Abstract
Background and purpose:
Although inorganic arsenite (AsIII) is toxic in humans, it has recently emerged as an effective chemotherapeutic agent for acute promyelocytic leukemia (APL). In humans and most animals, AsIII is enzymatically methylated in the liver to weakly toxic dimethylarsinic acid (DMAsV) that is a major pentavalent methylarsenic metabolite. Recent reports have indicated that trivalent methylarsenicals are produced through methylation of AsIII and participate in arsenic poisoning. Trivalent methylarsenicals may be generated as arsenical–glutathione conjugates, such as dimethylarsinous glutathione (DMAsIIIG), during the methylation process. However, less information is available on the cytotoxicity of DMAsIIIG.
Experimental approach:
We synthesized and purified DMAsIIIG using high performance TLC (HPTLC) methods and measured its cytotoxicity in rat liver cell line (TRL 1215 cells).
Key results:
DMAsIIIG was highly cytotoxic in TRL 1215 cells with a LC50 of 160 nM. We also found that DMAsIIIG molecule itself was not transported efficiently into the cells and was not cytotoxic; however it readily became strongly cytotoxic by dissociating into trivalent dimethylarsenicals and glutathione (GSH). The addition of GSH in micromolar physiological concentrations prevented the breakdown of DMAsIIIG, and the DMAsIIIG-induced cytotoxicity. Physiological concentrations of normal human serum (HS), human serum albumin (HSA), and human red blood cells (HRBC) also reduced both the cytotoxicity and cellular arsenic uptake induced by exposure to DMAsIIIG.
Conclusions and implications:
These findings suggest that the significant cytotoxicity induced by DMAsIIIG may not be seen in healthy humans, even if DMAsIIIG is formed in the body from AsIII.
Keywords: arsenic, arsenite, dimethylarsenic, dimethylarsinous glutathione, dimethylarsinic acid, trivalent methylarsenic, trivalent dimethylarsenic, GSH, methylation, acute promyelocytic leukemia
Introduction
Arsenic is a metalloid that is widely distributed in the environment in inorganic trivalent (inorganic arsenite; AsIII) or pentavalent (inorganic arsenate; AsV) forms (Morton and Dunnette, 1994), and its high toxicity is well known. Epidemiological studies have provided clear evidence that inorganic arsenicals are human carcinogens (NRC, 1999); however, AsIII has recently emerged as an outstanding chemotherapeutic agent with remarkable efficacy for certain human cancers such as acute promyelocytic leukemia (APL) (Chen et al., 1997; Shen et al., 1997). In humans and numerous experimental animals, AsIII is enzymatically methylated in the liver to organic arsenicals such as monomethylarsonic acid (MMAsV) and dimethylarsinic acid (DMAsV) (Yamauchi and Yamamura, 1979; Buchet et al., 1980). MMAsV and DMAsV are the major organic pentavalent arsenic metabolites found in human urine after exposure to inorganic arsenicals. It is believed that the methylation of inorganic arsenicals results in a reduction in general toxicity, as indicated by increases in the values for LD50 and the in vitro lethal concentration in 50% of a population (LC50) (Sakurai et al., 1998, 2002). However, recent studies have increasingly suggested that the methylation of inorganic arsenicals is not a universal detoxification metabolic reaction. Wang et al. (2004) reported that trivalent dimethylarsenicals were found in the urine collected from APL patients undergoing AsIII treatment. The synthetic trivalent dimethylarsenicals, such as iododimethylarsine (DMAsIIII), were more cytotoxic in vitro than inorganic arsenicals and pentavalent methylarsenicals (Styblo et al., 2000; Mass et al., 2001). Recent evidence has suggested that trivalent methylarsenicals may be generated as arsenical–glutathione (GSH) conjugates, such as dimethylarsinous glutathione (DMAsIIIG), in the human body (Hayakawa et al., 2005); however, due to its instability, little information is available on the cytotoxicity of DMAsIIIG (Styblo et al., 2000; Hayakawa et al., 2005).
In this study, we synthesized and purified DMAsIIIG using a high-performance TLC (HPTLC) method and observed the cytotoxicity of the synthesized DMAsIIIG using rat liver cells. The liver is a major site of arsenic methylation. We found that DMAsIIIG itself was not transported efficiently into the cells and was not cytotoxic; however, it readily became highly cytotoxic in a neutral solution by a chemical conversion to the trivalent dimethylarsenite ion (DMAsIII+) and/or dimethylarsinous acid (DMAsIIIOH). The addition of GSH in micromolar physiological concentrations inhibited this chemical change and prevented the cytotoxicity of trivalent dimethylarsenicals.
Methods
Cell culture
The TRL 1215 cell line is a rat epithelial liver cell line originally derived from the liver of 10-day-old Fisher F344 rats (Idoine et al., 1976). TRL 1215 cells were cultured in William's E medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U ml−1 penicillin G and 100 μg ml−1 streptomycin under a humidified atmosphere of 5% CO2/95% air at 37°C.
Arsenic analysis
Preparation of arsenic samples
Arsenicals in samples from HPTLC or cellular arsenicals in TRL 1215 cells exposed to arsenicals were analyzed by atomic absorption spectrophotometry (AAS), HPLC-inductively coupled argon plasma (ICP) MS (HPLC-ICP MS) or fast atom bombardment MS (FAB-MS). TRL 1215 cells which were grown in flat-bottomed 75-cm2 tissue culture flask to confluence (8 × 107 cells/flask) and incubated with arsenicals were isolated by trypsinization and resuspended in 1 ml of ice-cold 0.25 M sucrose solution that included 50 mM Tris-HCl (pH 7.5), 10 mM potassium chloride, 5 mM magnesium chloride, 1 mM dithiothreitol and 1 mM phenylmethanesulfonyl fluoride, and sonicated on ice using MICROSON ultrasonic homogenizer (Model XL2007, Misonix Inc., Farmingdale, NY, USA). The degree of cell rupture was checked by phase-contrast microscopy and 95% cell rupture was sufficient. The suspension of ruptured cells was centrifuged at 2000 g for 10 min, and divided into the supernatant (cytoplasm) and the pellet (cell membrane and nucleus). The pellet was suspended again in 1 ml of ice-cold 0.25 M sucrose solution, placed on ice-cold 1 M sucrose and centrifuged at 1500 g for 10 min. The cell membrane at the 0.25 M sucrose/1 M sucrose interface was collected with a pipette, rinsed with 0.25 M sucrose solution and resuspended in 1 ml of distilled water. Three milliliters of nitric acid and 1 ml of sulfuric acid were then added to the solutions of cytoplasm or cell membrane, heated at 240°C until sulfur trioxide was visible, and the digested solutions were neutralized with ammonium hydroxide.
