Gruwel et al., 1995), indicating that small changes in [Na+]i may have a substantial effect on the Na+-K+-ATPase activity. In the present study, ACh and ionomycin produced different patterns of hyperpolarization, indicating that the response to ACh is more complex and cannot simply be mimicked by an overall elevation of [Ca2+]i in endothelial cells and probably reflects endoplasmic reticulum Ca2+ refilling. Previously it has been shown (Bondarenko, 2004) that NCX blockers and, importantly, external Na+ withdrawal during the plateau phase of the hyperpolarization substantially inhibit the sustained endothelial hyperpolarization in response to ACh in rat aorta, suggesting that Na+ influx activates the reversed NCX and possibly other Na+ extrusion mechanisms following stimulation by ACh in the endothelial cells. This hypothesis is supported by results obtained with regard to several Na+ entry pathways described in endothelial cells: various nonselective cationic channels including store-operated channels (Nilius and Droogmans, 2001) and the sodium–hydrogen exchanger (Fleming et al., 1994). Moreover, recent data show that endothelial cells from rat and human arteries are equipped with functional nicotinic ACh receptors that are Na+ permeant (Macklin et al., 1998; Bruggmann et al., 2002). Collectively, these data support the view that intracellular Na+ loading may underpin Na+-K+-ATPase stimulation by ACh in rat aortic endothelium.
In conclusion, the results obtained demonstrate that Na+-K+-ATPase inhibition either by ouabain or withdrawal of external K+ attenuates the sustained component of endothelial hyperpolarization to ACh in rat aorta. These data indicate that Na+-K+-ATPase stimulation is at least partially involved in the ACh-induced hyperpolarization of endothelial cells. The precise mechanisms underlying this observation cannot be deducted from the present study, but may involve Na+ loading into endothelial cells. Because a maintained hyperpolarization of endothelial cells is of primary importance in the smooth muscle hyperpolarization and relaxation, the results obtained suggest that suppression of endothelium-dependent relaxation by inhibitors of the Na+-K+-ATPase may be attained not only by their action on the smooth muscle Na+-K+-ATPase, but also on the endothelial Na+-K+-ATPase. Therefore, the physiological role of the Na+-K+-ATPase in endothelial cells may not only encompass a maintenance of electrochemical gradient for Na+ and K+ and contribution towards the resting membrane potential, but also a fine tuning of the electrochemical gradient for Ca2+ during stimulation with ACh.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- EDHF
endothelium-derived hyperpolarizing factor
- EGTA
ethyleneglycol tetraacetate
- HEPES
4-(2-hydroxyethyl)-1-piperazineethyl-sulphonic acid
- [Na+]i
intracellular Na+ concentration
- L-NMMA-
NG-monomethyl-L-arginine monoacetate
- NO
nitric oxide
Conflict of interest
The authors state no conflict of interest.
References
- Blanco G, Mercer RW. Isozymes of the Na,K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bondarenko A. Sodium–calcium exchanger contributes to membrane hyperpolarization of intact endothelial cells from rat aorta during acetylcholine stimulation. Br J Pharmacol. 2004;143:9–18. doi: 10.1038/sj.bjp.0705866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K, Quast U. Differences in the K+-channels opened by cromakalim, acetylcholine and substance P in rat aorta and porcine coronary artery. Br J Pharmacol. 1991;102:585–594. doi: 10.1111/j.1476-5381.1991.tb12217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990;259:H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Bruggmann D, Lips KS, Pfeil U, Haberberger R, Kummer W. Multiple nicotinic acetylcholine receptor α-subunits are expressed in the arterial system of the rat. Histochem Cell Biol. 2002;118:441–447. doi: 10.1007/s00418-002-0475-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Cheung DW. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992;70:257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- Domotor E, Abbott NJ, Adam-Vizi V. Na+-Ca2+ exchange and its implications for calcium homeostasis in primary cultured rat brain microvascular endothelial cells. J Physiol. 1999;515:147–155. doi: 10.1111/j.1469-7793.1999.147ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XH, Komiyama Y, Nishimura N, Masuda M, Takahashi H. Nanomolar level of ouabain increases intracellular calcium to produce nitric oxide in rat aortic endothelial cells. Clin Exp Pharmacol Physiol. 2004;31:276–283. doi: 10.1111/j.1440-1681.2004.03995.x. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Marin J, Encabo A, Alonso MJ, Balfagon G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen Pharmacol. 1999;33:35–41. doi: 10.1016/s0306-3623(98)00259-6. [DOI] [PubMed] [Google Scholar]
- Fleming I, Hecker M, Busse R. Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ Res. 1994;74:1220–1226. doi: 10.1161/01.res.74.6.1220. [DOI] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruwel ML, Alves C, Schrader J. Na+-K+-ATPase in endothelial cell energetics: 23Na nuclear magnetic resonance and calorimetry study. Am J Physiol. 1995;268:H351–H358. doi: 10.1152/ajpheart.1995.268.1.H351. [DOI] [PubMed] [Google Scholar]