AAS
The aqueous solutions containing arsenicals prepared from HPTLC samples or TRL 1215 cells exposed to arsenicals were made in a volume of 8 ml with distilled water, and 1 ml of hydrochloric acid, 0.5 ml of 20% ascorbic acid and 0.5 ml of 20% potassium iodide were added to the solutions. Arsenic in the solutions was analyzed by hydride generation coupled with AAS (Ohta et al., 2004; Sakurai et al., 2005) using SpestraAA-220 (Varian Australia Pty Ltd, Mulgrave, Victoria, Australia). The cellular arsenic content was expressed as nanograms of arsenicals per milligram of cellular protein, as determined by BCA protein assay (Pierce Co., Rockford, IL, USA), or expressed as nM calculated using total cell numbers and estimated cell density (2.4 × 10−9 cm3/cell) as measured using a hemacytometer counting chamber (Mizoguchi and Hara, 1996; Sakurai et al., 2005).
HPLC-ICP MS
Arsenicals extracted from TLC were also analyzed by HPLC-ICP MS and FAB-MS. For HPLC-ICP MS analysis (Shraim et al., 2001; Hayakawa et al., 2005; Sakurai et al., 2005), 10 μl of a sample solution was applied to a reversed-phase C18 column (Inertsil ODS; GL Sciences Inc., Tokyo, Japan) connected to an L-6200 Inteligent pump (Hitachi Ltd, Ibaraki, Japan) with a mobile phase of 5 mM tetrabutylammonium hydroxide, 3 mM malonic acid and 5% methanol at flow rate of 1 ml min−1. The outlet of the HPLC system was coupled directly to the inlet of the ICP MS (ELAN 6000, Perkin-Elmer Co., Norwalk, CT, USA), and signals at m/z 75 and 77 (corresponding to arsenicals and ArCl, respectively) were monitored.
Assay for cytotoxicity
TRL 1215 cells were plated on flat-bottomed 96-well tissue culture plates (1 × 104 cells per 200 μl per well) and allowed to adhere to the plate for 24 h at 37°C, at which time the medium was removed and replaced with fresh medium containing the various test compounds, including arsenicals. Cells were then incubated with test compounds for an additional 1–48 h at 37°C. After incubation, cells were washed twice with warmed phosphate-buffered saline (pH 7.4) to remove nonadherent dead cells, and cell viability was determined by the AlamarBlue assay. The AlamarBlue assay is similar to 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT assay and measures the metabolic integrity of cells (Ohta et al., 2004; Sakurai et al., 2005). Briefly, after incubations with test samples and replacement with 200 μl per well fresh media, 20 μl per well AlamarBlue solution (Iwaki Grass Co., Chiba, Japan) was added directly to the 96-well plates, incubated for 4 h at 37°C, and the absorbance at 570 nm (referenced to 630 nm) was measured by a microplate reader model 550 (Bio-Rado Laboratories, Hercules, CA, USA). Data are expressed as percent metabolic integrity using the values from control cells as 100%. Similar results were obtained when cytotoxicity of arsenicals was determined by the counting of viable cells under microscopy (data not shown).
Statistics
The data represent the mean±s.e.m. of three or more determinations. Statistical evaluations where appropriate were carried out with analysis of variance (ANOVA) followed by Dunnett's multiple comparison test or Student's t-test. A value of P<0.05 was considered significant in all cases.
Reagents
Sodium arsenite (AsIII) and sodium arsenate (AsV) were purchased from Sigma Chemical Co. (St Louis, MO, USA). DMAsV sodium salt was purchased from Calbiochem Biosciences Inc. (La Jolla, CA, USA). MMAsV was purchased from Tri Chemical Co. (Yamanashi, Japan). These purchased arsenicals were recrystallized twice, and their purities were >99.9% as determined by gas chromatography/mass spectrometry (GC/MS) (Sakurai et al., 2004a). Endotoxin contamination of these arsenicals was not detected (<0.0000003%, wt/wt) using the endotoxin-specific limulus test (Seikagaku Co., Tokyo, Japan). GSH, oxidized form of GSH (GSSG), cysteine, N-acetyl-L-cysteine (NAC), human serum albumin (HSA; >99.0%) were purchased from Sigma. Bovine serum albumin (BSA) (>98.0%) was purchased from Wako Pure Chemical Industries Ltd (Osaka, Japan). Human serum (HS) was purchased from Chemicon International Inc. (Temecula, CA, USA). FBS was purchased from Thermo Electron Co. (Melbourne, Australia).
DMAsIIIG was synthesized from DMAsV and GSH by incubating in distilled water for 1 h at 37°C (Scott et al., 1993; Kala et al., 2000), and separated by HPTLC method (see details in Results section). HPTLC was performed on 0.1 mm precoated silica gel HPTLC plates (Merck KgaA, Darmstadt, Germany) with a developing solvent of ethyl acetate:acetic acid:water (3:2:1), and iodine vapor was used for the visual detection of DMAsIIIG (Sakurai et al., 2002). Separated DMAsIIIG was collected from the HPTLC plate with silica gel and stored at −85°C. DMAsIIIG was extracted by ice-cold distilled water before experimental use and centrifuged at 20 000 g for 5 min at 4°C to remove silica gel. Supernatants were then filtered through 0.20 μm filters and used as an aqueous solution of DMAsIIIG.
Results
Preparation of DMAsIIIG by HPTLC
It has been reported that GSH nonenzymatically reduces DMAsV to DMAsIIIG in water (Scott et al., 1993; Kala et al., 2000). In order to determine the likelihood of DMAsIIIG production, 10 mM DMAsV was incubated with or without 10–50 mM GSH in distilled water for 1 h at 37°C. After incubation, these mixtures were applied to an HPTLC plate and developed using ethyl acetate:acetic acid:water (3:2:1). Separated compounds were detected with iodine vapor. As shown in Figure 1a, GSH (lane 1, relative mobility (Rf)=0.33) and GSSG (lane 2, Rf=0.06) spots were detected with iodine vapor; however, DMAsV was not detected under these experimental conditions (lane 3). A spot of the putative DMAs-GSH conjugate DMAsIIIG was detected with iodine vapor at a different position from the GSH and GSSG spots (Figure 1a, lanes 4–12, Rf=0.49) after incubating DMAsV with GSH. Moreover, arsenical was only detected from this putative DMAsIIIG spot on the HPTLC plate as determined by AAS (Figure 1b). The spot density of the putative DMAsIIIG was dependent on the mixed GSH concentrations (lanes 4–12), and the spot density of the remaining GSH markedly increased when DMAsV was incubated with GSH at GSH:DMAsV molar ratios greater than 3 (Figures 1a and c). Similar results were observed when DMAsV and GSH were reacted in phosphate buffer (pH=7.4) for 1 h at 37°C (data not shown). A putative DMAsIIIG was extracted from an HPTLC sample (Figure 1a, lanes 4–12) by using distilled water, applied to another HPTLC plate, and separated. As shown in Figure 1d, a spot of the extracted DMAsIIIG (lane 5) appeared at the same position as that of the putative DMAsIIIG (lane 4) produced by the mixture of DMAsV and GSH. A GSSG spot was also detected when the extracted DMAsIIIG was separated on the HPTLC plate (lane 5).
Figure 1.
DMAsV readily combines with GSH in water. (a) DMAsV (10 mM) was incubated with or without 10–50 mM GSH in distilled water for 1 h at 37°C. After incubation, aliquots (5 μl) of these mixtures were spotted on a HPTLC plate and developed using ethyl acetate: acetic acid: water (3:2:1). Lane 1, 10 mM GSH; lane 2, 10 mM GSSG; lane 3, 10 mM DMAsV; lanes 4–12, 10 mM DMAsV plus 10 (lane 4), 15 (lane 5), 20 (lane 6), 25 (lane 7), 30 (lane 8), 35 (lane 9), 40 (lane 10), 45 (lane 11) or 50 mM (lane 12) GSH. Separated compounds were visually detected with iodine vapor. (b) The compounds separated in (a, lane 8) were extracted at every 1 cm length (area) from the development starting point by distilled water, and the arsenic content in each area was measured using AAS. Results are expressed as the arithmetic mean±s.e.m. of three separate experiments. (c) Changes in visible spot density for the remaining GSH in (a, lanes 4–12) are expressed as percentages of the spot density of 10 mM GSH alone (100%) (a, lane 1). (d) Purification of DMAsIIIG. Putative DMAsIIIG separated in (a, lane 8) was extracted from the HPTLC plates using distilled water and purified. Five *microliters of the 10 mM purified putative DMAsIIIG (arsenic content was measured by AAS) was then spotted onto a new HPTLC plate (d, lane 5); 5 μl of 10 mM GSH (d, lane 1), 10 mM GSSG (d, lane 2), 10 mM DMAsV (d, lane 3) and 10 mM DMAsV incubated with 30 mM GSH for 1 h at 37°C (d, lane 4) were also spotted. These compounds were developed using ethyl acetate:acetic acid:water (3:2:1) and visually detected with iodine vapor.
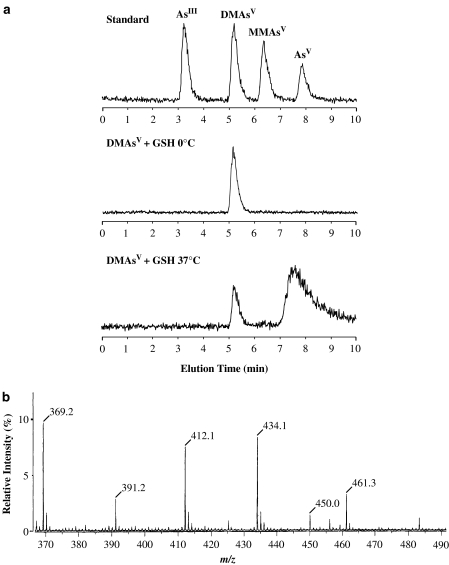
We subsequently identified the chemical species of the putative DMAsIIIG by HPTLC-ICP MS (Figure 2a) and FAB-MS (Figure 2b). As shown in Figure 2a, when 10 mM DMAsV was incubated with 30 mM GSH for 1 h at 0°C, the DMAsV was unchanged; however, when DMAsV was incubated with GSH for 1 h at 37°C, more than 85% of the DMAsV was converted to an unknown arsenic compound that is possibly DMAsIIIG. This reaction was very similar to those observed using the HPTLC method (Figure 1). We also analyzed the chemical form of the putative DMAsIIIG extracted from the HPTLC (Figure 1d, lane 4) by FAB-MS. As shown in Figure 2b, this was confirmed to be DMAsIIIG. These data imply that DMAsV combines with GSH under physiological conditions (pH=7.4, 37°C) at molar ratios of DMAsV:GSH=1:3 and is converted to DMAsIIIG that can be detected by iodine vapor on HPTLC plates.
Figure 2.
Arsenic analysis. (a) HPLC-ICP MS chromatograms of standard arsenic solutions (upper figure; arsenic concentrations are 10 ppb); 10 mM DMAsV incubated with 30 mM GSH in distilled water for 1 h at 0°C (middle figure) or 37°C (lower figure). (b) FAB-MS spectrum of purified DMAsIIIG. DMAsV (10 mM) was incubated with 30 mM GSH in distilled water for 1 h at 37°C. After incubation, aliquots of this mixture were spotted onto an HPTLC plate and developed using ethyl acetate:acetic acid:water (3:2:1). Separated DMAsIIIG was extracted using distilled water and purified. The FAB-MS of purified DMAsIIIG employed positive-ion mode and showed signals at m/z 412.1 [M+H]+, m/z 434.1 [M+Na]+ and m/z 450.0 [M+K]+. Other signals originating from the glycerol matrix were observed at m/z 369.2 [4 M+H]+, m/z 391.2 [4 M+Na]+ and m/z 461.3 [5 M+H]+.