- Gupta S, McArthur C, Grady C, Ruderman NB. Stimulation of vascular Na+-K+-ATPase activity by nitric oxide: a cGMP-independent effect. Am J Physiol. 1994;266:H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- Hirano S, Agata N, Hara Y, Iguchi H, Shirai M, Tone H, et al. A possible mechanism of endothelium-dependent relaxation induced by pirarubicin and carbachol in rat isolated aorta. J Pharm Pharmacol. 1992;44:244–249. doi: 10.1111/j.2042-7158.1992.tb03591.x. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kanmura Y, Kuriyama H. A23187 increases calcium permeability of store sites more then of surface membranes in the rabbit mesenteric artery. J Physiol. 1985;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Dusting GJ. Endothelium-dependent vasorelaxation independent of nitric oxide and K+ release in isolated renal arteries of rats. Br J Pharmacol. 2001;132:1558–1564. doi: 10.1038/sj.bjp.0703965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Nuttall AL, Zhao H, Dai CF, Guan BC, Si JQ, et al. Electrical coupling and release of K+ from endothelial cells co-mediate ACh-induced smooth muscle hyperpolarization in guinea-pig inner ear artery. J Physiol. 2005;564:475–487. doi: 10.1113/jphysiol.2004.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Seol GH, Liang GH, Kin JA, Suh SH. Na+-K+ pump activation inhibits endothelium-dependent relaxation by activating the forward mode of Na+/Ca2+ exchanger in mouse aorta. Am J Physiol. 2005;289:H2020–H2029. doi: 10.1152/ajpheart.00908.2004. [DOI] [PubMed] [Google Scholar]
- Laskey RE, Adams DJ, Johns A, Rubanyi GM, Van Breemen C. Membrane potential and Na+-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990;265:2613–2619. [PubMed] [Google Scholar]
- Lee YH, Ahn DS, Song HJ, Kim YH, Kim HS, Ahn SH, et al. Effects of Na+, K+-pump inhibitors on acetylcholine-induced relaxation in the rabbit aorta. Yonsei Med J. 1992;33:8–13. doi: 10.3349/ymj.1992.33.1.8. [DOI] [PubMed] [Google Scholar]
- Li L, Van Breemen C. Na+-Ca2+ exchange in intact endothelium of rabbit cardiac valve. Circ Res. 1995;76:396–404. doi: 10.1161/01.res.76.3.396. [DOI] [PubMed] [Google Scholar]
- Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- Marin J, Redondo J. Vascular sodium pump: endothelial modulation and alterations in some pathological processes and aging. Pharmacol and Ther. 1999;84:249–271. doi: 10.1016/s0163-7258(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:415–459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Oike M, Droogmans G, Casteels R, Nilius B. Electrogenic Na+-K+ transport in human endothelial cells. Pflug Arch. 1993;424:301–307. doi: 10.1007/BF00384356. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H, Hargittai PT, Lieberman EM, Mustafa SJ. Effect of ouabain on adenosine receptor-mediated hyperpolarization in porcine coronary artery smooth muscle. Eur J Pharmacol. 1997;322:185–190. doi: 10.1016/s0014-2999(96)00992-2. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Feletou M, Thollon C, Vilaine JP, Duhault J, Vanhoutte PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br J Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport RM, Schwartz K, Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985;110:203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Sage SO, Van Breemen C, Cannell MB. Sodium–calcium exchange in cultured bovine pulmonary artery endothelial cells. J Physiol. 1991;440:569–580. doi: 10.1113/jphysiol.1991.sp018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake N, Shibata M, Shibata S. The involvement of KCa, KATP and KV channels in vasorelaxing responses to acetylcholine in rat aortic rings. Gen Pharmacol. 1997;28:453–457. doi: 10.1016/s0306-3623(96)00238-8. [DOI] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Hattori Y, Fukao M, Watanabe H, Akaishi Y, Sato A, et al. Role of endothelial Ni2+-sensitive Ca2+ entry pathway in regulation of EDHF in porcine coronary artery. Am J Physiol. 2001;280:H730–H737. doi: 10.1152/ajpheart.2001.280.2.H730. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Koller A. Increase in endothelial Ca2+ activate KCa channels and elicit EDHF-type arteriolar dilation via gap junctions. Am J Physiol. 2002;282:H1760–H1767. doi: 10.1152/ajpheart.00676.2001. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Marchenko SM, Sage SO. Cytosolic calcium concentration in resting and stimulated endothelium of excised intact rat aorta. J Physiol. 1995;489:309–317. doi: 10.1113/jphysiol.1995.sp021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Voorde J, Vanheel B. EDHF-mediated relaxation in rat gastric small arteries: influence of ouabain/Ba2+ and relation to potassium ions. J Cardiovasc Pharmacol. 2000;35:543–548. doi: 10.1097/00005344-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Vanheel B, Van De Voorde J, Leusen I. Contribution of nitric oxide to the endothelium-dependent hyperpolarization in rat aorta. J Physiol. 1994;475:277–284. doi: 10.1113/jphysiol.1994.sp020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AH, Richards GR, Burnham MP, Feletou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol. 2002;136:918–926. doi: 10.1038/sj.bjp.0704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. Hyperpolarisation of rat mesenteric endothelial cells by ATP-sensitive K+ channel openers. Eur J Pharmacol. 2000;397:279–290. doi: 10.1016/s0014-2999(00)00271-5. [DOI] [PubMed] [Google Scholar]
- Zahler R, Sun W, Ardito T, Kashgarian M. Na-K-ATPase α-isoform expression in heart and vascular endothelia: cellular and developmental regulation. Am J Physiol. 1996;270:C361–C371. doi: 10.1152/ajpcell.1996.270.1.C361. [DOI] [PubMed] [Google Scholar]