Cytotoxicity of purified DMAsIIIG in TRL 1215 cells
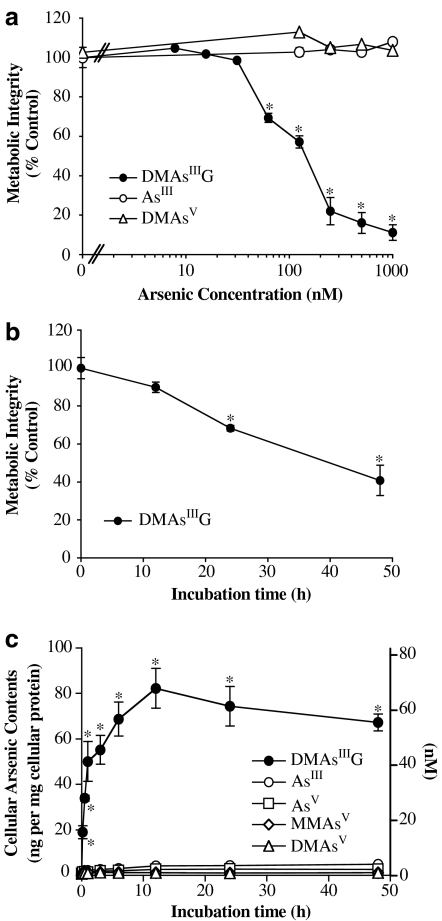
The cytotoxicity of the purified DMAsIIIG extracted from an HPTLC spot was compared with that of inorganic trivalent AsIII and pentavalent DMAsV using rat liver TRL 1215 cells. It was found that DMAsIIIG showed strong cytotoxicity at submicromolar levels; its LC50 value after exposure of TRL 1215 cells for 48 h was 160 nM (Figure 3a). When cells were exposed to 200 nM DMAsIIIG, a significant number of cells died after only 24 h exposure (Figure 3b). In contrast, AsIII and DMAsV exhibited no cytotoxicity up to concentrations of 1 μM and up to 48 h exposure (Figure 3a). The uptake of DMAsIIIG into TRL 1215 cells was also markedly higher than that of other inorganic and pentavalent methyl arsenicals such as AsIII, AsV, MMAsV, or DMAsV. As shown in Figure 3c, when cells were exposed to 200 nM DMAsIIIG for 48 h at 37°C, the cellular arsenic content increased quickly from 10 min and reached a peak at 12 h exposure, when cell death was still low (Figure 3b), of 82.3±9.0 ng mg−1 cellular protein (69.1±7.3 nM; n=3), as determined by AAS. This high cellular arsenic content was retained until 48 h. However, when cells were exposed to 200 nM AsIII or DMAsV for 48 h at 37°C, the cellular arsenic content was only 4.9±0.4 or 1.3±0.1 ng mg−1 cellular protein (4.1±0.4 or 1.1±0.1 nM; n=3), respectively.
Figure 3.
Cytotoxicity and cellular uptake of purified DMAsIIIG in TRL 1215 cells. (a) TRL 1215 cells were exposed to various concentrations of DMAsIIIG, AsIII or DMAsV for 48 h at 37°C, and cellular viability was then assessed by the AlamarBlue assay. (b) TRL 1215 cells were exposed to 200 nM DMAsIIIG for 0-48 h at 37°C, and cellular viability was assessed by the AlamarBlue assay. (c) TRL 1215 cells were exposed to 200 nM AsIII, AsV, MMAsV, DMAsV or DMAsIIIG for 0–48 h at 37°C, and time course of cellular arsenic contents were measured using AAS. Cellular arsenic contents are expressed as ng per mg cellular protein or nanomolar. All results are expressed as the arithmetic mean±s.e.m. of three separate experiments each performed in triplicate (n=9). *P<0.001, in comparison to the control cells incubated with the medium alone.
DMAsIIIG is an unstable chemical
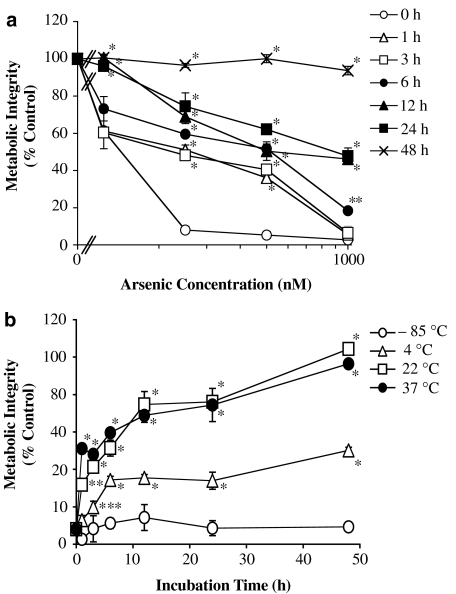
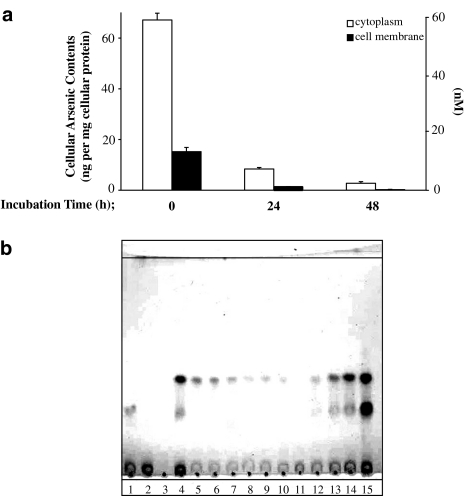
The purified DMAsIIIG extracted from HPTLC was preincubated in water for 1–48 h at 37°C, and its cytotoxicity was then observed. As shown in Figure 4a, preincubation reduced the cytotoxicity of DMAsIIIG in a time-dependent manner. Preincubation for 1 h at 37°C or more significantly reduced, and a 48 h preincubation completely abolished, the cytotoxicity induced by DMAsIIIG. As shown in Figure 4b, DMAsIIIG-induced cytotoxicity was significantly reduced by preincubation in water at a temperature greater than 4°C; however, the cytotoxicity was retained after a preincubation at −85°C. Figure 5 shows that preincubation of DMAsIIIG in water for 24 or 48 h at 37°C also reduced its cellular uptake into TRL 1215 cells. Most arsenicals accumulated in the cytoplasm of the cells. As shown in Figure 5b, a DMAsIIIG HPTLC spot also disappeared after preincubation in water at 37°C in a time-dependent manner. However, the addition of GSH prevented this and a visible DMAsIIIG HPTLC spot was retained in a GSH concentration-dependent manner even after a 48 h preincubation at 37°C (lanes 12–15; similar results are also shown in Figure 8, lanes 7 and 8).
Figure 4.
DMAsIIIG is an unstable chemical; DMAsIIIG-induced cytotoxicity was reduced by preincubation. (a) DMAsIIIG alone was preincubated in distilled water for 0–48 h at 37°C. TRL 1215 cells were exposed to various concentrations of these preincubated DMAsIIIG for 48 h at 37°C, and cellular viability was then assessed by the AlamarBlue assay. (b) DMAsIIIG was preincubated in distilled water for 0–48 h at −85, 4, 22 or 37°C. TRL 1215 cells were exposed to 500 nM of these preincubated DMAsIIIG for 48 h at 37°C, and cellular viability was then assessed by the AlamarBlue assay. All results are expressed as the arithmetic mean±s.e.m. of three separate experiments each performed in triplicate (n=9). *P<0.001, in comparison to the cells exposed to the same concentrations of DMAsIIIG without preincubation, **P<0.01, ***P<0.05.
Figure 5.
DMAsIIIG is an unstable chemical. (a) Cellular uptake of DMAsIIIG was reduced by preincubation. DMAsIIIG was preincubated in distilled water for 24 or 48 h at 37°C. TRL 1215 cells were exposed to 200 nM of these preincubated DMAsIIIG for 48 h at 37°C. After exposure, cellular arsenic contents were measured using AAS and are expressed as ng per mg cellular protein or nanomolar. Results are expressed as the arithmetic mean±s.e.m of three separate experiments each performed in triplicate (n=9). (b) The chemical form of DMAsIIIG was altered by preincubation. 10 mM DMAsIIIG was preincubated with or without 1–30 mM GSH in distilled water for 0–48 h at 37°C. After preincubation, aliquots (5 μl) of these mixtures were spotted on an HPTLC plate that was developed using ethyl acetate:acetic acid:water (3:2:1), and the separated spots were detected with iodine vapor. Lane 1, 10 mM GSH; lane 2, 10 mM GSSG; lane 3, 10 mM DMAsV; lane 4, 10 mM DMAsV incubated with 30 mM GSH for 1 h at 37°C; lane 5, 10 mM DMAsIIIG; lanes 6–11, 10 mM DMAsIIIG alone preincubated in distilled water for 1 (lane 6), 3 (lane 7), 6 (lane 8), 12 (lane 9), 24 (lane 10) or 48 h (lane 11) at 37°C; lanes 12–15, 10 mM DMAsIIIG preincubated in distilled water with 1 (lane 12), 3 (lane 13), 10 (lane 14) or 30 mM (lane 15) GSH for 48 h at 37°C.
Figure 8.
Effects of GSH, HS or HSA on the chemical form of DMAsIIIG. DMAsIIIG (10 mM) was incubated with or without 10 mM GSH, 50% HS, or 50 g l−1 HSA for 6 or 48 h at 37°C. After incubation, aliquots (5 μl) of these mixtures were spotted onto an HPTLC plate that was developed using ethyl acetate:acetic acid:water (3:2:1), and the separated spots were detected with iodine vapor. Lane 1, 10 mM GSH; lane 2, 10 mM GSSG; lane 3, 10 mM DMAsV; lane 4, 10 mM DMAsIIIG; lanes 5–6, 10 mM DMAsIIIG preincubated in distilled water for 6 (lane 5) or 48 h (lane 6) at 37°C; lanes 7–8, 10 mM DMAsIIIG incubated with 10 mM GSH for 6 h (lane 7) or 48 h (lane 8) at 37°C; lane 9, 50% HS; lanes 10–11, 10 mM DMAsIIIG incubated with 50% HS for 6 h (lane 10) or 48 h (lane 11) at 37°C; lane 12, 50 g l−1 HSA; lanes 13–14, 10 mM DMAsIIIG incubated with 50 g l−1 HSA for 6 h (lane 13) or 48 h (lane 14) at 37°C.
Exogenous GSH prevented DMAsIIIG-induced cytotoxicity by reducing cellular uptake of DMAsIIIG
Figure 6 shows that exogenous GSH prevented DMAsIIIG-induced cytotoxicity in TRL 1215 cells, either at millimolar concentrations (Figure 6a) or at 20 μM, GSH significantly inhibited cytotoxicity induced by 100 nM DMAsIIIG (Figure 6b). Similar effects were observed with other thiol reagents, such as cysteine and N-acetyl-L-cysteine (NAC) (Figure 6a), but was not observed with GSSG (data not shown). Figure 7 shows that exogenous GSH also inhibited the uptake of DMAsIIIG into TRL 1215 cells. When cells were incubated with 200 nM DMAsIIIG for 48 h at 37°C, exogenous GSH decreased the cellular uptake of DMAsIIIG in a dose-dependent manner, and 5 mM GSH resulted in almost complete inhibition (Figures 7a and b). In contrast, exogenous GSH did not affect the cellular uptake of other arsenic compounds, such as AsIII and DMAsV, at any concentration (Figure 7a).
Figure 6.
Effects of GSH, cysteine or NAC on DMAsIIIG-induced cytotoxicity. (a) TRL 1215 cells were exposed to various concentrations of DMAsIIIG for 48 h at 37°C in the presence or absence of 5 mM GSH, Cys or NAC, and cellular viability was then assessed by the AlamarBlue assay. (b) TRL 1215 cells were exposed to 100 nM or 1 μM DMAsIIIG for 48 h at 37°C in the presence or absence of various concentrations of GSH, and cellular viability was then assessed. All results are expressed as the arithmetic mean±s.e.m. of three separate experiments each performed in triplicate (n=9). *P<0.001 in comparison to the control cells incubated with the medium alone, **P<0.05. †P<0.001 compared with the cells exposed to the same concentrations of DMAsIIIG alone, ††P<0.01.
Figure 7.
Effect of GSH on cellular uptake of arsenicals. (a) TRL 1215 cells were exposed to 500 nM AsIII, 500 nM DMAsV or 200 nM DMAsIIIG for 48 h at 37°C in the presence or absence of 1 or 5 mM GSH, and the cellular arsenic contents were then measured by AAS. (b) TRL 1215 cells were exposed to 200 nM DMAsIIIG for 48 h at 37°C in the presence or absence of various concentrations of GSH, and cellular arsenic contents were then measured by AAS. Cellular arsenic contents are expressed as ng per mg cellular protein (a) or nanomolar (a and b). All results are expressed as the arithmetic mean±s.e.m. of three separate experiments each performed in triplicate (n=9). *P<0.001 in comparison to the cells exposed to 200 nM DMAsIIIG alone.
Serum, serum albumin and red blood cells prevented DMAsIIIG-induced cytotoxicity by reducing the cellular uptake of DMAsIIIG
Table 1 shows the effects of the addition of HS, HSA, or human red blood cells (HRBC) on DMAsIIIG-induced cytotoxicity in TRL 1215 cells. HS, HSA and HRBC significantly inhibited DMAsIIIG-induced cytotoxicity at concentrations similar to those found in vivo in normal human blood. These blood components also decreased the cellular uptake of DMAsIIIG into TRL 1215 cells. As shown in Figure 8, an HPTLC spot of DMAsIIIG disappeared following preincubation with either HS or HSA at 37°C for more than 6 h (lanes 9–14). Similar results were obtained by the addition of FBS and BSA (data not shown).
Table 1.
Effects of HS, HSA, and HRBC on the cytotoxicity (LC50) and cellular uptake of DMAsIIIG
| LC50 (nM) |
Cellular arsenic contents (nM) |
||
|---|---|---|---|
|
DMAsIIIG |
|||
| 100 nM | 200 nM | ||
| DMAsIIIG alone | 160.3±12.3 | 42.7±0.6 | 62.7±1.2 |
| DMAsIIIG+HS | 710.0±75.1* | 19.4±0.5* | 45.8±0.7* |
| DMAsIIIG+HSA | >1000* | 18.1±0.3* | 38.6±0.4* |
| DMAsIIIG+HRBC | 965.0±12.2* | 8.0±0.2* | 11.4±0.8* |
Abbreviations: DMAsIIIG, dimethylarsinous glutathione; HS, human serum; HSA, human serum albumin; HRBC, human red blood cells.
TRL1215 cells were exposed to DMAsIIIG in the presence or absence of HS (50%), HSA (50 g l−1) or HRBC (5 × 1012 cells l−1) for 48 h at 37°C. Cellular viability and cellular arsenic contents were then measured. Results are expressed as the arithmetic mean±s.e.m. of three separate experiments each performed in triplicate (n=9).
P<0.001, in comparison to the cells exposed to DMAsIIIG alone.
Discussion
Recently, an inorganic arsenical – AsIII – was found to be effective in inducing complete remission in patients with APL (Chen et al., 1997; Shen et al., 1997). Multiple AsIII injections (10 mg day−1) for at least 28 consecutive days are required to induce complete remission (Shen et al., 1997). Methylated arsenicals are formed from inorganic arsenicals by enzymatic methylation (Hindmarsh and McCurdy, 1986) and accumulate in the human body during AsIII treatment as a chemotherapeutic agent (Wang et al., 2004; Fukai et al., 2006). Arsenic intoxication also occurs widely through the consumption of contaminated well water or foods containing inorganic arsenicals (Morton and Dunnette, 1994; NRC, 1999); therefore, the accumulation of the metabolites of inorganic arsenicals is likely to occur. It was recently reported that toxic trivalent dimethylarsenic compounds might be produced through the methylation of inorganic arsenicals (Wang et al., 2004), and that the cytotoxicity of a synthetic trivalent dimethylarsenic compound – DMAsIIII – was higher than that of inorganic arsenicals (Styblo et al., 2000; Mass et al., 2001). Trivalent dimethylarsenical is thought to be generated as an arsenical-GSH conjugate – DMAsIIIG – in the human body. Hayakawa et al. (2005) recently reported that inorganic arsenicals were metabolized to DMAsIIIG in the cells by a putative arsenic methyltransferase – Cyt 19 (AS3MT) – and might be excreted from the cells in the human body as DMAsIIIG. However, the toxicity of DMAsIIIG has not been established. Here, we studied in detail the in vitro cytotoxicity of synthetic DMAsIIIG compared with those of AsIII and pentavalent DMAsV using rat liver TRL 1215 cells.
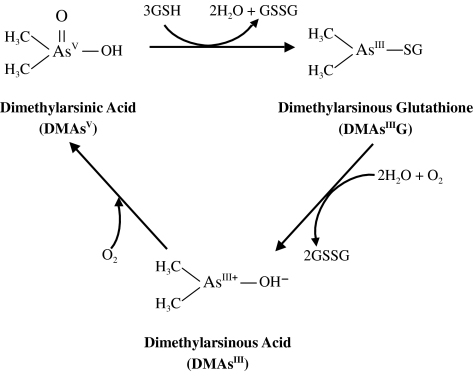
Scott et al. (1993) reported that DMAsV might combine with GSH in a molar ratio of DMAsV:GSH=1:3 and that DMAsIIIG was formed in vitro (Figure 9). In this study, we confirmed the formation of the DMAs-GSH conjugate DMAsIIIG during the incubation of 10 mM DMAsV with 30 mM GSH for 1 h at 37°C using HPLC-ICP MS and FAB-MS analysis. We readily purified this chemical by an HPTLC method and observed its in vitro cytotoxicity using rat liver TRL 1215 cells. Purified DMAsIIIG was much more cytotoxic than either AsIII or DMAsv; and its LC50 value was 160 nM. However, this action of purified DMAsIIIG was readily reduced by preincubation in water at temperatures greater than 4°C. The high cellular arsenic uptake of DMAsIIIG and a visible DMAsIIIG HPTLC spot were also greatly reduced by preincubation. Under our experimental conditions, only GSH and/or GSH-conjugated chemicals could be visually detected by iodine vapor on an HPTLC plate; however, a DMAsV spot could not be detected. Thus, the GSH molecule might readily be removed from DMAsIIIG during the preincubation period. The released DMAsIII+ might subsequently be converted in water to DMAsIIIOH and finally be oxidized to the weakly toxic pentavalent dimethylarsenic compound DMAsV (Figure 9).
Figure 9.
Putative chemical reactions of dimethylarsenic compounds with GSH.
The submicromolar LC50 values of DMAsIIIG determined in this study are very important in the elucidation of the actual role of the metabolic methylation of arsenicals in the human body, because other major human arsenic metabolites, including inorganic arsenicals, do not show cytotoxicity in mammalian cells at the submicromolar level (Sakurai et al., 1998, 2002). Additionally, it has been reported that the mean blood concentration of total arsenical was in the submicromolar range in relapsed APL patients, in whom complete remission had been induced by AsIII treatment (Fukai et al., 2006); similar levels were found in the blood of patients with chronic arsenic poisoning in Inner Mongolia, China and northeastern Taiwan who continued to drink well water containing high concentrations of inorganic arsenicals (Pi et al., 2000; Wu et al., 2003). Interestingly, exogenous thiol agents, such as GSH, cysteine and NAC, greatly decreased both the cytotoxicity and cellular arsenic contents induced by DMAsIIIG exposure. The addition of GSH inhibited the preincubation-induced disappearance of the visible DMAsIIIG spot on HPTLC plates. It is generally believed that the large GSH molecule is not transported efficiently into cells (De Flora et al., 2001). Therefore, these thiol agents may maintain the molecular form of DMAsIIIG so that DMAsIIIG itself cannot subsequently be transported into cells. The significant cytotoxicity induced by DMAsIIIG could be the result of the dissociation of the conjugate into trivalent dimethylarsenic compounds and GSH, the former probably becoming DMAsIII+ and/or DMAsIIIOH, before being transported into cells (Thompson, 1993; Dopp et al., 2004, 2005) (Figure 9). We showed in this study that a GSSG spot appeared when purified DMAsIIIG was applied and developed again on a new HPTLC plate, indicating that the GSH molecule readily dissociates from DMAsIIIG and is converted to oxidized GSH. The plasma GSH concentrations remain at micromolar levels in healthy humans (Donner et al., 1998; Kokcam and Naziroglu, 1999), and we showed in this study that exogenous GSH at micromolar levels prevented submicromolar DMAsIIIG-induced cytotoxicity. In addition, the physiological concentrations of normal HS, HSA and HRBC significantly reduced both the cytotoxicity and cellular arsenic contents induced by DMAsIIIG exposure. These findings suggest that the significant cytotoxicity of DMAsIIIG may never be manifest in humans even if DMAsIIIG is enzymatically formed from inorganic arsenicals in the human body. HS and HSA could not prevent the disappearance of a DMAsIIIG spot during the preincubation on HPTLC plates. The reason why serum and serum albumin inhibit the cytotoxicity of DMAsIIIG is unclear; however, it is suggested that they specifically reduce the cytotoxicity by combining protein thiols with the DMAsIII+ ion or by facilitating the oxidation of DMAsIII+ to DMAsV.
GSH may be a key molecule in preventing chronic arsenic toxicity. Clearly, if GSH levels are reduced, inorganic arsenicals are more toxic and induce complete necrosis in mammalian cells (Sakurai et al., 1998, 2002). GSH depletion causes an essentially minor, nontoxic, human monomethylarsenic metabolite – MMAsV – to become toxic (Sakurai et al., 2004a, 2005). DMAsV appears unable to induce apoptosis after GSH depletion (Sakurai et al., 1998, 2002, 2004b) that allows the survival of damaged abnormal cells. Furthermore, the strongly toxic DMAsIIIG may exert its cytotoxicity by dissociating into DMAsIII+ and GS− and by being transported into cells after depletion of blood GSH levels. GSH may be also a cofactor in the enzymatic methylation of inorganic arsenicals in humans (Zakharyan et al., 2001; Hayakawa et al., 2005). These findings suggest that GSH depletion may trigger the disruption of the arsenic detoxifying system and induce various arsenic toxicities in humans. In fact, reduced nonprotein sulfhydryl levels in peripheral blood, often seen as an indicator of GSH levels, were observed in chronic arsenic poisoning patients in Inner Mongolia, China (Pi et al., 2000). Further research will be required in order to determine the role of metabolic methylation and GSH in arsenic toxicity in APL patients who are injected with AsIII as a chemotherapeutic agent and/or in chronic arsenic poisoning in those who regularly ingest inorganic arsenic-contaminated well water.
Acknowledgments
We express our thanks to Ms Chihiro Kawata, Mr Kouichirou Matsuda, Ms Tomoe Sakoda and Mr Hiroki Soejima (Faculty of Pharmaceutical Sciences, Tokushima Bunri University) for their valuable technical assistance.
Abbreviations
- AAS
atomic absorption spectrophotometry
- APL
acute promyelocytic leukemia
- AsIII
inorganic arsenite
- AsV
inorganic arsenate
- DMAsIII+
trivalent dimethylarsenite ion
- DMAsV
dimethylarsinic acid
- DMAsIIIG
dimethylarsinous glutathione
- DMAsIIII
iododimethylarsine
- DMAsIIIOH
dimethylarsinous acid
- FAB
fast atom bombardment
- FBS
fetal bovine serum
- GC
gas chromatography
- GSH
glutathione
- GSSG
oxidized form of glutathione
- HPTLC
high-performance TLC
- HRBC
human red blood cells
- HS
human serum
- HSA
human serum albumin
- ICP
inductively coupled argon plasma
- LC50
in vitro lethal concentration in 50% of a population
- MMAsV
monomethylarsonic acid
- MS
mass spectrometry
- NAC
N-acetyl-L-cysteine
- Rf
relative mobility
Conflict of interest
The authors state no conflict of interest.
References
- Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1980;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- De Flora S, Izzotti A, D'Agostini F, Balansky RM. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- Donner MG, Klein GK, Mathes PB, Schwandt P, Richter WO. Plasma total homocysteine levels in patients with early-onset coronary heart disease and a low cardiovascular risk profile. Metaboism. 1998;47:273–279. doi: 10.1016/s0026-0495(98)90256-6. [DOI] [PubMed] [Google Scholar]
- Dopp E, Hartmann LM, Florea AM, von Recklinghauesn U, Pieper R, Shokouhi B, et al. Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicol Appl Pharmacol. 2004;201:156–165. doi: 10.1016/j.taap.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Dopp E, Hartmann LM, von Recklinghauesn U, Florea AM, Rabieh S, Zimmermann U, et al. Forced uptake of trivalent and pentavalent methylated and inorganic arsenic and its cyto-/genotoxicity in fibroblasts and hepatoma cells. Toxicol Sci. 2005;87:46–56. doi: 10.1093/toxsci/kfi218. [DOI] [PubMed] [Google Scholar]
- Fukai Y, Hirata M, Ueno M, Ichikawa N, Kobayashi H, Saitoh H, et al. Clinical pharmacokinetic study of arsenic trioxide in an acute promyelocytic leukemia (APL) patient: speciation of arsenic metabolites in serum and urine. Biol Pharm Bull. 2006;29:1022–1027. doi: 10.1248/bpb.29.1022. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- Hindmarsh JT, McCurdy F. Clinical and environmental aspects of arsenic toxicity. Crit Rev Clin Lab Sci. 1986;23:315–347. doi: 10.3109/10408368609167122. [DOI] [PubMed] [Google Scholar]
- Idoine JB, Elliott JM, Wilson MJ, Weisburger EK. Rat liver cells in culture: effect of storage, long-term culture, and transformation on some enzyme levels. In Vitro. 1976;12:541–553. doi: 10.1007/BF02797437. [DOI] [PubMed] [Google Scholar]
- Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, et al. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- Kokcam I, Naziroglu M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin Chim Acta. 1999;289:23–31. doi: 10.1016/s0009-8981(99)00150-3. [DOI] [PubMed] [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, et al. Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Hara S. Effect of fatty acid saturation in membrane lipid bilayers on simple diffusion in the presence of ethanol at high concentrations. J Ferment Bioeng. 1996;81:406–411. [Google Scholar]
- Morton WE, Dunnette DA.Health effects of environmental arsenic Arsenic in the Environment 1994John Wiley & Sons, New York; 17–34.In: Nriagu JO (ed)Vol. II [Google Scholar]
- NRC . Arsenic in the Drinking Water. National Research Council, National Academy Press, Washington, DC; 1999. [Google Scholar]
- Ohta T, Sakurai T, Fujiwata K. Effects of arsenobetaine, a major organic arsenic compound in seafood, on the maturation and functions of human peripheral blood monocytes, macrophages and dendritic cells. Appl Organomet Chem. 2004;18:431–437. [Google Scholar]
- Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, et al. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic Biol Med. 2000;28:1137–1142. doi: 10.1016/s0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Kaise T, Matsubara C. Inorganic and methylated arsenic compounds induce cell death in murine macrophages via different mechanisms. Chem Res Toxicol. 1998;11:273–283. doi: 10.1021/tx9701384. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Kojima C, Ochiai M, Ohta T, Sakurai MH, Waalkes MP, et al. Cellular glutathione prevents cytolethality of monomethylarsonic acid. Toxicol Appl Pharmacol. 2004a;195:129–141. doi: 10.1016/j.taap.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Ochiai M, Kojima C, Ohta T, Sakurai MH, Takada NO, et al. Role of glutathione in dimethylarsinic acid-induced apoptosis. Toxicol Appl Pharmacol. 2004b;198:354–365. doi: 10.1016/j.taap.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Ochiai M, Kojima C, Ohta T, Sakurai MH, Takada NO, et al. Preventive mechanism of cellular glutathione in monomethylarsonic acid-induced cytolethality. Toxicol Appl Pharmacol. 2005;206:54–65. doi: 10.1016/j.taap.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Qu W, Sakurai MH, Waalkes MP. A major human arsenic metabolite, dimethylarsinic acid, requires reduced glutathione to induce apoptosis. Chem Res Toxicol. 2002;15:629–637. doi: 10.1021/tx0101604. [DOI] [PubMed] [Google Scholar]
- Scott N, Hatlelid KM, Mackenzie NE, Carter DE. Reactions of arsenic (III) and arsenic (V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- Shraim A, Hirano S, Yamauchi H. Extraction and speciation of arsenic in hair using HPLC-ICP MS. Anal Sci. 2001;17 Suppl:i1729–i1732. [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, Lecluyse EL, Hamilton GA, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rats and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Thompson DJ. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–114. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou J, Lu X, Gong Z, Le XC. Arsenic speciation in urine from acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Chem Res Toxicol. 2004;17:95–103. doi: 10.1021/tx0341714. [DOI] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Ho IC, Chen CJ, Lee TC. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environ Health Perspect. 2003;111:1429–1438. doi: 10.1289/ehp.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Yamamura Y. Dynamic change of inorganic arsenic and methylarsenic compounds in human urine after oral intake as arsenic trioxide. Ind Health. 1979;17:79–83. [Google Scholar]
- Zakharyan RA, Sampayo-Reyes A, Healy SM, Tsaprallis G, Board PG, Liebler DC, et al. Human monomethylarsonic acid (MMAV) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol. 2001;14:1051–1057. doi: 10.1021/tx010052h. [DOI] [PubMed] [Google Scholar]